Abstract
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), is characterized by chronic relapsing inflammation of the gastrointestinal tract. Delivery of orally administered drugs to the colon is highly desirable for the treatment of UC, as it improves their efficacy while reducing systemic toxicity. However, the targeting of oral drugs to the colon, which is located at the distal end of the gastrointestinal tract, is difficult due to physiological challenges, biochemical barriers, and environmental barriers, including those associated with mucus and epithelium. Recent preclinical studies have indicated that nanoparticle-based drug delivery systems (DDS) may be promising tools for targeted delivery to the colon, with potentially effective outcomes in the treatment of UC. This review highlights general considerations and limitations for oral drug delivery to the colon. Further, this review provides a systematic evaluation of synthetic nanoparticle-based DDS, and emerging naturally derived nanoparticles (eg, extracellular vesicles and plant-derived nanoparticles). These novel nanoparticle-based treatment strategies for UC may offer the opportunity for the practical translation of nanoparticle formulas into the clinic.
Keywords: IBD therapy, ulcerative colitis, nanoparticles, oral drug delivery systems
INTRODUCTION
Inflammatory bowel disease (IBD) is a group of chronic relapsing disorders of the gastrointestinal (GI) tract that are characterized pathologically by intestinal inflammation and epithelial injury.1 Crohn’s disease (CD) and ulcerative colitis (UC) are the 2 major types of IBD. CD affects the entire length of the GI tract, from the oral mucosa to the anus, with the terminal ileum being the most affected part in 90% of patients.2 UC characteristically involves only the large bowel; it begins in the rectum and progressively extends to the proximal colon, and some patients with severe disease experience a tropism for the appendix.3
To date, the etiology of IBD is not completely understood.4 Conventional medication for IBD therapy comprises anti-inflammatory drugs (eg, 5-aminosalicyclic acid and corticosteroids) and immunosuppressive agents (eg, azathioprine, 6-mercaptopurine, methothexate, ciclosporin-A and tacrolimus).5 The emergence of monoclonal antibodies as biological therapies has significantly increased the treatment options for IBD in recent years. In 1998, the tumor necrosis factor (TNF)–α antibody inflixmab was the first biological to be approved by the US Food and Drug Administration (FDA) for the therapy of severe, active, and fistulizing CD.6 Since then, further TNF-α antibodies for IBD treatment, such as adalimumab or certolizumab, have made it to the market, with more still in the development pipelines.7 In addition, other antibodies such as ustekinumab and natalizumab targeting IL-12/IL-23 and adhesion molecules also have been suggested as therapeutic options in IBD (Table 1).8 However, there is still a large unmet need for novel therapeutic approaches as many patients do not respond to the clinically approved drugs, including TNF blockers and vedolizumab.9–11
TABLE 1:
Monoclonal Antibody–Based Biological Therapies for IBD
| Structure | Drug name | Indications | Target(s) | Route |
|---|---|---|---|---|
 |
Infliximab (75% human, 25% mouse) | CD and UC | TNF-α | Intravenous |
 |
Adalimumab (100% human) | CD and UC | TNF-α | Subcutaneous injection |
 |
Golimumab (100% human) | UC | TNF-α | Subcutaneous injection |
 |
Certolizumab pegol (humanized Fab fragment) | CD | TNF-α | Subcutaneous injection |
 |
Ustekinumab (100% human) | CD | IL-12 and IL-23 | Subcutaneous injection |
 |
Natalizumab (humanized) | CD | α4β1and α4β7 | Intravenous |
 |
Vedolizumab (humanized) | CD and UC | α4β7 | Intravenous |
IBD predominantly affects the colon, and consequently colon-targeted drug delivery systems have received significant attention for IBD therapy. The mechanisms used in traditional drug delivery approaches can be generally divided into (1) approaches involving pH-dependent coating polymers, (2) time-dependent approaches, and approaches based on (3) pro-drugs and (4) polysaccharides. As shown in Table 2, these approaches have been extensively investigated, and the FDA has approved several of them for clinical application. However, these traditional colon-targeting approaches may vary in specificity and release profile; some of them continuously release the drug throughout the gastrointestinal tract before the delivery system arrives in the colon, reducing drug availability and increasing the likelihood of systemic adverse effects. These deficiencies emphasize the need for novel drug delivery systems that maximize the release of the drug at the inflamed colon without affecting normal tissues, thereby reducing the adverse effects of the drug.
TABLE 2:
Current Delivery Systems for Drugs Approved by the FDA for IBD Treatment by Oral Adminisration
| Drug | Brand Names | Formulation | Mechanism(s) |
|---|---|---|---|
| Aminosalicylate (5-ASA) | Azulfidine | 5-ASA linked to Sulfapyridine by azo-bond | Enzymatic reduction |
| Asacol | 5-ASA coated with Eudragit-S | pH-responsive | |
| Asacol HD | 5-ASA coated with Eudragit-S | pH-responsive | |
| Apriso | 5-ASA coated with Eudragit-L100, polyacrylate-dispersion, povidone K, simeticone | pH-responsive | |
| Colazal | 5-ASA linked to 4-aminobenzoyl-β-alanine by azo-bond | Enzymatic reduction | |
| Colazide | 5-ASA linked to 4-aminobenzoyl-β-alanine by azo-bond | Enzymatic reduction | |
| Claversal | 5-ASA coated with Eudragit-L | pH-responsive | |
| Calitoflak | 5-ASA coated with Eudragit-L100 | pH-responsive | |
| CODES | Polysaccharide coating coupled with a pH-sensitive polymer coating | pH-responsive + bacteria degradation | |
| Dipentum | 5-ASA dimer linked by azo-bond | Enzymatic reduction | |
| Lialda | 5-ASA coated with Multi Matix system with lipophilic and hydrophilic matrices | pH-responsive + time-delayed | |
| Mezavant | 5-ASA coated with Multi Matix system with lipophilic and hydrophilic matrices | pH-responsive + time-delayed | |
| Pentasa | 5-ASA microgranules coated in ethylcellulose | Time-dependent | |
| Salazopyrin | 5-ASA linked to Sulfapyridine by azo-bond | Enzymatic reduction | |
| Salofalk | 5-ASA coated with Eudragit-L | pH-responsive | |
| SalofalkGranu-Stix | 5-ASA coated with Eudragit-L100, polyacrylate-dispersion, povidone K, simeticone | pH-responsive | |
| Budesonide | Entocort EC | Eudragit-L coated beads with ethyl cellulose matrix | pH-responsive + time-dependent |
| Uceris | Multi Matix system using a Eudtagit-S coating a matrix core containing | pH-responsive + time-delayed | |
| Beclomethasone | Clipper | Eudtagit-L 100–55 coated tablet | pH-responsive |
The development of new technologies has expanded opportunities for IBD treatment. In particular, nanoparticles may be promising tools for the targeted delivery of drugs to specific sites of the inflamed colon by employing different mechanisms (Fig. 1). Nanoparticle-based drug delivery systems (DDS) provide several substantial advantages, including (1) they provide high local drug concentration at the site of disease for prolonged pharmacological activities and maximized drug efficacy; (2) nanoparticle-based targeted delivery may prevent or reduce drug degradation and loss of efficacy before reaching the site of action; (3) targeted drug delivery in IBD has the potential to reduce dosing frequency and minimize systemic side effects. This article provides a comprehensive overview of current and emerging nanoparticle-based drug delivery systems for colon-targeted delivery and for treatment of UC. Use of these novel nanoparticle-based UC treatment strategies may offer the opportunity for the practical translation of nanoparticle formulas into the clinic.
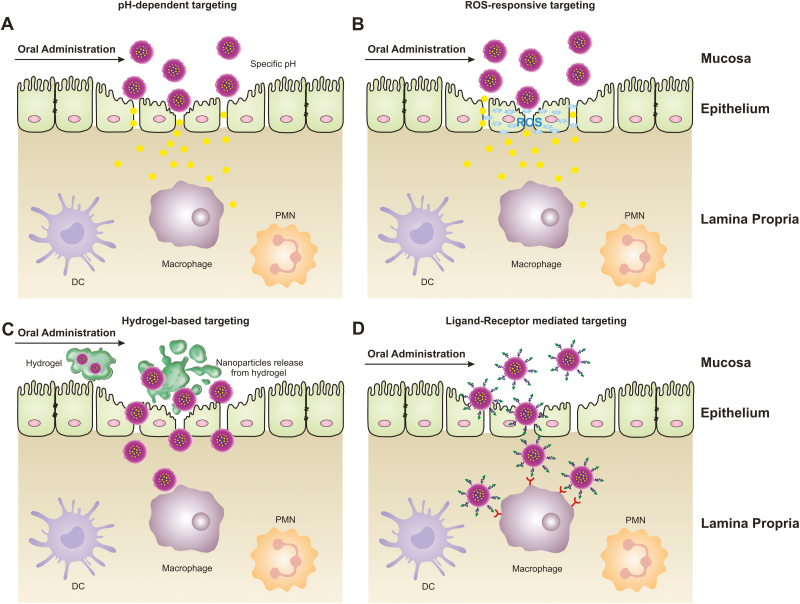
FIGURE 1.
Nanoparticle-based delivery strategies for ulcerative colitis (UC). Nanoparticles employ different targeting mechanisms to cross the epithelial barrier and reach the UC site. When delivered by such a system, nanoparticles can achieve a high local drug concentration, experience less drug degradation and loss of efficacy before reaching the site of action, and minimize systemic drug side effects. A, Following oral administration, nanoparticles target the epithelium of the inflamed colon based on its specific pH. B, Following oral administration, nanoparticles target the epithelium of the inflamed colon based on its specific level of reactive oxygen species (ROS). C, Following oral administration, nanoparticles are delivered to the inflamed colon in a hydrogel. D, Following oral administration, nanoparticles target the inflamed colonic epithelium by a ligand-receptor interaction. Abbreviation: PMN, polymorphonuclear leukocyte.
NANOPARTICLES
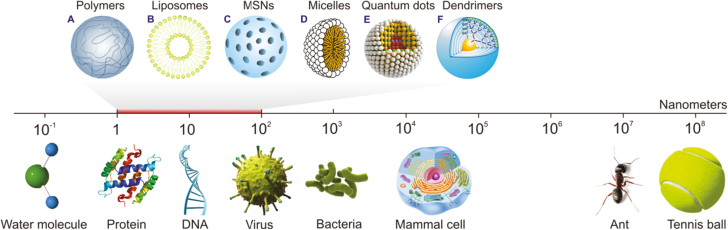
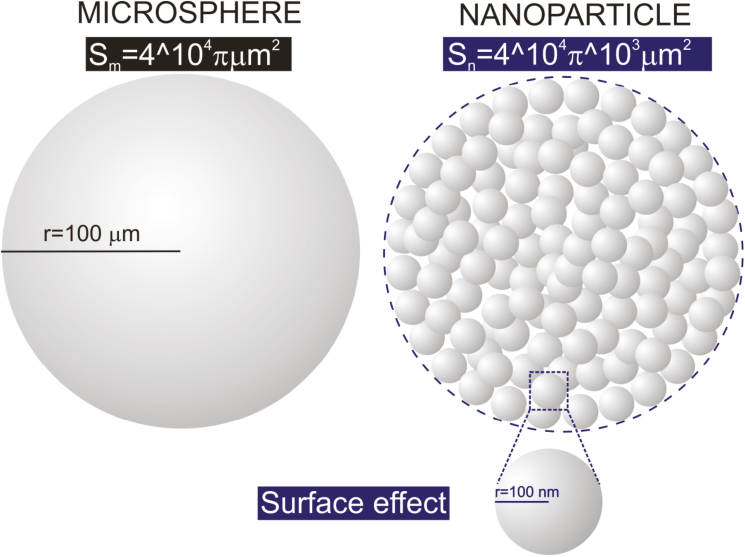
Nanoparticles (NPs) are generally defined as any particulate material with at least 1 dimension lying in the range of 1–100 nm. They can be composed of 1 or more species of atoms (or molecules) and can exhibit a wide range of size-dependent properties.12 Given their size range, nanoparticles bridge the gap between small molecules and bulk materials in terms of their energy state. To better conceptualize the nano-scale, consider that the diameter of a water molecule is about 0.3 nm, making it one of the smallest of all molecules, whereas most viruses are 20 to 400 nm in diameter, and mammalian cells tend to be about 104 nm (Fig. 2).13 The size of a nanoparticle greatly depends on the process used for its synthesis. Two approaches have been used to prepare ultrafine particles: the breakdown (top-down) method, in which an external force is used to break a solid into smaller particles; and the build-up (bottom-up) method, in which gas or liquid atoms are assembled into nanoparticles via atomic transformations or molecular condensation.14 Nanoparticles have several unique properties that are not found in their bulk counterparts, including a high surface-to-volume ratio (Fig. 3), a high surface energy, and unique mechanical, thermal, electrical, magnetic, and optical behaviors. These unique properties make nanoparticles suitable for a wide range of applications, such as in biology and medicine.15–17 Their chemical reactivity and dispersibility in various solvents can be regulated by modifying their surface with desired functional groups, enabling them to suit particular applications.
FIGURE 2.
Illustration comparing the sizes of various particles and the main units of living entities. A, Polymeric nanoparticles are solid polymeric matrices. B, Liposomes are vesicles formed from a phospholipid bilayer that mimics the cell membrane structure. C, Mesoporous silica nanoparticles (MSNs) comprise a solid framework with a porous structure and large surface area; different functional groups may be attached at the surface to target the drug moiety to a particular site. D, Micelles comprise an aggregate (or supramolecular assembly) of surfactant molecules dispersed in a liquid colloid. A typical micelle in aqueous solution forms an aggregate with the hydrophilic “head” regions in contact with the surrounding solvent, sequestering the hydrophobic single-tail regions in the center of the micelle. E, Quantum dots are semiconductor particles that are so small (several nanometers) that their optical and electronic properties differ from those of larger particles. F, Dendrimers are highly branched, star-shaped macromolecules with nanometer-scale dimensions. They are defined by 3 components: a central core, an interior dendritic structure (the branches), and an exterior surface with functional surface groups.
FIGURE 3.
Illustration comparing the surface-to-volume ratios of a micronanoparticle (Sm) and a nanoparticle (Sn). Most nanoparticles are spherical and thus have a surface-to-volume ratio of 3/r, where r is the radius. As r decreases, the surface-to-volume ratio increases.
GENERAL CONSIDERATION FOR TARGETED DRUG DELIVERY TO THE COLON
pH and Enzymes
The biochemical microenvironment encountered by orally administered nanoparticle-based drug delivery systems includes the different pH values and enzymatic activities within the GI tract. Each segment of the GI tract maintains its own characteristic pH, from the acidic stomach lumen (pH 1–3) required for digestion, to the mildly alkaline duodenum and ileum (pH 6.6–7.5) required for neutralization, to neutral (pH 7–8) in the colon.18 Drugs may be susceptible to different pH values, resulting in, for example, their oxidation, deamination, or hydrolysis, thus potentially causing loss of activity.19 For example, the extreme acidity in the stomach can rapidly degrade many peptide- and protein-based drugs, and drugs such as erythromycin, penicillin, and omeprazole.
Drugs are also vulnerable to enzymes present throughout the GI tract, such as salivary amylase in the mouth, pepsin and gastric lipase in the stomach, and trypsin in the intestine. The colon harbors more than 400 different species of aerobic and anaerobic microorganisms.20 These bacteria contain several hydrolytic and reductive metabolizing enzymes, which are responsible for the degradation of di-, tri-, and polysaccharides.21 Therefore, polysaccharides such as chitosan, guar gum, and pectin are commonly employed as drug delivery carriers for agents targeting the colon.
Mucus
The mucus layer is the first physical barrier encountered by orally administered drugs.22 Mucus is a hydrogel complex composed of carbohydrates, proteins (including antibodies), lipids, bacterial debris, and inorganic salts.23 The barrier properties of mucus are rooted in its dense network of mucin fibers, which contain highly glycosylated, negatively charged segments. In addition, mucins contain regularly spaced hydrophobic domains, which bind hydrophobic particles with high avidity.24 Although mucus does not directly act as a barrier to the oral delivery of many small and large therapeutic molecules, encapsulation of these drugs within nanoparticle carriers may improve their oral bioavailability.25 The ability of a carrier to interact with mucus can determine whether the drug can reach and be absorbed by epithelial cells. Orally delivered nanoparticles can (1) remain bound to the chyme, transiting rapidly through the GI tract and being rapidly eliminated through the feces; (2) bind to loosely adherent mucus, remaining bound until the mucus is cleared; or (3) penetrate the mucus, remaining bound to firmly adherent mucus or entering the intestinal epithelium.
Epithelium
The harsh acidic environment and presence of enzymes in the stomach make this organ infeasible for drug absorption.26 In contrast, the intestinal epithelium, consisting of various types of cells and structures, is highly absorptive. The epithelium constituting intestinal villi consists mainly of enterocytes and goblet cells. Enterocytes control the passage of macromolecules and pathogens, while allowing the digestive absorption of dietary nutrients.27 Goblet cells secrete the mucus gel layer, a viscous fluid composed primarily of high–molecular weight glycoproteins (mucins) suspended in a solution of electrolytes. In addition, M cells, located within the epithelium of Peyer’s patches, are composed of enterocytes and a few goblet cells. These cells are tightly connected by tight junctions, composed of claudins, occludins, and junction adhesion molecules, forming a strong barrier that hinders the passage of molecules and pathogens.28
Under physiological conditions, transport via the paracellular route is limited because of the very small surface area of the intercellular spaces and the tightness of the junctions between epithelial cells.29 Intestinal permeability may be modified by IBD, especially UC, allowing nanoparticles to be more easily transported through the intestinal epithelial barrier.30
Transcellular transport of nanoparticles involves transcytosis, in which particles are taken up by cells. This begins with an endocytic process that takes place at the cell apical membrane. Subsequently, nanoparticles are transported through the cells and released at the basolateral pole, where they may interact with immune cells in the submucosal layer. However, because enterocytes have low endocytic activity, transport efficiency via this route is usually very low. In addition, the transport of nanoparticles by this route depends on (1) the physicochemical properties of the particles, including their size, zeta potential, and surface hydrophobicity and the presence of a ligand at the particle surface; (2) the physiology of the GI tract; and (3) the animal model used.31
P-Glycoprotein Efflux Bump
P-glycoprotein (P-gp) is an efflux membrane transporter that functions as an ATP-dependent drug efflux bump at the apical surface of cells.32 P-glycoprotein may act as a hydrophobic vacuum cleaner, extracting hydrophobic substrates that enter the membrane lipid bilayer from the lumen back to the extracellular medium, before they reach the cytoplasm. These hydrophobic substrates can include anticancer agents, immunosuppressants, steroid hormones, calcium channel blockers, beta-adrenoreceptor blockers, and cardiac glycosides. P-glycoprotein is highly expressed in the epithelial cells of the small intestine, suggesting the importance of this protein’s role in limiting the oral bioavailability of certain drugs.33 For example, many anticancer drugs, including vincristine and paclitaxel, are substrates of P-glycoproteins and not optimally available by the oral route.34
Microbiota
The microbiota affects the physiological functions of the GI tract, from maintaining local barrier homeostasis to systemically regulating metabolism, inflammation, immunity, and other functions.35, 36 Most of the vast intestinal microbiome resides in the anaerobic colon and fulfills its energy needs by fermenting various substrates that were not digested in the small intestine (eg, di-, tri-polysaccharides, mucopolysaccharides). To utilize this roughage as a source of carbon, the anaerobic bacteria produce a wide range of reductive and hydrolytic enzymes. They react to the constantly changing mixture of complex carbohydrates entering the colon by recognizing a variety of substrates and producing the appropriate digestive enzymes.37 Thus, drug delivery systems can be designed to include prodrugs and biodegradable polymers that will be specifically degraded by the enzymes of the microbiota. The biodegradable polymers used in nanoparticle-based drug delivery systems (eg, naturally occurring polysaccharides obtained from plants, animals, algae, and microbes) are broken down by the colonic microbiota to simple saccharides.38, 39 It is also notable that the vast microbiota can change the redox potential, which is an expression of total metabolic and bacterial activity.40 Thus, microbiota-induced changes in the redox potential could be used as a highly specific mechanism for colon-targeted drug delivery.
COLON SYSTEMS TARGETED BY SYNTHETIC NANOPARTICLES
Recently developed synthetic nanoparticle-based drug delivery systems have displayed features making them suitable for the oral delivery of drugs targeting the colon.41 Nanoparticles can overcome physiological barriers and deliver drugs to sites of inflammation directly. These nanoparticles can be used therapeutically as drug carriers, dissolving, entrapping, or encapsulating the active substance (a drug or biologically active material). Alternatively, active substances can absorb onto or be attached to nanoparticles. The advantages of nanoparticle delivery systems over conventional colon-targeted approaches include improved efficacy, reduced toxicity, and enhanced bio-distribution.42 This section reviews current orally administered nano-delivery systems for UC, involving different strategies for the delivery of targeted drugs to inflamed colon tissue (Fig. 1).
pH-Dependent Nano-Delivery Systems
Nanoparticles responsive to stimuli have been developed to further improve the stability of orally administered drugs. For example, pH-sensitive nanoparticles are made of materials, such as acrylates and anionic polymers, that are insoluble at low pH and dissolve and/or swell at higher pH.43 Their pH sensitivity can enable these nanoparticles to retain and possibly protect their cargo under the acidic conditions prevalent in the stomach and small intestine, allowing drug release in the higher pH environment of the colon.44 Most pH-sensitive materials have been found to be safe and have been used in the manufacture of gastro-resistant solid dosage forms, some of which are already available commercially.45
The most commonly used pH-dependent coating polymers for oral delivery are methacrylic acid copolymers (Eudragit). Varying the composition of their side chains can alter the pH at which they are soluble.46 For example, pH-sensitive nanoparticles loaded with budesonide (BSD), a topically active corticosteroid, were prepared using mixtures of poly-(lactic-co-glycolic) acid (PLGA) and methacrylate copolymer (Eudragit S100). These nanospheres showed strong pH-dependent drug release properties at acidic and neutral pH, followed by a sustained release phase at pH 7.4. Animal experiments in a trinitrobenzene sulfonic acid (TNBS)–induced model of colitis showed that BSD-loaded nanospheres had greater therapeutic efficacy than BSD alone. These nanospheres showed stronger and more specific adhesion to ulcerated and inflamed rat mucosal colonic tissue in vivo and lower systemic toxicity than conventional enteric-coated microparticles.47 Moreover, drug release from Eudragit S100–coated liposomes was significantly lower in solutions that simulated the pH conditions of the stomach (pH 1.4) and small intestine (pH 6.3), but was equivalent to uncoated control at pH 7.8, indicating that this liposome formulation displayed appropriate pH responsive release characteristics. The coating layer was unable to withstand the additional challenge of bile salts, suggesting that its stability may be adversely affected in vivo, resulting in premature degradation of the liposomes and release of the drug in the duodenum.48
Another interesting pH-responsive nano-system is based on ε-polylysine-coated mesoporous silica nanoparticles (SPL-Pred-MCM).49 A large amount (ca. 34% w/w) of prednisolone was loaded into 3-aminopropyl-functionalized mesoporous silica nanoparticles (MCM-NH2), with prednisolone release targeted to the colon by coating the nanoparticles with succinylated ε-polylysine (SPL). An in vitro study demonstrated that SPL-Pred-MCM selectively released prednisolone at the pH prevalent in the colon (pH 5.5–7.4), but not at the more acidic conditions of the stomach (pH 1.9) and small intestine (pH 5.0). Moreover, SPL-Pred-MCM could deliver cargo intracellularly to immune cells (RAW 264.7 macrophages) and intestinal epithelial cells (LS 174T and Caco-2 adenocarcinoma cell lines), suggesting that this drug delivery system is highly promising for diseases such as IBD and colorectal cancer.
Despite the promising results of these pH-dependent nano-delivery systems targeting the colon, major concerns include the inherent interindividual and intra-individual differences in pH, and the change in luminal pH due to disease state should draw our attention. Thus, when designing a delivery system to target the colon based on pH alone, researchers should consider the individual inflammation state for better disease therapy.
ROS-Responsive Nano-Delivery Systems
Drug carriers that respond to changes in redox potential may also show clinical efficacy in the treatment of UC.50 Oxidative stress has been defined as an imbalance between the generation of reactive oxygen species (ROS) and the decrease in antioxidant defense systems.51 Oxidative stress is a particular characteristic of inflammatory reactions, because inflammatory cells such as neutrophils and macrophages produce large amounts of ROS.52 UC has been associated with the overproduction of ROS. For example, mucosal ROS concentrations are 10- to 100-fold higher in biopsies taken from sites of patients with than without UC, with ROS concentrations correlating with disease progression.53 By taking advantage of the pathological conditions of IBD, redox-responsive nano-delivery systems hold enormous potential for IBD therapy.
Orally administered thioketal nanoparticles (TKNs) have been used to target delivery of TNF-α siRNA to sites of intestinal inflammation.54 TKNs are formed from the polymer poly-(1, 4-phenyleneacetone dimethylene thioketal) (PPADT), which is selectively degraded in response to ROS. Complexing TNF-α siRNA with cationic species, such as DOTAP, enhances TNF-α siRNA transfection stability, mucosal transport, cellular internalization, and endosomal escape. 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) is a cationic lipid that can complex with TNF-α siRNA and form DOTAP/siRNA. TKNs orally administered to a murine model of UC were degraded in response to the abnormally high levels of ROS specific to sites of intestinal inflammation. This resulted in the release of siRNA targeting the proinflammatory cytokine TNF-α, thereby diminishing TNF-α mRNA levels in the colon and protecting mice from UC.
Similarly, nitroxide radical–containing nanoparticles (RNPo), composed of an amphiphilic block copolymer, methoxy-poly(ethylene glycol)-b-poly (4-[2,2,6,6-tetramethyl-piperidine-1-oxyl]oxymethylstyrene (MeO-PEG-b-PMOT), were developed for delivery of low–molecular weight TEMPOL.55 Oral administration of these particles resulted in their specific accumulation in the colons of mice with colitis. The hydrophobic segment of this copolymer possesses stable nitroxide radicals, which can scavenge ROS, suggesting that RNPo may be an important therapeutic agent for the treatment of UC.
Although ROS-responsive nano-delivery systems represent a promising approach for the treatment of UC,56, 57 several obstacles have limited the application of ROS-responsive systems, including (1) the instability of the drug delivery carriers in the harsh, acidic, and enzyme-rich environment of the upper GI tract; (2) premature release, which lowers the drug concentration at inflamed colon sites and triggers undesirable side effects; and (3) the ability to target only 1 or 2 ROS. To mitigate these problems, future work should seek to design multifunctional delivery systems that use multiple delivery strategies (eg, both pH and ligand-receptor interactions) and/or that respond to numerous ROS.
Hydrogel-Based Nano-Delivery Systems
The primary goals in the development of drug delivery systems are to protect therapeutically active molecules from premature degradation, enhance the efficacy of these drugs, and minimize their side effects. Ideally, controlled-release systems can meet these criteria by maintaining the drug concentration within a therapeutic window over an extended period of time, minimizing dosage and frequency of administration.58 Hydrogels are a particularly appealing type of drug delivery system and have been used in many branches of medicine, including IBD.59 Hydrogels are composed of a large amount of water and a cross-linked polymer network. The high water content (typically 70%–99%) provides physical similarity to biological tissues, providing hydrogels with excellent biocompatibility and the ability to easily encapsulate hydrophilic drugs. Moreover, because most hydrogels are formed in aqueous solutions, the risks of drug (eg, recombinant proteins and monoclonal antibodies) denaturation and aggregation upon exposure to organic solvents are minimal.60
A recently developed hydrogel, using ions (Ca2+ and SO42-) that cross-linked chitosan and alginate, was found to be sensitive to the environment of the colon.61 This hydrogel was used to encapsulate nanoparticles containing the anti-inflammatory tripeptide Lys-Pro-Val (KPV). Under the protection of the hydrogel, the particles were able to pass through the stomach and small intestine and were specifically degraded in the inflamed colon. Use of these improved NPs in a hydrogel system significantly reduced colitis symptoms in a DSS model of colitis, accompanied by a reduction in myeloperoxidase (MPO) activity and histologic alterations. Moreover, a 1200-fold lower dose was sufficient to ameliorate mucosal inflammation in vivo when compared with free KPC in solution. Nanoparticles containing CD98 siRNA embedded in this hydrogel were also used to treat IBD.62 Oral administration of CD98 siRNA/polyethyleneimine (PEI) nanoparticles encapsulated in this hydrogel reduced CD98 expression in mouse colonic tissue and decreased DSS-induced colitis. Another study confirmed that this hydrogel system yielded excellent outcomes.63
Active Targeting-Dependent Nano-Delivery Systems
Active targeting, or the intentional homing of NPs to inflamed or diseased sites, may result in more accurate spatial localization, increasing the therapeutic efficacy of a drug while eliminating its adverse effects on normal tissue.64 The presence of a ligand on the surface of an NP can facilitate binding to specific molecules overexpressed on the surface of targeted cells, triggering receptor-mediated endocytosis.16, 65 Most research on active targeting-dependent nano-delivery systems has focused on the parenteral route of administration, targeting various conditions, including cancers, infections, and inflammation.66 The positive outcomes of this research led to the use of this strategic approach for orally administered nano-delivery systems. Various ligands have been attached to nano-delivery carriers to improve the efficiency of oral delivery and therapeutic outcomes. During the inflammatory process of UC, certain ligands/receptors are overexpressed on the surfaces of some cells (eg, colonic epithelial cells and immune cells), and thus may serve as molecular targets for anchoring drug delivery systems via specific interactions. For example, the expression of the glycoprotein CD98 is upregulated on colonic epithelial cells and immune cells (eg, macrophage cells) in the inflamed state of UC.63 These ligands have generally included saccharides (eg, mannose, galactose, and hyaluronic acid) and adhesive proteins such as monoclonal antibody fragments and lectins.
Saccharide ligands
Saccharides are sources of nutrition that play important roles in various biological processes, including intracellular signaling, bioadhesion, cell migration, inflammation, and cancer development and metastasis. Intestinal epithelial cells and immune system cells possess a variety of saccharide receptors, such as mannose, galactose, and hyaluronic acid receptors.64 The presence of saccharide moieties on the surface of nanoparticles may enhance their transport characteristics.
The mannose receptor is a 175-kDa transmembrane protein primarily expressed on macrophage membranes. Because macrophages are abundant in inflamed colon tissue, the mannose receptor may be a candidate for active targeting in the treatment of UC.67 For example, active targeting ability was assessed using mannosylated PLGA nanoparticles loaded with ovalbumin (OVA).68 Ex vivo uptake studies in colons affected by colitis showed that the ratio of accumulation of mannosylated PLGA nanoparticles in inflamed over healthy tissue was significantly higher than the ratio of accumulation of trimethylchitosan (TMC), PLGA, and Eudragit S100 nanoparticles. These findings were attributed to targeting by mannosylated PLGA nanoparticles of mannose receptors present on intestinal inflammatory cells, including macrophages and dendritic cells, infiltrating the inflamed tissues. In addition, nanoparticles made of a mannosylated bioreducible cationic polymer (PPM), sodium triphosphate (TPP), and TNF-α siRNA (TPP-PPM/siTNF NPs) were synthesized by electrostatic interactions and found to have enhanced siRNA condensation capacity and significant macrophage-targeting ability.69 Further ex vivo experiments indicated that these nanoparticles markedly inhibited TNF-α secretion by tissue with DSS-induced colitis. Importantly, flow cytometry showed that TPP-PPM/siTNF NPs were efficiently taken up by macrophages (29.5%), but not by epithelial cells.
Another potential target for nanodelivery systems is the galactose receptor (macrophage galactose lectin [MGL]), a transmembrane cell surface receptor on activated macrophages. For example, galactosylated trimethyl chitosan-cysteine (GTC) nanoparticles have been synthesized as activated macrophage-targeting carriers for oral siRNA administration.70 GTC nanoparticles loaded with siRNA were prepared by ionic gelation with tripolyphosphate (TPP) or hyaluronic acid (HA). Cell binding, cellular uptake, cytotoxicity, and the gene knockdown efficiency of GTC nanoparticles in vitro have been assessed using lipopolysaccharide (LPS)-activated macrophage cell line RAW 264.7. Mapk4 siRNA-loaded GTC nanoparticles effectively inhibited the activation of TNF-α in vitro and suppressed colonic inflammation in vivo by improving body weight loss, colon length shortening, and MPO activity.
HA has also been used as an active ligand in nano-delivery systems. HA is composed of N-acetylglucosamine and D-glucuronic acid disaccharide units and is generally considered a nontoxic, biodegradable natural acidic polysaccharide.16 HA binds with high affinity to its receptor CD44, which has been reported to be overexpressed on tumor cells and activated inflammatory cells in colitis tissues. An HA-functionalized polymeric nanoparticle loaded with CD98 siRNA and the anti-inflammatory agent curcumin (CUR; HA-siCD98/CUR-NPs) was found to have combined effects against UC, in that it protected the mucosal layer and alleviated inflammation both in vitro and in vivo.71 In addition, HA-functionalized polymeric nanoparticles loaded with KPV (HA-KPV-NPs) were found to be effective against UC by accelerating mucosal healing and alleviating inflammation.72 Overall, these studies indicate that active targeting mediated by saccharide ligands is a promising approach to enhance the accumulation and uptake of therapeutic drugs by inflamed tissues.
Antibodies as ligands
Antibodies target specific antigens expressed only on the surface of diseased cells, or to a much greater extent on diseased than on healthy cells. These properties allow antibodies to be conceptually exploited to courier nanoparticles and their cargo, enabling selective targeting and delivery.73, 74
Transferrin receptor (TfR) is a carrier protein that facilitates cellular uptake of iron from the plasma glycoprotein transferrin by receptor-medicated endocytosis. TfR expression was found to be elevated in the basolateral and apical membranes of enterocytes present in the colonic mucosa of patients with UC and in the colonocytes of rats with induced colitis.75 Thus, TfR may be a candidate targeting receptor for drug delivery to tissue affected by colitis. Ex vivo binding studies, testing the ability of nano-liposomes with conjugated anti-TfR antibody to target inflamed regions of the colon, showed that, compared with nonspecific immunoliposomes, anti-TfR immunoliposomes exhibited significantly greater accumulation in the mucosa of rats with DNBS-induced colitis. These findings indicate that mucosal inflammation can be targeted by nano-liposomes expressing anti-TfR, a target resulting from the inflammation-dependent, apical, elevated expression of the receptor.
Another type of receptor suitable for active targeting is CD98, which is overexpressed on the luminal side of the inflamed colonic epithelium, both in mice with colitis and in patients with CD.76 NPs have been loaded with CD98 Fab’-bearing quantum dots (Fab’-NPs) and with PEGylated quantum dots (PEG-NPs), with cellular uptake experiments demonstrating that Fab’-NPs were efficiently internalized into Colon-26 and RAW 264.7 cells through the CD98-mediated endocytosis pathway. Moreover, the targeting effect of CD98 Fab’ markedly increased its cellular uptake efficiency compared with that of control PEG-NPs, and ex vivo studies showed much more effective accumulation in colitis tissue of Fab’-NPs than of PEG-NPs.77 An orally delivered hydrogel encapsulating NPs loaded with CD98 siRNA and with single-chain CD98 antibodies (scCD98) conjugated onto its surface has also been developed.63 The scCD98-functionalized nanoparticles without siCD98 had high affinity for CD98-overexpressing cells. The scCD98-functionalized, siCD98-loaded nanoparticles significantly reduced levels of CD98 in Colon-26 cells and RAW 264.7 macrophages and reduced the synthesis of pro-inflammatory cytokines (eg, TNF-α, IL-6, and IL-12). Oral administration of scCD98-functionalized, siCD98-loaded nanoparticles to mice with colitis reduced colon expression of CD98 and the severity of colitis compared with control mice, based on loss of body weight, myeloperoxidase activity, production of inflammatory cytokines, and histological analysis. Approximately 24.1% of colonic macrophages (CD11b+CD11c−F4/80+) in these mice had taken up fluorescently labeled siRNA-loaded nanoparticles within 12 hours of administration.
Ligands used for active targeting of nano-delivery systems are listed in Table 3. Overall, these studies indicate that active targeting is a very promising approach for enhancing drug accumulation and uptake into inflamed tissue in patients with IBD. Additional in vivo studies are required to assess the efficacy and stability of various targeted ligands and delivery systems in animal models of UC.
TABLE 3:
NP-Based Drug Delivery System Targeting the Inflamed Colon
| Nps Delivery System | Drug | Targeting Mechanism | Delivery Route | Colitis Model | Pros/Cons | Ref. |
|---|---|---|---|---|---|---|
| PLGA/Eudragit S100 NPs | Curcumin | pH-dependent | Oral | DSS mice | This delivery system significantly decreased neutrophil infiltration and TNF-α secretion while maintaining the colonic structure similar to the control group in a murine DSS-induced colitis model. | 46 |
| PLGA/Eudragit S100 NPs | Budesonide | pH-dependent | Oral | TNBS mice | The nanospheres loaded with Budesonide showed significant anti-inflammatory effects in the TNBS-induced colitis rat model. This therapeutic response could be related, first, to the higher drug levels that reached the colon and, second, to the specific adhesion and uptake of the nanospheres at the site of inflammation. | 47 |
| PLGA/Eudragit S100 NPs | Budesonide | pH-dependent | Oral | TNBS, DSS, and oxazolone mice | This nanoparticle delivery system may improve the anti-inflammatory efficacy of budesonide in terms of endoscopical, histological, and biochemical parameters in comparison with the free drug. Moreover, such nanoparticle delivery via oral administration can be further improved by implementing pH-sensitive release characteristics, eg, using appropriate coating. | 117 |
| ε‑polylysine-coated MSNs | prednisolone | pH-dependent | In vitro | RAW 264.7, LS 174T, and Caco-2 cell lines | This mesoporous silica nanoparticle (MSN) delivery system offers a highly promising and novel drug delivery system for diseases of the colon such as inflammatory bowel disease. However, the authors only propose its use in vivo and did not show any in vivo data of colitis models. | 49 |
| PPADT/DOTAP NPs | TNF-α siRNA | ROS-responsive | Oral | DSS mice | Orally administered PPADT/DOTAP NPs loaded with siRNA against the proinflammatory cytokine TNF-α diminish TNF-α messenger RNA levels in the colon and protect mice from ulcerative colitis. This delivery system showed great potential for clinical application. | 54 |
| RNPo | TEMPOL or mesalamine | ROS-responsive | Oral | DSS mice | Orally administered RNPO accumulates specifically in the colons of mice with colitis and is more effective in reducing inflammation than low–molecular weight TEMPOL or mesalamine. RNPO might be developed for treatment of patients with ulcerative colitis. | 55 |
| MeO-PEG-b- PMOT block copolymer NPs | TEMPOL | ROS-responsive | Oral | DSS mice | Oral administration of RNPO did not change any composition of bacteria in feces, which strongly suggests a protective effect of RNPO on healthy environments in intestinal microflora. RNPO may become an effective and safe medication for treatment of UC. | 118 |
| Tpl/OxbCD NPs | TEMPOL | ROS-responsive | Oral | DSS and TNBS mice | Oral administration of Tpl/OxbCD NPs notably mitigated manifestations relevant to colitis and significantly suppressed expression of proinflammatory mediators. Accordingly, by scavenging multiple components of ROS, Tpl/OxbCD NPs may effectively reduce ulcerative colitis in mice and could be intensively developed as a translational nanomedicine for the management of IBD. | 57 |
| Chitosan-coated PLGA NPs | Lyophilized probiotic extract | Enzyme- responsive | Oral | TNBS rat | This nanoparticle-encapsulated form is very effective in the control of colitis. Regarding the safety of this compound, further studies should be conducted in patients with IBD. | 119 |
| PLA NPs | Tripeptide KPV | Hydrogel-based | Oral | DSS mice | By using NPs, KPV can be delivered at a concentration that is 12,000-fold lower than that of KPV in free solution, but with similar therapeutic efficacy. Administration of encapsulated drug-loaded NPs is a novel therapeutic approach for IBD. | 61 |
| PEI/PLA NPs | CD98 siRNA | Hydrogel-based | Oral | DSS mice | This hydrogel-based delivery system showed therapeutic effect with colon-homing capabilities and the ability to directly release “molecularly specific” CD98 siRNA in colonic cells, thereby decreasing colitis. | 62 |
| TPP-PPM NPs | TNF-α siRNA | Mannose- mannose receptor | Ex vivo | DSS mice | This delivery system suggests that nontoxic TPP-PPM/siRNA NPs can be exploited as efficient, macrophage-targeted carriers for IBD therapy. However, the authors did not test its effect on this colitis model. | 69 |
| Mannosylated PLGA NPs | OVA | Mannose- mannose receptor | Oral | DSS mice | This delivery system only showed enhanced accumulation of OVA in inflamed colon tissue. | 68 |
| GTC/TPP NPs | Map4k4 siRNA | Galactose- galactose receptor | Oral | DSS mice | Oral administration of GTC/TPP NPs containing siMap4k4 significantly improved DSS-induced body weight loss, colon length shortening, and increase of myeloperoxidase activity. This study would provide an effective approach for oral siRNA delivery in the treatment of IBD. | 70 |
| PLGA NPs | CD98 siRNA and curcumin | HA-CD44 | Oral | DSS mice | This delivery system shows the promising capability of codelivered siCD98 and CUR for boosting conventional monotherapy via this novel nanotherapeutic agent, which offers a structurally simple platform for orally administered delivery of drugs to target cells in UC therapy. | 71 |
| CD98 Ab-urocanic acid/ PEI NPs | CD98 siRNA | CD98 Ab-CD98 | Oral | DSS and CD4+CD45RBhigh T cell transfer mice | Nanoparticles containing surface CD98 antibody and loaded with siCD98 reduce expression of this protein by colonic epithelial cells and macrophages, and oral administration decreases the severity of colitis in mice. | 63 |
| F4/80 Ab-PLA- PEG NPs | TNF-α siRNA | F4/80 Ab- F4/80 | Oral | DSS mice | Fab’-bearing PLA-PEG NPs are powerful and efficient nanosized tools for delivering siRNAs into colonic macrophages. | 120 |
| Immunoliposomes | Transferrin-TfR | Ex vivo | DNBS rat | The results only showed that anti-TfR immunoliposomes accumulated significantly better in the mucosa of DNBS-induced rats than the accumulation of nonspecific immunoliposomes, and there are no therapeutic results. | 75 | |
| Lectin-conjugated PLGA-NPs | Betamethasone | Lectin- lectin receptor | Oral | TNBS mice | Targeted NPs using lectins, especially with PNA, as stably targeting moiety in the GI tract seems to be a very promising tool in the future treatment of IBD. | 121 |
| Ultra-small solid archaeolipid NPs | Bexamethasone | TPA-scavenger receptors class A | In vitro | J774A1 cells | Because of their structural and pharmacodynamic features, SAN-Dex may be suitable for oral targeted delivery to inflamed mucosa. However, there are only in vitro results. | 122 |
| PLGA/PLA- PEG-FA NPs | 6-shogaol | FA-folate receptor | Oral | DSS mice | The results demonstrate a convenient, orally administered 6-shogaol drug delivery system that effectively targets colitis tissue, alleviates colitis symptoms, and accelerates colitis wound repair. This system may represent a promising therapeutic approach for treating IBD. | 65 |
COLON TARGETING BY NATURALLY DERIVED NANOPARTICLES
Although synthetic nanoparticles for the treatment of UC have achieved some preclinical success, these nanoparticles have 2 major limitations. First, the potential in vivo toxicity of each constituent of these nanoparticles must be evaluated before clinical applications; and second, the scale of production is limited. In contrast, nanoparticles derived from natural sources are considered safe and cost-effective and may overcome the limitations of synthetic nanoparticles. Extracellular vesicles (EVs) isolated from mammalian cells and plant-derived nanoparticles isolated from edible plants have recently shown great preclinical promise for the treatment of UC.78, 79 These findings suggest that naturally derived nanoparticles may provide a new approach for the treatment of UC. This section will summarize the development of naturally derived nanoparticles and their applications to the treatment of UC.
Extracellular Vesicles
Over the past 2 decades, EVs have been identified as important mediators of intercellular communication, enabling functional transfer of bioactive molecules between cells. Consequently, it is becoming increasingly clear that these vesicles contribute to the pathogenesis of various human diseases, providing opportunities for therapeutic applications.80
EVs can be defined as cell-secreted phospholipid bilayer–bound structures that are present in body fluids, generated by an evolutionarily conserved process, and secreted by all types of human cells tested to date.81 Generally, EVs can be categorized into 3 subtypes—exosomes, microvesicles, and apoptotic vesicles—based on their morphological, biochemical, and biogenic parameters. Apoptotic vesicles are formed from the plasma membrane when cells are dying, with the contents of each cell partitioned into different membrane-enclosed vesicles. Hence, apoptotic vesicles, which contain cytoplasmic organelles and fragmented nuclei, are generally larger particles (1–5 μm) than exosomes (50–150 nm) and microvesicles (100–1000 nm). The latter not only show a partially overlapping size distribution, but their biogenesis pathways are very similar.82 The main difference between their pathways of formation is that exosomes are generated by the exocytosis of multivesicular bodies, whereas microvesicles are generated by the outward budding and fusion of the plasma membrane. Although their contents likely differ, both exosomes and microvesicles are enriched in subsets of proteins, mRNA, and noncoding RNA, such as microRNAs (miRNAs), which are derived from the parental cells. Some types of EVs have also been reported to contain extrachromosomal DNA.83
Naturally occurring immunosuppressive EVs may have therapeutic potential in patients with IBD. For example, EVs produced by intestinal epithelial cells (IECs) with high levels of transforming growth factor beta 1 (TGF-β1) showed immunosuppressive activity.84 Transfer of these EVs into mice with dextran sulfate sodium (DSS)–induced colitis prevented the development of colitis by inducing regulatory T cells and immunosuppressive dendritic cells (DCs). These findings indicated that EVs from IECs participate in maintaining the immune balance of the intestinal tract. In addition, exosomes derived from interleukin-10 (IL-10)-treated bone marrow–derived DCs could suppress TNBS-induced colitis. Further investigation showed that the therapeutic effects of IL-10 exosomes were associated with a downregulation of expression of IL-2, IFN-γ, and TNF-α mRNAs and an upregulation of IL-10 mRNA in colon tissue, and an upregulation of regulatory T cells in colonic lamina propria.85 These results indicated that EVs were effective nanocarriers for the delivery of biological agents for the treatment of IBD.
Regenerative medicine focuses on the restoration of lost, damaged, and aging cells and tissues in the human body.84 Mesenchymal stem/stromal cells (MSCs) from bone marrow, the umbilical cord, and adipose tissues are of great interest in regenerative therapy of tissues damaged by various pathological conditions. The chief therapeutic attributes of MSCs are their ability to migrate to injured sites, promote functional recovery, and modulate immune responses.86 Several recent studies have shown that EVs secreted by MSCs also have the ability to serve as therapeutic tools in the repair of tissue injuries, including in colitis.87 Thus, MSC-EVs are likely to become a more efficient cell-free therapeutic approach, which may overcome the obstacles associated with the use of native or engineered stem cells. A study investigating whether EVs derived from bone mesenchymal stem cells (BMSCs) alleviated TNBS-induced colitis found that BMSC-EVs containing various proteins, mRNAs, and microRNAs could attenuate the severity of colitis by reducing NF-κBp65, TNF-α, iNOS, COX-2, and IL-1β mRNA and protein levels and by increasing IL-10 expression.88 In addition, exosomes secreted by human umbilical cord mesenchymal stem cells (hucMSCs) were found able to relieve the severity of DSS-induced colitis by increasing the expression of IL-10 and by decreasing the expression of TNF-α, IL-1β, IL-6, iNOS, and IL-7.89 Taken together, these interesting results indicate that MSC-EVs have theoretical advantages over intact MSCs and may, in the future, be preferable to whole cells in regenerative medicine.
Plant-Derived Nanoparticles
There is great interest in determining whether plant-derived nanoparticles can play a role in interspecies communication and have a direct beneficial effect on human diseases, especially intestinal inflammation.90–92 Orally administered nanoparticles from grapes (GELNs), containing proteins, lipids, and microRNA, were found to pass through the gut and be taken up by intestinal stem cells.93 Furthermore, these nanoparticles seemed resistant to degradation by saliva, the acidic environment of the stomach, and the highly active proteolytic enzymes present along the intestinal tract. These findings suggested that edible plant-based nanoparticles can be orally delivered to the intestine and taken up by intestinal cells, where they may exert activities such as intestinal regeneration. Edible plant-derived nanoparticles that naturally target colonic tissues and have anti-inflammatory properties may therefore represent a novel natural and nontoxic delivery system that could easily be scaled up for the treatment of patients with digestive tract diseases such as IBD.
Grapefruit-derived nanovesicles (GDNs) have been found to be selectively taken up by intestinal macrophages and to ameliorate DSS-induced colitis in mice.94 The anti-inflammatory effects of GDNs were mediated by upregulating the production of heme oxygenase-1 (HO-1) and downregulating the production of IL-1β and TNF-α in intestinal macrophages. These GDNs were found to be biocompatible, biodegradable, and stable across a wide range of pH values, suggesting that they could be used to develop novel oral drug delivery systems. For example, the anti-inflammatory drug methotrexate (MTX) was incorporated into GDNs, with the encapsulated drug showing lower toxicity and markedly greater therapeutic effects against DSS-induced colitis in mice than free MTX. These findings indicated that GDNs can serve as immune modulators in the intestine and promote homeostasis of intestinal macrophages. These nanoparticles thus show potential for use in oral delivery systems for small molecule drugs that can attenuate inflammatory responses in human diseases.
Ginger has been used medically as a digestive aid in traditional medicine. Key therapeutically active constituents of ginger include 6-gingerol and 6-shogaol, which have been shown to have anti-oxidation, anti-inflammatory, and anticancer activities.95 We recently found that delivery of ginger-derived compounds in nanoparticles may be a more efficient method of targeting colon tissue than providing the herb as a food or supplement.96, 97
The ginger-derived nanoparticles (GDNPs) used in our previous work were ~230 nm in diameter. Using animal models of IBD, we found that these GDNPs targeted the colon efficiently and were absorbed mainly by cells in the lining of the intestines, the site of IBD-associated inflammation. GDNPs contain high levels of lipids, a few proteins, ~125 miRNAs, and large amounts of bioactive constituents of ginger. GDNPs are mainly taken up by intestinal epithelial cells (IECs) and macrophages and are nontoxic. Oral administration of GDNPs increases the survival and proliferation of IECs, reduces the concentrations of proinflammatory cytokines (TNF-α, IL-6, and IL-1β), and increases the concentrations of anti-inflammatory cytokines (IL-10 and IL-22) in colitis models, suggesting that GDNPs can attenuate damaging factors while promoting healing effects. The advantages of ginger include its nontoxicity and cost-effectiveness. Ginger root is converted into GDNPs by use of a blender, high-speed centrifugation, and ultrasonic dispersion of ginger juice to break it up into single pellets. Additionally, GDNPs reduce acute colitis and prevent chronic colitis and colitis-associated cancer. Moreover, the particles enhance intestinal repair by increasing the survival and proliferation of the cells that make up the lining of the colon, while concomitantly lowering the production of proteins that promote inflammation.
These findings indicate that plant-derived nanoparticles may represent a novel, natural delivery mechanism for IBD prevention and treatment. Moreover, these plant-derived nanoparticles may overcome the limitations common to synthetic nanoparticles, such as their potential toxicity and the limited scale of production.
CONCLUSIONS AND PERSPECTIVE
This review has described drug delivery strategies based on synthetic nanoparticles, extracellular vesicles, and plant-derived nanoparticles, and their potential for the treatment of IBD. Significant progress has been made toward developing a truly selective drug delivery system that targets the site of inflammation in UC. However, nanotechnological approaches could also be adapted to Crohn’s disease by targeting drug-loaded nanoparticles to both the small intestine (an additional site of this disease) and the large intestine. The challenges to targeting and retaining nanoparticle-loaded drugs in the small intestine include the need to (1) specifically release nanoparticles from the hydrogel to the small intestine and/or (2) decorate the nanoparticles with ligands specific to receptors expressed mostly in epithelial cells of the small intestine. Our group and others are currently investigating delivery systems that could specifically target and deliver nanoparticle-loaded drugs to the small intestine. Ideally, a therapeutic NP-based system should target its cargo specifically to the inflamed site, thereby enhancing the therapeutic efficacy while reducing side effects. Various targeting modalities, such as ultrasound-assisted delivery98 and micro-needle-based systems,99, 100 can be used to augment the targeting of NPs. Biomimetic nanoparticles employing components derived from human immune cells, such as neutrophils,101, 102 platelets,103–105 and macrophages,106, 107 can mimic the endogenous inflammation-targeting mechanisms of these cells to selectively target inflamed vasculature in animal models.108, 109 Such biomimetic nanoparticles combine the unique functionalities of cellular components and the capability of nano-engineering to effectively deliver therapeutic agents. This approach could be applied to targeted delivery in IBD.
Attention should also be given to smart nanoparticle-based delivery systems that offer the potential for both diagnostic and therapeutic functions (ie, “nanotheranostics”) for IBD.110 Complex nanoparticle-based delivery systems can deliver more than 1 drug to produce combination therapies, perform multimodal imaging, or operate in theranostic space by simultaneously providing diagnostic and therapeutic capabilities. New and more sophisticated nanosystem designs that respond to changes in pH and the enzymatic environment can become bio-activatable upon recognizing changes in physiology or the disease state. In this context, the various parameters associated with the IBD microenvironment could be used to trigger drug release. External triggers, such as light or applied electromagnetic fields, can also be used to activate nanoparticles; this could allow for even more sophisticated nanoparticle designs and programmed drug release in the future. Nanoshells,111 dendrimers,112 liposomes,113, 114 and nanogels115, 116 have all shown the potential to be ideal for these types of applications, as they display imaging properties and can serve as nanocarriers.
Unfortunately, multiple barriers may limit the successful clinical translation of nanotheranostics. To address the potential issues, investigators should seek to gain an in-depth understanding of nanoparticle-IBD interactions and the potential for cooperation between diagnosis and therapy. More effort should also be directed at the large-scale synthesis of nanotheranostics, the long-term assessment of their toxicity, and the establishment of relevant regulatory protocols. Only then can we hope to deliver this exciting technology at the patient’s bedside for effective and personalized therapy.
Supported by: This study was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institutes of Health (RO1DK-107739, RO1DK-116306, RO1DK-071594 to D. Merlin), and the VA Merit Award (BX002526) to D. Merlin. M. Zhang is the recipient of a Research Fellowship Award from the Crohn’s and Colitis Foundation of America. D. Merlin is a recipient of a Research Career Scientist Award from the Department of Veterans Affairs. No writing assistance was utilized in the production of this manuscript.
Conflicts of interest: The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
REFERENCES
- 1.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–42. [DOI] [PubMed] [Google Scholar]
- 2.Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590–605. [DOI] [PubMed] [Google Scholar]
- 3.Ordás I, Eckmann L, Talamini M, et al. . Ulcerative colitis. Lancet. 2012;380:1606–19. [DOI] [PubMed] [Google Scholar]
- 4.Bilsborough J, Targan SR, Snapper SB. Therapeutic targets in inflammatory bowel disease: current and future. Am J Gastroenterol. 2016;3:27–37. [Google Scholar]
- 5.Neurath MF. Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol. 2017;14:269–78. [DOI] [PubMed] [Google Scholar]
- 6.Arora Z, Shen B. Biological therapy for ulcerative colitis. Gastroenterol Rep (Oxf). 2015;3:103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dretzke J, Edlin R, Round J, et al. . A systematic review and economic evaluation of the use of tumour necrosis factor-alpha (TNF-α) inhibitors, adalimumab and infliximab, for Crohn’s disease. Health Technol Assess. 2011;15:1–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nielsen OH. New strategies for treatment of inflammatory bowel disease. Front Med (Lausanne). 2014;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colombel JF, Sandborn WJ, Reinisch W, et al. ; SONIC Study Group Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–95. [DOI] [PubMed] [Google Scholar]
- 10.Feagan BG, Rutgeerts P, Sands BE, et al. ; GEMINI 1 Study Group Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 11.Colombel JF, Rutgeerts PJ, Sandborn WJ, et al. . Adalimumab induces deep remission in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:414–22.e5. [DOI] [PubMed] [Google Scholar]
- 12.Tomalia DA, Khanna SN. A systematic framework and nanoperiodic concept for unifying nanoscience: hard/soft nanoelements, superatoms, meta-atoms, new emerging properties, periodic property patterns, and predictive Mendeleev-like nanoperiodic tables. Chem Rev. 2016;116:2705–74. [DOI] [PubMed] [Google Scholar]
- 13.Laroui H, Wilson DS, Dalmasso G, et al. . Nanomedicine in GI. Am J Physiol Gastrointest Liver Physiol. 2011;300:G371–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viscido A, Capannolo A, Latella G, et al. . Nanotechnology in the treatment of inflammatory bowel diseases. J Crohns Colitis. 2014;8:903–18. [DOI] [PubMed] [Google Scholar]
- 15.Zhang MZ, Yu Y, Yu RN, et al. . Tracking the down-regulation of folate receptor-α in cancer cells through target specific delivery of quantum dots coupled with antisense oligonucleotide and targeted peptide. Small. 2013;9:4183–93. [DOI] [PubMed] [Google Scholar]
- 16.Zhang M, Xu C, Wen L, et al. . A hyaluronidase-responsive nanoparticle-based drug delivery system for targeting colon cancer cells. Cancer Res. 2016;76:7208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molinaro R, Corbo C, Martinez JO, et al. . Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat Mater. 2016;15:1037–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collnot EM, Ali H, Lehr CM. Nano- and microparticulate drug carriers for targeting of the inflamed intestinal mucosa. J Control Release. 2012;161:235–46. [DOI] [PubMed] [Google Scholar]
- 19.Agoram B, Woltosz WS, Bolger MB. Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Adv Drug Deliv Rev. 2001;50:S41–67. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg M, Gomez-Orellana I. Challenges for the oral delivery of macromolecules. Nat Rev Drug Discov. 2003;2:289–95. [DOI] [PubMed] [Google Scholar]
- 21.Philip AK, Philip B. Colon targeted drug delivery systems: a review on primary and novel approaches. Oman Med J. 2010;25:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao B, Merlin D. Oral colon-specific therapeutic approaches toward treatment of inflammatory bowel disease. Expert Opin Drug Deliv. 2012;9:1393–407. [DOI] [PubMed] [Google Scholar]
- 23.Liu M, Zhang J, Shan W, et al. . Developments of mucus penetrating nanoparticles. J Pharm Sci. 2015;10:275–82. [Google Scholar]
- 24.Yang M, Lai SK, Yu T, et al. . Nanoparticle penetration of human cervicovaginal mucus: the effect of polyvinyl alcohol. J Control Release. 2014;192:202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lundquist P, Artursson P. Oral absorption of peptides and nanoparticles across the human intestine: opportunities, limitations and studies in human tissues. Adv Drug Deliv Rev. 2016;106:256–76. [DOI] [PubMed] [Google Scholar]
- 26.Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target?Nat Rev Gastroenterol Hepatol. 2017;14:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.des Rieux A, Fievez V, Garinot M, et al. . Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release. 2006;116:1–27. [DOI] [PubMed] [Google Scholar]
- 28.González-Mariscal L, Nava P, Hernández S. Critical role of tight junctions in drug delivery across epithelial and endothelial cell layers. J Membr Biol. 2005;207:55–68. [DOI] [PubMed] [Google Scholar]
- 29.Shakweh M, Ponchel G, Fattal E. Particle uptake by peyer’s patches: a pathway for drug and vaccine delivery. Expert Opin Drug Deliv. 2004;1:141–63. [DOI] [PubMed] [Google Scholar]
- 30.Cuvelier CA, Quatacker J, Mielants H, et al. . M cells are damaged and increased in number in inflamed human ileal mucosa. Eur J Morphol. 1993;31:87–91. [PubMed] [Google Scholar]
- 31.Florence AT. Issues in oral nanoparticle drug carrier uptake and targeting. J Drug Target. 2004;12:65–70. [DOI] [PubMed] [Google Scholar]
- 32.Johnstone RW, Ruefli AA, Smyth MJ. Multiple physiological functions for multidrug transporter P-glycoprotein?Trends Biochem Sci. 2000;25:1–6. [DOI] [PubMed] [Google Scholar]
- 33.Gavhane YN, Yadav AV. Loss of orally administered drugs in GI tract. Saudi Pharm J. 2012;20:331–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amin ML. P-glycoprotein inhibition for optimal drug delivery. Drug Target Insights. 2013;7:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicholson JK, Holmes E, Kinross J, et al. . Host-gut microbiota metabolic interactions. Science. 2012;336:1262–67. [DOI] [PubMed] [Google Scholar]
- 36.Blander JM, Longman RS, Iliev ID, et al. . Regulation of inflammation by microbiota interactions with the host. Nat Immunol. 2017;18:851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams BA, Grant LJ, Gidley MJ, et al. . Gut fermentation of dietary fibres: physico-chemistry of plant cell walls and implications for health. Int J Mol Sci. 2017;18:2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, Sang Y, Feng J, et al. . Polysaccharide-based micro/nanocarriers for oral colon-targeted drug delivery. J Drug Target. 2016;24:579–89. [DOI] [PubMed] [Google Scholar]
- 39.Qiao H, Fang D, Chen J, et al. . Orally delivered polycurcumin responsive to bacterial reduction for targeted therapy of inflammatory bowel disease. Drug Deliv. 2017;24:233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng H, Powell JE, Steele MI, et al. . Honeybee gut microbiota promotes host weight gain via bacterial metabolism and hormonal signaling. Proc Natl Acad Sci U S A. 2017;114:4775–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hua S, Marks E, Schneider JJ, et al. . Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: selective targeting to diseased versus healthy tissue. Nanomedicine. 2015;11:1117–32. [DOI] [PubMed] [Google Scholar]
- 42.Date AA, Hanes J, Ensign LM. Nanoparticles for oral delivery: design, evaluation and state-of-the-art. J Control Release. 2016;240:504–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu L, Yao W, Rao Y, et al. . pH-responsive carriers for oral drug delivery: challenges and opportunities of current platforms. Drug Deliv. 2017;24:569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gugulothu D, Kulkarni A, Patravale V, et al. . pH-sensitive nanoparticles of curcumin-celecoxib combination: evaluating drug synergy in ulcerative colitis model. J Pharm Sci. 2014;103:687–96. [DOI] [PubMed] [Google Scholar]
- 45.Bai XY, Yan Y, Wang L, et al. . Novel pH-sensitive hydrogels for 5-aminosalicylic acid colon targeting delivery: in vivo study with ulcerative colitis targeting therapy in mice. Drug Deliv. 2016;23:1926–32. [DOI] [PubMed] [Google Scholar]
- 46.Beloqui A, Coco R, Memvanga PB, et al. . pH-sensitive nanoparticles for colonic delivery of curcumin in inflammatory bowel disease. Int J Pharm. 2014;473:203–12. [DOI] [PubMed] [Google Scholar]
- 47.Makhlof A, Tozuka Y, Takeuchi H. pH-sensitive nanospheres for colon-specific drug delivery in experimentally induced colitis rat model. Eur J Pharm Biopharm. 2009;72:1–8. [DOI] [PubMed] [Google Scholar]
- 48.Barea MJ, Jenkins MJ, Gaber MH, et al. . Evaluation of liposomes coated with a pH responsive polymer. Int J Pharm. 2010;402:89–94. [DOI] [PubMed] [Google Scholar]
- 49.Nguyen CT, Webb RI, Lambert LK, et al. . Bifunctional succinylated ε-polylysine-coated mesoporous silica nanoparticles for pH-responsive and intracellular drug delivery targeting the colon. ACS Appl Mater Interfaces. 2017;9:9470–83. [DOI] [PubMed] [Google Scholar]
- 50.Talaei F, Atyabi F, Azhdarzadeh M, et al. . Overcoming therapeutic obstacles in inflammatory bowel diseases: a comprehensive review on novel drug delivery strategies. Eur J Pharm Sci. 2013;49:712–22. [DOI] [PubMed] [Google Scholar]
- 51.Birben E, Sahiner UM, Sackesen C, et al. . Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piechota-Polanczyk A, Fichna J. The role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:605–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simmonds NJ, Allen RE, Stevens TR, et al. . Chemiluminescence assay of mucosal reactive oxygen metabolites in inflammatory bowel disease. Gastroenterology. 1992;103:186–96. [DOI] [PubMed] [Google Scholar]
- 54.Wilson DS, Dalmasso G, Wang L, et al. . Orally delivered thioketal nanoparticles loaded with TNF-α-siRNA target inflammation and inhibit gene expression in the intestines. Nat Mater. 2010;9:923–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vong LB, Tomita T, Yoshitomi T, et al. . An orally administered redox nanoparticle that accumulates in the colonic mucosa and reduces colitis in mice. Gastroenterology. 2012;143:1027–36.e3. [DOI] [PubMed] [Google Scholar]
- 56.Vong LB, Mo J, Abrahamsson B, et al. . Specific accumulation of orally administered redox nanotherapeutics in the inflamed colon reducing inflammation with dose-response efficacy. J Control Release. 2015;210:19–25. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Q, Tao H, Lin Y, et al. . A superoxide dismutase/catalase mimetic nanomedicine for targeted therapy of inflammatory bowel disease. Biomaterials. 2016;105:206–21. [DOI] [PubMed] [Google Scholar]
- 58.Sharpe LA, Daily AM, Horava SD, et al. . Therapeutic applications of hydrogels in oral drug delivery. Expert Opin Drug Deliv. 2014;11:901–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016;1:16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oliva N, Conde J, Wang K, et al. . Designing hydrogels for on-demand therapy. Acc Chem Res. 2017;50:669–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laroui H, Dalmasso G, Nguyen HT, et al. . Drug-loaded nanoparticles targeted to the colon with polysaccharide hydrogel reduce colitis in a mouse model. Gastroenterology. 2010;138:843–53.e1. [DOI] [PubMed] [Google Scholar]
- 62.Laroui H, Geem D, Xiao B, et al. . Targeting intestinal inflammation with CD98 siRNA/PEI-loaded nanoparticles. Mol Ther. 2014;22:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiao B, Laroui H, Viennois E, et al. . Nanoparticles with surface antibody against CD98 and carrying CD98 small interfering RNA reduce colitis in mice. Gastroenterology. 2014;146:1289–300.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang X, Wu W. Ligand-mediated active targeting for enhanced oral absorption. Drug Discov Today. 2014;19:898–904. [DOI] [PubMed] [Google Scholar]
- 65.Zhang M, Xu C, Liu D, et al. . Oral delivery of nanoparticles loaded with ginger active compound, 6-shogaol, attenuates ulcerative colitis and promotes wound healing in a murine model of ulcerative colitis. J Crohn's Colitis. 2018;12(2):217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang M, Xiao B, Wang H, et al. . Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy. Mol Ther. 2016;24:1783–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.He C, Yin L, Tang C, et al. . Multifunctional polymeric nanoparticles for oral delivery of TNF-α siRNA to macrophages. Biomaterials. 2013;34:2843–54. [DOI] [PubMed] [Google Scholar]
- 68.Coco R, Plapied L, Pourcelle V, et al. . Drug delivery to inflamed colon by nanoparticles: comparison of different strategies. Int J Pharm. 2013;440:3–12. [DOI] [PubMed] [Google Scholar]
- 69.Xiao B, Laroui H, Ayyadurai S, et al. . Mannosylated bioreducible nanoparticle-mediated macrophage-specific TNF-α RNA interference for IBD therapy. Biomaterials. 2013;34:7471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang J, Tang C, Yin C. Galactosylated trimethyl chitosan-cysteine nanoparticles loaded with Map4k4 siRNA for targeting activated macrophages. Biomaterials. 2013;34:3667–77. [DOI] [PubMed] [Google Scholar]
- 71.Xiao B, Zhang Z, Viennois E, et al. . Combination therapy for ulcerative colitis: orally targeted nanoparticles prevent mucosal damage and relieve inflammation. Theranostics. 2016;6:2250–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiao B, Xu Z, Viennois E, et al. . Orally targeted delivery of tripeptide KPV via hyaluronic acid-functionalized nanoparticles efficiently alleviates ulcerative colitis. Mol Ther. 2017;25:1628–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peer D, Karp JM, Hong S, et al. . Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–60. [DOI] [PubMed] [Google Scholar]
- 74.Schroeder A, Heller DA, Winslow MM, et al. . Treating metastatic cancer with nanotechnology. Nat Rev Cancer. 2011;12:39–50. [DOI] [PubMed] [Google Scholar]
- 75.Harel E, Rubinstein A, Nissan A, et al. . Enhanced transferrin receptor expression by proinflammatory cytokines in enterocytes as a means for local delivery of drugs to inflamed gut mucosa. PLoS One. 2011;6:e24202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nguyen HT, Dalmasso G, Torkvist L, et al. . CD98 expression modulates intestinal homeostasis, inflammation, and colitis-associated cancer in mice. J Clin Invest. 2011;121:1733–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiao B, Yang Y, Viennois E, et al. . Glycoprotein CD98 as a receptor for colitis-targeted delivery of nanoparticle. J Mater Chem B. 2014;2:1499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang M, Viennois E, Xu C, et al. . Plant derived edible nanoparticles as a new therapeutic approach against diseases. Tissue Barriers. 2016;4:e1134415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Robbins PD, Dorronsoro A, Booker CN. Regulation of chronic inflammatory and immune processes by extracellular vesicles. J Clin Invest. 2016;126:1173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buzas EI, György B, Nagy G, et al. . Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol. 2014;10:356–64. [DOI] [PubMed] [Google Scholar]
- 81.Stremersch S, De Smedt SC, Raemdonck K. Therapeutic and diagnostic applications of extracellular vesicles. J Control Release. 2016;244:167–83. [DOI] [PubMed] [Google Scholar]
- 82.Koniusz S, Andrzejewska A, Muraca M, et al. . Extracellular vesicles in physiology, pathology, and therapy of the immune and central nervous system, with focus on extracellular vesicles derived from mesenchymal stem cells as therapeutic tools. Front Cell Neurosci. 2016;10:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Dommelen SM, Vader P, Lakhal S, et al. . Microvesicles and exosomes: opportunities for cell-derived membrane vesicles in drug delivery. J Control Release. 2012;161:635–644. [DOI] [PubMed] [Google Scholar]
- 84.Jiang L, Shen Y, Guo D, et al. . EpCAM-dependent extracellular vesicles from intestinal epithelial cells maintain intestinal tract immune balance. Nat Commun. 2016;7:13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang X, Meng S, Jiang H, et al. . Exosomes derived from interleukin-10-treated dendritic cells can inhibit trinitrobenzene sulfonic acid-induced rat colitis. Scand J Gastroenterol. 2010;45:1168–77. [DOI] [PubMed] [Google Scholar]
- 86.Rani S, Ryan AE, Griffin MD, et al. . Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther. 2015;23:812–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Y, Tian J, Tang X, et al. . Exosomes released by granulocytic myeloid-derived suppressor cells attenuate DSS-induced colitis in mice. Oncotarget. 2016;7:15356–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang J, Liu XX, Fan H, et al. . Extracellular vesicles derived from bone marrow mesenchymal stem cells protect against experimental colitis via attenuating colon inflammation, oxidative stress and apoptosis. PLoS One. 2015;10:e0140551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mao F, Wu Y, Tang X, et al. . Exosomes derived from human umbilical cord mesenchymal stem cells relieve inflammatory bowel disease in mice. Biomed Res Int. 2017;2017:5356760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang M, Merlin D. Curcuma longa-derived nanoparticles reduce colitis and promote intestinal wound repair by inactivating the NF-κB pathway. Gastroenterology. 2017;152:S567. [Google Scholar]
- 91.Mu J, Zhuang X, Wang Q, et al. . Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol Nutr Food Res. 2014;58:1561–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Deng Z, Rong Y, Teng Y, et al. . Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell AMP-activated protein kinase. Mol Ther. 2017;25:1641–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ju S, Mu J, Dokland T, et al. . Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol Ther. 2013;21:1345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang B, Zhuang X, Deng ZB, et al. . Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol Ther. 2014;22:522–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shukla Y, Singh M. Cancer preventive properties of ginger: a brief review. Food Chem Toxicol. 2007;45:683–90. [DOI] [PubMed] [Google Scholar]
- 96.Zhang M, Viennois E, Prasad M, et al. . Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang M, Collins JF, Merlin D. Do ginger-derived nanoparticles represent an attractive treatment strategy for inflammatory bowel diseases?Nanomedicine (Lond). 2016;11:3035–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schoellhammer CM, Schroeder A, Maa R, et al. . Ultrasound-mediated gastrointestinal drug delivery. Sci Transl Med. 2015;7:310ra168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Traverso G, Schoellhammer CM, Schroeder A, et al. . Microneedles for drug delivery via the gastrointestinal tract. J Pharm Sci. 2015;104:362–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zaric M, Lyubomska O, Touzelet O, et al. . Skin dendritic cell targeting via microneedle arrays laden with antigen-encapsulated poly-D,L-lactide-co-glycolide nanoparticles induces efficient antitumor and antiviral immune responses. ACS Nano. 2013;7:2042–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kang T, Zhu Q, Wei D, et al. . Nanoparticles coated with neutrophil membranes can effectively treat cancer metastasis. ACS Nano. 2017;11:1397–411. [DOI] [PubMed] [Google Scholar]
- 102.Gao J, Wang S, Wang Z. High yield, scalable and remotely drug-loaded neutrophil-derived extracellular vesicles (EVs) for anti-inflammation therapy. Biomaterials. 2017;135:62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Burnouf T, Burnouf PA, Wu YW, et al. . Circulatory-cell-mediated nanotherapeutic approaches in disease targeting. Drug Discov Today. In press. [DOI] [PubMed] [Google Scholar]
- 104.Wei X, Gao J, Fang RH, et al. . Nanoparticles camouflaged in platelet membrane coating as an antibody decoy for the treatment of immune thrombocytopenia. Biomaterials. 2016;111:116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hu CM, Fang RH, Wang KC, et al. . Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526:118–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xuan M, Shao J, Dai L, et al. . Macrophage cell membrane camouflaged au nanoshells for in vivo prolonged circulation life and enhanced cancer photothermal therapy. ACS Appl Mater Interfaces. 2016;8:9610–18. [DOI] [PubMed] [Google Scholar]
- 107.Molinaro R, Corbo C, Martinez JO, et al. . Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat Mater. 2016;15:1037–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang Z, Li J, Cho J, et al. . Prevention of vascular inflammation by nanoparticle targeting of adherent neutrophils. Nat Nanotechnol. 2014;9:204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chu D, Gao J, Wang Z. Neutrophil-mediated delivery of therapeutic nanoparticles across blood vessel barrier for treatment of inflammation and infection. ACS Nano. 2015;9:11800–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen H, Zhang W, Zhu G, et al. . Rethinking cancer nanotheranostics. Nat Rev Mater. 2017;2:201724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao R, Han X, Li Y, et al. . Photothermal effect enhanced cascade-targeting strategy for improved pancreatic cancer therapy by gold nanoshell@mesoporous silica nanorod. ACS Nano. 2017;11:8103–13. [DOI] [PubMed] [Google Scholar]
- 112.Wang H, Huang Q, Chang H, et al. . Stimuli-responsive dendrimers in drug delivery. Biomater Sci. 2016;4:375–390. [DOI] [PubMed] [Google Scholar]
- 113.Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65:36–48. [DOI] [PubMed] [Google Scholar]
- 114.Torchilin VP. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov. 2014;13:813–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hassan S, Prakash G, Ozturk A, et al. . Evolution and clinical translation of drug delivery nanomaterials. Nano Today. 2017;15:91–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen S, Bian Q, Wang P, et al. . Photo, pH and redox multi-responsive nanogels for drug delivery and fluorescence cell imaging. Polym Chem. 2017;8:6150–7. [Google Scholar]
- 117.Ali H, Weigmann B, Neurath MF, et al. . Budesonide loaded nanoparticles with pH-sensitive coating for improved mucosal targeting in mouse models of inflammatory bowel diseases. J Control Release. 2014;183:167–77. [DOI] [PubMed] [Google Scholar]
- 118.Vong LB, Yoshitomi T, Morikawa K, et al. . Oral nanotherapeutics: effect of redox nanoparticle on microflora in mice with dextran sodium sulfate-induced colitis. J Gastroenterol. 2014;49:806–13. [DOI] [PubMed] [Google Scholar]
- 119.Saadatzadeh A, Atyabi F, Fazeli MR, et al. . Biochemical and pathological evidences on the benefit of a new biodegradable nanoparticles of probiotic extract in murine colitis. Fundam Clin Pharmacol. 2012;26:589–98. [DOI] [PubMed] [Google Scholar]
- 120.Laroui H, Viennois E, Xiao B, et al. . Fab’-bearing siRNA tnfα-loaded nanoparticles targeted to colonic macrophages offer an effective therapy for experimental colitis. J Control Release. 2014;186:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moulari B, Béduneau A, Pellequer Y, et al. . Lectin-decorated nanoparticles enhance binding to the inflamed tissue in experimental colitis. J Control Release. 2014;188:9–17. [DOI] [PubMed] [Google Scholar]
- 122.Higa LH, Jerez HE, de Farias MA, et al. . Ultra-small solid archaeolipid nanoparticles for active targeting to macrophages of the inflamed mucosa. Nanomedicine (Lond). 2017;12:1165–75. [DOI] [PubMed] [Google Scholar]