Abstract
Background
The aim of this study was to analyze the differences in vaginal microecological factors and genital tract infections among pregnant women of different ages.
Material/Methods
This study included 751 pregnant women from West China Second University Hospital, Sichuan University, China, from January 2015 to April 2017. After gram staining, the vaginal microecological factors of these cases were observed, including vaginal cleanliness, lactobacillus number, bacterial density, flora diversity, dominant bacteria, pH, clue cells, Candida species, and Trichomonas vaginalis.
Results
There was no significant difference in bacterial density, flora diversity, vaginal cleanliness, or lactobacillus number among pregnant women of different age groups. Of the 32.62% of pregnant women who had genital tract infections, the incidence of bacterial vaginosis, Candida albicans infection, non-albicans Candida infection, and T. vaginalis infection were 20.91%, 14.91%, 4.26%, and 1.73%, respectively. The amalgamative incidence of bacterial vaginosis was 9.19%. The incidence of non-albicans Candida infection in the optimum reproductive age group was higher than in the older age group (P=0.0433). The incidence of T. vaginalis infection in the younger age group was higher than in the optimum reproductive age group and higher than in the older age group (P=0.0010 and P=0.0041).
Conclusions
The microecological status of pregnant women was basically the same as that of normal women. The most frequent genital tract infection was bacterial vaginosis. While bacterial vaginosis is amalgamative with vulvovaginal candidiasis and T. vaginalis infection, there was no significant difference in vaginal microecological observations among pregnant women in different age groups except that the non-albicans Candida infection incidence in the optimum reproductive age group and the T. vaginalis infection incidence in the younger age group was higher than in the other groups.
MeSH Keywords: Age Groups, Microbiota, Pregnant Women, Reproductive Tract Infections
Background
The vagina is a sensitive and complicated microecosystem, consisting of anatomic structures, microorganisms, local immunity, and endocrine regulation functions [1]. The bacterial colonization of the vagina is usually a mixed population, with anaerobes the dominant bacteria. Vaginal microbiomes are mutually antagonistic and interdependent, keeping a dynamic balance, regulated by the endocrine system and local immune system, and affected by the internal environment of the vagina. Estrogen level, Lactobacillus species (spp.), local immunity, and vaginal pH value play important roles in maintaining the microecological balance of the vagina [2]. Pregnancy is known to be a time when the vagina is prone to various vaginal infections. Changes in physiological hormones affect the vaginal microecological environment, composition and proportion of microorganism in the vaginal microecosystem, and the pH value of the vagina. In addition, immunosuppression that occurs during pregnancy reduces body immunity so that opportunistic infections, such as vulvovaginal candidiasis (VVC) and Trichomonas vaginitis (TV) infection, increase, thus contributing to the vaginal microecosystem’s fragile balance [3].
Few studies have investigated the characteristics of the vaginal microecosystem and genital tract infection (GTI) in pregnant women. Some clinical studies have shown that for pregnant women, it is common for their vagina to have normal pH values (3.8 to 4.5) and Lactobacillus spps., but the exact proportion of Lactobacillus spp. and the vaginal microecosystem factors of importance to pregnant women is still unclear [4]. Various kinds of vaginitis may result in abortion, intrauterine infection, fetal growth retardation, premature rupture of membranes, preterm labor, low birth weight, puerperal infection, and other adverse pregnancy outcomes. Severe illness and rapidly progressing illness can even lead to cervical cancer and other diseases, which may result in an adverse impact on both maternity and fetal health [5,6]. Thus, to learn more about the vaginal microecological status and GTI characteristics of pregnant women is of great significance for early diagnosis and treatment of vaginitis or amalgamative infections. This study analyzed characteristics of the vaginal microecosystem and GTIs of 751 pregnant women seen at our hospital between January 2015 and April 2017.
Material and Methods
Patients
The study was carried out from January 2015 to April 2017 in the Obstetrics and Gynecology Department of West China Second University Hospital, Sichuan University, China. The detection of vaginal cleanliness, white blood cells, lactobacillus number, Candida spp. (spore, blastospore, and pseudohyphae), Trichomonas vaginalis, clue cells, bacterial density, flora diversity, dominant bacteria, and vaginal pH value were performed in the Department of Laboratory Medicine of the same hospital. All participants signed informed consent. All procedures and protocols of this study were approved by the Ethics Committee of our hospital (Medical Research 2013, No.28), within which the work was undertaken and that it conforms to the provisions of the Declaration of Helsinki.
A total of 751 pregnant women were enrolled in this study. Based on age, they were divided into 4 groups: the younger age group (13–24 years old), the optimum reproductive age group (25–30 years old), the older age group (31–34 years old), and the oldest age group (35–43 years old). Participants were surveyed about their age, status of their pregnancy, marital status, the nature of their vaginal discharge (color, secretion amount, itching, and perineal dysuria), symptoms, past medical history, and treatment history before gynecological examination. The inclusive criteria were as follows: natural conception, intrauterine pregnancy proven by B-ultrasound, and no hormones, antibiotic, immunosuppressive agents use in the past 2 weeks, no sexual life, and their history of vulva/vaginal medication in the last 3 days. Women who had pregnancy complications, such as hypertension, diabetes mellitus, surgical complications, placenta previa, placental abruption, miscarriage, or preterm labor, were excluded from the study [3].
Vaginal discharge samples of these 751 patients were collected on sterile long handle scrapers. Some of these samples were made into smears, and the rest were collected on sterile cotton swabs (Medical Apparatus and Instruments Factory of Yangzhou Chuangxin, Jiangsu, China). All swabs and smears were sent to the Department of Laboratory Medicine of our hospital for fungal morphologic observation and vaginal pH determination.
Methods
Microecological observation and vaginal pH determination
Vaginal smears were subjected to gram staining. The staining procedure was previously described by Dai et al. [7]. After gram staining, the microecological observations including vaginal cleanliness, white blood cells, lactobacillus number, Candida spp. (spore, blastospore, and pseudohyphae), T. vaginalis, clue cells, bacterial density, flora diversity, and dominant bacteria of vaginal smears were observed by 2 microbiologists under an oil immersion field microscope. These observations were consistent and followed the guidelines of both the National System for External Quality Assessment (NSEQA) and the College of American Pathologists (CAP) [8]. Vaginal pH value was determined by the precise pH test paper method.
Diagnostic criteria
Bacterial density refers to the density and distribution of microhabitat in a specimen. In our study, as observed under optical microscope, bacterial density was divided into 4 grades: grade I (1+): 1–9; grade II (2+): 10–99; grade III (3+): 100 and above; and grade IV: (4+): bacteria clustered full of vision. Flora diversity refers to the amount of all the types of bacteria in a smear, as observed in a high view microscopic field (1000x). Flora diversity was also divide into 4 grades: grade I (1+): 1–3 kinds of flora; grade II (2+): 4–6 kinds of flora; grade III (3+): 7–10 kinds of flora; and grade IV (4+): more than 10 kinds of flora. According to The Expert Consensus on Clinical Application of Vaginal Microecosystem Assessment of Cooperative Group of Infectious Diseases in the Department of Obstetrics and Gynecology of the Chinese Medical Association [9], normal vaginal microecosystem was defined as follows: vaginal cleanliness was grade I; bacterial density was level II–III; flora diversity was level II–III, dominant bacteria were Lactobacillus spp. with normal function (the production of H2O2 is normal) and vaginal pH value of 3.8–4.5. Microecological imbalances can be diagnosed when any one of these factors (e.g., vaginal cleanliness, bacterial density, flora diversity, dominant bacteria, pH value, and lactobacillus function) is abnormal.
The criteria of vaginal cleanliness were as follows [10]: grade I was a large number of large gram-positive rods (indicative of Lactobacillus spp.) [11], vaginal epithelial cells, and no other bacteria observed with WBC 0~5/HP under microscopy. Grade II was some Lactobacillus spp. and vaginal epithelial cells, some pus cells, and other bacteria observed under microscopy with WBC 10~15/HP. Grade III was a small amount of Lactobacillus spp., a large number of pus cells and other bacteria observed under microscopy with WBC 15~30/HP. Grade IV was no Lactobacillus spp. but pus cells and other bacteria observed under microscopy with WBC more than 30/HP. Grade I~II means normal vaginal cleanliness, while grade III~IV means abnormal vaginal cleanliness with inflammation.
The diagnosis criteria of TV were as follows [12]: after gram staining, T. vaginalis was seen as a flagellate mono-cell parasite that was a bit larger than a white blood cell. It was pear shaped or polymorphic shaped; an oval nucleus was located at 1/3 the body front; beside the nucleus there was an axon and 4 flagellums; however, with gram staining it was not observed very clearly. TV was diagnosed after T. vaginalis was observed in vaginal smears.
The diagnosis criteria of VVC were as follows [13]: after gram staining, Candida spores appeared to be gram-positive oval cells that were smaller than erythrocytes, with a diameter of 2~6 μm; blastospores were spores with sprouts, which is arranged in double, while pseudohyphae were extending germ tubes of the blastospores. VVC was diagnosed after Candida spores, blastospores, and/or pseudohyphae were observed in vaginal smears. Candida albicans infection was diagnosed after Candida spores, blastospores and pseudohyphae were observed in vaginal smears. Non-albicans candida (NAC) infection was diagnosed after only Candida spores or blastospores were observed in vaginal smears.
Clue cells are epithelial cells covered with small gram-negative or gram-variable rods (indicative of Gardnerella vaginalis). The 4 diagnosis criteria for bacterial vaginosis (BV) have been reported: clue cell percentage over 20%, pH value over 4.5, presence of homogeneous vaginal discharge, and positive whiff test were [13]. In our study, BV was diagnosed using 3 of these 4 criteria: clue cell percentage over 20%, pH value over 4.5, and presence of homogeneous vaginal discharge.
The observed characteristic of Candida species, clue cells, and T. vaginalis all highly agreed with the descriptions given in several previous studies [12,13].
Statistical analysis
The data was analyzed by SPSS Statistics ver.17.0 (SPSS Inc., Chicago, IL, USA). The χ2 test was used to analyze the differences in microecological factors such as vaginal cleanliness, Lactobacillus spp., Candida spp., T. vaginalis, clue cells, bacterial density, flora diversity, dominant bacteria, pH value. P<0.05 was considered statistically significant and P<0.01 was considered extremely statistically significant in all statistical analysis [14].
Results
General data of the pregnant women
There were 751 pregnant women enrolled in this study, the age ranged from 13 to 43 years old. Five women were excluded based on the inclusive criteria described earlier. The age distribution of study participants is showed in Figure 1. They were primarily middle-aged women (mean ±SD 29.75±4.85 years, with a near normal distribution). Participants were divided into 4 groups according to age: the younger age (13–24 years old) group (n=92, 12.25%); the optimum reproductive age (25–30 years old) group (n=356, 47.40%); the older age (31–35 years old) group (n=220, 29.29%); and the oldest age (older than 35 years of age) group (n=83, 11.06%). The age of pregnant women mainly ranged from 25 to 30 years (Figure 1).
Figure 1.
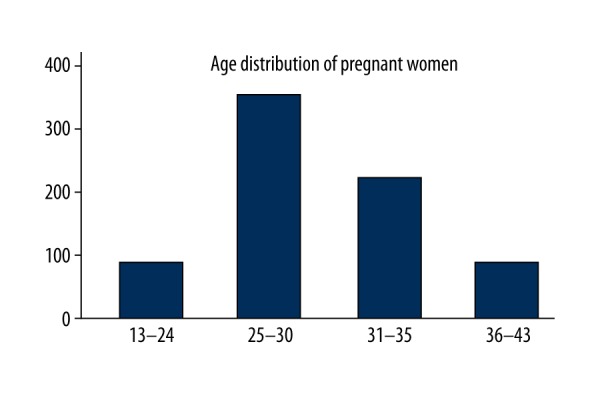
Age distribution of 751 pregnant women.
Analysis of vaginal microecological factors of the pregnant women
Table 1 shows that pregnant women with normal vaginal cleanliness (I–II grade) accounted for 61.65% of cases (463 out of 751 cases). Those who had normal lactobacillus numbers (3+ to 4+) accounted for 76.30% of cases (573 out of 751 cases); of which in 570 cases (75.90%), the Lactobacillus spp. was the dominant bacteria; bacterial density with basically normal distribution, in which grade II–III accounted for 85.09% of cases (639 out of 751 cases). Flora diversity grade I–II accounted for 86.55% of cases (650 out of 751 cases). The incidence of GTIs for these pregnant women was 32.62% of cases (245 out of 751 cases). Among this group, the incidences of BV (clue cell percentage >20%, pH >4.5 and presence of homogeneous vaginal discharge), Candida albicans infection, NAC infection, and TV were 20.91%, 14.91%, 4.26%, and 1.73%, respectively. The amalgamative incidence of BV with Candida albicans, NAC, and T. vaginalis infection was 9.19% (69 out of 751 cases), which accounted for 43.95% of BV infected pregnant women. Among 144 cases of Candida spp. infected pregnant women, Candida albicans infection accounted for 77.78% of cases (112 out of 144 cases) and NAC infection accounted for the rest of the 22.2% cases (32 out of 144 cases). Those pregnant women with pH <4.5 accounted for 73.10% of cases (549 out of 751 cases.
Table 1.
An overall analysis of the vaginal microecological status of 751 pregnant women.
| Microecological factors | Grade, cases (percentage) | Total cases | ||||
|---|---|---|---|---|---|---|
| Vaginal cleanliness | I grade | II grade | III grade | IV grade | ||
| 14 (1.86) | 463 (61.65) | 260 (35.95) | 14 (1.86) | 751 | ||
| Lactobacillus spp. | <1/oif | 1–5/oif | 6–30/oif | >30/oif | ||
| 114 (15.18) | 64 (8.51) | 335 (44.60) | 238 (31.70) | 751 | ||
| Blastospore | Absent | Present | ||||
| 607 (80.83) | 144 (19.17) | 751 | ||||
| Pseudohypha | Absent | Present | ||||
| 639 (83.09) | 112 (14.91) | 751 | ||||
| Trichomonas vaginalis | Absent | Present | ||||
| 738 (98.27) | 13 (1.73) | 751 | ||||
| Clue cells percentage over 20%1 | Absent | Present | ||||
| 594 (79.09) | 157 (20.91) | 751 | ||||
| Homogeneous vaginal discharge | Absent | Present2 | ||||
| 585 (77.90) | 166 (22.10) | 751 | ||||
| pH | 3.8–4.0 | 4.4 | >4.53 | |||
| 192 (25.56) | 357 (47.54) | 202 (26.90) | 751 | |||
| Bacterial density | I grade | II grade | III grade | IV grade | ||
| 27 (3.60) | 329 (43.81) | 310 (41.28) | 85 (11.32) | 751 | ||
| Flora diversity | I grade | II grade | III grade | IV grade | ||
| 315 (41.94) | 335 (44.61) | 63 (8.39) | 38 (5.06) | 751 | ||
| Dominant bacteria | Absent | Lactobacillus spp. | Gram-negative rods | Gram-positive coccus | Candida spp. | |
| 19 (2.53) | 570 (75.90) | 157 (20.90) | 3 (0.40) | 2 (0.27) | 751 | |
| Causal bacteria4 | Absent | Gram-variable rods only | Candida albicans5 | Non-albicans candida5 | Trichomonas vaginalis | |
| 506 (67.38) | 88 (11.72) | 112 (51)6 | 32 (9)7 | 13 (9)8 | 751 | |
The total cases of BV were the sum of single and amalgamative BV infection (157 cases, 20.91%);
These cases included all cases that clue cells percentage was over 20% in our study. Homogeneous vaginal discharge is not a microecological item, but a diagnostic criterion of BV;
These cases also included all cases that clue cells percentage was over 20% in our study;
The total cases of BV, VVC and TV were 245 (32.62%);
Candida albicans infection can be diagnosed after all Candida spores, blastospores and pseudohyphae were observed in vaginal smears. Non-albicans candida (NAC) infection can be diagnosed after only Candida spores, blastospores were observed in vaginal smears;
112 cases were Candida albicans (14.91%), 51 cases were amalgamative infection of Candida albicans and BV (6.79%);
32 cases were NAC (4.26%), 9 cases were amalgamative infection of NAC and BV (1.20%);
13 cases were Trichomonas vaginalis (1.73%), of which 9 were amalgamative infected with BV, accounting for 1.20%.
Table 2 shows that there was no significant difference in bacterial density, flora diversity, vaginal cleanliness, lactobacillus number, or vaginal pH value among each age group of pregnant women. Table 3 shows that there was no significant difference for these microecological factor between comparisons of any 2 age groups. Also, there was no significant difference in the incidence of Candida albicans infection, NAC infection, TV, BV, and the amalgamative incidence of BV infection among groups. Table 4 shows that there was no significant difference in the positive rates of Candida spp., T. vaginalis, and BV among the different age groups of pregnant women. However, Table 5 shows that there was a significant difference in the incidence of NAC between the optimum reproductive age group and older age group (P=0.0433). There was a significant difference in the incidence of TV between the younger age group and the optimum reproductive age group (P=0.0010); also, the younger age group and the older age group (P=0.0041).
Table 2.
Analysis of total difference in vaginal cleanliness, Lactobacillus, bacteria density, flora diversity and vaginal pH in pregnant women of different ages.
| Age groups of the pregnant women | Cases | Microecological factors | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaginal cleanliness | Bacteria density | Flora diversity | Lactobacillus number | Vaginal pH | |||||||||||||||
| I grade | II grade | III grade | IV grade | I grade | II grade | III grade | IV grade | I grade | II grade | III grade | IV grade | 1+ | 2+ | 3+ | 4+ | ≤4.5 | >4.5 | ||
| A: younger group | 92 | 3 | 55 | 30 | 4 | 5 | 34 | 42 | 11 | 39 | 38 | 11 | 4 | 14 | 9 | 34 | 35 | 67 | 25 |
| B: optimum reproductive group | 356 | 4 | 216 | 132 | 4 | 12 | 165 | 140 | 39 | 155 | 156 | 32 | 13 | 55 | 31 | 166 | 104 | 260 | 96 |
| C: older group | 220 | 4 | 141 | 72 | 3 | 7 | 93 | 96 | 24 | 87 | 103 | 15 | 15 | 29 | 20 | 96 | 75 | 163 | 57 |
| D: oldest group | 83 | 3 | 51 | 26 | 3 | 3 | 37 | 32 | 11 | 34 | 38 | 5 | 6 | 16 | 4 | 39 | 24 | 59 | 24 |
| Total | 751 | 14 | 463 | 260 | 14 | 27 | 329 | 310 | 85 | 315 | 335 | 63 | 38 | 114 | 64 | 335 | 238 | 549 | 202 |
| χ2 | 10.6651 | 4.2427 | 7.4336 | 7.2665 | 0.2857 | ||||||||||||||
| P | 0.2994 | 0.8950 | 0.5920 | 0.6094 | 0.9627 | ||||||||||||||
Table 3.
Analysis of the differences of Lactobacillus number, bacteria density, vaginal cleanliness, flora diversity and vaginal pH value of the pregnant women of each two age groups.
| Comparison of the pregnant women of each two age groups | Microecological factors | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vaginal cleanliness | Bacteria density | Flora diversity | Lactobacillus number | Vaginal pH value | ||||||
| χ2 | P | χ2 | P | χ2 | P | χ2 | P | χ2 | P | |
| A: B | 6.8068 | 0.0783 | 3.0584 | 0.3827 | 0.8933 | 0.8270 | 3.4664 | 0.3251 | 0.0016 | 0.9681 |
| A: C | 3.3687 | 0.3382 | 1.4296 | 0.6986 | 3.2719 | 0.3516 | 1.2106 | 0.7504 | 0.0536 | 0.8170 |
| A: D | 0.1170 | 0.9897 | 1.5193 | 0.6778 | 2.5363 | 0.4687 | 3.9974 | 0.2617 | 0.0657 | 0.7978 |
| B: C | 1.5198 | 0.6777 | 1.1358 | 0.7684 | 4.3777 | 0.2236 | 1.8106 | 0.6126 | 0.0779 | 0.7802 |
| B: D | 5.8635 | 0.1184 | 0.3798 | 0.9444 | 2.8544 | 0.4146 | 1.8902 | 0.5955 | 0.1288 | 0.7197 |
| C: D | 2.4867 | 0.4777 | 0.7639 | 0.8581 | 0.1165 | 0.9898 | 3.5418 | 0.3154 | 0.2781 | 0.5979 |
A – younger group, B – optimum reproductive group, C – older group D – oldest group.
Table 4.
The total differences in the positive cases of Candida spp., Trichomonas vaginalis and BV among the pregnant women of different age groups.
| Age groups of the pregnant women | Cases | Candida spp. | Trichomonas vaginalis | Total BV infection@ | Amalgamative BV infection | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Candida spores and Blastospore only$ | Pseudohyphae (not only) | ||||||||||
| Present | Absent | Present | Absent# | Present | Absent | Present | Absent | Present | Absent | ||
| A: younger group | 92 | 4 | 76 | 12 | 80 | 4 | 88 | 18 | 74 | 10 | 82 |
| B: optimum reproductive group | 356 | 20 | 279 | 57 | 299 | 5 | 351 | 75 | 281 | 33 | 323 |
| C: older group* | 220 | 5 | 188 | 27 | 193 | 3 | 217 | 45 | 175 | 16 | 204 |
| D: oldest group | 83 | 3 | 64 | 16 | 67 | 1 | 82 | 19 | 64 | 10 | 73 |
| Total | 751 | 32 | 607 | 112 | 639 | 13 | 738 | 157 | 594 | 69 | 682 |
| χ2 | 3.8142 | 3.0461 | 4.2273 | 0.3306 | 2.0957 | ||||||
| P | 0.1485 | 0.3845 | 0.1208 | 0.9542 | 0.5528 | ||||||
Because the positive cases (frequencies) of Non-albicans candida(NAC) and Trichomonas vaginalis was less than the theoretical frequencies (5) in the older group and the oldest group, the two groups were combined into C group (old group,31 to 43 y old);
NAC infection can be diagnosed after only Candida spores, blastospores were observed in vaginal smears. Because the frequencies of NAC were less than the theoretical frequencies in the oldest group, the two groups were combined into C group;
Candida albicans infection can be diagnosed after all Candida spores, blastospores and pseudohyphae were observed in vaginal smears. Non-albicans candida (NAC) infection can be diagnosed after only Candida spores, blastospores were observed in vaginal smears;
These cases included the cases that the Candida spores and blastospores were present and that all Candida spp. was absent;
BV infection cases included the cases that clue cell percentage was over 20%, pH value was over 4.5 and presence of homogeneous vaginal discharge.
Table 5.
Analysis of the differences of the incidence of VVC, TV, BV of the pregnant women of each two age groups.
| Comparison of the pregnant women of each two age groups | The differences of GTIs incidence | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-albicans candida infection | Candida albicans infection | Trichomonas vaginitis | Total BV infection | Amalgamative BV infection | ||||||
| χ2 | P | χ2 | P | χ2 | P | χ2 | P | χ2 | P | |
| A: B | 0.3035 | 0.5817 | 0.4942 | 0.4821 | 10.9136 | 0.0010 | 0.1003 | 0.7515 | 0.2156 | 0.6424 |
| A: C | 2.7966 | 0.0845 | 0.0352 | 0.8511 | 8.2472 | 0.0041 | 0.0318 | 0.8584 | 1.0987 | 0.2946 |
| A: D | 0.3832 | 0.5359 | 1.2616 | 0.2614 | 3.0376 | 0.0814 | 0.2896 | 0.5905 | 0.0599 | 0.8067 |
| B: C | 4.0845 | 0.0433 | 1.5256 | 0.2168 | 0.7901 | 0.3741 | 0.0310 | 0.8603 | 0.6967 | 0.4039 |
| B: D | 2.0186 | 0.1554 | 0.5178 | 0.4718 | 0.5347 | 0.4647 | 0.1331 | 0.7152 | 0.5881 | 0.4432 |
| C: D | 0.7690 | 0.3805 | 2.4280 | 0.1192 | 0.6538 | 0.4188 | 0.2148 | 0.6430 | 1.7520 | 0.1856 |
A – younger group; B – optimum reproductive group; C – older group; D – oldest group.
Discussion
In this study, the vaginal cleanliness of pregnant women was mainly grade I–II, and the pH value was normal. Lactobacillus number was 3+ to 4+, while Lactobacillus spp. was the dominant bacteria in 75.90% of the pregnant women, which was consistent with results from a previously reported study [9]. The bacterial density was mainly at grade II–III, which was basically the same as the reference value of women of childbearing age. Flora diversity was mainly grade I–I, which was lower than the reference grade of aforementioned women (grade II–III) [9]. Among the 32.62% of GTI incidences in the pregnant women, the incidences of BV, Candida albicans infection, NAC infection, and TV were basically consistent with previous reports [15]. The amalgamative incidence of BV with VVC and TV was 9.19%, which accounted for 43.95% of the total BV infected women, which was similar to what Li et al. previously reported [15]. The main pathogen of VVC of pregnant women was Candida albicans, which corresponded to previous reports in the literature [16].
Considering the differences in the vaginal microecological factors and the incidence of different GTIs among pregnant women of different ages, we divided the participants into 4 groups according to their age, and then analyzed the differences. Our study showed that there was no significant difference in vaginal cleanliness, bacterial density, flora diversity, or lactobacillus number among the age groups or between 2 age groups. For GTIs, there was no significant difference in the incidence of Candida albicans infection, NAC infection, TV, BV, or amalgamative infection of BV among the age groups. However, the incidence of NAC infection in the optimum reproductive age group was significantly higher than in the older age group, and the incidence of TV in the younger age group was significantly higher than in the optimum reproductive age group and the older age group.
The change in hormone levels in pregnant women, especially the increase of estrogen levels, leads to the accumulation of glycogen in vaginal epithelial cells. Then, the increase of lactic acid by the decomposition of glycogen by Lactobacillus spp. leads to the decrease of vaginal pH and imbalance in the vaginal microecosystem, which favors anaerobic pathogens in an acidic environment and the adhesion and infection of pathogens [17]. A variety of vaginal inflammations can lead to adverse pregnancy outcomes, which can do harm to the mother and fetus. So, early diagnosis and treatment of vaginitis is better for maternal and fetus health. During pregnancy, BV-related pathogens can produce a variety of lipids and proteins which can degrade cervical mucus and digest fetal membrane, thus decreasing the thickness and elasticity of fetal membranes, which can lead to premature rupture of membranes and chorioamnionitis. The bacterial biproducts cause the membrane and exuviates to release arachidonic acid, which promotes the synthesis of prostaglandins, then uterine contractions, and finally can lead to premature labor [18]. Candida spp. can infect the fetal membrane; cause a decrease of local tension of the fetal membrane, and even premature rupture in serious cases [16]. TV can lead to adverse pregnancy outcomes such as miscarriage, intrauterine infection, fetal growth restriction, premature rupture of membranes, premature labor, low birth weight infants, and puerperium infections [19].
To reduce the harm of this disease to maternal and fetus health, drugs are the optimal treatment. Related studies [20] suggest that pregnant women are more sensitive to drugs. So, not only efficiency, but also drug safety should be taken into consideration to avoid adverse effects on fetal development. In gestational VVC, it is recommended to administer local treatment but not oral administration of the whole body. To treat gestational TV, systemic administration should be the priority; local vaginal treatment should be used together. Sexual partners of these pregnant women should be treated and advised to avoid unprotected sex before cure [21]. The treatment of pregnant women with BV is mainly based on topic administration of metronidazole, with live Lactobacillus probiotics to recover the vaginal microecosystem. For mixed infections caused by multiple pathogens, comprehensive treatment should be carried out for all pathogens. More attention should be placed on repair of the mucous membrane and supplement probiotics while killing or inhibiting the pathogens in order to improve the pregnancy vaginal microecosystem, so that the incidence of premature labor and chorioamnionitis of GTI women can be reduced, and the risk of amniotic infection can be decreased [22].
Our study had some limitations. First, the diagnosis of VVC would be better if based on microscopic examination of wet smear or fungal culture of vaginal discharges [23,24]. In our study, the diagnosis of VVC in the 751 cases was only based on the characteristics of vaginal discharges and morphological examination under a microscope. Second, we tried to find the difference in vaginal microecosystem and incidence of GTIs among different age groups but to find a smaller difference, even no difference in microecological factors, the sample and research participants should be enlarged in follow-up studies. Third, the methods in this study had some limitations. Group B Streptococcus (GBS) plays an important role in perinatal infection and may lead to many perinatal adverse outcomes. In the future, more advanced methods or technology such as specialty agar or polymerase chain reaction (PCR) should be adapted to further studies in vaginal microecology and GTI of pregnant women.
Conclusions
The microecological status of pregnant women was basically the same as that of normal women. While bacterial vaginosis is amalgamative with vulvovaginal candidiasis and T. vaginalis infection, there was no significant difference in vaginal microecological observations among pregnant women in different age groups except that the non-albicans Candida infection incidence in the optimum reproductive age group and the T. vaginalis infection incidence in the younger age group was higher than in the other groups.
Acknowledgements
We would like to extend sincere gratitude and thanks to the staff of the Department of Laboratory Medicine, the Outpatient Department of Obstetrics and Gynecology of the West China Second University Hospital, Sichuan University, China, and the investigators of Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education. Also, we acknowledge with deep gratitude the assistance of Dr. Cheng Xi (Chengdu Medical College, Sichuan, China) for critical reading of the manuscript.
Abbreviations
- GTI
genital tract infection
- spp.
species
- TV
Trichomonas vaginalis
- VVC
vulvovaginal candidiasis
- BV
bacterial vaginosis
- NAC
non-albicans candida
- NSEQA
National System for External Quality Assessment
- CAP
College of American Pathologists
- GBS
group B Streptococcus
- PCR
polymerase chain reaction
Footnotes
Source of support: Support Program of Science & Technology Department of Sichuan Province, China (Program No. 2013SZ0037)
Conflict of interest
None.
References
- 1.Liao QP. Female vaginal micro ecosystem and vaginal microecological evaluation. J Pract Obstet Gynecol. 2010;26(2):81–83. [Google Scholar]
- 2.Xie LX, Jia HJ, Hu LN, et al. A study on the distribution of vaginal microflora in 800 women and its influencing factors. Progr Obstet Gynecol. 2008;17(2):81–86. [Google Scholar]
- 3.Fan L, Wu JC, Ren ML. Analysis of vaginal micro ecological status of pregnant women. Jiangsu Med J. 2011;37(2):205–7. [Google Scholar]
- 4.Hitti J, Hillier SL, Agnew KJ, et al. Vaginal indicators of amniotic fluid infection in preterm labor. Obstet Gynecol. 2001;97(2):21l–19. doi: 10.1016/s0029-7844(00)01146-7. [DOI] [PubMed] [Google Scholar]
- 5.Deng FW. Analysis of the distribution of pathogenic bacteria and drug resistance in urinary tract infection. Chin J Nosocomiol. 2012;22(17):3890–92. [Google Scholar]
- 6.Hackenhaar AA, Albernaz EP. Prevalence and associated factors with hospitalization for treatment of urinary tract infection during pregnancy. Rev Bras Uinecol Obstet. 2013;3(5):199–204. doi: 10.1590/s0100-72032013000500002. [DOI] [PubMed] [Google Scholar]
- 7.Dai QK, Hu LN, Jiang YM, et al. An epidemiological survey of bacterial vaginosis, vulvovaginal candidiasis and trichomoniasis in the Tibetan area of Sichuan Province, China. Eur J Obstet Gynecol Reprod Biol. 2010;150:207–9. doi: 10.1016/j.ejogrb.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 8.Hu Z, Zhou W, Mu L, et al. Identification of cytolytic vaginosis versus vulvovaginal candidiasis. J Low Genit Tract D. 2015;19:152–55. doi: 10.1097/LGT.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 9.Chinese Medical Association Obstetrics and Gynecology Branch Infectious Diseases Collaborative Group. Expert consensus on clinical application of vaginal microecosystem assessment. Chin J Obstet Gynecol. 2016;51(10):721–23. [Google Scholar]
- 10.Zhu BZ, Wen YQ, Xiang ZR. Vaginal cleanliness judgment standard of confusion and advice. Lab Med Clin. 2012;9(3):342–43. [Google Scholar]
- 11.El Sayed Zaki M, Raafat D, El Emshaty W, et al. Correlation of Trichomonas vaginalis to bacterial vaginosis: A laboratory-based study. J Infect Dev Ctries. 2010;4(3):156–63. doi: 10.3855/jidc.434. [DOI] [PubMed] [Google Scholar]
- 12.Fan SR, Zhang HP. 2010 United States Centers for Disease Control vaginitis treatment guidelines. J Chin General Pract. 2011;14(8):821–22. [Google Scholar]
- 13.van Schalkwyk J, Yudin MH, Yudin MH, et al. Vulvovaginitis: screening for and management of trichomoniasis, vulvovaginal candidiasis, and bacterial vaginosis. J Obstet Gynaecol Cancer. 2015;37(3):266–76. doi: 10.1016/S1701-2163(15)30316-9. [DOI] [PubMed] [Google Scholar]
- 14.Sun YW. Prophylactic medicine. fourth edition. Beijing: People’s Medical Publishing House; 2012. [Google Scholar]
- 15.Li XD, Tong F, Zhang XJ, et al. Incidence and risk factors of bacterial vaginosis among pregnant women: A prospective study in Maanshan City, Anhui Province, China. J Obstet Gynaecol Res. 2015;41(8):1214–22. doi: 10.1111/jog.12704. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Zhang CY, Liu XF. Analysis of the distribution of pathogenic bacteria and drug resistance of female reproductive system during pregnancy. Chin J Hum Sexual. 2017;26(1):101–3. [Google Scholar]
- 17.Sun WP. Study on vaginal changes of Lactobacillus and pH in healthy pregnant women. Chin J Microecol. 2011;23(3):264–66. [Google Scholar]
- 18.Wang LJ, An XL, Deng HJ, et al. Significance of early screening and treatment for bacterial vaginosis during pregnancy. Chin J Hum Sexual. 2015;24(9):101–3. [Google Scholar]
- 19.He FY. Lower genital tract infections and pregnancy outcomes. Matern Child Health Care Chin. 2012;27(2):312–13. [Google Scholar]
- 20.Deng Q, Huang LC, Jiang DD, et al. Lactobacillus combined metronidazole to treat trichomonas vaginitis clinical analysis. Chin J Hum Sexual. 2016;25(1):122–25. [Google Scholar]
- 21.Chinese Medical Association Obstetrics and Gynecology Branch Infectious Diseases Collaborative Group. Trichomonas vaginitis diagnosis and treatment guidelines (draft) Chin J Obstet Gynecol. 2011;46(4):318–18. [Google Scholar]
- 22.Cui WH, Feng L, Song WH, et al. In regulating pregnancy vaginal Lactobacillus micro ecological imbalance of special function. Chin J Eugen Genet. 2013;21(2):121–22. [Google Scholar]
- 23.Owen MK, Clenney TL. Management of vaginitis. Am Fam Physician. 2004;70:2125–32. [PubMed] [Google Scholar]
- 24.Landers DV, Wiesenfeld HC, Heine RP, et al. Predictive value of the clinical diagnosis of lower genital tract infection in women. Am J Obstet Gynecol. 2004;190:1004–10. doi: 10.1016/j.ajog.2004.02.015. [DOI] [PubMed] [Google Scholar]


