Abstract
Unlike the behavioral effects planarians display when exposed to cocaine, amphetamines, cathinones, ethanol and sucrose, effects of opioid receptor agonists, especially mu opioid receptor agonists, are poorly defined in these flatworms. Here, we tested the hypothesis that planarians exposed to a selective mu opioid receptor agonist, DAMGO (0.1, 1, 10 μM), would display a triad of opioid-like effects (place conditioning, abstinence-induced withdrawal, and motility changes). DAMGO was selected versus morphine because of its greater mu opioid receptor selectivity. In place conditioning and abstinence experiments, the planarian light/dark test (PLDT) was utilized (i.e., planarians are placed into a petri dish containing water that is split into light and dark compartments and time spent in the compartments is determined). Planarians conditioned with DAMGO (1 μM) spent more time on the drug-paired side compared to water controls. In abstinence experiments, planarians exposed to DAMGO for 30 min were removed and then placed into water, where light avoidance (e.g. defensive responding) and depressant-like effects (i.e., decreased motility) were quantified. Compared to water controls, DAMGO-withdrawn planarians spent less time in the light (10 μM) and displayed decreased motility (1, 10 μM). Acute DAMGO exposure (1 μM) produced hypermotility that was antagonized by naltrexone (1, 10, 100 μM). In contrast, acute exposure to the kappa opioid receptor agonist U50,488H (0.1, 1, 10 μM) resulted in decreased motility. Our results show that a mu opioid agonist produces mammalian-like behavioral responses in planarians that may be related to addiction and suggest opioid-like behavioral effects are conserved in invertebrates.
Keywords: mu opioid, DAMGO, naltrexone, planarians, invertebrate, withdrawal, addiction, anxiety, place preference, CPP
Introduction
Flatworms called planarians are the simplest living animals having bilateral symmetry and a CNS with cephalization (Pagan, 2014). Planarians express and utilize mammalian-like neurotransmitter systems, such as dopamine, serotonin, acetylcholine, GABA, and glutamate (Nishimura et al., 2010). Moreover, planarians exposed to different classes of addictive substances, including psychostimulant drugs, ethanol and common table sugar, display mammalian-like behavioral responses that include: changes in motility and expression of stereotyped movements during acute exposure; abstinence-induced withdrawal responses including decreased motility and anxiogenic-like responding that can be characterized by the planarian light/dark test (PLDT); and environmental place conditioning (EPC) resembling conditioned place preference (CPP) in rodents that may be indicative of a drug-seeking or anxiolytic-type phenotype (Pagán et al., 2008, 2009; Kusayama and Watanabe, 2000; Palladini et al., 1996; Raffa and Rawls, 2008; Ramoz et al., 2012; Zhang et al., 2013; Tallarida et al., 2014; Nayak et al., 2016, Zwede et al., 2018).
While the hallmark feature of planarians is a remarkable capacity to regenerate, another defining phenomenon is a tendency, similar to rodents, to spend more time in dark versus light environments (Zewde et al., 2018). This behavioral phenomenon is called negative phototaxis, or light avoidance, and may indicate defensive responding related to an anxiogenic phenotype (Davidson et al., 2011). This negative phototaxis is evident in drug-naïve planarians but is further altered by addictive substances administered in conditioning or abstinence paradigms (Zewde et al., 2018; Nayak et al., 2016). Negative phototaxis is reduced (i.e., planarians spend more time in the light compartment) following conditioning sessions in which an addictive substance is paired with the light compartment (Hutchinson et al., 2015; Tallarida et al., 2014). However, during abstinence from prior cocaine or ethanol exposure, planarians display enhanced negative phototaxis (i.e., spend less time in the light) (Nayak et al., 2016).
Among agents that act at the opioid receptor subtypes (mu, kappa and delta), kappa opioid agonists and antagonists are best characterized in terms of planarian behavioral responses. There is strong evidence that planarians display abstinence-induced and naloxone-precipitated withdrawal responses following discontinuation of chronic exposure to the kappa opioid agonist U-50,488 (Raffa et al., 2003; Umeda et al., 2004). The withdrawal response quantified in those studies was reduced motility, which was determined by placing a piece of graph paper beneath a transparent petri dish containing the planarian and subsequently counting the number of grids crossed over a finite time period (Raffa et al., 2003, 2013; Raffa and Rawls, 2008; Rawls et al., 2006, 2007, 2008). Behavioral effects of different classes of addictive substances in planarians are also blocked by opioid receptor antagonists. For example, a cannabinoid receptor agonist, WIN 55212-2, produces dose-dependent stereotyped motor behavior in planarians that is antagonized by co-exposure to cannabinoid or opioid receptor antagonists (Buttarelli et al., 2002). In addition, spontaneous discontinuation of chronic nicotine exposure produces abstinence-induced withdrawal (reduced motility) that is antagonized by a nonselective opioid receptor antagonist (naloxone), a selective mu opioid antagonist or a selective delta opioid antagonist but not by a selective kappa opioid antagonist (Raffa et al., 2013). Information about effects of mu opioid agonists in planarians is especially lacking, but planarians do display reduced motility following a 2-h exposure to morphine (Murugan and Persinger, 2014).
For our studies, we selected DAMGO, which is a synthetic opioid peptide that displays high selectivity for mu relative to delta and kappa opioid receptors (Kd values = 1.18, 1,430, and 213 nM for human mu, delta, and kappa opioid receptors, respectively) (Zhao et al., 2003). Although morphine is the prototypical opioid agonist and standard against which all opioids are evaluated, it displays agonist activity at kappa and delta, as well as mu, opioid receptors. Since our goal here was to characterize behavioral effects produced by a mu opioid agonist, without significant contamination from kappa or delta receptor activation, we selected DAMGO versus morphine or other mixed opioid agonists. We used established behavioral assays to determine if planarians exposed to DAMGO display a triad of mu opioid-like effects that are well defined in mammals. These effects are: (1) rewarding or anxiolytic effects assessed by a place conditioning assay resembling rodent CPP; (2) abstinence-induced withdrawal effects including decreased motility (i.e., depressant-like response) and increased defensive responding (i.e., anxiogenic-like response akin to responses rodents display in the elevated plus maze or light/dark box assays); and (3) change in motility during acute exposure.
Experimental Procedures
Subjects, drugs and experimental conditions
Planarians (Dugesia dorotocephala) were purchased from Carolina Biological Supply (Burlington, NC, USA). Upon arrival in the laboratory, planarians were maintained in the aqueous solution provided by Carolina Biological Supply, acclimated to room temperature (21 °C), and tested within 3 days of receipt. DAMGO and the kappa agonist U50,488 hydrochloride was purchased from Tocris Biosciences (St. Louis, MO, USA). Naltrexone hydrochloride (NTX) and the kappa opioid receptor antagonist, nor-Binaltorphimine dihydrochloride (nBNI), were purchased from Sigma-Aldrich (St. Louis, MO, USA). Morphine sulfate was provided by NIDA Drug Supply (Bethesda, MD, USA). All drugs were dissolved in spring water, with stock and working solutions prepared daily, and concentrations were based on prior planarian work and Kd values of DAMGO (Tallarida et al., 2014; Zhao et al., 2003). Behavioral experiments were conducted between 10 AM and 4 PM. Behavioral responses were quantified by a trained observer with a stopwatch who was blinded to drug treatment. Planarians are large enough (average length about 3–15 mm) so that behavior can be observed and quantified with the naked eye, so behavioral responses were quantified without the aid of a microscope. Experiments and behavioral observations were conducted in ‘real time’, and no videotaping was used.
Abstinence-induced withdrawal experiments: PLDT (planarian light/dark test)
The PLDT capitalizes on the tendency of planarians to spend more time in dark versus light environments and can quantify defensive responding manifested by abstinence from chronic drug exposure (Zewde et al., 2018; Nayak et al., 2016). An individual planarian was removed from its home jar and placed into a secondary jar (identical to home jar) containing DAMGO (0.1, 1, 10 μM) or water for 30 min (total of 46 planarians). Planarians were then removed and placed at the midline of a petri dish (5.5 cm diameter) containing water. A sleeve of black construction paper covered one half of the dish on the top, bottom and vertical sides to create a dark and ‘ambient’ light environment. Each planarian was given free access to roam the ‘ambient light’ and dark sides of the dish, and time spent in the light was recorded for 10 min.
Abstinence-induced withdrawal experiments: Motility assay
Decreased motility following discontinuation of chronic exposure to an addictive substance is a feature of abstinence-induced withdrawal in planarians (Rawls et al., 2006, 2007, 2008; Raffa et al., 2003, 2008). Individual planarians were removed from their home jars and placed for 30 min into a secondary jar (identical to home jar) containing DAMGO (0.1, 1, 10 μM) or water (total of 72 planarians). Planarians were then removed and placed for 5 min into a transparent petri dish containing water (i.e., DAMGO exposure was 30 min and DAMGO abstinence was 5 min). The dish was placed over graph paper with gridlines spaced 0.5 cm apart and motility counts were quantified as the number of gridlines that planarians crossed in 5 min (Raffa and Valdez, 2001).
Environmental place conditioning (EPC) experiments
EPC experiments followed a design similar to that described for the light/dark test above but consisted of 3 phases (pretest, conditioning, and post-test). For the pretest, planarians were placed at the midline of a petri dish containing only water that was split into light and dark compartments, and time spent in each compartment was recorded over 10 min. Planarians were then immediately conditioned for 30 min with DAMGO (0.1, 1, 10 μM) or water in the least-preferred environment (determined from pretest and designated as DAMGO-paired side) followed by 30 min with water in the preferred environment (total of 47 planarians). Immediately following DAMGO conditioning a post-test was conducted in which planarians were placed at the midline of a petri dish containing only water and allowed free access to roam both the light and dark sides of the dish for 10 min (time spent in the DAMGO-paired compartment was determined). The difference in time spent in the DAMGO-paired environment (post-test minus pre-test times) were quantified and graphed. To investigate a role for kappa opioid receptors in the effects of DAMGO, separate EPC experiments using a total of 16 planarians were conducted as described above except that planarians were conditioned with a fixed concentration of DAMGO (10 μM) either by itself or in combination with nBNI (1 μM).
Acute effects of DAMGO and naltrexone (NTX) on motility
We assessed acute effects of DAMGO on planarian motility and, in combination with NTX, a role for mu opioid-like receptors in the DAMGO-induced response. Individual planarians were removed from their home jars and placed for 5 min into a petri dish containing DAMGO (0 or 1 μM) by itself or in combination with NTX (1, 10, or 100 μM) (total of 96 planarians). The dish was placed over graphing paper with gridlines spaced 0.5 cm apart and motility counts were quantified as the number of gridlines crossed in 5 min. Separate experiments, using the same experimental design, tested effects of morphine (0, 0.1. 1, 10 μM) (total of 48 planarians) or U50,488 (0, 0.1. 1, 10 μM) (total of 48 planarians), a selective kappa opioid receptor agonist.
Data analysis
Comparisons of group means (± S.E.M.) were evaluated by one-way ANOVA or two-way ANOVA (NTX x DAMGO). In cases of a significant main effect, differences between individual groups were identified with a Bonferroni post-hoc test. Whenever data did not comply with assumptions of parametric testing, the non-parametric Kruskal-Wallis test was performed followed by paired comparisons (Dunn’s test). P < 0.05 was considered significant in all cases.
Results
DAMGO exposure causes environmental place conditioning (EPC) (Fig. 1)
Fig. 1. DAMGO produced environmental place conditioning (EPC).
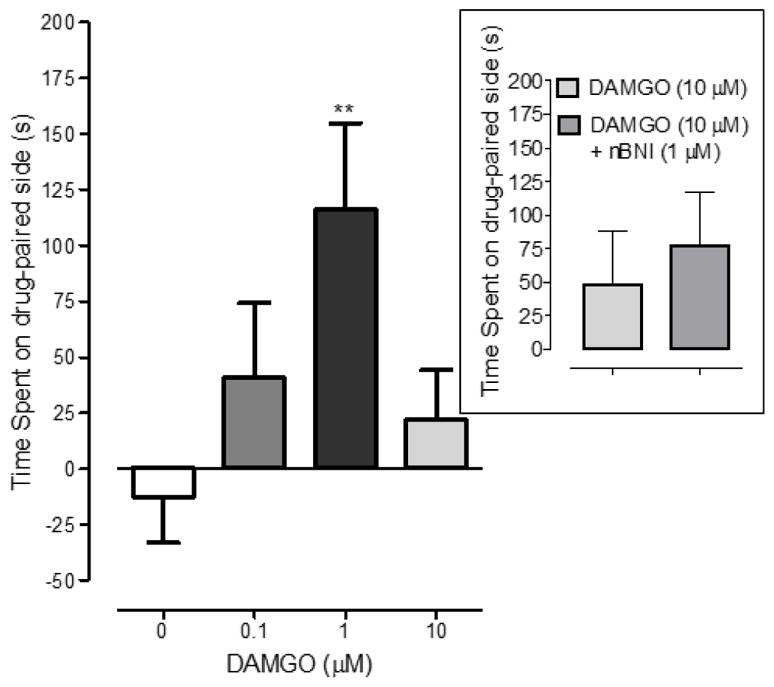
Planarians were conditioned with DAMGO (0.1, 1, 10 μM) and time spent on the DAMGO-paired side was quantified and graphed (+ S.E.M.). N=11–12 planarians/group. **p < 0.01 compared to water control (0 μM). (Box) Planarians were conditioned with the highest concentration of DAMGO (10 μM) by itself or in combination with the kappa opioid antagonist nBNI (1 μM). Time spent on the DAMGO-paired side was quantified and graphed (+ S.E.M.). N=8 planarians/group.
One-way ANOVA identified a main effect on place conditioning [F(3, 46) = 3.455, p < 0.05]. Post-hoc analysis revealed that planarians conditioned with 1 μM DAMGO spent a greater amount of time (116.0 ± 38.6) in the drug-paired compartment compared to DAMGO-naïve planarians exposed only to water (−12.5 ± 20.6) (p < 0.01). For lower (0.1 μM) and higher (10 μM) concentrations of DAMGO, the time spent in the DAMGO-paired compartment was not significantly different from water-exposed controls (p > 0.05). In separate experiments in which planarians were conditioned with DAMGO (10 μM) by itself or with nBNI (1 μM), time spent in the DAMGO-paired compartment was not significantly different between the two groups (p > 0.05, Student’s t-test) (Fig. 1, Box).
DAMGO abstinence decreases time spent in the light (Fig. 2A)
Fig. 2. DAMGO-withdrawn planarians display abstinence-induced withdrawal responses. (A).
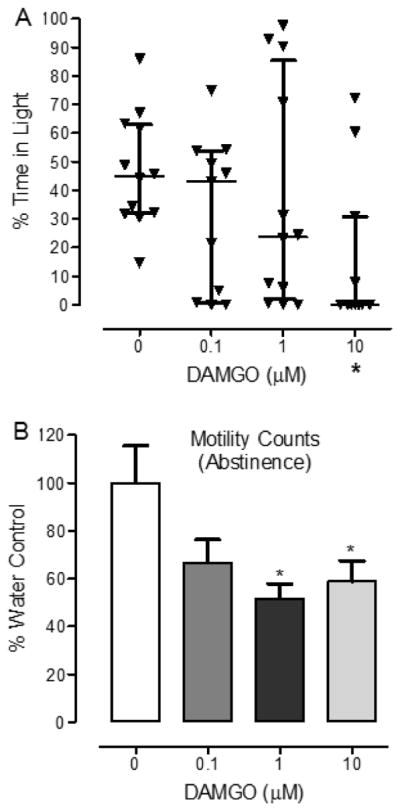
Abstinence from DAMGO exposure enhances light avoidance (defensive responding). Planarians were exposed to different concentrations of DAMGO (0, 0.1, 1, 10 μM) for 30 min and then removed and placed into a petri dish containing water that was split evenly into light and dark sides. Time spent in the light was measured over 10 min. Data was presented as a scatter plot showing the median percentage of time spent in the light (with interquartile range) over the 10-min interval. N=11–12 planarians/group. *p < 0.05 compared to water control (0 μM). (B) Abstinence from DAMGO exposure decreases motility. Planarians were exposed to different concentrations of DAMGO (0, 0.1, 1, 10 μM) for 30 min and them removed and placed into a petri dish containing water. Motility counts were quantified as the number of gridlines that planarians crossed in 5 min. Data were presented as percentage of water control (+S.E.M.) over the 5-min interval. N=18 planarians/group. *p < 0.05 compared to water control (0 μM).
In the PLDT there was a significant difference in percentage of time spent in the light across groups [Kruskal-Wallis test, H=8.455, p < 0.05]. Planarians exposed to DAMGO (10 μM) for 30 min and then withdrawn and placed at the midline of a petri dish (split evenly into light and dark sides) containing water spent less time in the light compared to DAMGO-naïve planarians exposed only to water (p < 0.05, Dunn’s test) (Fig. 2A). During abstinence from a 30-min exposure to lower concentrations of DAMGO (0.1, 1 μM), the percentage of time planarians spent in the light versus dark compartments compared to water-exposed controls was not significantly different (p > 0.05).
DAMGO abstinence reduces planarian motility (Fig. 2B)
One-way ANOVA revealed a main effect on planarian motility ([F(3, 71) = 4.003, p < 0.01]. Planarians exposed to DAMGO concentrations of 1 μM or 10 μM for 30 min and then withdrawn and placed into a petri dish containing water displayed decreased motility compared to DAMGO-naïve planarians exposed only to water (p < 0.05) (Fig. 2B). Abstinence from exposure to a concentration of 1 μM DAMGO reduced motility to 51.6 ± 6.3 percentage of water control whereas motility was reduced to 51.6 ± 6.3 percentage of water control during abstinence from 10 μM DAMGO. Abstinence from the lowest concentration of DAMGO (1 μM) (66.7 ± 9.6 percentage of control) did not significantly alter motility counts relative to water-exposed controls (p > 0.05). Experiments controlling for potential toxicity to DAMGO showed that continuous exposure to DAMGO (1 μM) (i.e., exposure to DAMGO during the 30 min exposure interval and during the 5-min test interval) did not reduce motility counts relative to water control planarians (p > 0.05) (data not shown).
Acute DAMGO exposure produces enhanced motility that is blocked by NTX (Fig. 3A)
Fig. 3. Acute DAMGO exposure produces hypermotility that is antagonized by NTX and the kappa opioid agonist U50,488 decreases motility. (A).
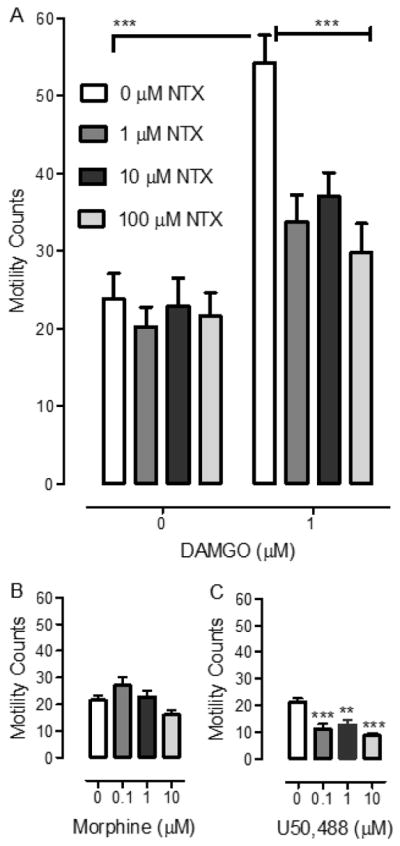
Planarians were exposed for 5 min to either DAMGO (1 μM) or water in combination with different concentrations of NTX (0, 1, 10, 100 μM). Motility was measured over the 5-min interval, and data were presented as mean number of motility counts (+ S.E.M.) over 5 min. N= 12 planarians/group. ***p < 0.001 compared to DAMGO (1 μM) by itself. (B) Morphine does not significantly affect planarian motility. Planarians were exposed for 5 min to morphine (0.1, 1, 10 μM) and motility was measured over 5 min. Data were presented as mean number of motility counts (+ S.E.M.) over 5 min. N=8 planarians/group. (C) U50,488 reduces planarian motility. Planarians were exposed for 5 min to U50,488 (0.1, 1, 10 μM) and motility was measured over 5 min. Data were presented as mean number of motility counts (+ S.E.M.) over 5 min. N=8 planarians/group. ***p < 0.001 compared to water control (0 μM).
A two-way ANOVA conducted on the NTX/DAMGO data set for acute motility revealed effects of DAMGO [F(1, 88) = 50.42, p < 0.0001] and NTX [F(3, 88) = 6.59, p < 0.001] and a significant interaction [F(3, 88) = 4.25, p < 0.01]. Bonferroni post-hoc analysis indicated that DAMGO (1 μM) produced greater motility during a 5-min acute exposure compared to drug-naïve planarians exposed to water (p < 0.001). In planarians exposed to a combination of DAMGO (1 μM) and NTX (1, 10 or 100 μM), motility was reduced compared to planarians exposed only to DAMGO (1 μM) (p < 0.001). In planarians exposed to NTX (1, 10 or 100 μM) by itself, motility was not different than in drug-naïve planarians exposed to water (p > 0.05).
Acute U50,588 exposure decreases motility whereas morphine has no effect (Figs. 3B–C)
In experiments examining effects of morphine (0.1, 1 or 10 μM) on planarian motility (Fig. 3B), one-way ANOVA revealed a main effect [F(3, 31) = 3.835, p < 0.05], but post-hoc analysis did not reveal any significant differences between individual groups (p > 0.05). For experiments testing effects of U50,488 (0.1, 1 or 10 μM) on planarian motility (Fig. 3C), one-way ANOVA indicated a main effect [F(3, 31) = 10.6, p < 0.0001]. Compared to drug-naïve planarians, planarians exposed to different concentrations of U50,488 [0.1 μM (p < 0.001), 1 μM (p < 0.01), 10 μM (p < 0.001)] displayed significantly reduced motility counts.
Discussion
We tested the hypothesis that planarians would display mu opioid-like opioid effects when exposed to DAMGO, a biosynthetic peptide with 500- to 1000-fold greater selectivity for mu relative to kappa and delta opioid receptors (Schnittler et al., 1990). That is, indeed, what we found. Specifically, planarians conditioned with DAMGO displayed environmental place conditioning (EPC), a response that resembles CPP displayed by rodents following conditioning with addictive substances. Planarians spontaneously withdrawn from chronic DAMGO exposure displayed decreased motility and enhanced defensive responding. Acute DAMGO exposure produced hypermotility that was antagonized by naltrexone, thus identifying activation of mu opioid-like receptors as a plausible underlying mechanism of DAMGO action.
The place conditioning produced by DAMGO in planarians is consistent with evidence that addictive substances from different classes of abused drugs, such as depressants (ethanol, benzodiazepines), stimulants (cocaine, amphetamines, synthetic cathinones), and natural rewards (table sugar), also produce EPC in planarians (Rawls et al., 2011; Ramoz et al., 2012; Zhang et al., 2013; Tallarida et al., 2014). The maximal environmental shift produced by DAMGO was 116 s and comparable in efficacy to cocaine, sucrose, and ethanol, which produced maximal environmental shifts of 100 s, 107 s, and 150 s, respectively, in published planarian studies (Tallarida et al., 2014; Zhang et al., 2013). DAMGO-induced place conditioning in planarians is consistent with rodent studies showing that different mu opioid receptor agonists (e.g. DAMGO, morphine, oxycodone) produce CPP (Bals-Kubik et al., 1990; Suzuki et al., 1991; Contarino et al., 1997; Nikura et al., 2013; Koek, 2016). It is worth mentioning that a biased design was in our EPC experiments. For rodent CPP studies that utilize biased paradigms, interpretation of data is challenging in cases in which a test compound has anxiolytic properties because it can be unclear if the preference shift produced by the compound is due to anxiolytic or rewarding effects. Thus, the DAMGO-induced place conditioning that we detected in planarians may be due rewarding or anxiolytic effects, or a combination of both effects.
The concentration-response curve for DAMGO in our planarian EPC experiments resembled an inverted-U shape in which a median concentration of 1 μM elicited the maximal shift while lower (0.1 μM) and higher (10 μM) concentrations produced weaker shifts. Inverted-U shape concentration-response curves are also produced by cocaine and sucrose in planarian EPC experiments and by cocaine and morphine in rodent CPP experiments (Tallarida et al., 2014; Zhang et al., 2013; Koek, 2016; Zakharova et al., 2009). Reasons for the inverted U-shape following DAMGO conditioning are unclear but may be related to the time course and sequence of our conditioning paradigm. During the conditioning phase planarians were first exposed to DAMGO for 30 min in the least-preferred environment and then immediately exposed to water for 30 min in the preferred, or opposite, environment. It is conceivable, especially in the case of the higher concentration (10 μM), that residual quantities of DAMGO remained during the ‘water conditioning session’, albeit at much lower concentrations. If so, the lingering quantities of DAMGO could have disrupted conditioning (i.e., the presence of DAMGO in both conditioning environments leads to less place conditioning and a weaker environmental shift). Alternatively, high concentrations of DAMGO may be less rewarding than lower concentrations. To discriminate between these possibilities, future studies should increase the time interval between DAMGO and water conditioning sessions, as was done, for example, to investigate CPP produced by high doses of the long-acting opioid buprenorphine (Tzschentke, 2004), and alternate, and counterbalance, the sequence of conditioning sessions for DAMGO and water.
Kappa opioid receptor involvement is another potential explanation for the descending limb of the DAMGO concentration-response curve. Mammalian studies have shown that kappa opioid receptor activation produces dysphoric effects and kappa opioid agonists cause conditioned place aversion (CPA), rather than conditioned place preference (Shippenberg and Herz, 1986). DAMGO does display high selectivity for mu opioid receptors relative to delta and kappa opioid receptors (Kd values = 1.18, 1,430, and 213 nM for human mu, delta, and kappa opioid receptors, respectively) (Zhao et al., 2003). Nonetheless, we still considered the possibility that DAMGO, especially when administered to planarians at higher concentrations, loses selectivity for mu opioid receptors, thereby producing weaker place conditioning that results from a blend of mu (rewarding) and kappa (dysphoric) opioid receptor activation. Since nBNI did not significantly enhance place conditioning produced by the highest concentration DAMGO in our experiments, it seems unlikely that kappa opioid receptor activation is a primary mechanistic reason for the descending limb of the DAMGO concentration-response curve.
We detected, and quantified, two abstinence-induced withdrawal responses in planarians following spontaneous discontinuation of chronic DAMGO exposure. One withdrawal response was decreased motility, an endpoint used previously to quantify planarian withdrawal during abstinence from cocaine, amphetamines, benzodiazepines, and opioids (Raffa and Valdez, 2001; Raffa et al., 2003, 2008; Rawls et al., 2006, 2007, 2008). Although ‘decreased planarian motility’ is a behavioral signature following spontaneous discontinuation of addictive substances, interpretation of its implication remains open. In the simplest sense the phenomenon can be defined as an abstinence-induced withdrawal response because the decrease in motility occurs specifically when drug exposure is discontinued but not in drug-naïve planarians or in planarians exposed acutely or continuously to the drug. Importantly, both in the case of DAMGO here and previously with other addictive substances such as nicotine, sugar and synthetic cathinones (Zhang et al., 2013; Raffa et al., 2013; Ramoz et al., 2012; Rawls et al., 2011), motility during continuous exposure (e.g. 30–60 min depending on the study) is not reduced relative to drug-naïve planarians. Because decreased motility was evident only during DAMGO abstinence but not during a 30-min DAMGO exposure, it is unlikely that toxicity resulting from prolonged DAMGO exposure contributed to the reduced motility that presented during abstinence, though entirely excluding toxicity-related impacts would require experiments at the cellular level. The presence of a withdrawal sign or symptom following discontinuation of drug exposure is the hallmark characteristic of physical dependence, but any relevance of planarian opioid withdrawal responses to the strong opioid physical dependence that develops in mammals is unknown. One additional possibility is that the reduced motility during DAMGO abstinence reflects a “depressive-like state” in planarians, similar to the immobility displayed by morphine-withdrawn rats in the forced swim test (Anraku et al., 2001).
The second withdrawal response during DAMGO abstinence was increased defensive responding quantified by the PLDT (planarian/light dark test) (Zewde et al., 2018; Nayak et al., 2016). The PLDT is founded on the tendency of planarians, when given an option, to spend more time in dark versus light environments, which is a response that applies to both drug-naïve planarians and planarians spontaneously withdrawn from chronic exposure to cocaine or alcohol (Nayak et al., 2016). Similar behavioral phenomena occur in mammals, as rats or mice withdrawn from chronic regimens of an addictive substance spend more time in dark (e.g. light/dark box) or closed (e.g. EPM assay) compartments (Bourin and Hascoët, 2003). Classical benzodiazepine anxiolytics are active in these mammalian models and reduce the anxiogenic-type response during drug abstinence (Paine et al., 2002; Walf and Frye, 2007). The enhanced planarian defensive responding that manifests during DAMGO abstinence may, therefore, be the invertebrate equivalent of an aversive state that exists in human drug abusers following discontinuation of chronic consumption of abused drugs and increases vulnerability to relapse (Koob and Kreek, 2007).
The magnitude of light avoidance in planarians across experiments can vary. This phenomenon is evident in Fig. 2A, as the percentage of time that drug-naïve planarians spent in the light compartment was greater than that previously observed (Zewde et al., 2018; Nayak et al., 2016). Two factors that influence the magnitude of light avoidance are the intensity of the light source and circadian rhythms. Predictably, the greater the intensity of the light, the greater the light avoidance. Since our experiments here were conducted in a behavioral room with standard lighting, the light avoidance detected in drug-naïve planarians was likely less than what would be produced by more intense light sources. Another factor is circadian dependence. We recently showed that time of day influences the percentage of time planarians spend in the light, with the greatest light avoidance occurring at 6 AM and the least light avoidance detected at midnight (Zewde et al., 2018). In fact, at midnight, planarians do not show any light avoidance at all as they actually spend a greater amount of time in the light. This opposite directional trend that manifests at midnight may be related to evidence that most planarians are nocturnal, which might account for increased exploration into the light compartment (Lombardo et al., 2011).
Sensitivity is an attractive feature of the planarian abstinence assay because a quantifiable effect is present during spontaneous opioid abstinence. Although a withdrawal syndrome can be detected in rodents following spontaneous discontinuation of opioid exposure, the administration of an opioid antagonist (e.g. naloxone or NTX) is often required to precipitate robust, quantifiable withdrawal signs and symptoms (e.g. wet dog shakes, lacrimation, teeth chattering, escape behavior, diarrhea, etc.). Aside from the decreased motility and enhanced defensive responding detected during DAMGO abstinence, no other overt withdrawal responses were observed. Yet, on the basis of prior work, such responses might be observed following abstinence from higher concentrations of DAMGO. For example, Pagán et al. (2009) demonstrated that nicotine produces four distinct planarian withdrawal responses: “HeadBops” (“nodding”-like movements while gliding at the bottom of the dish), “HeadSwings” (head rotation in the absence of gliding while the tail is fixed to the bottom of the dish), “TailTwists” (bending of the tail tip) and “Corkscrews” (spiral rotation while floating/swimming.
Acute DAMGO exposure increased planarian motility, and it did so at the same concentration that produced the most robust place conditioning in the EPC assay. The enhanced motility produced by DAMGO was significantly inhibited by the opioid receptor antagonist NTX, suggesting that DAMGO activated opioid receptors, most likely mu since three different concentrations of NTX were effective, to increase motility. Since NTX did not affect motility when tested by itself, the existence of an endogenous opioid tone that regulates motility in planarians is unlikely. Consistent with a previous report testing effects of DAMGO and the opioid antagonist naloxone (Passarelli et al., 1999) in planarians, neither DAMGO nor NTX caused stereotyped motor behaviors such as C-shapes or screw-like hyperkinesias. DAMGO, as well as the closely related analog DAGO, consistently produce locomotor activation following acute exposure in mice (Mickley et al., 1990; Michael-Titus et al., 1989) and rats (Vezina et al., 1987; Locke and Holtzman, 1986; Shim et al., 2014). Morphine, in contrast, produces complex effects on locomotor activity in rodents that are species- and dose-dependent, as well as bimodal in some cases, with low doses that are more selective for mu opioid receptors often enhancing locomotor activation and higher doses that also activate kappa and delta opioid receptors reducing locomotor activity (Brady and Holtzman, 1981; Murphy et al., 2001). To probe a similar mu versus kappa opioid effect on planarian motility, we conducted follow-up experiments testing effects of morphine or U50,488, a highly selective kappa opioid agonist, on motility. Morphine, unlike DAMGO, did not enhance motility and, at the highest concentration tested (10 μM), even caused a non-significant reduction in motility. U50,488 reduced planarian motility at all concentrations tested. These cumulative results with mechanistically different opioid agonists suggest that mu opioid receptor activation, as in the case of DAMGO, enhances motility whereas kappa opioid receptor activation, as in the case of U50,488H, reduces motility.
The planarian model offers advantages that enable it to complement existing vertebrate model systems, and in some cases, to more efficiently study drug-induced behavioral effects that are methodologically challenging to investigate in higher-order species. Planarians do utilize key neurotransmitter systems that underlie behavioral effects of drugs of abuse (Pagán et al., 2014; Raffa and Rawls, 2008). Studying behavioral effects of addictive substances in planarians offers the ability to measure behavioral output in a sensitive, reproducible, quantifiable, cost-effective, and time-effective manner and to minimize interpretive complications related to complicated pharmacokinetic factors such as drug absorption, distribution, metabolism, elimination, and blood-brain-barrier passage. Because planarians lack a blood-brain barrier (Pagán et al., 2014), it is possible to identify the drug concentration that reaches receptor targets as opposed to just dose, which is less amenable to rigorous quantitative analysis. Chronic drug exposure paradigms, including the study of drug combinations and poly-drug interactions, are easily accomplished by soaking planarians in a drug concentration within a petri dish. This feat is accomplished easily due to the aquatic nature of planarians, thus offering a distinct advantage over mammalian models that rely on multiple injections or mini-pumps to achieve constant drug concentrations. The impact of higher-order confounding influences, such as the effects of handling, familiarity of the testing environment, strain, stress from procedures, and anticipation of drug administration, may also be minimized in planarians. From a translational perspective, the planarian model may be adapted to the in vivo screening of very small amounts of novel synthetic compounds, which are available in limited quantity, for their potential as abuse-deterrent and CNS-active therapeutics. A limitation of the planarian model is the relative lack of information regarding its genome. Although more information about the genetic makeup of planarians continues to emerge (Grohme et al., 2018), it remains difficult at the cellular level to effectively identify receptor subtypes that contribute to planarian behavioral responses.
In conclusion, the present study showed that a selective mu opioid receptor agonist produces a triad of mammalian-like effects in planarians. The effects included place conditioning suggestive of rewarding or anxiolytic effects, or both; abstinence-induced withdrawal suggestive of depressant- and anxiogenic-like phenotypes; and hypermotility that was blocked by an opioid antagonist. Our results suggest that some opioid-induced behavioral effects are conserved in invertebrates and that planarians offer a complementary model for studying behavioral effects of addictive substances.
Research Highlights.
DAMGO was used to investigate mu opioid-like effects in planarians.
DAMGO produced place conditioning in planarians.
Acute DAMGO exposure produced an enhancement of planarian motility that was antagonized by naltrexone.
DAMGO abstinence produced planarian withdrawal responses (defensive responding and decreased motility).
Acknowledgments
This study was funded by National Institutes of Health grants R25DA033270 (NIDA/OD) and P30 DA013429 (NIDA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anraku T, Ikegaya Y, Matsuki N, Nishiyama N. Withdrawal from chronic morphine administration causes prolonged enhancement of immobility in rat forced swimming test. Psychopharmacology (Berl) 2001;157:217–220. doi: 10.1007/s002130100793. [DOI] [PubMed] [Google Scholar]
- Bals-Kubik R, Shippenberg TS, Herz A. Involvement of central mu and delta opioid receptors in mediating the reinforcing effects of beta-endorphin in the rat. Eur J Pharmacol. 1990;175:63–69. doi: 10.1016/0014-2999(90)90153-w. [DOI] [PubMed] [Google Scholar]
- Bourin M, Hascoët M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- Brady LS, Holtzman SG. Locomotor activity in morphine-dependent and post-dependent rats. Pharmacol Biochem Behav. 1981;14:361–370. doi: 10.1016/0091-3057(81)90403-2. [DOI] [PubMed] [Google Scholar]
- Buttarelli FR, Pontieri FE, Margotta V, Palladini G. Acetylcholine/dopamine interaction in planaria. Comp Biochem Physiol C Toxicol Pharmacol. 2000;125:225–231. doi: 10.1016/s0742-8413(99)00111-5. [DOI] [PubMed] [Google Scholar]
- Contarino A, Zanotti A, Drago F, Natolino F, Lipartiti M, Giusti P. Conditioned place preference: no tolerance to the rewarding properties of morphine. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:589–594. doi: 10.1007/pl00004988. [DOI] [PubMed] [Google Scholar]
- Davidson C, Prados J, Gibson CL, Young AM, Barnes D, Sherlock R, Hutchinson CV. Shedding light on photosensitive behaviour in brown planaria (Dugesia Tigrina) Perception. 2011;40:743–746. doi: 10.1068/p6949. [DOI] [PubMed] [Google Scholar]
- Grohme MA, Schloissnig S, Rozanski A, Pippel M, Young GR, Winkler S, Brandl H, Henry I, Dahl A, Powell S, Hiller M, Myers E1, Rink JC. The genome of Schmidtea mediterranea and the evolution of core cellular mechanisms. Nature. 2018;554:56–61. doi: 10.1038/nature25473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson CV, Prados J, Davidson C. Persistent conditioned place preference to cocaine and withdrawal hypo-locomotion to mephedrone in the flatworm planaria. Neurosci Lett. 2015;593:19–23. doi: 10.1016/j.neulet.2015.03.021. [DOI] [PubMed] [Google Scholar]
- Koek W. Morphine-induced conditioned place preference and effects of morphine pre-exposure in adolescent and adult male C57BL/6J mice. Psychopharmacology (Berl) 2016;233:2015–2024. doi: 10.1007/s00213-014-3695-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusayama T, Watanabe S. Reinforcing effects of methamphetamine in planarians. Neuroreport. 2000;11:2511–2513. doi: 10.1097/00001756-200008030-00033. [DOI] [PubMed] [Google Scholar]
- Locke KW, Holtzman SG. Behavioral effects of opioid peptides selective for mu or delta receptors. II. Locomotor activity in nondependent and morphine–dependent rats. J Pharmacol Exp Ther. 1986;238:997–1003. [PubMed] [Google Scholar]
- Lombardo P, Giustini M, Miccoli FP, Cicolani B. Fine-scale differences in diel activity among nocturnal freshwater planarias (Platyhelminthes: Tricladida) J Circadian Rhythms. 2011;9:2. doi: 10.1186/1740-3391-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael-Titus A, Dourmap N, Costentin J. MU and delta opioid receptors control differently the horizontal and vertical components of locomotor activity in mice. Neuropeptides. 1989;13:235–242. doi: 10.1016/0143-4179(89)90076-0. [DOI] [PubMed] [Google Scholar]
- Mickley GA, Mulvihill MA, Postler MA. Brain mu and delta opioid receptors mediate different locomotor hyperactivity responses of the C57BL/6J mouse. Psychopharmacology (Berl) 1990;101:332–337. doi: 10.1007/BF02244050. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Lam HA, Maidment NT. A comparison of morphine-induced locomotor activity and mesolimbic dopamine release in C57BL6, 129Sv and DBA2 mice. J Neurochem. 2001;79:626–635. doi: 10.1046/j.1471-4159.2001.00599.x. [DOI] [PubMed] [Google Scholar]
- Murugan NJ, Persinger MA. Comparisons of responses by planarian to micromolar to attomolar dosages of morphine or naloxone and/or weak pulsed magnetic fields: revealing receptor subtype affinities and non-specific effects. Int J Radiat Biol. 2014;90:833–840. doi: 10.3109/09553002.2014.911421. [DOI] [PubMed] [Google Scholar]
- Nayak S, Roberts A, Bires K, Tallarida CS, Kim E, Wu M, Rawls SM. Benzodiazepine inhibits anxiogenic-like response in cocaine or ethanol withdrawn planarians. Behav Pharmacol. 2016;27:556–558. doi: 10.1097/FBP.0000000000000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niikura K, Ho A, Kreek MJ, Zhang Y. Oxycodone-induced conditioned place preference and sensitization of locomotor activity in adolescent and adult mice. Pharmacol Biochem Behav. 2013;110:112–116. doi: 10.1016/j.pbb.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K, Kitamura Y, Taniguchi T, Agata K. Analysis of motor function modulated by cholinergic neurons in planarian Dugesia japonica. Neuroscience. 2010;168:18–30. doi: 10.1016/j.neuroscience.2010.03.038. [DOI] [PubMed] [Google Scholar]
- Pagán OR, Rowlands AL, Azam M, Urban KR, Bidja AH, Roy DM, Feeney RB, Afshari LK. Reversal of cocaine-induced planarian behavior by parthenolide and related sesquiterpene lactones. Pharmacol Biochem Behav. 2008;89:160–170. doi: 10.1016/j.pbb.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Pagan OR, Rowlands AL, Fattore AL, Coudron T, Urban KR, Bidja AH, Eterović VA. A cembranoid from tobacco prevents the expression of nicotine-induced withdrawal behavior in planarian worms. Eur J Pharmacol. 2009;615:118–124. doi: 10.1016/j.ejphar.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagán OR. The First Brain: The Neuroscience of Planarians. Oxford University Press; 2014. [Google Scholar]
- Paine TA, Jackman SL, Olmstead MC. Cocaine-induced anxiety: alleviation by diazepam, but not buspirone, dimenhydrinate or diphenhydramine. Behav Pharmacol. 2002;13:511–523. doi: 10.1097/00008877-200211000-00001. [DOI] [PubMed] [Google Scholar]
- Palladini G, Ruggeri S, Stocchi F, De Pandis MF, Venturini G, Margotta V. A pharmacological study of cocaine activity in planaria. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1996;115:41–45. doi: 10.1016/s0742-8413(96)00053-9. [DOI] [PubMed] [Google Scholar]
- Passarelli F, Merante A, Pontieri FE, Margotta V, Venturini G, Palladini G. Opioid-dopamine interaction in planaria: a behavioral study. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1999;124:51–55. doi: 10.1016/s0742-8413(99)00048-1. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Baron S, Bhandal JS, Brown T, Song K, Tallarida CS, Rawls SM. Opioid receptor types involved in the development of nicotine physical dependence in an invertebrate (Planaria) model. Pharmacol Biochem Behav. 2013;112:9–14. doi: 10.1016/j.pbb.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Stagliano GW, Ross G, Powell JA, Phillips AG, Ding Z, Rawls SM. The kappa-opioid receptor antagonist nor-BNI inhibits cocaine and amphetamine, but not cannabinoid (WIN 52212-2), abstinence-induced withdrawal in planarians: an instance of 'pharmacologic congruence'. Brain Res. 2008;1193:51–56. doi: 10.1016/j.brainres.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffa RB, Valdez JM. Cocaine withdrawal in Planaria. Eur J Pharmacol. 2001;430:143–145. doi: 10.1016/s0014-2999(01)01358-9. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Rawls SM. A model for drug action and abuse. Austin, TX: Landes Bioscience; 2008. [Google Scholar]
- Ramoz L, Lodi S, Bhatt P, Reitz AB, Tallarida C, Tallarida RJ, Raffa RB, Rawls SM. Mephedrone ("bath salt") pharmacology: insights from invertebrates. Neuroscience. 2012;208:79–84. doi: 10.1016/j.neuroscience.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Baron S, Ding Z, Roth C, Zaveri N, Raffa RB. Nociceptin attenuates methamphetamine abstinence-induced withdrawal-like behavior in planarians. Neuropeptides. 2008;42:229–237. doi: 10.1016/j.npep.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Cavallo F, Capasso A, Ding Z, Raffa RB. The beta-lactam antibiotic ceftriaxone inhibits physical dependence and abstinence-induced withdrawal from cocaine, amphetamine, methamphetamine, and clorazepate in planarians. Eur J Pharmacol. 2008;584:278–284. doi: 10.1016/j.ejphar.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Gomez T, Raffa RB. An NMDA antagonist (LY 235959) attenuates abstinence-induced withdrawal of planarians following acute exposure to a cannabinoid agonist (WIN 55212-2) Pharmacol Biochem Behav. 2007;86:499–504. doi: 10.1016/j.pbb.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Patil T, Tallarida CS, Baron S, Kim M, Song K, Ward S, Raffa RB. Nicotine behavioral pharmacology: clues from planarians. Drug Alcohol Depend. 2011;118:274–279. doi: 10.1016/j.drugalcdep.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Rodriguez T, Baron DA, Raffa RB. A nitric oxide synthase inhibitor (L-NAME) attenuates abstinence-induced withdrawal from both cocaine and a cannabinoid agonist (WIN 55212-2) in Planaria. Brain Res. 2006;1099:82–87. doi: 10.1016/j.brainres.2006.04.103. [DOI] [PubMed] [Google Scholar]
- Schnittler M, Liebmann C, Schrader U, Schulze HP, Neubert K, Repke H. [3H]naloxone as an opioid receptor label: analysis of binding site heterogeneity and use for determination of opioid affinities of casomorphin analogues. Biomed Biochim Acta. 1990;49:209–218. [PubMed] [Google Scholar]
- Shim I, Stratford TR, Wirtshafter D. Dopamine is differentially involved in the locomotor hyperactivity produced by manipulations of opioid, GABA and glutamate receptors in the median raphe nucleus. Behav Brain Res. 2014;261:65–70. doi: 10.1016/j.bbr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A. Differential effects of mu and kappa opioid systems on motivational processes. NIDA Res Monogr. 1986;75:563–66. [PubMed] [Google Scholar]
- Suzuki T, Funada M, Narita M, Misawa M, Nagase H. Pertussis toxin abolishes mu- and delta-opioid agonist-induced place preference. Eur J Pharmacol. 1991;205:85–88. doi: 10.1016/0014-2999(91)90774-k. [DOI] [PubMed] [Google Scholar]
- Tallarida CS, Bires K, Avershal J, Tallarida RJ, Seo S, Rawls SM. Ethanol and cocaine: environmental place conditioning, stereotypy, and synergism in planarians. Alcohol. 2014;48:579–586. doi: 10.1016/j.alcohol.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Reassessment of buprenorphine in conditioned place preference: temporal and pharmacological considerations. Psychopharmacology (Berl) 2004;172:58–67. doi: 10.1007/s00213-003-1626-4. [DOI] [PubMed] [Google Scholar]
- Umeda S, Stagliano GW, Borenstein MR, Raffa RB. A reverse-phase HPLC and fluorescence detection method for measurement of 5-hydroxytryptamine (serotonin) in Planaria. J Pharmacol Toxicol Methods. 2005;51:73–76. doi: 10.1016/j.vascn.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Vezina P, Kalivas PW, Stewart J. Sensitization occurs to the locomotor effects of morphine and the specific mu opioid receptor agonist, DAGO, administered repeatedly to the ventral tegmental area but not to the nucleus accumbens. Brain Res. 1987;417:51–58. doi: 10.1016/0006-8993(87)90178-8. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Estradiol decreases anxiety behavior and enhances inhibitory avoidance and gestational stress produces opposite effects. Stress. 2007;10:251–260. doi: 10.1080/00958970701220416. [DOI] [PubMed] [Google Scholar]
- Zakharova E, Miller J, Unterwald E, Wade D, Izenwasser S. Social and physical environment alter cocaine conditioned place preference and dopaminergic markers in adolescent male rats. Neuroscience. 2009;163:890–897. doi: 10.1016/j.neuroscience.2009.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zewde AM, Yu F, Nayak S, Tallarida C, Reitz AB, Kirby LG, Rawls SM. PLDT (planarian light/dark test): an invertebrate assay to quantify defensive responding and study anxiety-like effects. J Neurosci Methods. 2018;293:284–288. doi: 10.1016/j.jneumeth.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Tallarida CS, Raffa RB, Rawls SM. Sucrose produces withdrawal and dopamine-sensitive reinforcing effects in planarians. Physiol Behav. 2013;112–113:8–13. doi: 10.1016/j.physbeh.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao GM, Qian X, Schiller PW, Szeto HH. Comparison of [Dmt1] DALDA and DAMGO in binding and G protein activation at mu, delta, and kappa opioid receptors. J Pharmacol Exp Ther. 2003;307:947–954. doi: 10.1124/jpet.103.054775. [DOI] [PubMed] [Google Scholar]


