Abstract
Background
The aim of this study was to explore the effect of baicalein on diabetic cardiomyopathy (DCM) rats and the mechanisms involved, and to determine the theoretical basis for clinical anti-tumor therapy.
Material/Methods
DCM rat model was induced with a single injection of streptozotocin. Then, DCM rats were treated with baicalein alone or co-treated with baicalein and PI3K/Akt inhibitor. Myocardial pathological changes were detected by HE and Masson staining. The activities of SOD, GSH-Px, and MDA in myocardial tissue were measured by biochemical tests. The levels of TNF-α, IL-1β, and cTn-I were examined by ELISA. NADP+/NADPH ratio was measured with the NADP+/NADPH assay kit. RT-PCR was used to detect the levels of PI3K and Akt. The levels of Bax, Bcl-2, Caspase-3, GSK-3β, PI3K, and Akt were detected by Western blot.
Results
Baicalein could improve pathological injury. SOD and GSH-Px activity decreased while the level of MDA increased in myocardial tissue. Baicalein treatment enhanced SOD activity in a dose-dependent manner but markedly reduced MDA. Similar changes were observed in both serum inflammatory factors and the NADP+/NADPH ratio. After adding PI3K-Akt inhibitor, the levels of PI3K and Akt mRNA expression were significantly decreased, but were not significantly different from the DCM group. Levels of Bcl-2, PI3K, p-GSK-3β/GSK-3β, and p-Akt were decreased in the DCM group, while the levels of Bax and Caspase-3 were obviously increased.
Conclusions
Baicalein can protect DCM rats against damage from oxidative stress and inflammation in myocardial tissue, and PI3K/Akt signaling pathway may be involved to mediating these effects.
MeSH Keywords: Diabetic Cardiomyopathies, Inflammation, Oxidative Stress
Background
Diabetic cardiomyopathy (DCM) is a myocardial dysfunction in diabetic humans or animals. It is defined pathologically as mononuclear or mixed cellular infiltration associated with myocyte necrosis and degeneration in the presence or absence of fibrosis [1]. DCM is characterized by heart muscle disorder in the absence of coronary artery disease and has symptoms of early-onset diastolic and late-onset systolic dysfunction [2]. Previous studies reported that reactive oxygen species, cardiac inflammation, accumulation of cardiac fibrosis, hyperglycemia, and apoptosis are all likely implicated in the pathophysiology of DCM [3,4]. Currently, there is no effective specific treatment available for DCM [5,6]. Several therapeutic approaches, such as inhibition of the renin-angiotensin-aldosterone-system and supplementation of endogenous antioxidants, have been proposed for the treatment of DCM5,6. A novel therapeutic strategy targeting the phosphoinositide 3-kinase PI3K/Akt signaling pathway has recently been tested in an experimentally induced DCM rat model and showed therapeutic potential [6,7]. The PI3K/Akt signaling pathway has been reported to be implicated in human malignancy; it can suppress inflammation and mediate oxidative stress [8–12]. Therefore, compounds with anti-inflammatory or antioxidant features may be beneficial for DCM.
Baicalein is a bioflavonoid originally isolated from the roots of Scutellaria baicalensis and Scutellaria lateriflora (2 flowering plants used in herbal medicine). It is part of the active ingredients of Sho-Saiko-To, a Japanese herbal supplement used to improve liver health. It has been reported that baicalein can act as an anti-inflammatory agent [13], inhibit certain types of lipoxygenases [14], and exhibit antimicrobial activity [15]. Since cardiac inflammation is an important contributor to the development of DCM, we suspect that baicalein may have beneficial effects in the treatment of DCM, which has not been reported until now.
Thus, the aim of the present study was to examine the effect of baicalein on streptozotocin (STZ)-induced DCM and to determine the potential underlying mechanisms in STZ-induced rats.
Material and Methods
Experimental animals
We purchased 4-week old, male, SPF Sprague-Dawley rats (100–120 g) from Ji’nan Peng Yue Experimental Animal Breeding Co., Ltd. (SCXK (Lu) 20140007) in China. All animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals. All experiments were approved by the Animal Care and Use Committee. A total of 70 rats were used, and 10 rats were fed a basal diet and served as the control group, while the other 60 were fed a high-sugar, high-fat diet prepared by adding 20% sucrose and 20% lard. After 8 weeks, insulin resistance was observed in the rats fed the high-sugar, high-fat diet. Streptozotocin (STZ) was then intraperitoneally injected into the rat (40 mg/kg). The concentrations of fasting blood glucose were then measured at 72 h and 7 d after injection. Rats with fasting blood glucose values equal to or higher than 11.6 μmol/L for both measurements were considered as the diabetic rats.
The DCM rats were then equally divided into 4 groups: the DCM group; the 100 mg/kg baicalein group; the 200 mg/kg baicalein group; and the 200 mg/kg and 0.3 mg/kg LY294002 group (PI3K-AKT inhibitor). After 16 weeks of treatment, blood collection by tail snipping was performed on all the rats after anaesthesia through intraperitoneal injection of pentobarbital at a dose of 50 mg/kg. Then rats were sacrificed by cervical dislocation. One part of the myocardial tissue was fixed in 4% paraformaldehyde and embedded in paraffin, and the other part was homogenized in physiological saline solution and the 10% homogenate was centrifuged at 4000 rpm for 15 min at 4°C to collect the supernatant for further analysis.
HE assay and Masson assay
The embedded tissue was then cut into 5-μm sections. Subsequent to dewaxing with xylene, hydration was performed using a series of graded concentrations of ethanol (100% ethanol for 5 min, 95% ethanol for 1 min, 80% ethanol for 5 min, 75% ethanol for 5 min, and distilled water for 2 min). H&E staining was performed using the routine method at room temperature for 12 min. Following dehydration, sections were treated twice with xylene at room temperature for 10 min. Then, tissue sections were sealed with neutral resin. After that, according the Masson Staining Kit (Beijing LEYBOLD Cable Technology Co. Ltd.) manual, a series of operations, including hydrochloric acid alcohol differentiation (30 s), tap water soaking for 15 min, staining with eosin stain (Beijing LEYBOLD Cable Technology Co. Ltd.) for 2 min, dehydration, transparentizing, and mounting, were done to make the sample ready for tests. The pathological changes of the tissue were observed using a light microscope (Olympus BX51, Japan Olympus Company, magnification, ×400).
Measurement of bio-markers of oxidative stress
The activity of superoxide dismutase (SOD) in myocardial tissue was measured by Xanthine Oxidase assay. The activity of glutathione peroxidase (GSH-Px) was measured by a colorimetric method. Thiobarbituric acid (TBA) assay was performed to detect malondialdehyde (MDA). The procedure followed the instructions of the manufacturer (R&D Systems, USA).
Serum cytokines analysis
The levels of TNF-α (ab100785, Abcam), IL-1β (ab100768, Abcam), and cTn-I (KE1457, ImmunoWay) in serum were measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (R&D Systems, USA).
Measurement of the NADP+/NADPH ratio
The measurements were carried out with duplicate samples. For each measurement, a 20-mg tissue sample was placed into a 1.5-mL EP tube, followed by addition of 100 μL NADPH or 100 μL NADP. The sample was then homogenized, heated at 60°C for 5 min, and mixed with 20 μL assay buffer and 100 μL NADP or 100 μL NADPH. After centrifugation at 14 000×g for 5 min, the supernatant was then saved for colorimetric measurement of the levels of NADP+ or NADPH. A series of standards were also prepared and measured colorimetrically. The standard curve derived was then used to calculate the ratio of NADP+/NADPH in the sample.
RT-PCR
Total RNA was extracted using a Trizol kit (Takara, Japan). Its purity and integrity were determined and the reverse transcription was performed using a reverse transcription kit (Takara, Japan). The Primers A 1 μL cDNA configuration reaction system was used to perform the PCR reaction using the SYBR Green kit. PCR primer sequences were PI3K: Forward: 5′-ACTTTGTGACCTTCGGCTTT-3′, Reverse: 5′-TACATTCCTGATCTTCCTCG-3′; AKT: Forward: 5′-TGCTCATTGAGAATGTCGCGTCTC-3′, Reverse: 5′-AGGCATTCCGC AGGAAGGTAAAGA-3′; β-actin: 5′-ACGGCCAGGT
CATCACTATTG-3′, Reverse: 5′-CCTGCTTGCTGATCCACATCT-3′. PCR amplifications were performed at 95°C for 3 min, followed by 30 cycles at 95°C for 1 min, 56°C for 40 s, and 72°C for 1 min. β-actin was used as an endogenous control to normalize differences. Melting curves were created to ensure that the production of non-specific products was avoided. Quantification was performed by normalizing the cycle threshold values to those of β-actin and analyzing results using the 2−ΔΔCt method. The band intensities were calculated using Image J software (ABI, USA).
Western blot analysis
Total protein was extracted by using Tissue Total Protein Lysis buffer (Sangon Biotech CO., Ltd., Shanghai, China) according to the manufacturer’s protocol. The protein samples were incubated at 0°C for 30 min, and then centrifuged at 10 000×g at 4°C for 8 min, and supernatants were then extracted. Protein concentrations were quantified using a BCA Protein Assay Reagent kit (Pierce; Thermo Fisher Scientific, Inc.). Protein samples (40-μg) were separated via 10% SDS-PAGE and transferred to a polyvinylidene fluoride membrane. The membrane was blocked with 5% skimmed milk for 1 h at room temperature, prior to an overnight incubation at 4°C with rabbit anti-rat Bax polyclonal antibody (dilution, 1: 500; cat. no. CPA1091; Cohesion, Ltd.), rabbit anti-rat bcl-2 monoclonal antibody (dilution, 1: 500; cat. no. CPA7391; Cohesion, Ltd.), rabbit anti-rat Caspase-3 polyclonal antibody (dilution, 1: 500; cat. no. CPA1140; Cohesion, Ltd.), rabbit anti-rat GSK-3β polyclonal antibody (dilution, 1: 500; cat. no. orb14419; Biorbyt, Ltd.), rabbit anti-rat p-GSK-3β polyclonal antibody (dilution, 1: 500; cat. no. orb222837; Biorbyt, Ltd.), rabbit anti-rat PI3K polyclonal antibody (dilution, 1: 500; cat, no. CPA1891; Cohesion, Ltd.), rabbit anti-rat t-Akt polyclonal antibody (dilution, 1: 500; cat. no. orb159895; Biorbyt Ltd.), and rabbit anti-rat p-Akt polyclonal antibody (dilution, 1: 500; cat. no. orb222951; Biorbyt, Ltd.). The membrane was then washed 3 times for 5 min with TBST (TBS with 1ml/l Tween-20). Finally, the membrane was incubated with horseradish peroxidase-conjugated secondary antibodies (dilution, 1: 5000; cat. no. orb345943; Biorbyt, Ltd.) for 2 h at room temperature, and then washed 3 times for 10 min with TBST. Imaging was performed using enhanced chemiluminescence (ECL) Prime Western Blotting Detection reagent (GE Healthcare, Chicago, IL, USA) in a dark room. The expression of the protein samples was standardized to β-actin, then band densities were scanned and quantified using Image J 2.1 software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
SPSS Statistics V19.0 was used for statistical analysis. The relevant data are expressed as the mean ± standard deviation. Multiple comparisons between more than 2 groups were performed by one-way analysis of variance followed by least-significant difference or Kruskal-Wallis tests. P<0.05 was considered to indicate a statistically significant difference.
Results
Histopathological analysis
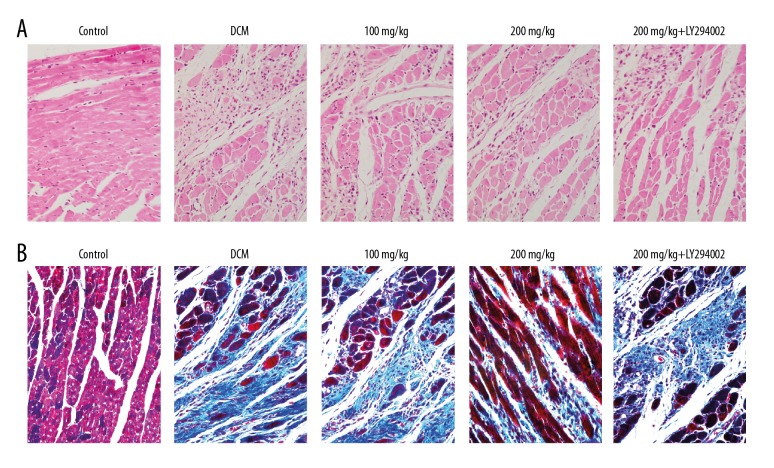
As shown in Figure 1A, in the control group, the normal myocardial cells were neatly and tightly arranged, with clear structure and less extracellular matrix. In the DCM group, hypertrophy and distortion were noted in the myocardial cells, which were irregularly arranged. In the baicalein treatment groups, the above pathological injury was improved to some extent. After adding PI3K-AKT inhibitor, there was no significant change in pathological injury compared with the model group. As shown in Figure 1B, compared with the control group, large blue collagen fibers were seen in the myocardial fibers of the DCM group, mainly distributed in the myocardial interstitium and the peripheral blood vessels. In the baicalein treatment groups, the above pathological injury was improved to some extent. After adding PI3K-AKT inhibitor, the pathological injury was not different from the DCM group.
Figure 1.
Pathological changes of myocardial tissue. (A) HE staining; (B) Masson staining. (×400).
Effect of baicalein on myocardial tissue oxidative stress parameters
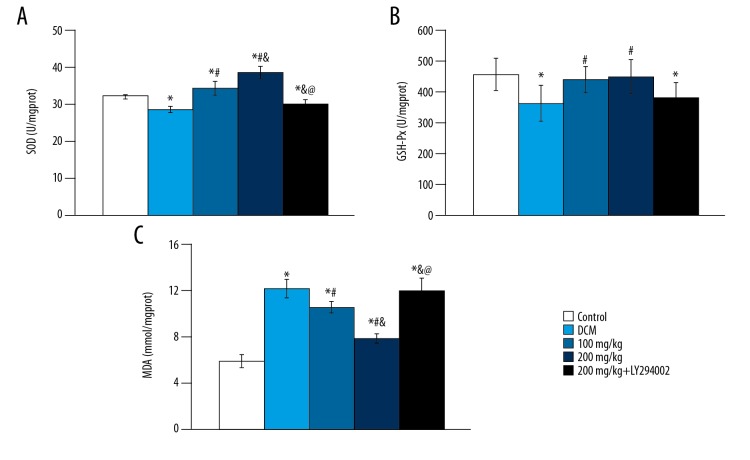
As shown in Figure 2, compared with control group, SOD and GSH-Px activity in DCM group were significantly decreased, while the content of MDA was significantly increased (p<0.05). In baicalein treatment groups, the activity of SOD increased significantly (p<0.05) in a dose-dependent manner, while the GSH-Px activity was not significantly different (p>0.05), and the MDA content decreased significantly (p<0.05). The activities of MDA, SOD, and GSH-Px were not significantly different from those in the DCM group after adding PI3K-AKT inhibitor (p>0.05), which indicated that the effect of baicalein was inhibited.
Figure 2.
Changes of SOD activity, GSH-Px activity and MDA level in different groups. (A) SOD activity; (B) GSH-Px activity; (C) MDA level. vs. control group, * p<0.05; vs. DCM group, # p<0.05; vs. 100 mg/kg group, & p<0.05; vs. 200 mg/kg group, @ p<0.05.
Effects of baicalein on serum cytokines
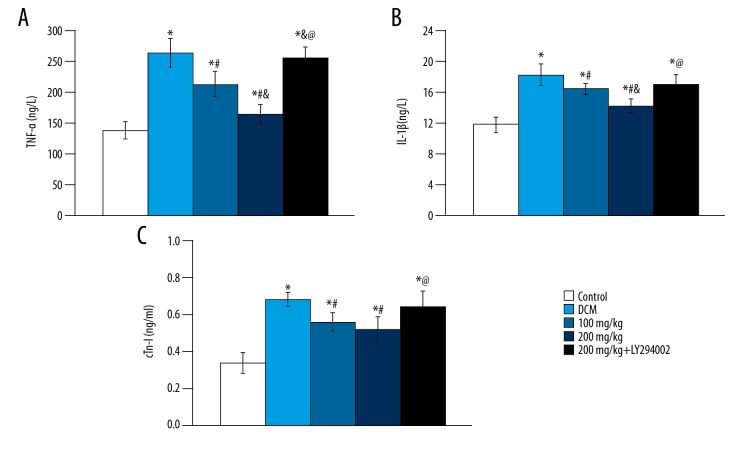
The levels of TNF-α, IL-1β, and cTn-I in serum were 264±23 ng/L, 18.2±1.4 ng/L, and 0.68±0.04 ng/ml, respectively, compared with the control group (138±14 ng/L, 11.9±0.9 ng/L, 0.34±0.06 ng/ml) were significantly increased (p<0.05). Compared with the DCM group, the levels of TNF-α, IL-1β, and cTn-I were significantly decreased (p<0.05), as were the levels of TNF-α, IL-1β, and cTn-I (p<0.05), in a dose-dependent manner (Figure 3). There was no significant difference in serum TNF-α, IL-1β, and cTn-I levels between the 2 groups (p>0.05) after adding PI3K-Akt inhibitor. Briefly, baicalein was shown to reduce the inflammatory response of DCM rats.
Figure 3.
Changes of TNF-α, IL-1β and cTn-I in different groups. (A) TNF-α; (B) IL-1β; (C) cTn-I. vs. control group, * p<0.05; vs. DCM group, # p<0.05; vs. 100 mg/kg group, & p<0.05; vs. 200 mg/kg group, @ p<0.05.
Effects of baicalein on NADP+/NADPH ratio
The ratio of NADP+/NADPH in DCM group was 2.2 times higher than that in the control group (p<0.05), which indicated that the activity of NADPH oxidase in the DCM group increased. The ratios of NADP+/NADPH in baicalein treatment groups were decreased by 27.28% and 83.33% (p<0.05), respectively, in a dose-dependent manner (Figure 4). The ratio of NADP+/NADPH increased with the PI3K-Akt inhibitor, which was not significantly different from the DCM group (p>0.05). Those results suggested that the protective effect of baicalein was inhibited.
Figure 4.
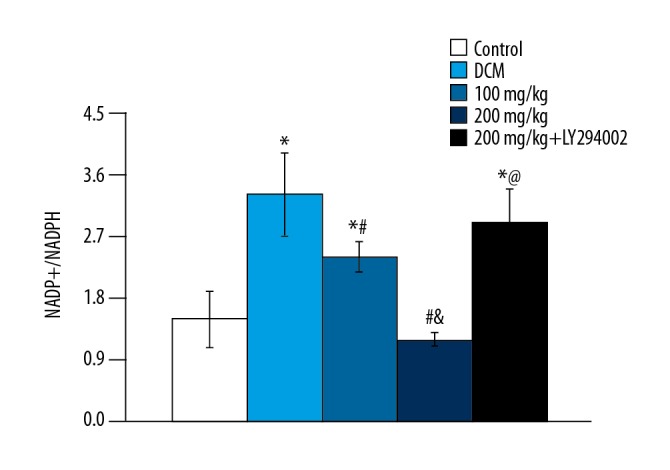
NADP+/NADPH ratios in different groups. vs. control group, * p<0.05; vs. DCM group, # p<0.05; vs. 100 mg/kg group, & p<0.05; vs. 200 mg/kg group, @ p<0.05.
Effect of baicalein on PI3K and Akt mRNA Expression
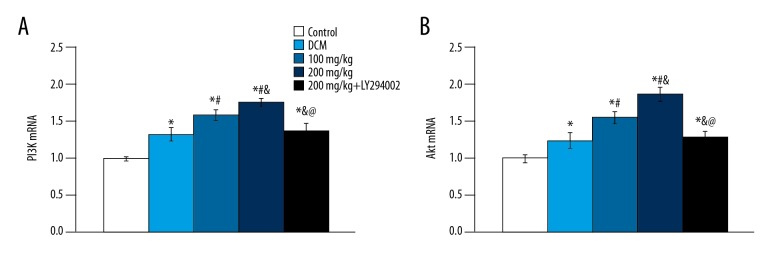
The expression of PI3K and Akt mRNA in the DCM group, 100 mg/kg group, and 200 mg/kg group were significantly higher than in the control group (p<0.05) (Figure 5), and levels in the baicalein treatment group were significantly higher than those in the DCM group (p<0.05). The expressions of PI3K and Akt mRNA were significantly decreased after adding PI3K-Akt pathway inhibitor, and there was no significant difference from the DCM group (p>0.05).
Figure 5.
PI3K and Akt mRNA expression in different groups. (A) PI3K mRNA; (B) Akt mRNA. vs. control group, * p<0.05; vs. DCM group, # p<0.05; vs. 100 mg/kg group, & p<0.05; vs. 200 mg/kg group, @ p<0.05.
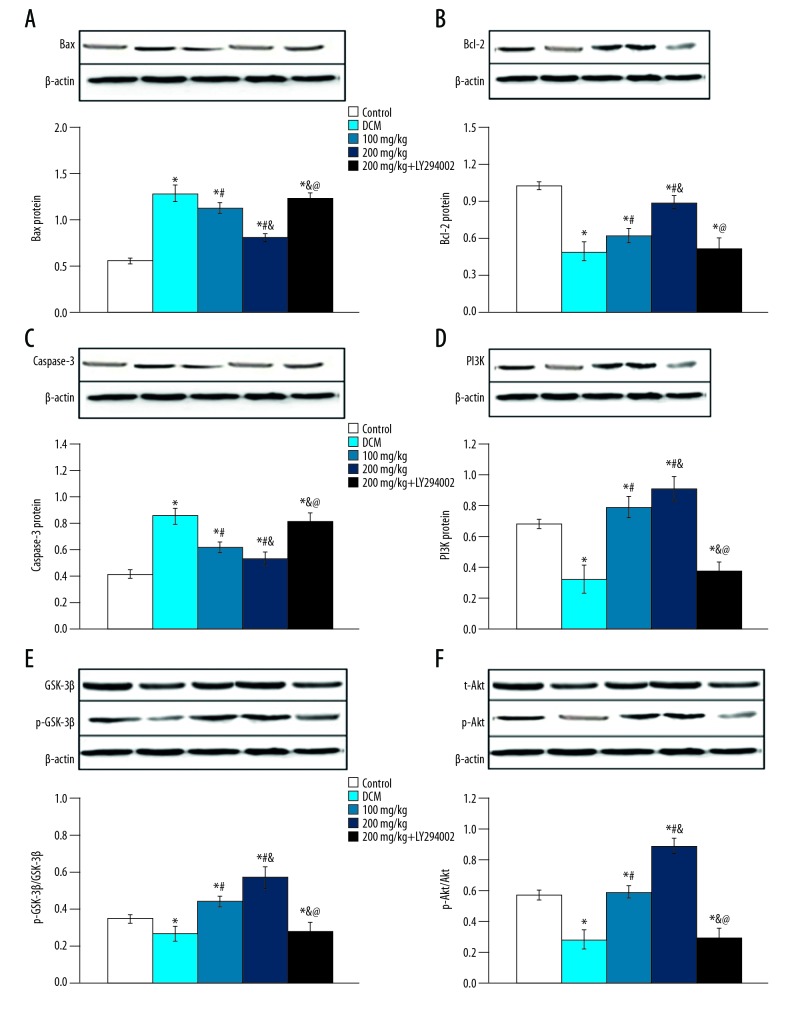
Effect of baicalein on Bax, Bcl-2, Caspase-3, GSK-3β, PI3K, and Akt protein expression
Compared with the control group, the expression of Bax and Caspase-3 protein in the DCM group increased, while the levels of Bcl-2, PI3K, p-GSK-3β/GSK-3β, and p-Akt expression were decreased (p<0.05). In baicalein treatment groups, the expressions of Bcl-2, PI3K, p-GSK-3β/GSK-3β, and p-Akt were significantly increased (p<0.05) and the levels of Bax and Caspase-3 expression were decreased. After adding PI3K-Akt inhibitor, the expression of Bax and Caspase-3 proteins were increased, while the expression of Bcl-2, PI3K, p-GSK-3β/GSK-3β, and p-Akt proteins were decreased (Figure 6). These results show that the effect of apoptosis protein expression is related to the PI3K/Akt signaling pathway.
Figure 6.
The protein expression in different group. (A) Bax; (B) Bcl-2; (C) Caspase-3; (D) PI3K; (E) GSK-3β; (F) Akt. vs. control group, * p<0.05; vs. DCM group, # p<0.05; vs. 100 mg/kg group, & p<0.05; vs. 200 mg/kg group, @ p<0.05.
Discussion
DCM is a disorder of the heart muscle in diabetic humans or animals. There are still no effective preventive or therapeutic approaches. In this study, a diabetic rat model was induced by a combination of STZ and high-energy dietary intake. Intraperitoneal injection of baicalein was used to observe the protective effect of baicalein on DCM and to explore its possible mechanism.
It was reported that oxidative stress plays an important role in the development of DCM [6]. Some enzymes, such as SOD and GSH, provide cellular protection against damage from oxygen-derived free radicals [16]. MDA is the end-product of the oxygen-derived free radicals and lipid oxidation, which reflects the damage caused by reactive oxygen species [17]. In the experiment, our results showed that DCM rats had decreased activity in both SOD and GSH-Px but had elevated levels of MDA. However, treatment with baicalein enhanced SOD activity in a dose-dependent manner and the MDA level decreased dramatically. Oxidative stress is an imbalance between the generation and elimination of reactive oxygen species (ROS), which is a key player in heart failure [18,19]. It has also been reported that NADPH oxidase plays a critical role in ROS generation [20,21]. The ratios of NADP+/NADPH in baicalein treatment groups were decreased in a dose-dependent manner. These biological parameters demonstrated baicalein can protect DCM rats by inhibition of oxidative stress.
Numerous studies suggested important roles of cardiac inflammation in the development of DCM [3,4]. Cardiac inflammation is characterized by increased levels of pro-inflammatory cytokines, and these pro-inflammatory cytokines are major contributors of DCM [22]. In the present study, we examined the serum levels of TNF-α, IL-1β, and cTn-I. We observed that DCM rats showed markedly elevated levels of serum inflammatory factors. HE and Masson staining showed baicalein can improve pathological injury. Those results indicate that baicalein can protect DCM rats by suppression of inflammation.
It was reported that the PI3K/Akt signaling pathway was related to DCM [7]. LY294002, a selective inhibitor of PI3K, was used in our study. HE and Masson staining showed that after adding PI3K-AKT inhibitor, the pathological injury was not different from the DCM group. Western blot analysis showed that after adding PI3K/Akt inhibitor, the expressions of Bax and Caspase-3 proteins were increased, while the expressions of Bcl-2, PI3K, p-GSK-3β/GSK-3β, and p-Akt proteins were decreased. This means that the effect of apoptosis protein expression is related to the PI3K/Akt signaling pathway. We also observed that co-treatment with baicalein and PI3K/Akt inhibitor offset the effect of baicalein on DCM, indicating that its effect of regulating oxidative stress and anti-inflammation was inhibited by PI3K/Akt inhibitor.
Conclusions
We conclude that baicalein shows promise as a therapy for DCM. Baicalein protected DCM rats by regulating oxidative stress parameters and inflammatory cytokines, and the PI3K/Akt signaling pathway mediated these effects.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Shimada K, Okabe TA, Mikami Y, et al. Therapy with granulocyte colony-stimulating factor in the chronic stage, but not in the acute stage, improves experimental autoimmune myocarditis in rats via nitric oxide. J Mol Cell Cardiol. 2010;49:469–81. doi: 10.1016/j.yjmcc.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Huynh K, McMullen J, Julius T, et al. Cardiac-specific IGF-1 receptor transgenic expression protects against cardiac fibrosis and diastolic dysfunction in a mouse model of diabetic cardiomyopathy. Diabetes. 2010;59:1512–20. doi: 10.2337/db09-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Westermann D, Walther T, Savvatis K, et al. Gene deletion of the kinin receptor B1 attenuates cardiac inflammation and fibrosis during the development of experimental diabetic cardiomyopathy. Diabetes. 2009;58:1373–81. doi: 10.2337/db08-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falcao-Pires I, Leite-Moreira A. Diabetic cardiomyopathy: Understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Fail Rev. 2012;17:325–44. doi: 10.1007/s10741-011-9257-z. [DOI] [PubMed] [Google Scholar]
- 5.Sahu BD, Mahesh Kumar J, Sistla R. Baicalein, a bioflavonoid, prevents cisplatin-induced acute kidney injury by up-regulating antioxidant defenses and down-regulating the MAPKs and NF-κB pathways. PLoS One. 2015;10:e0134139. doi: 10.1371/journal.pone.0134139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huynh K, Bernardo B, McMullen J, Ritchie R. Diabetic cardiomyopathy: Mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol Ther. 2014;142:375–415. doi: 10.1016/j.pharmthera.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Yu W, Wu J, Cai F, et al. Curcumin alleviates diabetic cardiomyopathy in experimental diabetic rats. PLoS One. 2012;7:e52013. doi: 10.1371/journal.pone.0052013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sourbier C, Lindner V, Lang H, et al. The phosphoinositide 3-kinase/Akt pathway: A new target in human renal cell carcinoma therapy. Cancer Res. 2006;66:5130–42. doi: 10.1158/0008-5472.CAN-05-1469. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson K, Anderson N. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–95. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 10.Hanada M, Feng J, Hemmings B. Structure, regulation and function of PKB/AKT-a major therapeutic target. Biochim Biophys Acta. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Schabbauer G, Tencati M, Pedersen B, et al. PI3K-Akt pathway suppresses coagulation and inflammation in endotoxemic mice. Arterioscler Thromb Vasc Biol. 2004;24:1963–69. doi: 10.1161/01.ATV.0000143096.15099.ce. [DOI] [PubMed] [Google Scholar]
- 12.Hambright H, Meng P, Kumar A, Ghosh R. Inhibition of PI3K/AKT/mTOR axis disrupts oxidative stress-mediated survival of melanoma cells. Oncotarget. 2015;6:7195–208. doi: 10.18632/oncotarget.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsieh CJ, Hall K, Ha T, et al. Baicalein inhibits IL-1β- and TNF-α-induced inflammatory cytokine production from human mast cells via regulation of the NF-κB pathway. Clin Mol Allergy. 2007;5:5. doi: 10.1186/1476-7961-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deschamps J, Kenyon V, Holman T. Baicalein is a potent in vitro inhibitor against both reticulocyte 15-human and platelet 12-human lipoxygenases. Bioorg Med Chem. 2006;14:4295–301. doi: 10.1016/j.bmc.2006.01.057. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Liu T, Wang K, et al. Baicalein inhibits Staphylococcus aureus biofilm formation and the quorum sensing system in vitro. PLoS One. 2016;11:e0153468. doi: 10.1371/journal.pone.0153468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaafi S, Afrooz Razm M, Hajipour B, et al. Anti-oxidative effect of lipoic acid in spinal cord ischemia/reperfusion. Med Princ Pract. 2011;20:19–22. doi: 10.1159/000319772. [DOI] [PubMed] [Google Scholar]
- 17.Qian H, Liu D. The time course of malondialdehyde production following impact injury to rat spinal cord as measured by microdialysis and high-pressure liquid chromatography. Neurochem Res. 1997;22:1231–36. doi: 10.1023/a:1021980929422. [DOI] [PubMed] [Google Scholar]
- 18.Rajesh M, Mukhopadhyay P, Batkai S, et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol. 2010;56:2115–25. doi: 10.1016/j.jacc.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Wang H, Hao P, et al. Inhibition of aldehyde dehydrogenase 2 by oxidative stress is associated with cardiac dysfunction in diabetic rats. Mol Med. 2011;17:172–79. doi: 10.2119/molmed.2010.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frantz S, Brandes R, Hu K, et al. Left ventricular remodeling after myocardial infarction in mice with targeted deletion of the NADPH oxidase subunit gp91PHOX. Basic Res Cardiol. 2006;101:127–32. doi: 10.1007/s00395-005-0568-x. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Wang X, Gong L, et al. Nox1 promotes colon cancer cell metastasis via activation of the ADAM17 pathway. Eur Rev Med Pharmacol Sci. 2016;20:4474–81. [PubMed] [Google Scholar]
- 22.Mano Y, Anzai T, Kaneko H, et al. Overexpression of human C-reactive protein exacerbates left ventricular remodeling in diabetic cardiomyopathy. Circ J. 2011;75:1717–27. doi: 10.1253/circj.cj-10-1199. [DOI] [PubMed] [Google Scholar]