Abstract
Background
Collagen type VI alpha 3 chain (COL6A3) has been proven to be a biomarker in the occurrence and development of bladder cancer, which is the most common malignant tumor in the urinary system. This study aimed to explore the effect and molecular mechanism of COL6A3 on EMT in vitro induced by TGF-β/Smad in bladder carcinoma.
Material/Methods
There were 42 patients included in the Kaplan-Meier survival analysis. A cell counting kit-8 (CCK-8) assay and an angiogenesis assay were used to measure cell proliferation and tube formation, respectively. Western blot analysis and quantitative reverse transcription-polymerase chain reaction (qPCR) were conducted for the proteins and mRNAs expression.
Results
COL6A3 was highly expressed in tissues and cells of bladder cancer. COL6A3 silencing could inhibit the cell proliferation and angiopoiesis. In addition, COL6A3 silencing obviously suppressed the levels of matrix metalloproteinase-2 (MMP2), Matrix metalloproteinase-9 (MMP9), and vimentin. On the contrary, the levels of epithelium-specific cell-cell adhesion molecule (E-cadherin) and tumor inhibitor of metalloproteinase-1 (TIMP-1) were significantly increased. Furthermore, we found that COL6A3 silencing reduced the activity of p-Smad2, p-Smad3, and transforming growth factor β (TGF-β).
Conclusions
COL6A3 could influence the viability and angiogenesis of bladder cancer cells. COL6A3 may have a certain relationship with the TGF-β/Smad-induced EMT process.
MeSH Keywords: Collagen Type VI, Epithelial-Mesenchymal Transition, Gallbladder Neoplasms, Transforming Growth Factor beta
Background
Bladder cancer is the one of the most common tumors in the urinary system, and even in the whole body. For urogenital tumors, its incidence ranks first in China [1]. Muscle invasive bladder cancer which is treated through radical cystectomy and adjuvant chemotherapy, still cannot meet the demand of the quality of life and prognosis. The 5-year survival rate is only 45–66% [2]. Therefore, it is very necessary to define the specific target genes and discuss the molecular mechanism of bladder cancer.
It has been found that epithelial-mesenchymal transition (EMT) is regarded as the key initiation step in tumor progression, metastasis, and multidrug resistance [3,4]. In the EMT process, epithelial cells lose their epithelial properties (E-cadherin) while obtain mesenchymal character (such as N-cadherin and vimentin). E-cadherin maintains normal intercellular connections and maintains epithelial cell polarity by participating in the regulation of calcium dependent cell adhesion. The reduction or deletion of E-cadherin expression is a landmark characteristic of the epithelial transformation of the tumor cells [5]. Matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) are reported to play a vital role in the development of tumor [6,7]. Moreover, MMP2 and MMP9 are overexpressed in transitional cell carcinoma of the bladder [8], and MMP9 may improve the invasion of tumor cells by induction of EMT [9]. Recently, massive studies have proved that the EMT process was induced by TGF-β via multiple pathways [10–14].
TGF-β is an interesting cytokine. It has been reported to be associated with the phenotypic regulation of cancer and the change of tumor microenvironment [15]. Smad proteins can be phosphorylated and activated by TGF-β signals, and then are translocated into the nucleus to induce the transcriptional activation of downstream genes [16–18]. Aberrant TGF-β/Samd signaling may lead to tumor metastasis, EMT, and DNA damage response [19–21]. In bladder cancer, highly phosphorylated Smad2 (intermediate molecule in TGF-β signaling) is highly correlated with the recurrence of highly invasive bladder cancer and leads to a sharp decline in survival [22]. TGF-β signaling pathway is involved in all aspects of psychological and physiological processes, including the progression of bladder cancer [23,24]. Moreover, the level of TGF-β1 secretion is shown to be associated with the active phenotype of bladder cancer cell lines [25].
COL6A3, which is involved in tumor grade, participates in cell anchoring and remodeling of the extracellular matrix (ECM). Stromal COL6A3 is reported to be involved in Hippo and Wnt signaling pathway to affect the tumor growth [26]. In addition, COL6A3 is found to be significantly upregulated in pancreatic ductal adenocarcinoma (PDA) [27,28]. In total, these results indicated that COL6A3 might have a specific function in human cancer. However, its function and regulation mechanism in bladder cancer has not been investigated. The current study aimed to identify the function of COL6A3 in the bladder cancer cells and explore the relationship between COL6A3 and TGF-β/Smad-induced EMT.
Material and Methods
Patient tissues
We collected 42 cases of bladder cancer tissues which were surgically resected and pathologically confirmed. Each case selected as the experimental groups had tissue specimens from: normal bladder mucosa away from the tumor site 5 cm, adjacent cancer tissue 1 cm away from the cancer site, and cancer tissue center. Chemotherapy and immunotherapy were not accepted before operation. Each specimen was stored in the refrigerator at −80°C. Informed consents were acquired from each patient, and this study was performed with the agreement of the Ethics Committee of Liaoning Cancer Hospital and Institute.
Cell culture
The SV-40 immortalized human uroepithelial cell line (SV-HUC-1) was purchased from the American Type Culture Collection (ATCC, Wiltshire, USA). T24 and 5637, the human bladder cancer cell lines, were obtained from Chinese Academy of Sciences Cell Bank (Shanghai, China). T24 as well as SV-HUC-1 cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum (FBS) while 5637 cells were grown in (RPMI) 1640 medium with 10% FBS, and then cells were cultured in an incubator with 5% CO2 at 37°C. All reagents were from Thermo Fisher Scientific, Waltham, MA, USA.
Cell transfection
Cells were seeded in 6-well plates at a density of 4×105 cells per well. The medium was replaced by Opti-MEM (Invitrogen) after 24 hours of culture. And 2 μg plasmid was transfected according to the Lipofectamine 2000 protocol (Invitrogen, Grand Island, NY, USA). After incubation for 48 hours, the cells were used for further study. Small interference RNAs (COL6A3-siRNA) and empty vector were purchased from Genepharma (Shanghai, China).
Angiogenesis assays
Matrigel (BD Biosciences, San Jose, CA, USA) was placed in a 4°C refrigerator for 12 hours for liquefaction, and then was added to each well of a 24-well plate and solidified in an incubator for 30 min. The T24 cells transfected by COL6A3-siRNA and empty vector were cultured at a concentration of 4×104/well in triplicates with control group. After 12 hours of culturing, the results were observed.
Cell counting kit-8 (CCK-8) assay
Cell viability was detected by a cell counting kit-8 (CCK-8) (Beyotime, Shanghai, China) assays. Cells in each group were plated in 96-well plates at a density of 3000 cells/well, followed by incubation on the new media for 12, 24, and 48 hours, respectively. CCK-8 was added and incubated for 4 hours. The absorbance was measured at 450 nm, 3 times in each group.
Quantitative reverse transcription-polymerase chain reaction (qPCR)
Total RNA was isolated according to the manufacturer’s instructions (Thermo Fisher Scientific), followed by the detection of purity and concentration. The amplification reactions were performed using the ABI 7900 system (Applied Biosystems, Carlsbad, CA, USA) with the following conditions: 95°C for 30 sec, 40 cycles at 95°C for 5 sec, and 60°C for 1 min. All primers were compared with BLAST, which were presented in Table 1. The data were analyzed using the 2−ΔΔCt method. Each reaction was performed 3 times.
Table 1.
List of qPCR primers.
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| COL6A3 | TCTCTTAAAATCAGTGCACAACG | AACTCTTTCAACAGAGGGAAGC |
| MMP2 | ATGACAGCTGCACCACTGAG | GCCTCGTATACCGCATCAAT |
| MMP9 | TACCGAGAAAGCCTATT | CACCTGGTTCAACTCACT |
| TIMP-1 | TTCCGACCTCGTCATCAGGG | ATTCAGGCTATCTGGGACCGC |
| E-cadherin | AACGCATTGCCACATACAC | GAGCACCTTCCATGACAGAC |
| Vimentin | ACAGGCTTTAGCGAGTTATT | GGGCTCCTAGCGGTTTAG |
| GAPDH | GGTGAAGGTCGGAGTCAACGG | CCTGGAAGATGGTGATGGGATT |
Western blot
Tissues and cells were lysed in the lysis buffer (Beyotime, Beijing). Total proteins were transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA) after separation by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Membranes were probed with rabbit monoclonal antibodies against TGF-β, Smad2/3, phospho-Smad2/3 (p-Smad2/3), vimentin, E-cadherin, MMP2, MMP-9, TIMP-1 (1: 1,000), and COL6A3 (1: 200, Abcam, Cambridge, MA, USA), and anti-bodies against GAPDH was used as a control, then with goat anti-rabbit secondary antibody. Blots were detected using a Bio-Rad Bioimaging system (Bio-Rad, Hercules, CA, USA). All experiments were performed 3 times.
Statistical analysis
All data values are expressed as mean ± standard deviation, and statistical analyses were performed using the SPSS software. Statistical evaluations were analyzed using the Student’s t-test. Multiple group comparisons were performed by ANOVA. A value of P<0.05 had statistical significance.
Results
COL6A3 was overexpressed in tissues and cells of bladder cancer
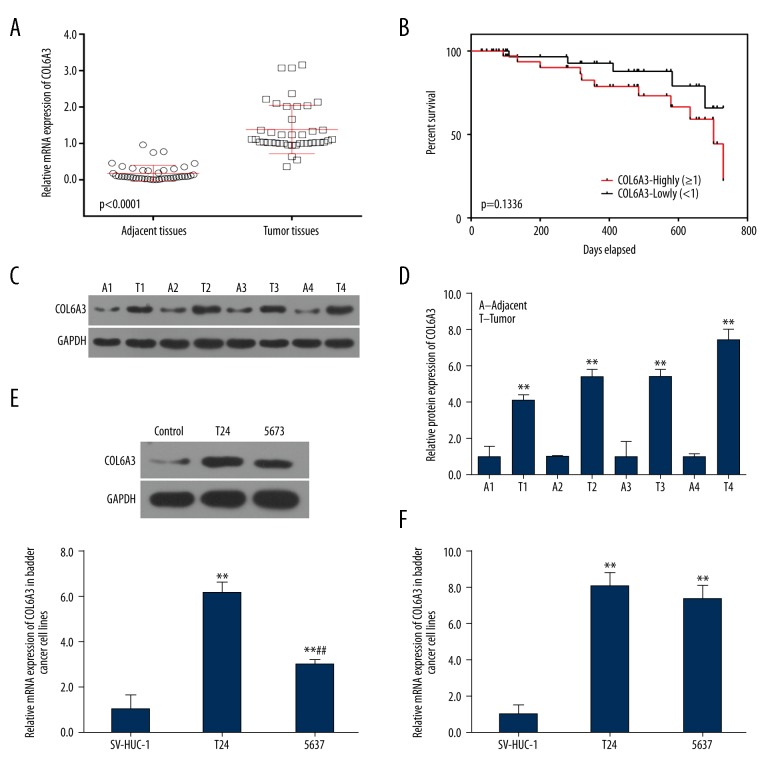
To ascertain the effect of COL6A3 on bladder cancer, the expression of COL6A3 in tumor tissues and their adjacent tissues was examined by quantitative reverse transcription-polymerase chain reaction (qPCR). The COL6A3 gene was higher expressed in the tumor tissues than that in the adjacent tissues (Figure 1A). Moreover, patients with high COL6A3 expression had low survival rate within 800 days after operation, while those with low COL6A3 expression showed opposite results. The results showed that COL6A3 was involved in cancer metastasis (P=0.1336, which might due to the insufficient sample size; Figure 1B). Furthermore, the expression of COL6A3 in 4 representative tumor tissues and their matched adjacent tissues were analyzed by western blot. And the result confirmed that COL6A3 was involved in bladder cancer (Figure 1C, 1D). The expression levels were also tested in T24 and 5637. The levels of protein expression in the T24 cell line were higher than that in the 5637 cell line (Figure 1E), and the trend of mRNA was the same (Figure 1F), indicating that T24 were the optimal cell lines for further study of bladder cancer. Given these results, COL6A3 was highly expressed in tissues and cells of bladder cancer.
Figure 1.
Expression of COL6A3 and survival situation. (A) Detection of the expression of COL6A3 in human bladder cancer tissues and adjacent tissues in 42 patients by qPCR. *** P<0.0001. (B) Using Kaplan-Meier method to show survival situation of patients with high or low level of COL6A3. The log-rank test was used to calculate the P value. Results were presented as mean ± SD from 3 independent experiments. (C) Western blot from 4 representative bladder cancer tumors (T1–T4) and adjacent tissues (A1–A4) showed expression of COL6A3 mainly in the tumors. (D) Protein expression of COL6A3 showed differential expression levels between individual tumors and showed higher levels in the tumor tissue than those in adjacent tissues. ** P<0.01 compared with adjacent tissues. (E) Representative of western blot showed that protein in T24 was higher than that in 5637. ** P<0.01 compared with SV-HUC-1, ## P<0.01 compared with T24. (F) Both cell lines expressed high levels of COL6A3 and T24 express higher levels of COL6A3. ** P<0.01 compared with SV-HUC-1, ## P<0.01 compared with T24.
Transfection with siRNA inhibit the expression of COL6A3 in bladder cancer cell line
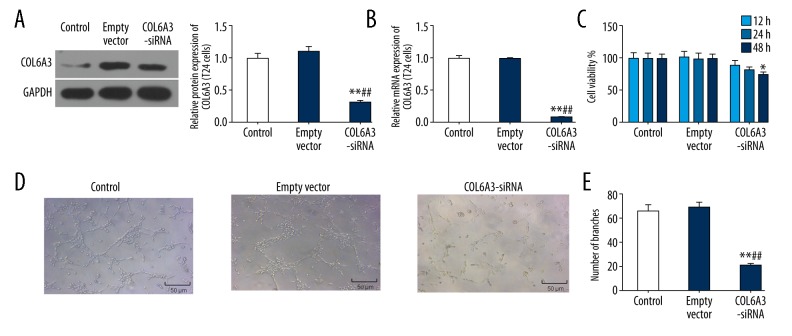
To explore the role of COL6A3 in bladder cancer, T24 cells were transfected with a COL6A3-siRNA and empty vector. The results demonstrated that the protein level of COL6A3 in COL6A3-siRNA group decreased to nearly 30% (P<0.01; Figure 2A) while the mRNA levels decreased to approximately 10% (P<0.01; Figure 2B), compared to cells without transfection.
Figure 2.
The role of COL6A3 in the biological behavior of bladder cancer cells. After transfection of T24 cells by COL6A3-siRNA, both the protein expression (A) and mRNA expression of COL6A3 (B) were significantly reduced. (C) Cell viability by CCK-8 assay. Interfered COL6A3 could significantly reduce the cell viability. (D) Angiogenesis showed that COL6A3 silencing could inhibit the formation of cable structures and reticular structures. (E) A significant decrease in the number of generated vessels when COL6A3 was downregulated conducted by angiogenesis experiment. * P<0.05, ** P<0.01 compared with control; ## P<0.01 compared with empty vector
COL6A3 enhanced cell proliferation
To understand the roles of COL6A3 on cell proliferation, we performed CCK-8 assays. Compared to negative control cells, the cell proliferation ability of the COL6A3-siRNA group decreased after COL6A3 downregulation, and the decline of cell proliferation was more obvious with the extension of time (P<0.05; Figure 2C).
Knockdown of COL6A3 suppressed the angiogenesis in vitro
In control and empty vector group, T24 cells gradually stretched, and connected each other into cords and network structure, forming luminal structures of various sizes and shapes. Nevertheless, tubes forming in the COL6A3-siRNA group was rare (Figure 2D). Furthermore, silencing COL6A3 notably reduced the number of generated blood vessels (P<0.01; Figure 2E).
COL6A3 promoted the EMT process
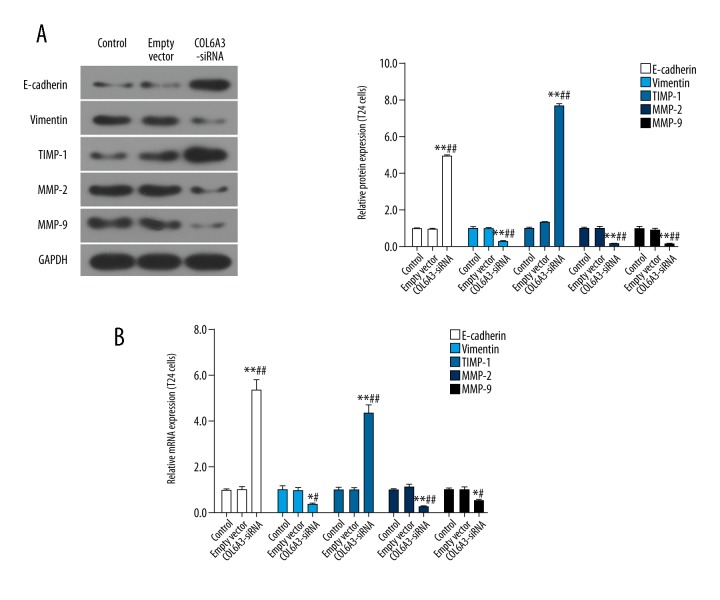
The EMT played a role in bladder carcinoma, so we detected EMT-related molecules by western blot to explore whether COL6A3 could affect EMT in bladder cancer cells. We found that the expression of vimentin was decreased significantly after COL6A3 interference, while the level of E-cadherin was the opposite. Furthermore, downregulation of COL6A3 reduced the levels of MMP2 and MMP-9 and increased TIMP-1 (Figure 3A). And the mRNA expression levels of these EMT-related molecules further verified these results (Figure 3B).
Figure 3.
The effect of COL6A3 on EMT related protein and mRNA. The MMP2, MMP9 and vimentin decreased significantly after COL6A3 interference, while the expression trend of E-cadherin and TIMP-1 is the opposite in the aspects of protein (A) and RNA (B). * P<0.05, ** P<0.01 compared with control; # P<0.05, ## P<0.01 compared with empty vector.
COL6A3 regulated the activity of TGFβ/Smad signaling pathway
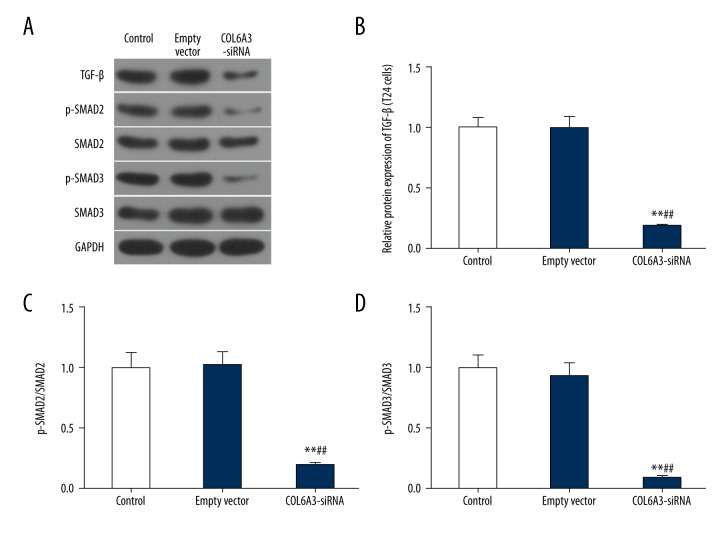
TGF-β receptor kinases could phosphorylate Smad2/3 to form a complex with Smad4, and nuclear translocation occurs to regulate gene expression, leading to the EMT [29]. To further analyze the mechanism of COL6A3 in bladder cancer, the protein levels of TGF-β, Smad2/3, and p-Smad2/3 were detected (Figure 4A). The expression of TGF-β showed a significant decrease with the knockdown of COL6A3 (Figure 4B). Interestingly, the expression levels of Smad2 and Smad3 protein were unchanged regardless of whether the COL6A3 expression interference, while the p-Smad2/3 were inhibited, so the p-Smad2/Smad2 and p-Smad3/Smad3 were appreciably decreased (Figure 4C, 4D).
Figure 4.
The effect of COL6A3 on the protein level of TGF-β/Smad pathway in bladder cancer cells. The expression level of Smad2 and Smad3 protein was similar (A), while the expression of TGF-β was decreased (B) after interference of COL6A3 expression. The phosphorylation of Smad2 (C) and Smad3 (D) faded after reducing the expression of COL6A3. ** P<0.01 compared with control, ## P<0.01 compared with empty vector.
Discussion
Bladder cancer is the most common urinary tract neoplasm. Although early radical cystectomy and targeted therapy are used, some concerns, such as high mortality and poor prognoses, still exist. Therefore, new target genes are urgently needed for this neoplasm.
It has been reported that COL6 is associated with cell anchoring, and can form a filamentous network with collagen types I and III and ECM remodeling to creates an environment for the tumor to flourish [30–32]. In our study, we confirmed that the expression of COL6A3 were upregulated in bladder cancer tissues. We also confirmed that COL6A3 was almost expressed exclusively in tumors, and could potentially serve as a new tumor marker [33]. In addition, patients with high expression level of COL6A3 had an extremely low survival rate as time passed. The levels of COL6A3 in tumor and adjacent tissues from 4 typical patients further verified that COL6A3 was involved in bladder cancers, which was accordance with a previous study [33]. In bladder cancer cells, we found that COL6A3 displayed a relatively high expression in T24 cells, compared with 5637 cells. When inhibiting the expression of COL6A3, we demonstrated that downregulated COL6A3 expression evidently decreased the proliferative abilities of bladder cancer cells. And this result was similar to that in gastric cancer [34]. Angiogenesis is the premise and foundation for tumor invasion and metastasis [35]. In this study, the number of regenerated vessels sank rapidly after COL6A3 expression was depressed. It has been previously reported that the high serum collagen is the result of high levels of degradation, angiogenesis, and remodeling in the ECM [36,37]. As a result, we speculated that interfered COL6A3 could inhibit the biological activities of bladder cancer cells and prevent cancer progression.
To understand the effects of COL6A3 on EMT in bladder cancer, the levels of EMT-related genes were detected. It has been proven that EMT involves the invasion and metastasis process of tumor [38,39], which can improve cell migration and invasion ability [40,41]. EMT is characterized by changes in the expression of adhesion molecules, the most important of which is the downregulation of E-cadherin [42]. E-cadherin is often related to cell migration. In addition, many biomarkers change in the process of EMT, such as the upregulation of N-cadherin, MMP2, MMP9, and vimentin. MMP2 and MMP9 could play a critical role in the metastasis of pancreatic cancer [43–45]. In our current study, COL6A3 silencing suppressed the expression of MMP-2, MMP-9, and vimentin, and then was involved in the inhibition of the EMT process in bladder cancer cells. These results suggested that COL6A3 may regulate the levels of EMT-related proteins to facilitate cell migration and metastasis in bladder cancer.
The aforementioned results confirmed that COL6A3 might play a certain role in bladder cancer. Moreover, we considered what mechanisms were regulated by COL6A3. There were many pathways that affect the EMT process of tumor, and TGF-β pathway was one of the core pathways to regulate cell proliferation and EMT process in the process of controlling organ development, tissue fibrosis, and cancer cell development [46]. Recently, the TGF-β/Smad2/3 signaling pathway has been reported to play critical roles in cancer metastasis and progression [47,48]. Therefore, we examined several related molecules and found that COL6A3 had a positive correlation with the expression of TGF-β as well as the p-Smad2/Smad2 and p-Smad3/Smad3 ratio in bladder cancer cells. TGF-β, which could phosphorylate and activate the Smad2/3, is involved in cell proliferation, production of ECM, and apoptosis [49,50]. Therefore, the carcinogenesis of COL6A3 in the bladder may partly be due to its relationship with the TGF-β signaling pathway. In this study, we found that COL6A3 silencing could lead to reduced levels of p-Smad2 and p-Smad3. Recently, the activation of TGF-β signaling has been reported to involve the EMT process and the risk of tumor metastasis [51–53]. These results revealed the possible molecular mechanisms of COL6A3 to promoted cell proliferation and the EMT process in bladder cells.
Conclusions
In total, our study identified that COL6A3 silencing significantly inhibited the cell proliferation, angiogenesis, and EMT process. In addition, we found that COL6A3 could suppress the molecules involved in TGF-β/Smad signaling pathway. These results may enhance our understanding of COL6A3 functions in bladder cells and encourage studies to explore possible mechanisms of TGF-β/Smad-induced EMT in bladder carcinomas.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Leow JJ, Martindoyle W, Rajagopal PS, et al. Adjuvant chemotherapy for invasive bladder cancer: A 2013 updated systematic review and meta-analysis of randomized trials. Eur Urol. 2014;66(1):42–54. doi: 10.1016/j.eururo.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–47. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Acloque H, Adams MS, Fishwick K, et al. Epithelial-mesenchymal transitions: The importance of changing cell state in development and disease. J Clin Invest. 2009;119(6):1438–49. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onder TT, Gupta PB, Mani SA, et al. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68(10):3645–54. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 6.Min KW, Kim DH, Do SI, et al. Expression patterns of stromal MMP-2 and tumoural MMP-2 and -9 are significant prognostic factors in invasive ductal carcinoma of the breast. APMIS. 2015;122(12):1196–206. doi: 10.1111/apm.12285. [DOI] [PubMed] [Google Scholar]
- 7.Vucemilo T, Skoko M, Sarcević B, et al. The level of serum pro-matrix metalloproteinase-2 as a prognostic factor in patients with invasive ductal breast cancer. Coll Antropol. 2014;38(1):135–40. [PubMed] [Google Scholar]
- 8.Hussein AA. The role of Matrix metalloproteinase-2 and -9 in situ hybridization in bladder cancer progression. Iraqi Journal of Medical Sciences. 2011;9:247–54. [Google Scholar]
- 9.Szarvas T, Vom DF, Ergün S, Rübben H. Matrix metalloproteinases and their clinical relevance in urinary bladder cancer. Nat Rev Urol. 2011;8(5):241–54. doi: 10.1038/nrurol.2011.44. [DOI] [PubMed] [Google Scholar]
- 10.Papageorgis P. TGFβ signaling in tumor initiation, epithelial-to-mesenchymal transition, and metastasis. J Oncol. 2015;2015(5):587193. doi: 10.1155/2015/587193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zavadil J, Böttinger EP. TGF-|[beta]| and epithelial-to-mesenchymal transitions. Oncogene. 2005;24(37):5764–74. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 12.Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Ann Rev Cell Dev Biol. 2011;27(1):347–76. doi: 10.1146/annurev-cellbio-092910-154036. [DOI] [PubMed] [Google Scholar]
- 13.Lamouille S, Derynck R. Cell size and invasion in TGF-β-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J Cell Biol. 2007;178(3):437–51. doi: 10.1083/jcb.200611146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 15.Pickup MW, Owens P, Moses HL. TGF-β, bone morphogenetic protein, and activin signaling and the tumor microenvironment. Cold Spring Harb Perspect Biol. 2017;9(5) doi: 10.1101/cshperspect.a022285. pii: a022285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massagué J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103(2):295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 17.Ikushima H, Miyazono K. TGFbeta signalling: A complex web in cancer progression. Nat Rev Cancer. 2010;10(6):415–24. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 18.Weiss A, Attisano L. The TGFbeta superfamily signaling pathway. Wiley Interdiscip Rev Dev Biol. 2013;2(1):47–63. doi: 10.1002/wdev.86. [DOI] [PubMed] [Google Scholar]
- 19.Barcellos-Hoff MH, Cucinotta FA. New tricks for an old fox: Impact of TGFβ on the DNA damage response and genomic stability. Sci Signal. 2014;7(341):re5. doi: 10.1126/scisignal.2005474. [DOI] [PubMed] [Google Scholar]
- 20.Derynck R, Muthusamy BP, Saeteurn KY. Signaling pathway cooperation in TGF-β-induced epithelial-mesenchymal transition. Curr Opin Cell Biol. 2014;31(31):56–66. doi: 10.1016/j.ceb.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucia C, Aiello FB. DNA repair and cytokines: TGF-β, IL-6, and thrombopoietin as different biomarkers of radioresistance. Front Oncol. 2016;6(10):175. doi: 10.3389/fonc.2016.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta S, Hau AM, Al-Ahmadie HA, et al. Transforming growth factor-β is an upstream regulator of mammalian target of rapamycin complex 2-dependent bladder cancer cell migration and invasion. Am J Pathol. 2016;186(5):1351–60. doi: 10.1016/j.ajpath.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pei M, Chen D, Li J, Wei L. Histone deacetylase 4 promotes TGF-beta1-induced synovium-derived stem cell chondrogenesis but inhibits chondrogenically differentiated stem cell hypertrophy. Differentiation. 2009;78(5):260–68. doi: 10.1016/j.diff.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Yao R, Lemon WJ, Wang Y, et al. Altered gene expression profile in mouse bladder cancers induced by hydroxybutyl(butyl)nitrosamine. Neoplasia. 2004;6(5):569–77. doi: 10.1593/neo.04223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hung TT, Wang H, Kingsley EA, et al. Molecular profiling of bladder cancer: Involvement of the TGF-beta pathway in bladder cancer progression. Cancer Lett. 2008;265(1):27–38. doi: 10.1016/j.canlet.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 26.Martianov I, Cler E, Duluc I, et al. TAF4 inactivation reveals the 3 dimensional growth promoting activities of collagen 6A3. PLoS One. 2014;9(2):e87365. doi: 10.1371/journal.pone.0087365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang CY, Wang J, Axellhouse D, et al. Clinical significance of serum COL6A3 in pancreatic ductal adenocarcinoma. J Gastrointest Surg. 2014;18(1):7–15. doi: 10.1007/s11605-013-2326-y. [DOI] [PubMed] [Google Scholar]
- 28.Arafat H, Lazar M, Salem K, et al. Tumor-specific expression and alternative splicing of the COL6A3 gene in pancreatic cancer. Surgery. 2011;150(2):306–15. doi: 10.1016/j.surg.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Islam SS, Mokhtari RB, El Hout Y, et al. TGF-β1 induces EMT reprogramming of porcine bladder urothelial cells into collagen producing fibroblasts-like cells in a Smad2/Smad3-dependent manner. J Cell Commun Signal. 2014;8(1):39–58. doi: 10.1007/s12079-013-0216-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lohi J, Leivo I, Oivula J, et al. Extracellular matrix in renal cell carcinomas. Histol Histopathol. 1998;13(3):785–96. doi: 10.14670/HH-13.785. [DOI] [PubMed] [Google Scholar]
- 31.Sethi T, Rintoul RC, Moore SM, et al. Extracellular matrix proteins protect small cell lung cancer cells againstapoptosis: A mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Med. 1999;5(6):662–68. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- 32.Sherman-Baust CA, Weeraratna AT, Rangel LB, et al. Remodeling of the extracellular matrix through overexpression of collagen VI contributes to cisplatin resistance in ovarian cancer cells. Cancer Cell. 2003;3(4):377–86. doi: 10.1016/s1535-6108(03)00058-8. [DOI] [PubMed] [Google Scholar]
- 33.Thorsen K, Sørensen KD, Bremseskildsen AS, et al. Alternative splicing in colon, bladder, and prostate cancer identified by exon array analysis. Mol Cell Proteomics. 2008;7(7):1214–24. doi: 10.1074/mcp.M700590-MCP200. [DOI] [PubMed] [Google Scholar]
- 34.Ao R, Guan L, Wang Y, Wang JN. Silencing of COL1A2, COL6A3 and THBS2 Inhibits Gastric Cancer Cell Proliferation, Migration and Invasion while Promoting Apoptosis through the PI3k-Akt Signaling Pathway. J Cell Biochem. 2017 doi: 10.1002/jcb.26524. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Li Q, Wang AY, Xu QG, et al. In-vitro inhibitory effect of EGFL7-RNAi on endothelial angiogenesis in glioma. Int J Clin Exp Pathol. 2015;8(10):12234–42. [PMC free article] [PubMed] [Google Scholar]
- 36.Öhlund D, Lundin C, Ardnor B, et al. Type IV collagen is a tumour stroma-derived biomarker for pancreas cancer. Br J Cancer. 2009;101(1):91–97. doi: 10.1038/sj.bjc.6605107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazouni C, Arun B, André F, et al. Collagen IV levels are elevated in the serum of patients with primary breast cancer compared to healthy volunteers. Br J Cancer. 2008;99(1):68–71. doi: 10.1038/sj.bjc.6604443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polyak K, Weinberg RA, Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat Rev Cancer. 9(4):265–73. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 39.Kang Y, Massagué J. Epithelial-mesenchymal transitions: Twist in development and metastasis. Cell. 2004;118(3):277–79. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 40.Luo M, Brooke M, Wicha MS. Epithelial-mesenchymal plasticity of breast cancer stem cells: Implications for metastasis and therapeutic resistance. Curr Pharm Des. 2015;21(10):1301–10. doi: 10.2174/1381612821666141211120604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Satoh K, Hamada S, Shimosegawa T. Involvement of epithelial to mesenchymal transition in the development of pancreatic ductal adenocarcinoma. J Gastroenterol. 2015;50(2):140–46. doi: 10.1007/s00535-014-0997-0. [DOI] [PubMed] [Google Scholar]
- 42.Yu H, Shen Y, Hong J, et al. The contribution of TGF-β in epithelial-mesenchymal transition (EMT): Down-regulation of E-cadherin via snail. Neoplasma. 2015;62(1):1–15. doi: 10.4149/neo_2015_002. [DOI] [PubMed] [Google Scholar]
- 43.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27(20):2192–206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andreolas C, Kalogeropoulou M, Voulgari A, et al. Fra-1 regulates vimentin during Ha-RAS-induced epithelial mesenchymal transition in human colon carcinoma cells. Int J Cancer. 122:1745–56. doi: 10.1002/ijc.23309. [DOI] [PubMed] [Google Scholar]
- 45.Shaul Y, Freinkman E, Comb W, et al. Dihydropyrimidine accumulation is required for the epithelial-mesenchymal transition. Cell. 2014;158(5):1094–109. doi: 10.1016/j.cell.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.TiradoRodriguez B, Ortega E, SeguraMedina P, HuertaYepez S. TGF-β: An important mediator of allergic disease and a molecule with dual activity in cancer development. J Immunol Res. 2014;2014(5):318481. doi: 10.1155/2014/318481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim TW, Michniewicz M, Bergmann DC, Wang ZY. Brassinosteroid regulates stomatal development by GSK3-mediated inhibition of a MAPK pathway. Nature. 2012;482(7385):419–22. doi: 10.1038/nature10794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu H, Luo H, Shen Z, et al. Transforming growth factor-β1 in carcinogenesis, progression, and therapy in cervical cancer. Tumour Biol. 2016;37(6):7075–83. doi: 10.1007/s13277-016-5028-8. [DOI] [PubMed] [Google Scholar]
- 49.Kamato D, Burch ML, Piva TJ, et al. Transforming growth factor-β signalling: role and consequences of Smad linker region phosphorylation. Cell Signal. 2013;25(10):2017–24. doi: 10.1016/j.cellsig.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 50.Gratchev A. TGF-β signalling in tumour associated macrophages. Immunobiology. 2016;222(1):75–81. doi: 10.1016/j.imbio.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 51.Zhao JJ, Hao S, Wang LL, et al. Long non-coding RNA ANRIL promotes the invasion and metastasis of thyroid cancer cells through TGF-β/Smad signaling pathway. Oncotarget. 2016;7(36):57903–18. doi: 10.18632/oncotarget.11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khan Z, Marshall JF. The role of integrins in TGFβ activation in the tumour stroma. Cell Tissue Res. 2016;365(3):657–73. doi: 10.1007/s00441-016-2474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moustakas A, Heldin CH. Mechanisms of TGFβ-induced epithelial-mesenchymal transition. J Clin Med. 2016;5(7) doi: 10.3390/jcm5070063. pii: E63. [DOI] [PMC free article] [PubMed] [Google Scholar]