Abstract
Background
Angiogenesis is an important component of wound healing and tissue repair. Kindlin-2 is an integrin-associated protein, encoded by the KINDLIN-2 gene, which has been shown to affect cell adhesion and migration of cells, including endothelial cells. The aim of this study was to use a mouse model of wound healing to evaluate the effects of expression of KINDLIN-2 on angiogenesis in wound healing in vivo.
Material/Methods
Thirty-six male C57BL/6 mice were studied in an established model that used a wound created on the back. Mice were divided randomly into three groups: the normal group (n=12) received injections of normal (0.9%) saline; the KINDLIN-2(−) group (n=12) received injections of adeno-associated virus with small interfering (si)RNA targeting the KINDLIN-2 gene (AAV-KINDLIN-2-siRNA); and the control (group (n=12) received injections of adeno-associated virus containing a scrambled RNA sequence (AAV-control-RNA). Wound healing was analyzed by biochemical examination of the exudates and histology. Evans blue dye was injected into the caudal vein of each mouse, two weeks after wound healing to assess neovascular permeability.
Results
Wound healing was significantly delayed in the KINDLIN-2 gene knockdown mice (AAV-KINDLIN-2-siRNA) compared with that of the normal group and the control group, and neovascular permeability was increased. In the AAV-KINDLIN-2-siRNA group, blood vessels were shorter and thinner compared with the normal group and the control group.
Conclusions
In a mouse model of wound healing, KINDLIN-2 gene knockdown inhibited wound healing, and increased neovascular permeability in vivo.
MeSH Keywords: Dependovirus; Neovascularization, Pathologic; Wound Healing
Background
Wound healing is a co-ordinated repair process used to restore the integrity of soft tissue and skin integrity and tissue barriers. The healing response can be divided into three overlapping phases, the inflammatory phase, the formation of new tissue elements that can include cell proliferation, and tissue remodeling [1,2]. The wound healing process also involves blood coagulation and angiogenesis, and if it occurs in the skin, re-epithelialization.
Angiogenesis is the development of new blood vessels and is a fundamental physiologic process that enables the supply of oxygen and nutrients to promote tissue repair and chronic inflammation. Angiogenic vessels initially consist of endothelial cells (ECs). New vessels in wounds sprout or form new vascular buds, either from pre-existing vessels or, more rarely, by the recruitment of circulating bone marrow-derived endothelial progenitor cells (stem cells) [2,3]. Although normal angiogenesis is part of the process of reparative wound healing and tissue repair, abnormal or excessive angiogenesis can result in diseases, such as diabetic retinopathy.
Kindlins are also known as fermitin family homolog-containing proteins and are a novel family of adaptor proteins that have key roles in multiple cell types and which promote the integrin cell signaling [4–7]. Three vertebrate kindlin family members include Kindlin-1, which is expressed in epithelial cells, Kindlin-2, which is expressed in most cells, and Kindlin-3, which is expressed by hematopoietic cells [4,8,9]. Kindlin-2 was the first of the Kindlin proteins to be discovered. Kindlin-2 is an integrin-associated protein, encoded by the KINDLIN-2 gene, which has been shown to affect cell adhesion and migration of cells, including endothelial cells, and is also expressed by cardiac muscle cells [10]. Kindlin-2 activates integrins by binding with the cytoskeletal protein, talin and recruits other proteins to adhere to and control integrin activation [10]. Kindlin-2 regulates cardiac myogenesis and myogenic differentiation, the formation of intercalated discs, and embryonic development [11–15]. Knockdown of expression of the KINDLIN-2 gene can be lethal in laboratory mice that are KINDLIN-2(−/−) [16]. KINDLIN-2(+/−) mice have been shown to exhibit a reduced density and growth of intra-tumor angiogenic vessels [17]. These findings suggest that Kindlin-2, encoded by the KINDLIN-2 gene, might regulate angiogenesis in wounds.
Therefore, the aim of this study was to use a mouse model of wound healing to evaluate the effects of knockdown of KINDLIN-2 on angiogenesis in wound healing in vivo. A commercially available adeno-associated virus (AAV) vector for small interfering (si)RNA delivery into murine cells was used for the gene knockdown and wound healing was evaluated by biochemical examination, tissue histology, and new vessel permeability.
Material and Methods
Ethical approval of the study protocol
All animal experiments were performed according to the recommendations of the Guide for the Care and Use of Laboratory Animals, National Institutes of Health (NIH) and conformed to the guidelines of the Declaration of Helsinki. The study was approved by the Ethics Committee of Zhongshan Hospital, which is affiliated to Fudan University.
Experimental groups
Mice were bred in an SPF-grade experimental animal room. All surgical experiments were carried out using sodium pentobarbital anesthesia. Thirty-six male C57BL/6 mice, between 8–9 weeks old, were purchased from SLAC Laboratory Animals (Shanghai, China). KINDLIN-2 mouse knockdown models were prepared as previously described [18–20].
Mice were divided randomly into three groups: the normal group (n=12) received injections of normal (0.9%) saline; the KINDLIN-2(−) group (n=12) received injections of adeno-associated virus with small interfering (si)RNA targeting the KINDLIN-2 gene (AAV-KINDLIN-2-siRNA); and the control (group (n=12) received injections of adeno-associated virus containing a scrambled RNA sequence (AAV-control-RNA). Then, 5×1010 v.g. of AAV-KINDLIN-2-siRNA (Jikai Biotechnology, Shanghai, China) or AAV-control-siRNA (Jikai Biotechnology, Shanghai, China) in 30 μL of normal saline was injected intradermally into the dorsal skin of mice; 30 μL of normal saline alone was used for the normal group. The wound model was evaluated 14 days after injection, following anesthesia using sodium pentobarbital.
Mouse wound model
The mouse wound model was created as previously described, and for all 36 mice included in the study [21,22]. Following the induction of anesthesia, the dorsal skin was shaved and disinfected using 75% ethanol. Skin wounds were created using a 6-mm skin punch and scissors. Five days after wounding, healing tissues were removed from some mice for further experiments, including immunofluorescence and Western blot, and from the remainder of the mice to evaluate wound healing and blood-vessel permeability. After four weeks, the mice were euthanized by cervical dislocation under anesthesia.
Western blot
Western blotting was performed as previously described [6]. Briefly, the wound tissues were weighed and lysed in lysis buffer. Proteins were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA). Then, the PVDF membranes were incubated with primary antibodies and horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology, Danvers, MA, USA). The blots were visualized using enhanced chemiluminescence (ECL) reagent. The antibodies used were a rabbit monoclonal antibody to Kindlin-2 (Sigma-Aldrich, Saint Louis, MO, USA) and a rabbit monoclonal antibody to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Cell Signaling Technology). ImageJ software (National Institutes of Health, Bethesda, MD, USA) was used to analyze blot density.
Immunofluorescence
Immunofluorescence analysis of tissue was performed as previously described [23,24]. Briefly, mouse skin tissues were sectioned using a cryostat microtome in a freezing chamber. First, the sections were permeabilized with 0.1% Triton X-100 for 30 min at room temperature. Sections were incubated in blocking solution for 60 min at room temperature. Immunostaining was done using primary antibodies that included a rat anti-CD31 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and a rabbit anti-Kindlin-2 antibody (Sigma-Aldrich, Saint Louis, MO, USA) with overnight incubation at 4°C, followed by incubation with the fluorescein isothiocyanate (FITC)-conjugated secondary antibody for 1 h at room temperature. Six samples were selected from each group of mice, and four image views were captured from each sample, by immunofluorescence microscopy.
Macroscopic analysis of the wound area
The wound area was photographed using a digital EOS40D camera (Canon, Tokyo, Japan) at one, three, six, and eight days post-wounding. The wound area was calculated using ImageJ software.
Evaluation of blood vessel permeability
Vascular permeability was measured as previously described [17]. Two weeks after the mouse skin wounds had healed, an intravenous injection of Evans Blue dye (EBD) 100 μL (Sigma-Aldrich, Saint Louis, MO, USA) and mustard oil was applied to the dorsal skin. Thirty minutes after injection with EBD, skin samples of similar size were removed, weighed, and photographed. Then, the EBD was extracted with 1 mL of formamide overnight at 60°C using constant agitation. The amount of EBD extracted was measured using a spectrophotometer at 610 nm. The skin was dried for 72 h at 60°C, weighed, and the absorbance was measured.
Statistical analysis
Data were presented as the mean ± standard error of the mean (SEM). Statistical analysis was performed using a Kolmogorov-Smirnov normality test. Between-group comparison of means was performed by one-way analysis of variance (ANOVA). P<0.05 was considered as statistically significant.
Results
The effects of KINDLIN-2 gene knockdown, expression of the Kindle-2 protein, and cell migration in the mouse skin wounds
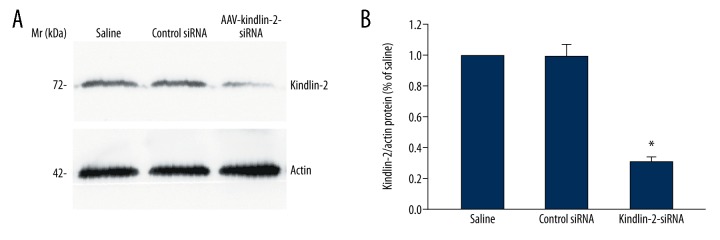
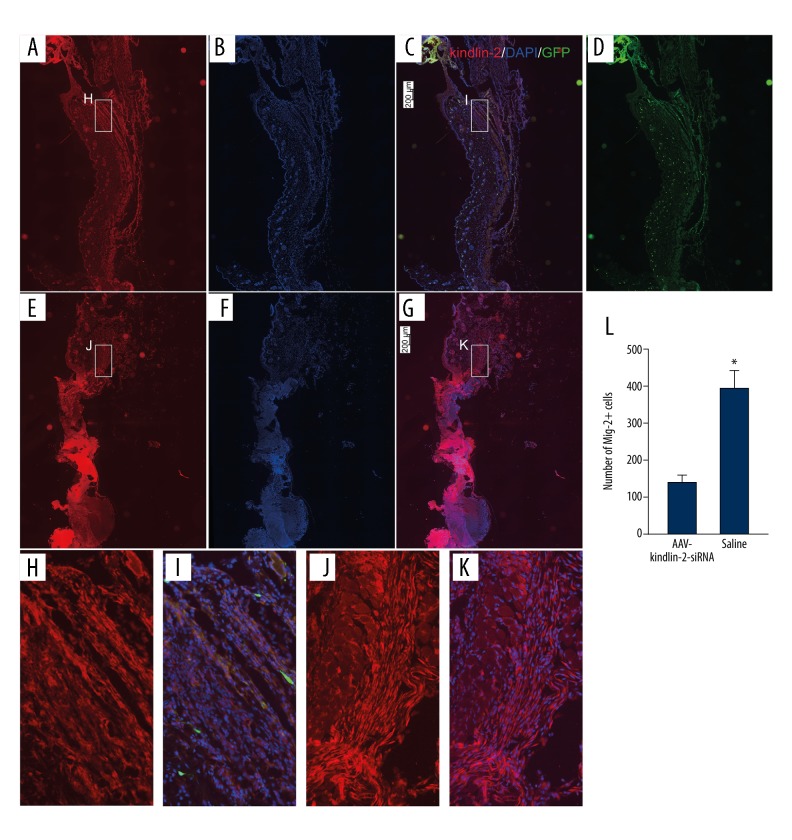
Western blotting (Figure 1) and immunofluorescence (Figure 2) were used to assess the levels of Kindlin-2 protein expression in the mouse skin samples in the three study groups. In the normal wound and control groups, there was no significant difference in expression levels of Kindlin-2 protein (P>0.05) (n=3) (Figure 1A). However, in the KINDLIN-2(−) group of mice that received injections of adeno-associated virus with small interfering (si)RNA targeting the KINDLIN-2 gene (AAV-KINDLIN-2-siRNA), there was significantly reduced expression of the Kindlin-2 protein when compared with the normal group (P<0.05) (n=3). Kindlin-2 protein was highly expressed in the normal group, and many Kindlin-2-positive cells (396±49 per field) migrated from the wound edge to the wound surface (Figure 2). However, in the KINDLIN-2(−) or AAV-KINDLIN-2-siRNA group, the number of Kindlin-2-positive cells was significantly reduced to 138±20 per field (P<0.05).
Figure 1.
The effects of KINDLIN-2 gene knockdown following intradermal injection of adeno-associated virus with small interfering (si)RNA targeting the KINDLIN-2 gene (AAV-KINDLIN-2-siRNA) on the expression of Kindlin-2 protein in mouse skin wounds (A) Western blot shows the expression of Kindle-2 and actin protein in mouse skin wounds in the groups injected with saline, AAV-KINDLIN-2-siRNA, and scrambled RNAi. (B) Intradermal injection of mouse skin wounds with AAV-KINDLIN-2-siRNA reduces the expression of Kindlin-2 protein in the wound. AAV-KINDLIN-2-siRNA – adeno-associated virus with small interfering (si)RNA targeting the KINDLIN-2 gene. Data are expressed as the mean ±SEM.
Figure 2.
The effects of KINDLIN-2 gene knockdown following intradermal injection of adeno-associated virus with small interfering (si)RNA targeting the KINDLIN-2 gene (AAV-KINDLIN-2-siRNA) on the migration of Kindlin-2 (+) cells in mouse skin wounds. (A–D) Intradermal injection of AAV-KINDLIN-2-siRNA reduced the number of KINDLIN-2(+) cells around the wounds and decreased the number of KINDLIN-2(+) cells (H, I) migrating from the wound edge into the wound. (E–G) KINDLIN-2(+) cells in the normal group with intradermal injection of saline; the KINDLIN-2(+) cells are shown around the wound surface. A large number of KINDLIN-2(+) cells are shown to migrate from the wound edge into the wound (J, K). Bars: 200 microns. Magnification: ×20. (L) The number of KINDLIN-2(+) cells migrating from the wound edge to the wound surface. AAV-KINDLIN-2-siRNA – adeno-associated virus with small interfering (si)RNA targeting the KINDLIN-2 gene. Data are expressed as the mean ±SEM.
The effects of KINDLIN-2 gene knockdown and wound healing in the mouse skin wounds
To determine the effects of Kindle-2 on wound healing, the mouse skin wounds in the three study groups were observed wounds for ten days. Wound healing was significantly delayed in the AAV-KINDLIN-2-siRNA group (or KINDLIN-2(−) group) (P<0.05 (n=6) (Figure 3). The percentage wound area on day-9 in the AAV-KINDLIN-2-siRNA group (24± 2%) was significantly greater when compared with the control group (7±4%) and with the normal group (5±2%) (P<0.05) (n = 6). There was no significant difference in wound healing between the control group and the normal group (P>0.05) (n=6). These findings indicated that in the mouse wound healing model, the expression of the KINDLIN-2 gene encoding the Kindle-2 protein was required for normal wound healing and that knockdown of the KINDLIN-2 gene resulted in delayed and less effective wound healing.
Figure 3.
Assessment of skin wound healing in mice with or without KINDLIN-2 gene knockdown following intradermal injection of adeno-associated virus with small interfering (si)RNA targeting the KINDLIN-2 gene (AAV-KINDLIN-2-siRNA). (A) Macroscopic appearance of wounds at days 1, 3, 6, and 9 after injection of AAV-KINDLIN-2-siRNA (the KINDLIN-2(−) group); injection of AAV-control-siRNA (the control group); and injection of normal saline (the normal group). (B) Wound healing is significantly delayed after treatment with AAV-KINDLIN-2-siRNA resulting in KINDLIN-2 gene knockdown, compared with injection of normal saline (the normal group) and injection of control-siRNA (the control group) (n=6). AAV-KINDLIN-2-siRNA – adeno-associated virus with small interfering (si)RNA targeting the KINDLIN-2 gene.
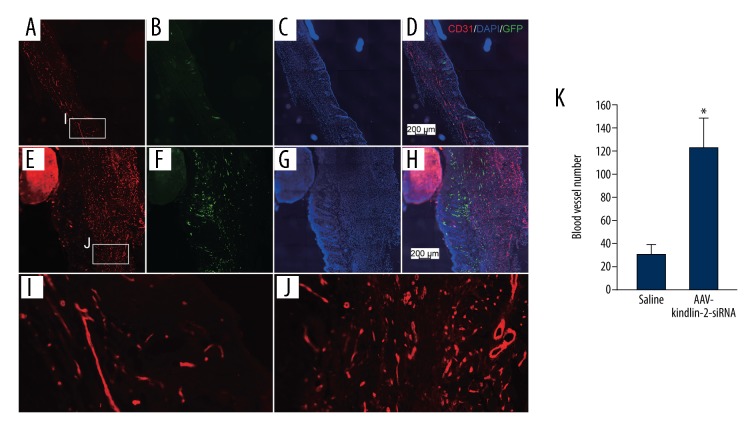
The effects of KINDLIN-2 gene knockdown and angiogenesis and vascular permeability in the mouse skin wounds
Angiogenesis plays a key role in the healing of skin wounds [25]. Kindlin-2, as a regulator of integrins, is essential for maintenance of the integrity and function of tissues in adults [26,27]. The role of the KINDLIN-2 gene and expression of its protein product, Kindlin-2 were evaluated. Five days after skin wounding, immunostaining of skin sections for endothelial cells was performed using an endothelial cell-specific antibody, CD31, which showed a significant increase of vessel numbers (123± 5 per field) in the AAV-KINDLIN-2-siRNA group compared with the normal group (30± 8 per field) (P<0.05) (n=3) (Figure 4). Also, in the AAV-KINDLIN-2-siRNA group, blood vessels were shorter and thinner, and dermal tissues were thicker. Two weeks (14 days) after the skin wounds had healed, the vascular permeability in healed wounds was assessed using Evans blue dye (EBD) staining (Figure 5). The permeability of blood vessels in the AAV-KINDLIN-2-siRNA group was significantly increased (to 159±25%) when compared with the normal group (P<0.05) (n=3). These findings indicated that in the mouse wound healing model, the expression of the KINDLIN-2 gene encoding the Kindle-2 protein was required for normal vascular permeability in angiogenesis and that knockdown of the KINDLIN-2 gene affected wound healing by disrupting neovascular permeability and not by increasing the number of angiogenic blood vessels.
Figure 4.
The number and morphology of angiogenic vessels in mouse wounds with KINDLIN-2 gene knockdown following intradermal injection of adeno-associated virus with small interfering (si)RNA targeting the KINDLIN-2 gene (AAV-KINDLIN-2-siRNA). Immunofluorescence using a labeled antibody to endothelial cells (CD31) (red) and cell nuclei stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). (A–D, I) Mouse skin wound angiogenesis in the normal group treated with an injection of normal (0.9%) saline. (E–H, J) Mouse skin wound angiogenesis in the KINDLIN-2(−) group treated with an injection of AAV-KINDLIN-2-siRNA. (K) Mouse skin wound angiogenesis in the KINDLIN-2(−) group treated with an injection of AAV-KINDLIN-2-siRNA showing the morphology of the new vessels. AAV-KINDLIN-2-siRNA – adeno-associated virus with small interfering (si)RNA targeting the KINDLIN-2 gene. Data are expressed as the mean ±SEM.
Figure 5.
Detection of neovascular permeability by Evans blue dye (EBD) injection. (A–C) Representative photographs of Evans blue dye (EBD) leakage from the dorsal skin wound vasculature. (D) Quantification of dorsal skin vasculature permeability. Injection of AAV-KINDLIN-2-siRNA shows an increase in the vasculature permeability. AAV-KINDLIN-2-siRNA – adeno-associated virus with small interfering (si)RNA targeting the KINDLIN-2 gene. All vascular permeability data are expressed as the mean ±SEM.
Discussion
Kindlin-2 is an integrin-associated protein, encoded by the KINDLIN-2 gene, which has been shown to affect cell adhesion and migration of cells, including endothelial cells. The aim of this study was to use a mouse model of wound healing to evaluate the effects of expression of KINDLIN-2 on angiogenesis in wound healing in vivo. The findings of this study showed that KINDLIN-2 gene knockdown inhibited wound healing, and increased endothelial cell permeability in vivo, and resulted in abnormal new vessel morphology.
Kindlin-2 is essential for embryonic development as Kindlin-2-deficient embryonic stem cells have shown reduced adhesion to various substrates of the extracellular matrix, such as laminin-111, laminin-332, and fibronectin, which can lead to embryo death [14]. Knockdown of the KINDLIN-2 gene that encodes Kindlin-2 has been reported to lead to a significant reduction in the invasive properties of cancer cells through nuclear factor kappa B (NF-κB)-dependent upregulation of expression of matrix metallopeptidase (MMP)-9 and MMP-2 [28].
Although a previously published study has shown that Kindlin-2 plays an important role in wound healing by regulating the function of fibroblasts [29], the role of Kindlin-2 in skin wound healing and angiogenesis has not been previously studied in detail. Kindlins and talins bind to the β-cytoplasmic tails of integrins for optimal integrin activation [25]. Kindlins have also been implicated in ‘outside-in’ signaling across integrins, and their capacity to regulate the function of integrin adhesion receptors has recently been studied [5,30,31].
Integrins, such as αvβ1, αvβ3, and αvβ5 are highly expressed in cells of the vasculature, especially by proliferating endothelial cells [32]. Disruption of binding of Kindlin-2 to the β-cytoplasmic tails of integrins can lead to defects in endothelial cell migration in vitro and to the developmental and tumor angiogenesis in vivo [33]. The hypothesis that drove this study was that expression of the KINDLIN-2 gene, and its protein Kindlin-2 regulated angiogenesis, vascular permeability, and wound healing by interacting with integrins, allowing interaction with β-/γ-catenin and actin filaments, linking vascular endothelial cadherin-based cell junctions to the actin cytoskeleton [34].
Angiogenesis is associated with cancer, diabetes mellitus-based complications, and inflammatory diseases. Research has shown that two alpha-v integrin pathways contribute to angiogenesis: one that depends on alpha vb3 and a second one that is potentiated by alpha vb5 [35].
Fibroblast growth factor receptor (FGFR) signaling is thought to be essential for vascular development. Fibroblast growth factor (FGF) signaling in endothelial cells is required for the response to injury but not for vascular homeostasis. Studies have shown that mice lacking FGFR1/2 in endothelial cells show a significant reduction in neovascular growth and tissue repair [35] and that FGF signaling is a key positive regulator in maintaining the integrity of endothelial cell barriers [36,37]. Previously published studies have also shown that antagonists to integrin αvβ3 suppress angiogenesis induced by FGFR2 [38].
Conclusions
Previously published studies support the findings of the present study, that the expression of the KINDLIN-2 gene and the Kindlin-2 protein induces normal angiogenesis, and aids the integrity of the endothelial cells and barriers related to integrin and fibroblast growth factor receptor (FGFR) signaling. According to the findings of the present study, combined with those of previously published studies, we propose that there is a Kindlin-2-integrin-FGF axis that regulates angiogenesis in wound healing. However, the mechanism of this pathway is not known and requires further study.
Footnotes
Source of support: The present study was supported by the National Key Research and Development program (Program 973; 2016YFC1100300) and by the National Natural Scientific Foundation of China (No. 81671915)
Conflict of interest
None.
References
- 1.Rege A, Thakor NV, Rhie K, et al. In vivo laser speckle imaging reveals microvascular remodeling and hemodynamic changes during wound healing angiogenesis. Angiogenesis. 2012;15(1):87–98. doi: 10.1007/s10456-011-9245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341(10):738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 3.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8(6):464–78. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 4.Plow EF, Qin J, Byzova T. Kindling the flame of integrin activation and function with Kindlins. Curr Opin Hematol. 2009;16(5):323–28. doi: 10.1097/MOH.0b013e32832ea389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montanez E, Ussar S, Schifferer M, et al. Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 2008;22(10):1325–30. doi: 10.1101/gad.469408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen C, Sun L, Zhu N, Qi FZ. Kindlin-1 contributes to EGF-induced re-epithelialization in skin wound healing. Int J Mol Med. 2017;39(4):949–59. doi: 10.3892/ijmm.2017.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harburger DS, Bouaouina M, Calderwood DA. Kindlin-1 and -2 directly bind the C-terminal region of beta integrin cytoplasmic tails and exert integrin-specific activation effects. J Biol Chem. 2009;284(17):11485–97. doi: 10.1074/jbc.M809233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malinin NL, Plow EF, Byzova TV. Kindlins in FERM adhesion. Blood. 2010;115(20):4011–17. doi: 10.1182/blood-2009-10-239269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bialkowska K, Ma YQ, Bledzka K, et al. The integrin co-activator Kindlin-3 is expressed and functional in a non-hematopoietic cell, the endothelial cell. J Biol Chem. 2010;285(24):18640–49. doi: 10.1074/jbc.M109.085746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma YQ, Qin J, Wu C, Plow EF. Kindlin-2 (Mig-2): A co-activator of beta3 integrins. J Cell Biol. 2008;181(3):439–46. doi: 10.1083/jcb.200710196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowling JJ, Dowling JJ, Vreede AP, et al. Kindlin-2 is required for myocyte elongation and is essential for myogenesis. BMC Cell Biol. 2008;9:36. doi: 10.1186/1471-2121-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowling JJ, Gibbs E, Russell M, et al. Kindlin-2 is an essential component of intercalated discs and is required for vertebrate cardiac structure and function. Circ Res. 2008;102(4):423–31. doi: 10.1161/CIRCRESAHA.107.161489. [DOI] [PubMed] [Google Scholar]
- 13.Hatcher CJ, Basson CT. Disrupted intercalated discs. Is Kindlin-2 required? Circ Res. 2008;102(4):392–94. doi: 10.1161/CIRCRESAHA.108.172171. [DOI] [PubMed] [Google Scholar]
- 14.Montanez E, Ussar S, Schifferer M, et al. Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 2008;22(10):1325–30. doi: 10.1101/gad.469408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu Y, Qi L, Wu J, et al. Kindlin 2 regulates myogenic related factor myogenin via a canonical Wnt signaling in myogenic differentiation. PLoS One. 2013;8(5):e63490. doi: 10.1371/journal.pone.0063490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harburger DS, Bouaouina Calderwood MD. Kindlin-1 and -2 directly bind the C-terminal region of beta integrin cytoplasmic tails and exert integrin-specific activation effects. J Biol Chem. 2009;284(17):11485–97. doi: 10.1074/jbc.M809233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pluskota E, Dowling JJ, Gordon N, et al. The integrin coactivator Kindlin-2 plays a critical role in angiogenesis in mice and zebrafish. Blood. 2011;117(18):4978–87. doi: 10.1182/blood-2010-11-321182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keswani SG, Balaji S, Le L, et al. Pseudotyped adeno-associated viral vector tropism and transduction efficiencies in murine wound healing. Wound Repair Regen. 2012;20(4):592–600. doi: 10.1111/j.1524-475X.2012.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jazwa A, Kucharzewska P, Leja J, et al. Combined vascular endothelial growth factor-A and fibroblast growth factor 4 gene transfer improves wound healing in diabetic mice. Genet Vaccines Ther. 2010;8:6. doi: 10.1186/1479-0556-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jakobsen M, Askou AL, Stenderup K, et al. Robust lentiviral gene delivery but limited transduction capacity of commonly used adeno-associated viral serotypes in xenotransplanted human skin. Human Gene Therapy Methods. 2015;26(4):123–33. doi: 10.1089/hgtb.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai SH, Tsao LP, Chang SH, et al. Pigment epithelium-derived factor short peptides facilitate full-thickness cutaneous wound healing by promoting epithelial basal cell and hair follicle stem cell proliferation. Exp Ther Med. 2017;14(5):4853–61. doi: 10.3892/etm.2017.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costa RA, Matos LBO, Cantaruti TA, et al. Systemic effects of oral tolerance reduce the cutaneous scarring. Immunobiology. 2016;221(3):475–85. doi: 10.1016/j.imbio.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Behrens J, Birchmeier W, Goodman SL, et al. Dissociation of Madin-Darby canine kidney epithelial cells by the monoclonal antibody anti-arc-1: Mechanistic aspects and identification of the antigen as a component related to uvomorulin. J Cell Biol. 1985;101(4):1307–15. doi: 10.1083/jcb.101.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corada M, Mariotti M, Thurston G, et al. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci USA. 1999;96(17):9815–20. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plow EF, Meller J, Byzova TV. Integrin function in vascular biology: A view from 2013. Curr Opin Hematol. 2014;21(3):241–47. doi: 10.1097/MOH.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 27.Larjava H, Plow EF, Wu C. Kindlins: Essential regulators of integrin signaling and cell-matrix adhesion. EMBO Rep. 2008;9(12):1203–8. doi: 10.1038/embor.2008.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang JR, Pan TJ, Yang H, et al. Kindlin-2 promotes invasiveness of prostate cancer cells via NF-kappaB-dependent upregulation of matrix metalloproteinases. Gene. 2016;576(1.3):571–76. doi: 10.1016/j.gene.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 29.He Y, Esser P, Schacht V, Bruckner-Tuderman L, Has C. Role of Kindlin-2 in fibroblast functions: implications for wound healing. J Invest Dermatol. 2011;131(1):245–56. doi: 10.1038/jid.2010.273. [DOI] [PubMed] [Google Scholar]
- 30.Feng C, Li YF, Yau YH, et al. Kindlin-3 mediates integrin alphaLbeta2 outside-in signaling, and it interacts with scaffold protein receptor for activated-C kinase 1 (RACK1) J Biol Chem. 2012;287(14):10714–26. doi: 10.1074/jbc.M111.299594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi X, Ma YQ, Tu Y, et al. The MIG-2/integrin interaction strengthens cell-matrix adhesion and modulates cell motility. J Biol Chem. 2007;282(28):20455–66. doi: 10.1074/jbc.M611680200. [DOI] [PubMed] [Google Scholar]
- 32.Felding-Habermann B, Cheresh MDA. Vitronectin and its receptors. Curr Opin Cell Biol. 1993;5(5):864–68. doi: 10.1016/0955-0674(93)90036-p. [DOI] [PubMed] [Google Scholar]
- 33.Liao Z, Kato H, Pandey M, et al. Interaction of Kindlin-2 with integrin beta3 promotes outside-in signaling responses by the alphaVbeta3 vitronectin receptor. Blood. 2015;125(12):1995–2004. doi: 10.1182/blood-2014-09-603035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pluskota E, Bledzka KM, Bialkowska K, et al. Kindlin-2 interacts with endothelial adherens junctions to support vascular barrier integrity. J Physiol. 2017;595(20):6443–62. doi: 10.1113/JP274380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedlander M, Brooks PC, Shaffer RW, et al. Definition of two angiogenic pathways by distinct alpha v integrins. Science. 1995;270(5241):1500–2. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- 36.Murakami M, Nguyen LT, Zhuang ZW, et al. The FGF system has a key role in regulating vascular integrity. J Clin Invest. 2008;118(10):3355–66. doi: 10.1172/JCI35298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murakami M, Sakurai T. Role of fibroblast growth factor signaling in vascular formation and maintenance: Orchestrating signaling networks as an integrated system. Wiley Interdiscip Rev Syst Biol Med. 2012;4(6):615–29. doi: 10.1002/wsbm.1190. [DOI] [PubMed] [Google Scholar]
- 38.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264(5158):569–71. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]