Abstract
Background
The course of inflammatory bowel disease (IBD) after liver transplantation (LT) for primary sclerosing cholangitis (PSC) is poorly understood. We describe the natural history of established IBD after LT (including risk of disease progression, colectomy, and neoplasia) and de novo IBD.
Methods
In a retrospective cohort, we identified all patients with PSC who underwent LT for advanced PSC at Mayo Clinic, Rochester, Minnesota. Risk factors were identified using multivariate Cox proportional hazard analysis.
Results
Three hundred seventy-three patients were identified (mean age, 47.5 ± 11.7 years; 64.9% male). Over a median (range) of 10 (5.5–17.1) years, 151 patients with PSC-IBD with an intact colon at the time of LT were studied. Post-LT, despite transplant-related immunosuppression, 56/151 (37.1%) required escalation of therapy, whereas 87 had a stable course (57.6%) and 8 patients (5.3%) improved. The 1-, 5-, and 10-year risks of progression of IBD were 4.0%, 18.5%, and 25.5%, respectively. On multivariate analysis, tacrolimus-based immunosuppression post-LT were associated with unfavorable course, and azathioprine use after LT was associated with improved course post-LT. Of 84 patients with no evidence of IBD at the time of LT, 22 (26.2%) developed de novo IBD post-LT. The 1-, 5-, and 10-year cumulative incidences of de novo IBD were 5.5%, 20.0%, and 25.4%, respectively. On univariate analysis, mycophenolate mofetil use after LT was associated with increased risk of de novo IBD, but azathioprine use after LT seemed to be protective.
Conclusions
The 10-year cumulative probability of IBD flare requiring escalation of therapy after LT for PSC was 25.5%, despite immunosuppression for LT. The 10-year cumulative risk of de novo IBD after LT for PSC was 25.4%. Transplant-related immunosuppression may modify the risk of de novo IBD, with an increased risk with mycophenolate and a decreased risk with azathioprine.
Keywords: inflammatory bowel disease, primary sclerosing cholangitis, liver tranpslant
INTRODUCTION
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease of possible autoimmune etiology, characterized by the destruction of the hepatic bile ducts due to progressive inflammation and fibrosis, leading to decompensated liver cirrhosis with a median time of survival ~12 years.1, 2 We previously reported that approximately 70%–80% of patients with PSC develop inflammatory bowel disease (IBD), especially ulcerative colitis (UC),3 whereas PSC occurs in 2%–8% of patients with UC and in 1%–3% of patients with Crohn’s disease.3–7 PSC-IBD is a unique phenotype characterized by extensive colitis with relative rectal sparing, backwash ileitis, mild histological inflammation and clinical course, and high risk of colorectal neoplasia.8–13
The course of IBD after liver transplantation (LT) is highly variable.14 In a systematic review of 14 studies on the course of IBD after LT for PSC, we observed that despite transplant-related immunosuppression, about 30% of patients had worsening of their established IBD; the remainder either had a stable course (39%) or improvement in disease course (31%).14 Active IBD at the time of LT,15 use of tacrolimus for immunosuppression,15 5-aminosalicylate (5-ASA) discontinuation after LT,15, 16 and active cigarette smoking have been variably associated with unfavorable IBD outcomes.17 Additionally, about 14%–30% of patients with PSC may develop de novo IBD after LT; risk factors remain largely unknown.14 Most previous studies on the course of IBD after LT are limited by a small sample size, low event rate, and short duration of follow-up, and used variable definitions of IBD disease.
Moreover, previous studies are unable to comprehensively examine patient-, disease-, and transplant-related factors associated with unfavorable IBD outcomes.
Hence, in this retrospective cohort study, we sought to determine: (a) the natural history of established IBD in patients with PSC-IBD after LT, including risk of disease progression, colectomy, and colorectal neoplasia; (b) risk factors associated with unfavorable IBD outcomes; and (c) the incidence, risk factors, and outcomes of de novo IBD after LT in patients with PSC.
METHODS
Patients
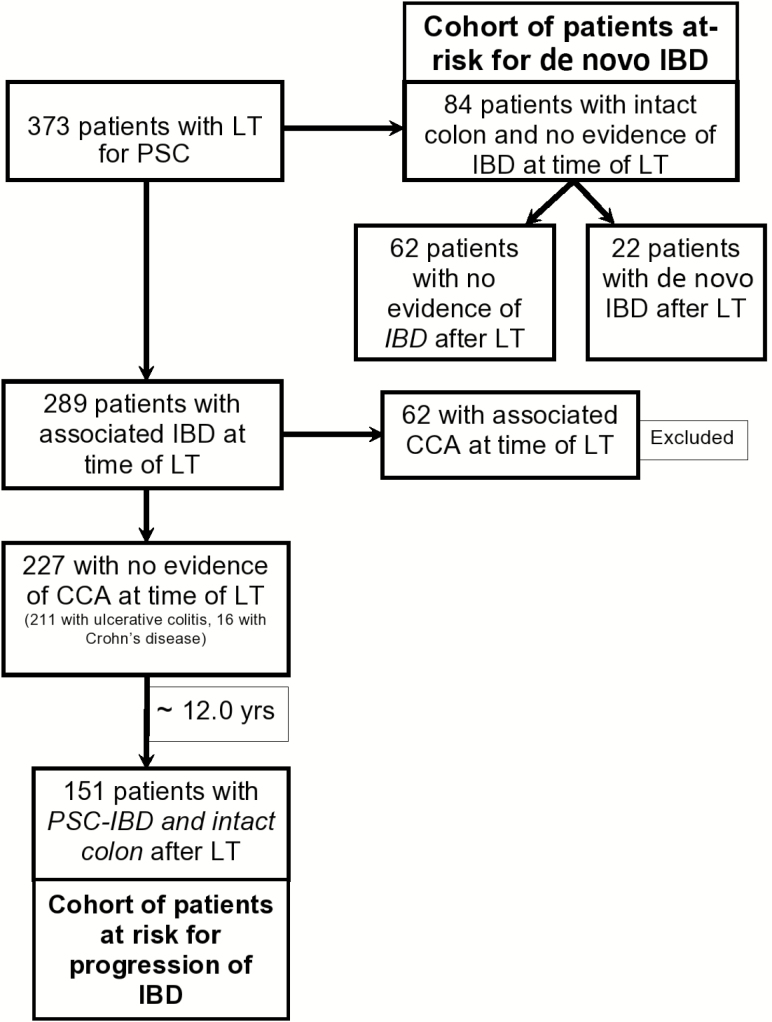
Through a prospectively maintained solid organ transplant registry, we identified 373 adult patients (>18 years) with PSC who underwent LT at Mayo Clinic Rochester between January 1984 and December 2012 and were followed through December 2015. From this cohort, we included patients with PSC, with or without associated IBD, who underwent LT for advanced PSC; we excluded patients who underwent LT for cholangiocarcinoma (n = 62) and those who died within 1 year after LT (n = 8). From these patients, we identified 2 separate cohorts of patients: (1) patients with established IBD at the time of LT, to study the natural history, outcomes, and risk factors associated with unfavorable IBD outcomes; and (2) patients without IBD at the time of LT, to study the incidence, risk factors, and outcomes of de novo IBD after LT. Patients who underwent colectomy before LT were excluded from this analysis (Fig. 1).
FIGURE 1.
Flow chart of the study cohorts.
Data Abstraction
Data collected included baseline demographics (age, sex, race), clinical variables (cigarette smoking status, comorbidities), transplant-related variables (allograft failure, type of donor, recurrent PSC, biliary strictures after LT, cytomegalovirus [CMV] infection, Epstein-Barr virus [EBV] infection, CMV mismatch, EBV mismatch, ABO blood type mismatch, sex mismatch, episodes of acute cellular rejection, chronic rejection, era of transplantation and retransplantation), associated IBD-related factors (subtype of IBD, IBD disease activity status at the time of and after LT, IBD treatment before and after transplant, and the presence vs absence of the colon at the time of LT), laboratory-related (renal function at the time of LT) and immune suppression–related variables (use of mycophenolate mofetil, azathioprine, prednisone, tacrolimus-, cyclosporine-, and sirolimus-based regimens) were abstracted systematically. At our center, immunosuppression protocols varied from cyclosporine, prednisone, and azathioprine before 1995, to a gradual shift to tacrolimus, prednisone, and mycophenolate mofetil after 1995. Current immunosuppression protocols included triple combination therapy for approximately 2 months. MMF is withdrawn between 2 and 4 months, and the steroids are tapered to none by 3 months from transplant, except for patients with early rejection or renal insufficiency. The majority were on monotherapy with tacrolimus or cyclosporine after 4 months, though patients with early rejection were maintained on dual therapy through year 1 and patients with renal insufficiency were maintained on dual therapy indefinitely with a lower tacrolimus target. Patients with PSC-autoimmune overlap with active hepatitis at the time of transplant were also typically maintained on dual therapy. Patients who developed biopsy-proven acute cellular rejection were treated with 3 intravenous boluses of methylprednisolone (1000 mg), followed by repeat biopsy to document resolution. Steroid-resistant rejection was treated with antithymocyte globulin (Thymoglobulin). IBD status did not influence the selection of these medications.
Outcomes
In patients with established IBD, “IBD disease progression” was defined as the need for escalation of IBD-related medical therapy or colectomy for medically refractory IBD, as compared with the pre-LT course, that is, progression from no therapy → 5-aminosalicylate therapy → use of immunomodulators (thiopurines, methotrexate) for IBD → biologic therapy (such as anti–tumor necrosis factor [anti-TNF] agents or vedolizumab). Intolerance to therapy was not considered progression. In addition, the development of colorectal neoplasia and cumulative risk of colectomy after LT was also estimated.
De novo IBD was defined as a new diagnosis of IBD in patients transplanted for PSC, with no prior diagnosis of IBD any time before LT. The diagnosis is made after ruling out other causes of post-LT colitis such as CMV colitis, infectious colitis, and mycophenolate colitis.
Statistical Analysis
The data were reported as means (±standard deviation), medians (interquartile range [IQR]), ranges, and categorical variables by counts and percentages as appropriate. The cumulative incidence rates of IBD progression, colectomy post-LT, de novo cancer, and de novo IBD were calculated for the entire follow-up period using Kaplan-Meier survival analysis for different cohorts. Patients were followed up until occurrence of disease progression, colectomy, death, or end of study periods. To identify risk factors (present at the time of LT) associated with IBD progression, colectomy, de novo cancer, and de novo IBD development, we performed univariate time-to-event analysis using the log-rank test based on predefined risk factors of interest: patient-related, IBD-related, immune suppression–related variables, and transplant-related. Variables that were significant (P < 0.10) on univariate analysis were then included in a multivariate Cox proportional hazard analysis to identify independent risk factors associated with unfavorable IBD course. All statistical analyses were conducted using JMP version 10 for Windows (SAS Institute Inc., Cary, NC, USA). P values <0.05 were considered statistically significant.
RESULTS
Patients With Established IBD at Time of Liver Transplantation
Of 373 patients who underwent LT for PSC, 151 with PSC-IBD formed the study cohort (Fig. 1). These patients were followed over a median (range) of 10 (5.5–17.1) years. Baseline IBD treatment at LT included no specific therapy in 66 patients, 5-ASA alone in 62 patients, corticosteroids and/or immunomodulators in 19 patients, and anti-TNF agents in 4 patients. Fifty-six (37.1%) patients had disease progression within a median interval of 3.2 years despite immunosuppression after LT, 87 (57.6%) patients had stable disease, and 8 (5.3%) patients improved. The baseline clinical and demographic characteristics for PSC-IBD patients after LT are shown in Table 1.
Table 1:
Comparison of Clinical and Demographic Characteristics of Patients With Different Courses of Inflammatory Bowel Disease After Liver Transplantation for Primary Sclerosing Cholangitis
| Characteristics | Progressed = 56 | Stable = 87 | Improved = 8 | De novo = 22 |
|---|---|---|---|---|
| Demographics | ||||
| Recipient age at LT, mean±SD, y | 44.9 ± 12.4 | 46.8 ± 11.3 | 50.8 ± 16.0 | 49.0 ± 12 |
| Male sex, No. (%) | 40 (71.4) | 58 (66.7) | 7 (87.5) | 12 (54.5) |
| Smoking at the time of LT, No. (%) | 1 (1.8) | 2 (2.3) | 0 (0.0) | 4 (18.2) |
| Alive at last follow-up, No. (%) | 41 (73.2) | 56 (64.4) | 7 (87.5) | |
| Median follow-up (IQR), y | 11.7 (6.9–17.5) | 12.4 (6.9–20.2) | 4.4 (3.6–8.0) | 11.7 (5.5–17.1) |
| Transplant-related variables | ||||
| Allograft failure, No. (%) | 14 (25.0) | 21 (24.1) | 0 (0.0) | 4 (18.2) |
| Living donor, No. (%) | 8 (14.3) | 7 (8.05) | 2 (25.0) | 7 (31.8) |
| Recurrent PSC, No. (%) | 21 (37.5) | 33 (37.9) | 0 (0.0) | 9 (40.9) |
| CMV infection, No. (%) | 14 (25.0) | 22 (25.3) | 2 (25.0) | 3 (13.6) |
| Retransplantation, No. (%) | 11 (19.6) | 18 (20.7) | 0 (0.0) | 2 (9.1) |
| Cancer post-LT, No. (%) | 19 (33.9) | 44 (50.6) | 3 (37.5) | 12 (54.6) |
| Acute cellular rejection, No. (%) | 27 (48.2) | 40 (46.0) | 5 (62.5) | 12 (54.6) |
| CMV mismatch, No. (%) | 22 (39.3) | 23 (26.4) | 1 (12.5) | 3 (13.6) |
| Era of LT (before 1995), No. (%) | 20 (35.7) | 48 (55.2) | 0 (0.0) | 6 (27.7) |
| Kidney dysfunction at LT (serum Creatinine > 1.3), No. (%) | 1 (1.8) | 9 (10.3) | 2 (25.0) | 5 (22.7) |
| WBC counts at LT per mm3, mean±SD | 6.5 ± 4.0 | 6.1 ± 2.9 | 5.4 ± 2.3 | 5.7 ± 1.6 |
| Neutrophils counts at LT, mean±SD, x10(9)/L | 3.8 ± 2.5 | 4.2 ± 2.1 | 3.9 ± 1.9 | 4.0 ± 1.7 |
| Hypoalbuminemia at LT (<3.5 g/dL), No. (%) | 31 (55.4) | 36 (41.4) | 7 (87.5) | 8 (36.4) |
| Immunosuppression | ||||
| Mycophenolate mofetil after LT, No. (%) | 29 (51.8 ) | 42 (48.3) | 7 (87.5) | 15 (68.1) |
| Azathioprine after LT, No. (%) | 17 (30.4) | 53 (61.0) | 1 (12.5) | 7 (31.8) |
| Prolonged corticosteroid use (>6 mo), No. (%) | 34 (60.7) | 59 (67.8) | 1 (12.5) | 6 (27.3) |
| Tacrolimus-based immunosuppression, No. (%) | 41 (73.2) | 40 (46.0) | 8 (100.0) | 16 (72.7) |
| Cyclosporine-based immunosuppression, No. (%) | 15 (26.8) | 45 (51.7) | 0 (0.0) | 6 (27.3) |
For patients with IBD progression, the mean age at the time of liver transplant was 44.9 ± 12.4 years and 40/56 (71.4%) were males. Higher rates of patients who developed worsening IBD activity after LT were exposed to tacrolimus. Patients with stable IBD disease post-LT were more likely to develop de novo cancers than others with unfavorable IBD disease.
Rate of Progression of IBD After LT
The cumulative incidence rates of IBD progression post-LT for PSC patients were 4.0%, 18.5%, and 25.5% at 1, 5, and 10 years, respectively. Thirty-three patients who were on no therapy or 5-ASA before LT now required IM and/or anti-TNF or surgery for medically refractory IBD. Immunosuppressive management of established IBD before and after LT is depicted in Table 2. Anti-TNF agents were used in 7 patients. Supplementary Table 1 demonstrates clinical and demographic characteristics for patients who received anti-TNF agents. At last follow-up, 6 of 7 patients (85.7%) treated with anti-TNF agents achieved clinical response and 3 (42.9%) achieved mucosal healing. Four patients developed serious infections (Escherichia coli, enteroaggregative E. coli, Serratia marcescens, and Rhodococcus equi) after starting anti-TNF agents, but none developed any malignancy.
Table 2:
Treatment of Inflammatory Bowel Disease After Liver Transplantation (as Compared With Pretransplant Therapy) in Patients With Primary Sclerosing Cholangitis-Related Inflammatory Bowel Disease and Intact Colon at Time of Liver Transplant
| Post-transplant Therapy for IBD | ||||||
|---|---|---|---|---|---|---|
| Pretransplant Therapy for IBD | None, No. (%) | 5-ASA, No. (%) | Corticosteroids/ Immunomodulators, No. (%) | Anti-TNF/Surgery, No. (%) | Total, No. (%) | |
| None | 34 | 17 | 8 | 7 | 66 (43.7) | |
| 5-ASA | 6 | 38 | 8 | 10 | 62 (41.1) | |
| Corticosteroids/Immunomodulators | 10 | 2 | 3 | 4 | 19 (12.6) | |
| Anti-TNF agents | 1 | 2 | - | 1 | 4 (2.6) | |
| 51 (33.8) | 59 (39.1) | 19 (12.6) | 22 (14.6) | 151 | ||
On univariate analysis, CMV mismatch (vs no mismatch; hazard ratio [HR], 2.20; 95% confidence interval [CI], 1.14–4.32; P = 0.019) and tacrolimus use after LT (vs cyclosporine; HR, 3.51; 95% CI, 1.84–7.20; P ≤ 0.001) were associated with IBD progression after LT, whereas renal dysfunction at the time of LT (HR, 0.20; 95% CI, 0.01–0.95; P = 0.041) and azathioprine use after LT (HR, 0.42; 95% CI, 0.23–0.76; P = 0.005) were protective against progressive IBD after LT. In a multivariate model that included CMV mismatch, tacrolimus use, azathioprine use after LT, and renal dysfunction at LT, azathioprine use (HR, 0.52; 95% CI, 0.30–0.87) was an independent risk factor associated with protection against IBD progression post-LT and tacrolimus use (HR, 4.02; 95% CI, 1.87–10.24) was an independent risk factor associated with disease progression. Neither active IBD at the time of LT (data not shown) nor 5-ASA use after LT demonstrated a statistically significant relationship (Table 3A).
TABLE 3A:
Risk Factors Associated With Progression of Existing Inflammatory Bowel Disease After Liver Transplantation
| IBD Progression | ||||
|---|---|---|---|---|
| Risk Factors | Univariate Analysis HR (95% CI) | P | Multivariate Analysis HR (95% CI) | P |
| Demographics | ||||
| Age at LT per y | 0.997 (0.97–1.02) | 0.805 | - | - |
| Sex (M:F) | 1.17 (0.64–2.26) | 0.623 | - | - |
| Smoking (ever:never) | 1.73 (0.70–3.67) | 0.216 | - | - |
| Transplant-related variables | ||||
| CMV infection (Y:N) | 1.46 (0.76–2.69) | 0.247 | - | - |
| CMV mismatch (Y:N) | 2.20 (1.14–4.32) | 0.019 | 1.10 (0.66–1.82) | 0.701 |
| Renal dysfunction at the time of LT (serum Creatinine > 1.3) | 0.20 (0.01–0.95) | 0.041 | 0.74 (0.34–1.45) | 0.283 |
| Type of LT (living donor vs orthotopic liver transplantation) | 2.36 (0.95–5.09) | 0.063 | ||
| Immunosuppression | ||||
| Tacrolimus-based immunosuppression (vs cyclosporine-based) | 3.51 (1.84–7.20) | <0.001 | 4.06 (1.87–10.24) | <0.001 |
| Mycophenolate mofetil use at LT (Y:N) | 1.40 (0.78–2.51) | 0.261 | - | - |
| Azathioprine use at LT (Y:N) | 0.42 (0.23–0.76) | 0.005 | 0.52 (0.30–0.87) | 0.014 |
| IBD-related variables | ||||
| Peritransplant 5-ASA use (Y:N) | 1.27 (0.48–2.79) | 0.60 | - | - |
Colectomy After LT for PSC-IBD Patients
Of 151 patients with PSC-IBD and intact colons at LT, 36 patients underwent colectomy after LT (15 for medically refractory IBD and 21 for colorectal neoplasia). The cumulative probabilities for colectomy-free survival after LT for PSC-IBD were 98.0%, 89.6%, and 79.7% at 1, 5, and 10 years, respectively. The overall colectomy-free survival rates in the IBD progression group were 98.2%, 85.0%, and 69.8% at 1, 5, and 10 years, respectively, compared with 97.7%, 92.9%, and 85.8% at 1, 5, and 10 years in the stable group (P = 0.02). On univariate analysis, progressive IBD (vs stable IBD; HR, 2.45; 95% CI, 1.27–4.84; P = 0.008) and hypoalbuminemia at the time of LT (<3.5 g/dL; HR, 7.40; 95% CI, 1.52–133.0; P = 0.008) were associated with increased risk of colectomy post-LT.
Cancer Development After LT in PSC-IBD Patients
Of 151 patients with PSC-IBD and intact colons at LT, 66 patients developed de novo cancer after LT over a median of 12 years. Fifty-nine cancers (28 nonmelanoma skin cancers and 31 nonskin cancers, with 7/31 being colorectal cancers) developed in 45 patients (50.6%) with a stable IBD course. Despite unfavorable IBD outcome and immunosuppression, 19 patients (33.9%) in the progressed group developed 26 cancers (16 nonmelanoma skin cancers, 10 nonskin cancers). Only 3 patients in the group with improved IBD activity post-LT developed 4 cancers (all were solid cancers).
The probabilities for survival free of nonskin cancer development after LT for PSC-IBD were 99.3%, 92.7%, 84.1%, and 79.5% at 1, 5, 10, and 20 years, respectively. The cumulative incidences of de novo colorectal cancer (not dysplasia) post-LT in PSC-IBD patients at 1, 5, 10, and 20 years after LT were 0.7%, 1.3%, 4.6%, and 25.4%, respectively. By univariate analysis, male sex (HR, 2.99; 95% CI, 1.37–7.50; P = 0.005), high neutrophil to lymphocyte ratio (NLR > 5.70) at the time of LT (HR, 3.21; 95% CI, 1.32–8.20; P = 0.010), and elevated INR at the time of LT (INR > 1.80; HR, 5.18; 95% CI, 1.47–14.40; P = 0.014) were significant factors associated with de novo nonskin malignancy development. In a multivariate model that included sex, colectomy post-LT, high INR, and high NLR, only elevated NLR at the time of LT (HR, 3.20; 95% CI, 1.28–8.41; P = 0.013) and elevated INR at the time of LT (HR, 4.19; 95% CI, 1.17–11.97; P = 0.031) were associated with increased risk. IBD status at the time of and after LT and the era of transplantation were not significantly associated with time to malignancy. Post-LT cancer development was not associated with decreased overall survival, increased recurrent PSC risk, or graft failure risk.
De Novo IBD After Liver Transplantation
Eighty-four patients without IBD at the time of LT formed the study cohort (mean age, 49 ± 12 years, 56% males). Over a median follow-up (IQR) of 11.7 (5.5–17.1) years after LT, 22 patients (26.2%) developed de novo IBD (20 patients with ulcerative colitis, 1 patient with Crohn’s disease, and 1 patient with indeterminate colitis). The most common presenting symptom was diarrhea (59.1%), followed by gastrointestinal bleeding (22.7%). The remainder of patients were asymptomatic at the time of diagnosis (18.2%). The cumulative incidences of de novo IBD at 1, 5, and 10 years after LT were 5.5%, 20.0%, and 25.4%, respectively. The majority of patients had a mild course requiring either no therapy (18.2%) or only 5-aminosalicylates (68.2%); only 3 patients required IBD-directed immunosuppression, but none required anti-TNF agents. Four patients underwent colectomy after LT for colorectal neoplasia, but none for intractable disease. Mycophenolate mofetil (MMF) use post-LT was associated with an increased risk of de novo IBD (HR, 3.3; 95% CI, 1.3–9.5), whereas azathioprine use was associated with decreased risk (HR, 0.2; 95% CI, 0.1–0.6). Due to a limited number of events, a multivariate Cox proportional hazard analysis could not be performed. CMV infection, CMV mismatch, and tacrolimus immunosuppression were not associated with development of de novo IBD after LT (Table 3B).
TABLE 3B:
Risk Factors Associated With De Novo Inflammatory Bowel After Liver Transplantation
| De Novo IBD | ||
|---|---|---|
| Risk Factors | Univariate Analysis HR (95% CI) | P |
| Demographics | ||
| Age at LT per year | 0.993 (0.961–1.027) | 0.666 |
| Sex (M:F) | 0.68 (0.27–1.74) | 0.411 |
| Smoking (ever:never) | 1.36 (0.24–2.74) | 0.622 |
| Transplant-related variables | ||
| CMV infection (Y:N) | 1.63 (0.37–5.13) | 0.474 |
| CMV mismatch (Y:N) | 1.53 (0.33–5.30) | 0.548 |
| Renal dysfunction at the time of LT (serum Creatinine > 1.3) | 0.57 (0.15–1.71) | 0.329 |
| Type of LT (living donor vs OLT) | 1.28 (0.28–4.34) | 0.714 |
| Immunosuppression | ||
| Tacrolimus-based immunosuppression (vs cyclosporine-based) | 1.23 (0.45–3.94) | 0.698 |
| Mycophenolate mofetil use at LT (Y:N) | 3.32 (1.34–9.43) | <0.001 |
| Azathioprine use at LT (Y:N) | 0.19 (0.13–0.57) | 0.003 |
OLT = orthotopic liver transplant.
DISCUSSION
In this study, we showed that up to 40% of patients with PSC-IBD after LT required escalation of IBD-related therapy, despite immunosuppression. About one-quarter of IBD-PSC patients required colectomy post-LT. Azathioprine use was an independent factor against IBD progression, and tacrolimus-based regimens had a higher risk of IBD-flare post-LT. De novo IBD developed in about one-quarter of patients within 12 years of transplantation and had a mild course in most patients. Azathioprine use seemed to be protective against de novo IBD. Nearly 40% required colectomy either before or after LT for intractable disease or advanced dysplasia or neoplasia.
Previous studies have shown conflicting data on the natural history of IBD post-LT for PSC.14, 18 In our study, about 37% of IBD patients had worsening disease activity post-LT despite being on an immunosuppressive regimen. Our data found that azathioprine use at the time of LT (for immunosuppression and IBD) was protective against worsening of IBD activity after LT. This supports the previous finding that the rate of IBD exacerbation in patients on azathioprine was lower than that of those who were not on azathioprine.19 We also found that patients who were immunosuppressed with tacrolimus-based regimens had a higher risk of IBD-flare post-LT. Previous studies reported that tacrolimus-based regimens were associated with increased risk of IBD-flare.15, 20 However, other studies have not shown that tacrolimus-based regimens increases the risk of IBD flare post-LT.21 Furthermore, shifting immunosuppressive treatment to cyclosporine-based regimens in such patients could increase the risk of acute rejection and graft loss.22 Prospective studies are needed to evaluate whether various immunosuppressive regimens increase the risk of progression of IBD.
In our study, 7 patients were treated with anti-TNF agents for refractory disease after LT. Clinical response was observed in more than 80%, and mucosal healing was achieved in more than 40%. Most of these patients developed recurrent PSC post-LT. Adverse events included infections in 4/7 (57.1%), but none developed any cancer. Our knowledge about the use of anti-TNF therapy for refractory IBD after LT is very limited given the paucity of the literature on its use.23–26 Patients treated with anti-TNF agents need careful monitoring for infections, autoimmune diseases, and cancers.27 Of note, 3 patients were treated with vedolizumab with success. Vedolizumab was reported to be safe and efficacious in the management of active IBD in patients post-LT, but further studies are needed.28, 29
Our study shows that the rates of de novo malignancies were higher in patients with a stable IBD course after LT for PSC. Sokol et al. found that colorectal cancer risk in PSC-IBD patients increased in patients with lower IBD disease activity and longer 5-ASA use.9 The reasons for increased risk of colorectal cancer and other cancers in patients with less active IBD after LT suggests that other factors are involved, such as the intensity of immunosuppression or other factors directly linked to PSC.
De novo IBD developed in one-quarter of patients without IBD at the time of LT. We advocate colonoscopy every 3–5 years in PSC patients without IBD, as 20% of patients diagnosed with de novo IBD were asymptomatic and approximately 20% developed colorectal cancer.30 The diagnosis of de novo IBD was confirmed by colonic and histologic features, and de novo IBD was distinguished from MMF-induced colitis by an expert gastrointestinal pathologist. Our pathologists were able to discriminate de novo IBD from mycophenolate colitis by the presence of crypt architecture distortion with lymphoplasmacytic-predominant lamina propria inflammation.31 CMV infection, CMV mismatch, tacrolimus use, and azathioprine-free regimens are reported to increase risk for de novo IBD after LT for PSC.32 We found that the risk increased with mycophenolate mofetil and decreased with azathioprine. However, our subgroup of patients who developed de novo IBD after LT for PSC is too small to draw definite conclusions.
We confirmed the results of our previous study that elevated INR and NLR at the time of LT were also found to be a risk factor for de novo cancer.33 We also noticed that serum hypoalbuminemia at the time of LT was a risk factor for colectomy after LT. The mechanism of low albumin in these patients could be related to significant intestinal leakage of protein-rich fluids secondary to mucosal injury in IBD patients.34 Serum hypoalbuminemia was reported to be a significant predictor of late mortality in patients on the liver transplant waiting list35 but was not studied extensively to predict IBD outcomes after LT. Albumin is a negative acute phase reactant and may have indicated higher inflammatory burden. Because this study was limited by the small number of included patients with PSC-IBD who underwent colectomy post-LT, further studies are needed to examine the prognostic utility of serum hypoalbuminemia with colectomy outcomes.
The main strengths of our study are the inclusion of a large number of PSC patients with PSC and IBD, which enabled us to assess variable outcomes. However, our study has some limitations. In addition to the retrospective nature of our study, we were not able to report disease duration, the frequency or chronicity of corticosteroids use, or the intensity of immunosuppression or immunomodulators in all patients for logistic reasons. In addition, we also could not perform multivariate Cox proportional hazards analysis to assess risk factors for de novo IBD and colectomy post-LT after LT due to the limited number of events.
In conclusion, 40% of PSC patients with IBD who underwent LT required escalation of medical therapy. Approximately 25% of IBD-PSC patients underwent colectomy after LT, and the risk increased with serum hypoalbuminemia at the time of LT. PSC patients who underwent LT in a different era and received azathioprine immunosuppression were associated with significantly favorable IBD disease. De novo IBD developed in 25% of patients after LT for PSC, and most of the patients had a mild course after LT.
SUPPLEMENTARY DATA
Supplementary data are available at Inflammatory Bowel Diseases online.
ACKNOWLEDGEMENTS
Supported by: We acknowledge support from National Cancer Institute (NCI) CA 170357 and the Mayo Clinic Center for Cell Signaling in Gastroenterology (NIDDK P30DK084567) and from the time Lapse NCI CA 170357. Dr. Singh is supported by the NLM training grant T15LM011271, the American College of Gastroenterology, and Crohn’s and Colitis Foundation of America, and has received research support from Pfizer.
Conflicts of interest: The authors have no financial or personal relationships that could present a potential conflict of interest.
Disclosures: Dr. Loftus has consulted for Takeda, Janssen, UCB Pharma, AbbVie, Amgen, Pfizer, Mesoblast, Eli Lilly, CVS Caremark, and Salix; and has received research support from Takeda, Janssen, UCB Pharma, AbbVie, Genentech, Seres Therapeutics, Amgen, Pfizer, Receptos, Celgene, Gilead, MedImmune, and Robarts Clinical Trials.
Author contributions: M. Mouchli reviewed medical records and data and participated in the writing of the paper. S. Singh reviewed medical records and data and participated in the writing of the paper. L. Boardman edited the manuscript. D. Bruining edited the manuscript. A. L. Lightner edited the manuscript. J. K. Heimbach edited the manuscript. C. B. Rosen edited the manuscript. B Hasan reviewed medical records and edited the manuscript. J. J. Poterucha edited the manuscript. K. D. Watt edited the manuscript. S. V. Kane edited the manuscript. L. E. Raffals edited the manuscript. E. V. Loftus, Jr., reviewed medical records and data and participated in the writing of the manuscript.
REFERENCES
- 1.Bambha K, Kim WR, Talwalkar J et al. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology. 2003;125:1364–69. [DOI] [PubMed] [Google Scholar]
- 2.Wiesner RH, Grambsch PM, Dickson ER et al. Primary sclerosing cholangitis: natural history, prognostic factors and survival analysis. Hepatology. 1989;10:430–36. [DOI] [PubMed] [Google Scholar]
- 3.Loftus EV Jr, Sandborn WJ, Lindor KD, Larusso NF. Interactions between chronic liver disease and inflammatory bowel disease. Inflamm Bowel Dis. 1997;3:288–302. [PubMed] [Google Scholar]
- 4.Olsson R, Danielsson A, Järnerot G et al. Prevalence of primary sclerosing cholangitis in patients with ulcerative colitis. Gastroenterology. 1991;100:1319–23. [PubMed] [Google Scholar]
- 5.Rasmussen HH, Fallingborg JF, Mortensen PB et al. Hepatobiliary dysfunction and primary sclerosing cholangitis in patients with Crohn’s disease. Scand J Gastroenterol. 1997;32:604–10. [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen HH, Fallingborg J, Mortensen PB et al. Primary sclerosing cholangitis in patients with ulcerative colitis. Scand J Gastroenterol. 1992;27:732–36. [DOI] [PubMed] [Google Scholar]
- 7.Schrumpf E, Elgjo K, Fausa O et al. Sclerosing cholangitis in ulcerative colitis. Scand J Gastroenterol. 1980;15:689–97. [DOI] [PubMed] [Google Scholar]
- 8.Loftus EV Jr, Harewood GC, Loftus CG et al. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sokol H, Cosnes J, Chazouilleres O et al. Disease activity and cancer risk in inflammatory bowel disease associated with primary sclerosing cholangitis. World J Gastroenterol. 2008;14:3497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jørgensen KK, Grzyb K, Lundin KE et al. Inflammatory bowel disease in patients with primary sclerosing cholangitis: clinical characterization in liver transplanted and nontransplanted patients. Inflamm Bowel Dis. 2012;18:536–45. [DOI] [PubMed] [Google Scholar]
- 11.Lindberg BU, Broomé U, Persson B. Proximal colorectal dysplasia or cancer in ulcerative colitis. The impact of primary sclerosing cholangitis and sulfasalazine: results from a 20-year surveillance study. Dis Colon Rectum. 2001;44:77–85. [DOI] [PubMed] [Google Scholar]
- 12.Kornfeld D, Ekbom A, Ihre T. Is there an excess risk for colorectal cancer in patients with ulcerative colitis and concomitant primary sclerosing cholangitis? A population based study. Gut. 1997;41:522–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soetikno RM, Lin OS, Heidenreich PA et al. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. 2002;56:48–54. [DOI] [PubMed] [Google Scholar]
- 14.Singh S, Loftus EV Jr, Talwalkar JA. Inflammatory bowel disease after liver transplantation for primary sclerosing cholangitis. Am J Gastroenterol. 2013;108:1417–1425. [DOI] [PubMed] [Google Scholar]
- 15.Verdonk RC, Dijkstra G, Haagsma EB et al. Inflammatory bowel disease after liver transplantation: risk factors for recurrence and de novo disease. Am J Transplant. 2006;6:1422–29. [DOI] [PubMed] [Google Scholar]
- 16.Navaneethan U, Choudhary M, Venkatesh PG et al. The effects of liver transplantation on the clinical course of colitis in ulcerative colitis patients with primary sclerosing cholangitis. Aliment Pharmacol Ther. 2012;35:1054–63. [DOI] [PubMed] [Google Scholar]
- 17.Cholongitas E, Papatheodoridis GV, Zappoli P et al. Combined HLA-DR and -DQ disparity is associated with a stable course of ulcerative colitis after liver transplantation for primary sclerosing cholangitis. Liver Transpl. 2007;13:552–57. [DOI] [PubMed] [Google Scholar]
- 18.Mosli M, Croome K, Qumosani K et al. The effect of liver transplantation for primary sclerosing cholangitis on disease activity in patients with inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2013;9:434–41. [PMC free article] [PubMed] [Google Scholar]
- 19.Haagsma EB, Van Den Berg AP, Kleibeuker JH et al. Inflammatory bowel disease after liver transplantation: the effect of different immunosuppressive regimens. Aliment Pharmacol Ther. 2003;18:33–44. [DOI] [PubMed] [Google Scholar]
- 20.Jørgensen KK, Lindström L, Cvancarova M et al. Immunosuppression after liver transplantation for primary sclerosing cholangitis influences activity of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:517–23. [DOI] [PubMed] [Google Scholar]
- 21.Ho GT, Seddon AJ, Therapondos G et al. The clinical course of ulcerative colitis after orthotopic liver transplantation for primary sclerosing cholangitis: further appraisal of immunosuppression post transplantation. Eur J Gastroenterol Hepatol. 2005;17:1379–85. [DOI] [PubMed] [Google Scholar]
- 22.McAlister VC, Haddad E, Renouf E et al. Cyclosporin versus tacrolimus as primary immunosuppressant after liver transplantation: a meta-analysis. Am J Transplant. 2006;6:1578–85. [DOI] [PubMed] [Google Scholar]
- 23.Sandhu A, Alameel T, Dale CH et al. The safety and efficacy of antitumour necrosis factor-alpha therapy for inflammatory bowel disease in patients post liver transplantation: a case series. Aliment Pharmacol Ther. 2012;36:159–65. [DOI] [PubMed] [Google Scholar]
- 24.Mohabbat AB, Sandborn WJ, Loftus EV Jr et al. Anti-tumour necrosis factor treatment of inflammatory bowel disease in liver transplant recipients. Aliment Pharmacol Ther. 2012;36:569–74. [DOI] [PubMed] [Google Scholar]
- 25.El-Nachef N, Terdiman J, Mahadevan U. Anti-tumor necrosis factor therapy for inflammatory bowel disease in the setting of immunosuppression for solid organ transplantation. Am J Gastroenterol. 2010;105:1210–11. [DOI] [PubMed] [Google Scholar]
- 26.Lal S, Steinhart AH. Infliximab for ulcerative colitis following liver transplantation. Eur J Gastroenterol Hepatol. 2007;19:277–80. [DOI] [PubMed] [Google Scholar]
- 27.Indriolo A, Ravelli P. Clinical management of inflammatory bowel disease in the organ recipient. World J Gastroenterol. 2014;20:3525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meszaros M, Pageaux GP, Altwegg R. Management of ulcerative colitis using vedolizumab after liver transplantation for primary sclerosing cholangitis. J Crohns Colitis. 2016;10:236. [DOI] [PubMed] [Google Scholar]
- 29.Hartery K, O’Reilly S, Houlihan D et al. Letter: vedolizumab for the management of inflammatory bowel disease in patients after liver transplantation for primary sclerosing cholangitis. Aliment Pharmacol Ther. 2017;45:376–78. [DOI] [PubMed] [Google Scholar]
- 30.Singh S, Watt KD. Long-term medical management of the liver transplant recipient: what the primary care physician needs to know. Mayo Clin Proc. 2012;87:779–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papadimitriou JC, Cangro CB, Lustberg A et al. Histologic features of mycophenolate mofetil-related colitis: a graft-versus-host disease-like pattern. Int J Surg Pathol. 2003;11:295–02. [DOI] [PubMed] [Google Scholar]
- 32.Åberg F, Abdulle A, Mäkelä A et al. Asymptomatic de novo inflammatory bowel disease late after liver transplantation for primary sclerosing cholangitis: a case report. Transplant Proc. 2015;47:2775–77. [DOI] [PubMed] [Google Scholar]
- 33.Mouchli MA, Singh S, Loftus EV Jr et al. Risk factors and outcomes of de novo cancers (excluding nonmelanoma skin cancer) after liver transplantation for primary sclerosing cholangitis. Transplantation. 2017;101:1859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Umar SB, DiBaise JK. Protein-losing enteropathy: case illustrations and clinical review. Am J Gastroenterol. 2010;105:43–9. [DOI] [PubMed] [Google Scholar]
- 35.Porrett PM, Baranov E, ter Horst M. Serum hypoalbuminemia predicts late mortality on the liver transplant waiting list. Transplantation. 2015;99:158–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.