Abstract
Background
Long noncoding RNAs (lncRNAs) have been implicated in several human cancers. The expression profile and underlying mechanism of the lncRNA MAP3K1-2 in gastric cancer (GC) are poorly understood.
Methods
Sixty-one patients with GC were recruited from Shanghai Baoshan Luo Dian Hospital (Shanghai, China). Tumor tissues and paired normal tissues (5 cm adjacent to the tumor) were obtained. Expression of lncRNA MAP3K1-2 in GC cell lines was examined using quantitative real-time polymerase chain reaction. Protein expression was detected using Western blot. Cell cycle analysis was assessed using flow cytometry. Cell proliferation was assessed using soft agar assays, and cell invasion was assessed using Transwell assays.
Results
The expression level of lncRNA MAP3K1-2 was upregulated in GC cells and markedly higher in poorly differentiated cell lines. Silencing treatment of lncRNA MAP3K1-2 significantly inhibited cell proliferation and invasion in GC. In addition, knockdown of lncRNA MAP3K1-2 significantly inhibited the function of important genes in the MAPK signaling pathway. Higher expression of lncRNA MAP3K1-2 was often associated with poorer prognosis in patients with GC.
Conclusions
lncRNA MAP3K1-2 is a critical effector in GC tumorigenesis and progression, representing novel therapeutic targets. High lncRNA MAP3K1-2 expression may serve as a novel independent prognostic marker for predicting the outcome of GC.
Keywords: gastric cancer, long noncoding RNA, MAP3K1-2
Background
Gastric cancer (GC) is one of the leading causes of cancer-related death worldwide and the most common gastrointestinal malignancy in East Asia.1,2 Despite recent improvements in both surgical and chemotherapeutic approaches, GC continues to have a poor prognosis owing to the lack of early symptoms. This results in advanced stage and metastatic stages, which limit the success of therapeutic strategies. The molecular mechanisms underlying GC progression and tumor metastasis remain poorly understood. Improved understanding of tumorigenesis and GC progression is indispensable for precision therapy.
Long noncoding RNAs (lncRNAs) are broadly defined as transcribed RNA molecules >200 nucleotides in length and lacking an open reading frame of significant length (<100 amino acids).3–6 Increasing evidence has shown that lncRNAs play critical roles in human disease, including stem cell development, pluripotency, cell growth, apoptosis in malignant tumors, and carcinogenesis.7–12 Several researchers have identified dysregulated expression of many lncRNAs in GC, thereby uncovering their potential roles in GC. Functioning as oncogenes or tumor suppressors, lncRNAs participate in several vital steps of GC occurrence and development via signaling pathways, crossing talk with microRNAs, and in the regulation of epithelial-to-mesenchymal transition. To date, several lncRNAs have been well characterized in GC, particularly HOTAIR, ANRIL, H19, GAPLINC, PVT1, and LINC00152.13 However, the detailed function of the majority of lncRNA in cancer cells still remains unclear.
In our study, we discovered an lncRNA termed lncRNA MAP3K1-2 in GC, which displayed remarkably higher expression levels in GC than in normal tissues. The expression level of lncRNA MAP3K1-2 was upregulated in GC cells. Silencing treatment of lncRNA MAP3K1-2 significantly inhibited cell proliferation and invasion in GC cells. lncRNA MAP3K1-2 is a novel prognostic marker of GC, and higher expression of lncRNA MAP3K1-2 is associated with poorer prognosis in GC patients.
Methods
Patients and tissue specimens
Sixty-one patients with GC were recruited, from February 2002 to September 2006, from Shanghai Baoshan Luo Dian Hospital (Shanghai, China). No local or systemic treatment was administered to these patients before surgery, and there was no co-occurrence of diagnosed cancers. GC tissues and paired normal tissues (5 cm adjacent to the tumor) were obtained during the operation. Written informed consent was obtained from each patient before any study-specific investigation was performed. The samples and related medical data were rendered anonymous, allowing their automated processing in the context of this research. This study was approved by the Ethical Committee of Shanghai Baoshan Luo Dian Hospital.
Cell lines
Human GC cell lines N87, SGC7901, MKN45, and HGC27 were purchased from ATCC. All cells were cultured in RPMI 1640 (Gibco, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA) at 37°C in 5% humidified CO2.
Quantitative real-time polymerase chain reaction
GC tissues and cells were dissected and submerged in RNAlater™ (Ambion, Austin, TX, USA) for 24 hours and then stored at −80°C until further use. RNA reverse transcription was performed using the TAKARA reverse transcriptase M-MLV kit according to the manufacturer’s protocol (Takara, Bio Inc, Kusatsu, Shiga, Japan). Quantitative real-time PCR was carried out using SYBR Premix Ex Taq II in 20 μL reaction volumes (TAKARA). Quantitative real-time PCR was performed on an ABI 7500 Real-Time PCR System (Applied Biosystems, Waltham, MA, USA). Each experiment was repeated three times, and the specificity of each PCR was confirmed by melt curve analysis. The expression of GAPDH was measured as an endogenous control for. Measurements for all the samples were normalized to those for the endogenous control.
Production of recombinant lentivirus
The lncRNA MAP3K1-2-specific short hairpin RNA (shRNA) templates were cloned into the pSIH-H1 shRNA Cloning and Expression Vector (System Biosciences, Palo Alto, CA, USA). Lentivirus particles were prepared by co-transfecting specific pSIH-H1 shRNA Vector with packaging plasmids (pMD2.G and psPAX2) into 293 T cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). The viruses were harvested 48 hours after transfection. This recombinant lentivirus was designated MAP3K1-2 lenti. NC lenti were constructed by a similar process. NC lenti contained scrambled shRNA fragment, an irrelevant shRNA with random nucleotides. MKN45 cell line was seeded in 6-well plates with 2×105 cells per well and supplied with 2 mL RPMI 1640 medium without serum and antibiotics. On the day of transfection, lentivirus was added in the serum-free medium at 37°C in 5% humidified CO2.
Colony formation assay
Colony formation assay was performed to monitor the colony formation ability of GC cells. A single-cell suspension was obtained through trypsinization and filtration through a 40 μm filter; the cells were then transplanted into a 6-well plate containing 0.35% soft agar. Colonies were observed under a phase-contrast microscope and stained with crystal violet. Each sample was analyzed in triplicate, and this experiment was performed three times.
Western blot
MKN45 cell line was cultured in RPMI 1640 medium supplemented with 10% FBS. After 48 hours, cells were harvested and washed thrice with PBS. Cell pellets were lysed in lysis buffer for 30 minutes on ice. After removing the insoluble material by centrifugation at 12,000 rpm for 10 minutes, 20 μg protein of each sample was separated by sodium dodecyl sulfate -polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The membranes were blocked with PBS buffer containing 5% nonfat dry milk for 2 hours at room temperature and incubated overnight at 4°C with mouse anti-human proliferating cell nuclear antigen (PCNA) (eBioscience, San Diego, CA, USA), mouse anti-human p38, phospho-p38, ERK1/2, phosphor-ERK1/2, JNK, and phosphor-JNK (Abcam, Cambridge, MA, USA), and then incubated. This was followed by incubation with horseradish peroxidase-linked goat anti-mouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1 hour at room temperature. Protein expression was detected using an electrogenerated chemiluminescence kit (Pierce Biotechnology, Rockford, IL, USA). GAPDH served as the loading control (Santa Cruz Biotechnology).
Cell cycle analysis
Cells were harvested and centrifuged at 1,000 rpm for 5 minutes and washed twice with PBS. Subsequently, the cell concentration was adjusted as 1×106 cells/mL. Then, 100 μL of 200 μg/mL DNase-free, RNaseA was added into 100 μL cell sample and incubated for 30 minutes at room temperature. Next, 100 μL of 1 mg/mL propidium iodide was added into the cell sample and incubated for 5 minutes at room temperature, and then the sample was analyzed by a flow cytometer (BD Biosciences, San Jose, CA, USA).
Transwell assay
Invasion analysis was conducted using an 8 μm Transwell chamber (Corning, NY, USA). RPMI 1640 medium was placed in an incubator for prewarming. A 200 μL nonserum cell suspension containing approximately 2×104 cells was seeded into the upper Transwell chambers with 50 μL of Matrigel (1:4; BD Biosciences) 48 hours after transfection. The lower chambers contained 500 μL of DMEM with 10% FBS. Liquid in the upper and lower chambers was discarded after incubation for 24 hours. Cells in the upper membrane were wiped gently with cotton, washed thrice with PBS, fixed with precooled paraformaldehyde for 30 minutes, stained with 0.1% crystalline violet for 10 minutes, and rinsed in running water. Transwell chambers were photographed and observed under an inverted optical microscope. The counting was repeated three times for each group.
Statistical analysis
All statistical analyses were performed with SPSS 21.0 software (SPSS, Chicago, IL, USA). Patients were classified into high and low lncRNA MAP3K1-2 expression groups, based on whether their lncRNA MAP3K1-2 expression levels were above or below the mean value (high expression group, n=31; low expression group, n=30). One-way analysis of variance and Student’s t-tests were performed for comparisons. The chi-squared test was used to analyze categorical variables. Survival data were evaluated using univariate and multivariate Cox regression analysis. P<0.05 was considered to represent statistically significant differences.
Results
Expression of lncRNA MAP3K1-2 in GC
We used quantitative real-time PCR analysis to assess lncRNA MAP3K1-2 expression in GC cell lines. As shown in Figure 1, lncRNA MAP3K1-2 expression was higher in GC cell lines SGC7901 (moderately differentiated), MKN45 (poorly differentiated), and HGC27 (undifferentiated) than that in the N87 (well differentiated) cell lines.
Figure 1.
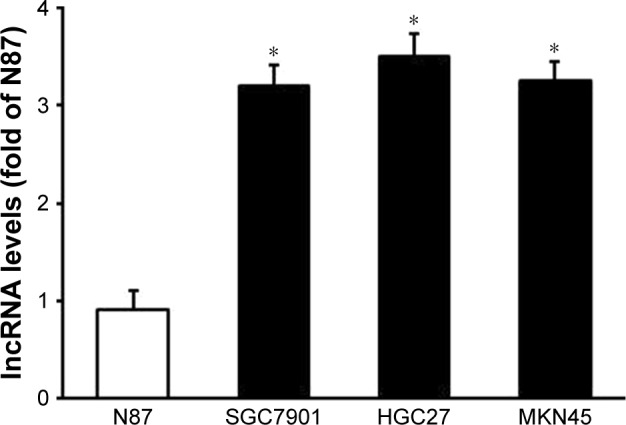
Expression of lncRNA MAP3K1-2 in gastric cancer cell lines N87, SGC7901, MKN45, and HGC27. Quantitative real-time polymerase chain reaction analysis showed high expression of lncRNA MAP3K1-2 in SGC7901, MKN45, and HGC27 cell lines. Data are presented as mean±SD from three independent experiments. *P<0.05, vs N87.
Abbreviation: lncRNA, long noncoding RNA.
As shown in Figure 2, we used quantitative real-time PCR analysis to assess lncRNA MAP3K1-2 expression in GC tissues. The expression of lncRNA MAP3K1-2 was significantly upregulated in the GC tissues compared with that in the paired adjacent normal tissues of the 61 patients with sporadic GC.
Figure 2.
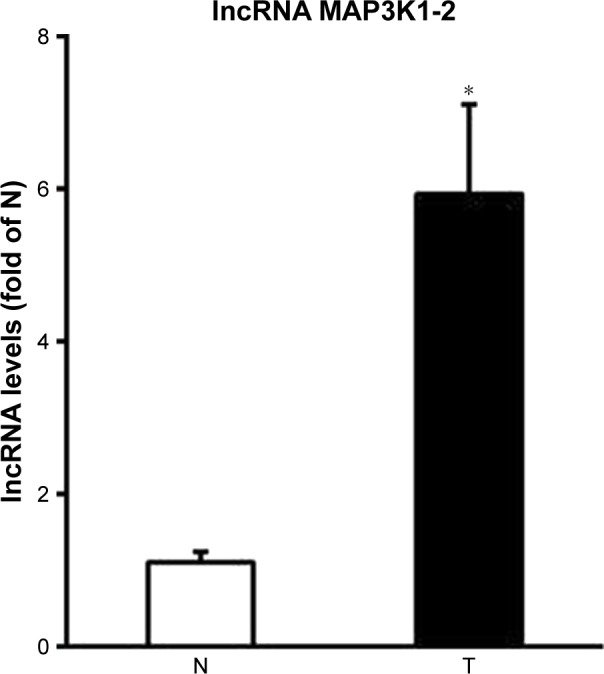
Expression of lncRNA MAP3K1-2 in tumor tissues (T) and paired adjacent normal tissues (N). Quantitative real-time polymerase chain reaction showed high expression of lncRNA MAP3K1-2 in gastric cancer tissues. Data are presented as mean±SD from three independent experiments. *P<0.05, vs N.
Abbreviation: lncRNA, long noncoding RNA.
Expression of lncRNA MAP3K1-2 after recombinant lentivirus transfection in MKN45
The MKN45 GC cell line was chosen for further mechanistic studies. Expression of lncRNA MAP3K1-2 was analyzed by quantitative real-time PCR in MKN45 cells with or without lentivirus transfection. As shown in Figure 3, lncRNA MAP3K1-2 expression level decreased in MKN45 with MAP3K1-2 lenti transfection, while lncRNA MAP3K1-2 expression level was still high in MKN45 with NC lenti transfection and MKN45 without lentivirus transfection. This result suggested that MAP3K1-2 lenti can successfully knockdown the expression level of lncRNA MAP3K1-2. We transfected MKN45 cells with MAP3K1-2 lenti to generate a cell line that stably expressed lncRNA MAP3K1-2 shRNA, as well as a cell line with stably knocked down lncRNA MAP3K1-2 expression.
Figure 3.
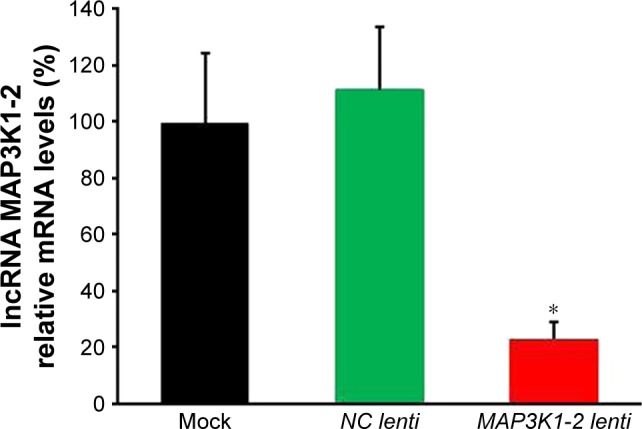
Efficacy of stable lncRNA MAP3K1-2 knockdown in MKN45. The expression level of lncRNA MAP3K1-2 in MKN45 after recombinant lentivirus transfection was determined by quantitative real-time polymerase chain reaction. lncRNA MAP3K1-2 expression level was decreased in MKN45 with MAP3K1-2 lenti transfection, while lncRNA MAP3K1-2 expression level was still high in MKN45 with NC lenti transfection and MKN45 without lentivirus transfection. *P<0.05 (mock: MKN45 without lentivirus transfection).
Abbreviation: lncRNA, long noncoding RNA.
lncRNA MAP3K1-2 promotes cell proliferation in GC cell line
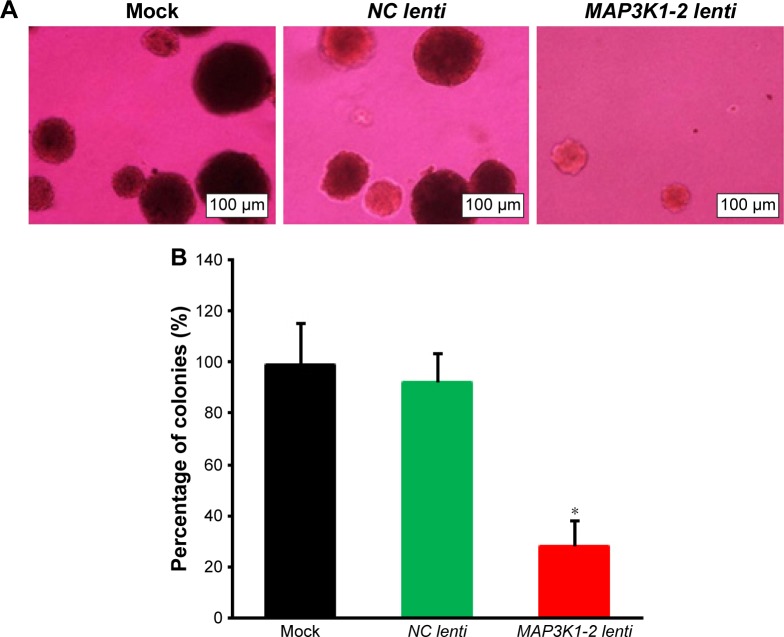
Soft agar assay showed that lncRNA MAP3K1-2 knockdown resulted in a significant decrease in colony formation abilities. lncRNA MAP3K1-2 knockdown significantly inhibited cell proliferation in MKN45 with MAP3K1-2 lenti transfection. lncRNA MAP3K1-2 promoted cell proliferation in MKN45 with NC lenti transfection and MKN45 without lentivirus transfection (Figure 4A and B).
Figure 4.
Effect of lncRNA MAP3K1-2 knockdown on the colony formation of MKN45. (A) Colony formation ability of MKN45 with or without recombinant lentivirus transfection was detected by soft agar colony formation assay. (B) Quantification of the results of Figure 4A. Results of colony formation assay showed that the colony formation rate of MKN45 with MAP3K1-2 lenti transfection was lower than the control group (P<0.05). Data are presented as mean±SD from three independent experiments. *P<0.05, vs NC lenti (mock: MKN45 without lentivirus transfection).
Abbreviation: lncRNA, long noncoding RNA.
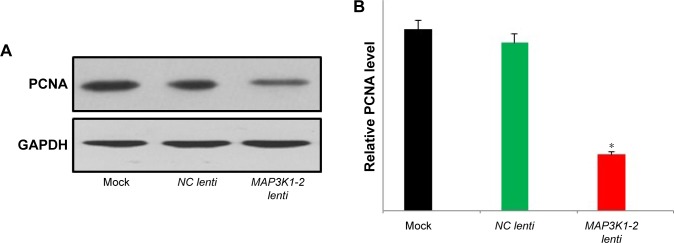
PCNA, which is present only in normal proliferating cells and tumor cells, is a key factor in DNA replication and cell cycle regulation. PCNA additionally plays an important role in cell proliferation and is a good indicator of this process. Expression of PCNA in MKN45 cells was evaluated by Western blot analysis. Protein was detected in MKN45 cells. The expression levels of PCNA were significantly lower in MKN45 cells with MAP3K1-2 lenti transfection than those in MKN45 cells with NC lenti transfection or without lentivirus transfection (Figure 5A and B).
Figure 5.
Western blot analysis of PCNA in MKN45 cells with or without lentivirus transfection. (A) Expression level of PCNA protein decreased in MKN45 with MAP3K1-2 lenti transfection. (B) Quantification of the results of the corresponding Western blot. Data are representative of results from three other experiments. *P<0.05 (mock: MKN45 without lentivirus transfection).
Abbreviation: PCNA, proliferating cell nuclear antigen.
Effect of lncRNA MAP3K1-2 on cell cycle
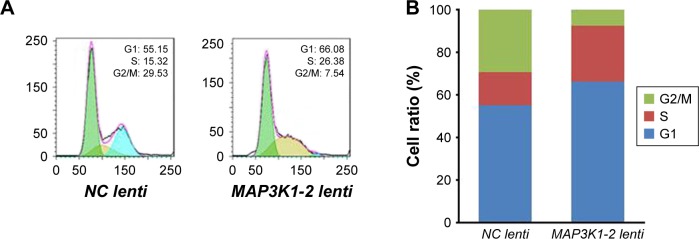
MKN45 cells were transfected with NC lenti and MAP3K1-2 lenti for 48 hours, and the cell cycle of these cells was then analyzed by flow cytometry. As shown in Figure 6A and B, the S phase of MKN45 with MAP3K1-2 lenti transfection was prolonged; lncRNA MAP3L1-2 knockdown induced cell cycle arrest of S phase.
Figure 6.
Effect of lncRNA MAP3K1-2 knockdown on cell cycle of MKN45. (A) Cell cycle of MKN45 with recombinant lentivirus transfection was detected by cytometry; (B) Quantification of the results of Figure 6A. The S phase of MKN45 with MAP3K1-2 lenti transfection was prolonged.
Abbreviation: PCNA, proliferating cell nuclear antigen.
Effect of lncRNA MAP3K1-2 on invasion of MKN45 cells
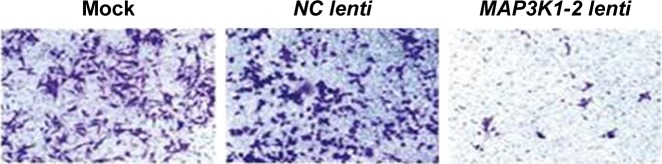
A large number of invasive cells were observed in the blank group and the NC lenti group; however, the number of invasive cells in the MAP3K1-2 lenti group was comparatively smaller (Figure 7). Compared with the blank group, the MAP3K1-2 lenti group had significantly decreased invasive cell numbers (P<0.05), while no visible difference between the blank group and the NC lenti group was noted (P>0.05).
Figure 7.
Comparison of invasion of MKN45 cells in mock, NC lenti group, and MAP3K1-2 lenti group detected by Transwell assay. MAP3K1-2 lenti group compared with mock and NC lenti group (mock: MKN45 without lentivirus transfection). Magnification ×100.
lncRNA MAP3K1-2 plays a role in the MAPK signaling pathway
As shown in Figure 8, we investigated whether lncRNA MAP3K1-2 plays an important role in inhibiting the function of some genes. P38, ERK1/2, and JNK are important genes in MAPK signaling pathway. Phosphorylation of p38, ERK1/2, and JNK was significantly inhibited after lncRNA MAP3K1-2 knockdown in MKN45 cells.
Figure 8.
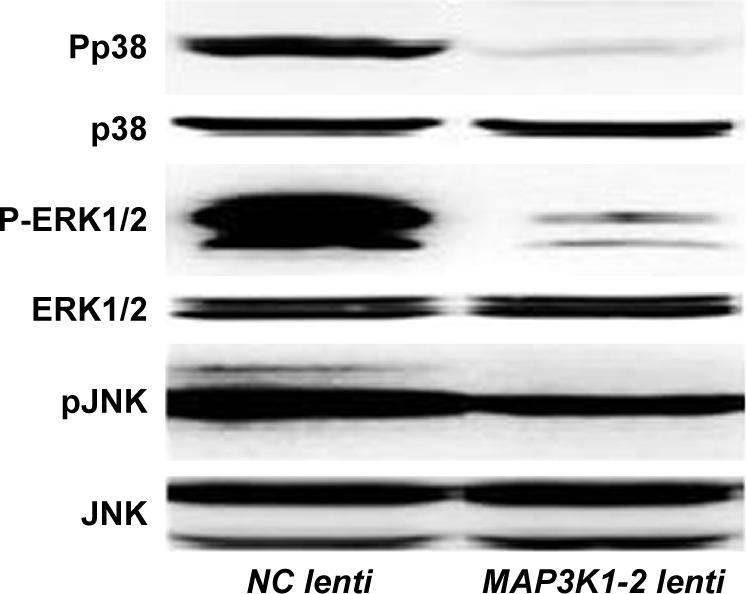
Western blot analysis of p38, ERK1/2, and JNK, and phosphorylation of p38, ERK1/2, and JNK in MKN45 cells with or without lentivirus transfection. Expression level of phosphorylation decreased in MKN45 with MAP3K1-2 lenti transfection. Data are representative of results from three other experiments.
Correlation between lncRNA MAP3K1-2 expression and clinicopathologic features
In this study, 41 men and 20 women with a mean age of 60 years (range, 37–92 years) were studied. Thirty-nine patients had lymphatic metastasis and eight patients had distant metastasis. We examined tissue specimens from all the 61 primary tumors. All deaths were attributable to GC. Patient characteristics are detailed in Table 1. High expression of lncRNA MAP3K1-2 was significantly correlated with tumor size, depth of invasion, presence of lymph node metastasis, and presence of distant metastasis (P<0.05). No significant difference was detected in distribution according to age, gender, and TNM stage between the two groups.
Table 1.
Relationships between lncRNA MAP3K1-2 expression and clinicopathological characteristics
| Characteristic | Number of patients | lncRNA MAP3K1-2 expression
|
P-value | |
|---|---|---|---|---|
| High (n=31) | Low (n=30) | |||
| Age (years) | 0.52 | |||
| ≥65 | 30 | 14 | 16 | |
| <65 | 31 | 17 | 14 | |
| Gender | 0.29 | |||
| Male | 41 | 23 | 18 | |
| Female | 20 | 8 | 12 | |
| Tumor size (cm) | 0.006 | |||
| ≥5 | 27 | 19 | 8 | |
| <5 | 34 | 12 | 22 | |
| Depth of invasion | 0.02 | |||
| T1+T2 | 8 | 1 | 7 | |
| T3+T4 | 53 | 30 | 23 | |
| TNM stage | 0.10 | |||
| I+II | 28 | 11 | 17 | |
| III+IV | 33 | 20 | 13 | |
| Lymph node metastasis | <0.01 | |||
| Present | 39 | 26 | 13 | |
| Absent | 22 | 5 | 17 | |
| Distant metastasis | 0.03 | |||
| Present | 8 | 7 | 1 | |
| Absent | 53 | 24 | 29 | |
Note: Bold values represent statistical significance, P<0.05.
Abbreviation: lncRNA, long noncoding RNA.
Relationship between lncRNA MAP3K1-2 and survival
In the univariate survival analysis (Table 2), there were no differences in survival with respect to age, sex, tumor size, TNM stage, and lymph node metastasis. It was identified that lncRNA MAP3K1-2 expression, depth of tumor invasion, and distant metastasis were significantly associated with prognosis in our study cohort of 61 GC patients. lncRNA MAP3K1-2 expression above the median was associated with decreased cancer-related survival. Furthermore, the multivariate analysis indicated that higher lncRNA MAP3K1-2 expression status remained a significant and independent risk factor for survival after adjusting for other factors (OR 5.81, P=0.006) (Table 3).
Table 2.
Univariate analysis of prognostic factors in 61 GC patients
| Parameters | Number of patients | Number of survivors | P-value |
|---|---|---|---|
| lncRNA MAP3K1-2 expression | <0.01 | ||
| High | 31 | 5 | |
| Low | 30 | 18 | |
| Age (years) | 0.08 | ||
| ≥65 | 30 | 8 | |
| <65 | 31 | 15 | |
| Gender | 0.41 | ||
| Male | 41 | 14 | |
| Female | 20 | 9 | |
| Tumor size | 0.25 | ||
| ≥5 cm | 27 | 11 | |
| <5 cm | 34 | 12 | |
| Depth of invasion | 0.02 | ||
| T1+T2 | 8 | 3 | |
| T3+T4 | 53 | 20 | |
| TNM stage | 0.20 | ||
| I+II | 28 | 11 | |
| III+IV | 33 | 12 | |
| Lymph node metastasis | 0.87 | ||
| Present | 39 | 12 | |
| Absent | 22 | 11 | |
| Distant metastasis | 0.02 | ||
| Present | 8 | 0 | |
| Absent | 53 | 23 |
Note: Bold values represent statistical significance, P<0.05.
Abbreviations: GC, gastric cancer; lncRNA, long noncoding RNA.
Table 3.
Multivariate Cox regression analysis for cancer-specific survival in 61 GC patients
| Variables | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| lncRNA MAP3K1-2 expression | 5.82 | 1.65–20.54 | 0.006 |
| Depth of invasion | 0.30 | 0.05–1.88 | 0.20 |
| Distant metastasis | 2.09 | 0.21–21.23 | 0.53 |
Note: Bold values represent statistical significance, P<0.05.
Abbreviations: GC, gastric cancer; lncRNA, long noncoding RNA.
Discussion
The carcinogenesis of GC is complex and consists of multiple processing steps involving numerous genetic and epigenetic alterations. Increasing evidence suggests that lncRNAs serve as key regulators of tumor initiation and development.14 As numerous lncRNAs have been identified as critical elements in cancer cell proliferation and metastasis, the role of lncRNAs in GC is receiving increased attention. Previous studies have confirmed that the dysregulation of lncRNAs participates in is involved in GC development.15 GAPLINC, which is highly expressed in GC, promotes cell proliferation and migration by regulating CD44 expression as a molecular decoy for miR211-3p.16 Recently, GAPLINC was recognized to promote GC cell proliferation by acting as a molecular sponge of miR-378 to modulate MAPK1 expression.17 Overexpression of H19 promotes GC cell proliferation and invasion via actively binding to ISM1.18 The H19/miR-675/RUNX1 pathway also regulates GC development.19 lncRNA HOXA11-AS displayed specific overexpression in GC but not in other cancers, and HOXA11-AS expression was associated with poor prognosis.20 HOTAIR promotes cell proliferation by completely sponging miR331-3p,21 whereas ANRIL promotes cell growth by epigenetically suppressing miR99a/449a in GC.22 GAS5 and MEG3 function as tumor suppressors in GC.23,24 However, the biological and molecular characteristics of most lncRNAs in the context of GC remain unknown.
Herein, we conducted lncRNA expression profiling of GC and adjacent tissue specimens using lncRNA chip. Several lncRNAs were differentially expressed between GC and adjacent normal tissues, including BC041451, lncRNA SPSB4-2, and lncRNA MAP3K1-2. In this study, we found that the expression level of lncRNA MAP3K1-2 in GC cells was high. No significant differences were found between moderately differentiated, poorly differentiated, and undifferentiated GC cells. lncRNA MAP3K1-2 knockdown by recombinant lentivirus transfection in GC cells inhibited cell proliferation in GC. PCNA, which is directly related to cell proliferation, is a DNA clamp that acts as a processivity factor for DNA polymerase δ in eukaryotic cells and is essential for replication.25 We investigated whether PCNA expression decreased in GC cells with MAP3K1-2 lenti transfection. lncRNA MAP3K1-2 knockdown induced cell cycle arrest in S phase, resulting in the inhibition of DNA replications. Moreover, the number of invasive cells after knockdown of MAP3K1-2 was comparatively smaller as indicated by the Transwell assay test. Thus, it can be seen that lncRNA MAP3K1-2 promotes cell proliferation and invasion in GC.
We also found that phosphorylation of p38, ERK1/2, and JNK in MKN45 cells was inhibited following lncRNA MAP3K1-2 knockdown, which may further influence cell proliferation, differentiation, motility, stress response, apoptosis, and survival via the MAPK signaling pathway. Abnormal regulation of this pathway also contributes to the pathogenesis of several malignancies. ERK1/2, JNK, p38, and ERK5 comprise the three main modules in this pathway, while ERK1/2 and JNK cascades are principal regulators of malignant phenotype.26,27 CASC2, a tumor suppressor gene, was first demonstrated in endometrial cancer. Its aberrant expression has been demonstrated in GC where it has been shown to exert its role via the inhibition of the MAPK pathway.28,29 Cross-talk between linc00483 and SPAG9 mRNA has been revealed that enhances the expression of the miR-30a-3p target gene SPAG9, and activating MAPKs promotes the proliferation and inhibits apoptosis among GC cells via competition for miR-30a-3p.30
Moreover, our results suggest that high expression of lncRNA MAP3K1-2 correlates with poorer prognosis in GC patients. lncRNA MAP3K1-2 expression above the median was associated with decreased cancer-related survival. Furthermore, the multivariate analysis indicated that higher lncRNA MAP3K1-2 expression status remained a significant and independent risk factor for survival after adjusting for other factors (OR 5.81, P=0.006). To further explore the early diagnostic value of lncRNA MAP3K1-2, a larger sample of GC tissue should be analyzed.
Although alterations of lncRNA MAP3K1-2 in gastric tumorigenesis are already a recognized phenomenon in our research, its functional role and molecular mechanism remain undetermined. Future research should aim to examine the expression of MAPKs pathway proteins such as FASL, DAXX, and ATF2.
Conclusion
In our study, lncRNA MAP3K1-2 was recognized as a critical effector in GC tumorigenesis and progression. High lncRNA MAP3K1-2 expression was also shown as a novel independent prognostic marker in GC. This observation paves the way for further clinical studies of significance of this molecule in a large sample of GC patients. Advances in the research of lncRNA MAP3K1-2 are expected to yield new diagnostic biomarkers as well as RNA-based therapeutic strategies.
Acknowledgments
This work was supported by a grant from Research project of Science and Technology Commission of Baoshan District of Shanghai (No 12-E-37).
Footnotes
Authors’ contributions
LW, M-WZ, and X-DG were responsible for the concepts and design of the study. M-WZ drafted the manuscript. X-DG, J-HY, and H-LL performed data and specimen acquisition. LW, M-WZ, H-LS, and X-JW carried out quantitative real-time PCR and lentivirus recombination experiments. J-HY and H-LL carried out soft agar assays and Transwell assays. Y-YG and B-HH carried out Western blotting and flow cytometry. LW and X-DG performed the statistical analysis. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Sugano K. Screening of gastric cancer in Asia. Best Pract Res Clin Gastroenterol. 2015;29(6):895–905. doi: 10.1016/j.bpg.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Guo X, Xia J, Deng K. Long non-coding RNAs: emerging players in gastric cancer. Tumour Biol. 2014;35(11):10591–10600. doi: 10.1007/s13277-014-2548-y. [DOI] [PubMed] [Google Scholar]
- 4.Nana-Sinkam SP, Croce CM. Non-coding RNAs in cancer initiation and progression and as novel biomarkers. Mol Oncol. 2011;5(6):483–491. doi: 10.1016/j.molonc.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhan A, Mandal SS. Long noncoding RNAs: emerging stars in gene regulation, epigenetics and human disease. ChemMedChem. 2014;9(9):1932–1956. doi: 10.1002/cmdc.201300534. [DOI] [PubMed] [Google Scholar]
- 6.Maruyama R, Suzuki H. Long noncoding RNA involvement in cancer. BMB Rep. 2012;45(11):604–611. doi: 10.5483/BMBRep.2012.45.11.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin Y, Yan P, Lu J, et al. Opposing Roles for the lncRNA Haunt and Its Genomic Locus in Regulating HOXA Gene Activation during Embryonic Stem Cell Differentiation. Cell Stem Cell. 2015;16(5):504–516. doi: 10.1016/j.stem.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Spurlock CF, Tossberg JT, Guo Y, et al. Expression and functions of long noncoding RNAs during human T helper cell differentiation. Nat Commun. 2015;6:6932. doi: 10.1038/ncomms7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang K, Liu CY, Zhou LY, et al. APF lncRNA regulates autophagy and myocardial infarction by targeting miR-188-3p. Nat Commun. 2015;6:6779. doi: 10.1038/ncomms7779. [DOI] [PubMed] [Google Scholar]
- 10.Montes M, Nielsen MM, Maglieri G, et al. The lncRNA MIR31HG regulates p16(INK4A) expression to modulate senescence. Nat Commun. 2015;6:6967. doi: 10.1038/ncomms7967. [DOI] [PubMed] [Google Scholar]
- 11.Yarmishyn AA, Kurochkin IV. Long noncoding RNAs: a potential novel class of cancer biomarkers. Front Genet. 2015;6:145. doi: 10.3389/fgene.2015.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi SJ, Wang LJ, Yu B, et al. LncRNA-ATB promotes trastuzumab resistance and invasion-metastasis cascade in breast cancer. Oncotarget. 2015;6(13):11652–11663. doi: 10.18632/oncotarget.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang XY, Pan HF, Leng RX, Ye DQ. Long noncoding RNAs: novel insights into gastric cancer. Cancer Lett. 2015;356(2 Pt B):357–366. doi: 10.1016/j.canlet.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Xu MD, Qi P, du X. Long non-coding RNAs in colorectal cancer: implications for pathogenesis and clinical application. Mod Pathol. 2014;27(10):1310–1320. doi: 10.1038/modpathol.2014.33. [DOI] [PubMed] [Google Scholar]
- 15.Sun W, Yang Y, Xu C, Xie Y, Guo J. Roles of long noncoding RNAs in gastric cancer and their clinical applications. J Cancer Res Clin Oncol. 2016;142(11):2231–2237. doi: 10.1007/s00432-016-2183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Y, Wang J, Qian J, et al. Long noncoding RNA GAPLINC regulates CD44-dependent cell invasiveness and associates with poor prognosis of gastric cancer. Cancer Res. 2014;74(23):6890–6902. doi: 10.1158/0008-5472.CAN-14-0686. [DOI] [PubMed] [Google Scholar]
- 17.Diao L, Wang S, Sun Z. Long noncoding RNA GAPLINC promotes gastric cancer cell proliferation by acting as a molecular sponge of miR-378 to modulate MAPK1 expression. Onco Targets Ther. 2018;11:2797–2804. doi: 10.2147/OTT.S165147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Yu B, Li J, et al. Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer. Oncotarget. 2014;5(8):2318–2329. doi: 10.18632/oncotarget.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhuang M, Gao W, Xu J, Wang P, Shu Y. The long non-coding RNA H19-derived miR-675 modulates human gastric cancer cell proliferation by targeting tumor suppressor RUNX1. Biochem Biophys Res Commun. 2014;448(3):315–322. doi: 10.1016/j.bbrc.2013.12.126. [DOI] [PubMed] [Google Scholar]
- 20.Sun M, Nie F, Wang Y, et al. LncRNA HOXA11-AS Promotes Proliferation and Invasion of Gastric Cancer by Scaffolding the Chromatin Modification Factors PRC2, LSD1, and DNMT1. Cancer Res. 2016;76(21):6299–6310. doi: 10.1158/0008-5472.CAN-16-0356. [DOI] [PubMed] [Google Scholar]
- 21.Liu XH, Sun M, Nie FQ, et al. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer. 2014;13:92. doi: 10.1186/1476-4598-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang EB, Kong R, Yin DD, et al. Long noncoding RNA ANRIL indicates a poor prognosis of gastric cancer and promotes tumor growth by epigenetically silencing of miR-99a/miR-449a. Oncotarget. 2014;5(8):2276–2292. doi: 10.18632/oncotarget.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun M, Jin FY, Xia R, et al. Decreased expression of long noncoding RNA GAS5 indicates a poor prognosis and promotes cell proliferation in gastric cancer. BMC Cancer. 2014;14:319. doi: 10.1186/1471-2407-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun M, Xia R, Jin F, et al. Downregulated long noncoding RNA MEG3 is associated with poor prognosis and promotes cell proliferation in gastric cancer. Tumour Biol. 2014;35(2):1065–1073. doi: 10.1007/s13277-013-1142-z. [DOI] [PubMed] [Google Scholar]
- 25.Cooper SE, Hodimont E, Green CM. A fluorescent bimolecular complementation screen reveals MAF1, RNF7 and SETD3 as PCNA-associated proteins in human cells. Cell Cycle. 2015;14(15):2509–2519. doi: 10.1080/15384101.2015.1053667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer. 2004;4(12):937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 27.Li R, Zhang L, Jia L, et al. Long non-coding RNA BANCR promotes proliferation in malignant melanoma by regulating MAPK pathway activation. PLoS One. 2014;9(6):e100893. doi: 10.1371/journal.pone.0100893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baldinu P, Cossu A, Manca A, et al. Identification of a novel candidate gene, CASC2, in a region of common allelic loss at chromosome 10q26 in human endometrial cancer. Hum Mutat. 2004;23(4):318–326. doi: 10.1002/humu.20015. [DOI] [PubMed] [Google Scholar]
- 29.Li P, Xue WJ, Feng Y, Mao QS. Long non-coding RNA CASC2 suppresses the proliferation of gastric cancer cells by regulating the MAPK signaling pathway. Am J Transl Res. 2016;8(8):3522–3529. [PMC free article] [PubMed] [Google Scholar]
- 30.Li D, Yang M, Liao A, et al. Linc00483 as ceRNA regulates proliferation and apoptosis through activating MAPKs in gastric cancer. J Cell Mol Med. 2018 May 15; doi: 10.1111/jcmm.13661. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]