Abstract
Endocannabinoids (eCBs) and neurotrophins, particularly brain-derived neurotrophic factor (BDNF), are potent neuromodulators found throughout the mammalian neocortex. Both eCBs and BDNF play critical roles in many behavioral and neurophysiological processes and are targets for the development of novel therapeutics. The effects of eCBs and BDNF are primarily mediated by the type 1 cannabinoid (CB1) receptor and the trkB tyrosine kinase receptor, respectively. Our laboratory and others have previously established that BDNF potentiates excitatory transmission by enhancing presynaptic glutamate release and modulating NMDA receptors. In contrast, we have shown that BDNF attenuates inhibitory transmission by inducing postsynaptic release of eCBs that act retrogradely to suppress GABA release in layer 2/3 of somatosensory cortex. Here, we hypothesized that BDNF also induces release of eCBs at excitatory synapses, which could have a mitigating or opposing effect on the direct presynaptic effects of BDNF. We found the highest levels of expression of CB1 and trkB and receptors in layers 2/3 and 5. Surprisingly, BDNF did not increase the frequency of spontaneous miniature excitatory postsynaptic currents (mEPSCs) onto layer 5 pyramidal neurons in somatosensory cortex, in contrast to its effects in the hippocampus and visual cortex. However, the effect of BDNF on mEPSC frequency in somatosensory cortex was unmasked by blocking CB1 receptors or disrupting eCB release. Thus, BDNF-trKB signaling regulates glutamate release in the somatosensory cortex via opposing effects, a direct presynaptic enhancement of release probability, and simultaneous postsynaptically-induced eCB release that decreases release probability via presynaptic CB1 receptors.
Keywords: endocannabinoids, glutamatergic, neocortex, neuromodulators, neurotrophins
1 |. INTRODUCTION
In the central nervous system, synapses are specialized sites of cell-to-cell contact that form the basic substrate of information transfer within neuronal networks. Chemical neurotransmission, be it excitatory or inhibitory, is regulated by a range of neuromodulators. It is now widely accepted that the proper and precise modulation of synapses is critical for brain development as well as many cognitive functions in the adult. In the mammalian neocortex, the endocannabinoid (eCB) system and neurotrophin signaling pathways have both been well characterized and identified as important regulators of synaptic activity and neuro-transmitter release. Extensive research within the past decade has firmly established the role of eCBs as retrograde messengers suppressing neurotransmitter release in either a transient or long-lasting manner at both inhibitory and excitatory terminals (Alger, 2012; Chevaleyre, Takahashi, & Castillo, 2006; Freund, Katona, & Piomelli, 2003; Kano, Ohno-Shosaku, Hashimotodani, Uchigashima, & Watanabe, 2009). Additionally, the eCB signaling system is now recognized as a regulator of various neural functions, including cognition, motor control, feeding behaviors and pain (Hillard, Weinlander, & Stuhr, 2012; Mechoulam & Parker, 2013). Further, the dysregulation of the eCB signaling system has been implicated in neuropsychiatric disorders such as depression and anxiety, providing a clear and understudied experimental model for potential therapeutic interventions. Likewise, neurotrophins, specifically brain-derived neurotrophic factor (BDNF), have been shown to modulate the efficacy of synaptic transmission and their expression at the synapse is activity dependent (Berninger & Poo, 1996; Katz & Shatz, 1996; Minichiello, 2009; Park & Poo, 2013).
Interestingly, multiple reports have described evidence for cross-talk between neurotrophin and eCB signaling. Specifically, Zhong et al. (2015) showed that BDNF in midbrain dopamine neurons regulates eCB responses, cocaine-induced synaptic plasticity, and associative learning by selectively knocking down BDNF expression in dopaminergic neurons. In the visual cortex, BDNF has been shown to oppose eCB-mediated forms of heterosynaptic long-term depression (LTD) at activated synapses by inducing homosynaptic long term potentiation (Huang, Yasuda, Sarihi, & Tsumoto, 2008). Furthermore, there is evidence of BDNF and its involvement as a modulator of eCB-mediated synaptic plasticity in the hippocampus (Roloff, Anderson, Martemyanov, & Thayer, 2010). Lastly, BDNF and eCB interactions have been demonstrated in mediating neuronal survival and protection against excitotoxicity (Khaspekov et al., 2004; Maison, Walker, Walsh, Williams, & Doherty, 2009).
BDNF may also be an important trigger for eCB synthesis and release. It is well established that eCB mobilization can be triggered by increases in intracellular calcium or activation of Gq coupled receptors and subsequent phospholipase C (PLC)-dependent increase in diacylglycerol (DAG), the precursor to 2-arachidonoylglycerol (2-AG) (Castillo, Younts, Chavez, & Hashimotodani, 2012; Hashimotodani, Ohno-Shosaku, & Kano, 2007). In addition, we have recently shown that BNDF/trkB signaling also triggers PLC-dependent eCB release. BDNF, acting through postsynaptic trkB receptors, induces 2-AG release from pyramidal neurons at neocortical inhibitory synapses, which in turn suppresses GABA release from presynaptic terminals (Lemtiri-Chlieh & Levine, 2010; Zhao & Levine, 2014). In fact, the suppressive effect of BDNF seems to be completely mediated by eCBs, because the effect of BDNF at these inhibitory synapses is completely prevented by blocking cannabinoid type 1 (CB1) receptors or interfering with eCB synthesis or release. Furthermore, we also showed that theta burst stimulation-induced release of endogenous BDNF can also trigger eCB synthesis and release resulting in inhibitory long-term depression (iLTD) (Zhao, Yeh, & Levine, 2015).
In the present studies, we explored whether BDNF can also trigger the release of eCBs at excitatory synapses. It is known that BDNF can directly potentiate glutamatergic neurotransmission by enhancing pre-synaptic release probability (Carmignoto, Pizzorusso, Tia, & Vicini, 1997; Lessmann & Heumann, 1998; Li, Zhang, Lester, Schuman, & Davidson, 1998; Schinder et al., 2000; Tyler & Pozzo-Miller, 2001), as well as enhancing postsynaptic NMDA receptor responsiveness (Crozier, Black, & Plummer, 1999; Levine, Crozier, Black, & Plummer, 1998; Lin et al., 1998; Madara & Levine, 2008). Conversely, eCB signaling decreases release probability at synapses throughout the neocortex. However, it is not known if BDNF induces eCB release at excitatory synapses. We hypothesized that, if it occurs, BDNF-induced eCB release at excitatory synapses would act to mitigate the direct effects of BDNF.
2 |. MATERIALS AND METHODS
2.1 |. Animal handling and slice preparation
All animal procedures were conducted using protocols approved by the University of Connecticut Institutional Animal Care and Use Committee. Postnatal Day 15–27 Swiss CD-1 (Charles River, Wilmington, MA) and C57BL/6J (The Jackson Laboratory, Bar Harbor, ME) mice were anesthetized by 3.5% isoflurane inhalation, followed by decapitation. Experiments were conducted on Swiss CD-1 mice except where noted. Whole brains were removed and immersed in ice-cold slicing solution containing (in mM) 110 choline chloride, 2.5 KCl, 1.25 NaH2PO4-H2O, 25 NaHCO3, 0.5 CaCl2, 7 MgCl2-6H2O, 25 D-glucose, 11.6 sodium ascorbate, and 3.1 sodium pyruvate, equilibrated with 95% O2-5% CO2 (pH 7.3, 310 ± 5 mosmol/kg). Transverse slices (300 μm) containing somatosensory cortex were cut with a Dosaka EM DTK-1000 vibratome (Kyoto, Japan) and transferred to an incubating chamber. Slices were then incubated for 15 min at 33–35 °C in carboxygenated incubating solution containing (in mM) 125 NaCl, 2.5 KCl, 1.25 NaH2PO4- H2O, 25 NaHCO3, 0.5 CaCl2, 3.5 MgCl2-6H2O, 25 D-glucose, 4 sodium lactate, 2 sodium pyruvate, and 0.4 ascorbic acid (pH 7.3, 310 ± 5 mosmol/kg) before being transferred to room temperature. Slices were then individually transferred to a recording chamber (room temperature) fixed to the stage of an Olympus BX51WI upright microscope fitted with an ×40 water-immersion objective lens (0.8 NA). The recording chamber was continuously perfused at 1.5–2.0 ml/min with carboxygenated artificial cerebrospinal fluid (aCSF) consisting of (in mM) 125 NaCl, 2.5 KCl, 1.25 NaH2PO4-H2O, 25 NaHCO3, 2 CaCl2, 2 MgCl2-6H2O, and 25 D-glucose (pH 7.3, 305 ± 5 mosmol/kg).
2.2 |. Electrophysiology
Whole cell recordings were obtained from layer 5 somatosensory cortex pyramidal neurons. Neurons were visually identified by their morphology and position under infrared differential interference contrast video microscopy. Patch electrodes (2–4 MΩ) were pulled from borosilicate glass capillaries using a Flaming/Brown P-97 micropipette puller (Sutter Instrument, Novato, CA). Pipette internal solution contained (in mM) 4 KCl, 125 K-Gluconate, 10 HEPES, 10 Phosphocreatine, 1 EGTA, 0.20 CaCl2, 4 Na2-ATP, and 0.30 Na-GTP.
The sodium equilibrium potential (ENa) with the use of the above internal and external solutions was close to +90 mV; thus EPSCs were recorded as inward currents. Cells were voltage clamped at −70 mV during recording. Miniature excitatory postsynaptic currents (mEPSCs) were isolated by bath perfusion of tetrodotoxin (TTX, 500 nM). All electrical currents were filtered at 2.9 kHz and digitized at >6 kHz using a HEKA EPC9 amplifier and ITC-16 digitizer (HEKA Elektronik, Darmstadt, Germany). Series resistance (Rs) was compensated up to 50% at 10–100 μs lag. Input resistance (Ri) was monitored with 10 mV (50 ms) hyperpolarizing voltage steps at the end of each sweep. Cells were rejected from analyses if Ri changed by > 15% or fell below 50 MΩ during the course of an experiment.
2.3 |. Chemicals
Unless otherwise stated, all drugs were obtained from Tocris Biosciences (Bristol, UK) and were delivered by bath perfusion. Drugs were first prepared as concentrated stock solution in solvents and stored at −20 °C. Stock solutions of WIN55–212,2, ANA-12, SR141716A, and AM404 were dissolved in 100% dimethyl sulfoxide (DMSO). The stock solution of BDNF was dissolved in 18 MΩ water. The stock solution of TTX was dissolved in aCSF. Drug stock solutions were diluted in aCSF on the day of recording to the final concentrations. The final concentration of DMSO did not exceed 0.1%, which by itself had no effect on synaptic transmission.
2.4 |. Immunohistochemistry
Immunohistochemical staining of tissue sections from perfusion-fixed mice has been described previously (Yeh et al., 2014). Briefly, animals were perfused transcardially with 4% paraformaldehyde/0.1 M sodium phosphate buffer (pH 7.4) after CO2 asphyxiation. After transcardial perfusion, brains were removed and postfixed in 4% paraformaldehyde/0.1 M sodium phosphate buffer (pH 7.4) overnight at 4 °C. Coronal sections (15 μm) through the somatosensory cortex were cut using a cryostat (Bright Instruments, Bedfordshire, UK) and immunostained with the appropriate antibodies. Sections were immunostained using the following antibodies: guinea pig polyclonal CB1 (1:500, generously provided by Dr. Ken Mackie, Indiana University), mouse monoclonal vGlut1 (1:500, Neuromab), rabbit polyclonal trkBH181 (1:100, Santa Cruz Biotechnology) and mouse monoclonal Map2 (1:1000, Sigma-Aldrich). Fluorescent secondary antibodies used were Jackson Immuno 488 donkey anti-guinea pig, Jackson Immuno Rhodamine donkey anti-mouse and Jackson Immuno Cy-5 donkey anti-rabbit. To confirm antibody specificity, we preincubated CB1 receptor and trkB receptor antibodies with their respective blocking peptides prior to overnight incubation on cryosectioned mouse brain tissue. In the case of both antibodies, the blocking peptides completely negated the fluorescent signals.
2.5 |. Image and data analysis
Immunolabeled samples were visualized using an Axiovert 200B with Apotome and Colibri LED illumination together with Axiovision software. Images were assembled in Adobe Photoshop CS6 with consistent quality adjustments for brightness, contrast and color balance. For mean fluorescence intensity, one mosaic image capturing a cortical column spanning the somatosensory cortex was taken from 3 consecutive coronal sections per animal included in the study. Fluorescence intensity values for each cortical laminae were then averaged and are reported as means ± SE. Confocal images were captured using a Zeiss LSM510 Meta confocal microscope. Off-line analysis of whole-cell patch clamp electrophysiological recordings was carried out using Clampfit 10 (Molecular Devices, Sunnyvale, CA) and Prism 6 (GraphPad Software, La Jolla, CA). Group data are reported as means ± SE. Statistical comparisons were made using one-way ANOVA and Dunnett’s multiple comparison test or paired Student’s t test for post hoc comparison. p <.05 was taken as a statistically significant effect.
3 |. RESULTS
3.1 |. Expression of trkB and CB1 receptors at excitatory terminals in mouse somatosensory cortex
We have previously shown that BDNF-trkB signaling triggers the release of eCBs at inhibitory synapses in layer 2/3 of mouse somato-sensory cortex (Lemtiri-Chlieh & Levine, 2010; Zhao & Levine, 2014), suggesting colocalization of CB1 and trkB receptors in this area. We have also shown that eCBs modulate excitatory synapses in layer 2/3 as well as layer 5 (Fortin & Levine, 2007). To determine the pattern of co-localization of CB1 receptors and trkB receptors across cortical layers, we performed immunohistochemical labeling of coronal sections of mouse somatosensory cortex at P21–24. Antibodies for the vesicular glutamate transporter 1 (vGlut1) and microtubule associated protein 2 (Map2) were used as markers of presynaptic terminals and dendritic processes, respectively. CB1 and trkB receptors both exhibit their highest levels of expression in layers 2/3 and 5 in the somatosensory cortex (Figure 1a), supporting functional interaction within these layers. We subsequently quantified this observation by measuring mean fluorescence intensity of CB1 receptor and trkB receptor protein expression within each cortical layer (Figure 1b).
FIGURE 1.
Type 1 cannabinoid (CB1) receptor and tropomyosin receptor kinase B (trkB) receptor expression in mouse somatosensory cortex. (a) Mosaic image showing immunohistochemical localization of CB1 receptors (green) and trkB receptors (red) in postnatal day (P) 23 coronal section of the somatosensory cortex. Images show the full extent of a cortical column, comprising layers 1–6 and the underlying white matter (WM). (b) Quantitative assessment of immunofluorescence intensity for CB1 receptor (top) and trkB receptor (bottom) protein across layers 1–6 in somatosensory cortex. n = 6 animals; values reported are means ± SE. (c) Top: higher magnification confocal image of layer 5 of the somatosensory cortex showing localization of CB1 receptor (green), trkB receptor (blue) and vesicular glutamate transporter 1 (vGlut1, red), a marker of excitatory presynaptic terminals. White arrows indicate synapses where CB1 receptor and vGlut1 puncta colocalize. Bottom: orthogonal projections of CB1 receptor (green), trkB receptor (blue) and microtubule associated protein 2 (Map2, red), a marker for dendritic processes. White arrows designate CB1 receptor puncta juxtaposed to Map2-positive dendrites. White asterisks in the bottom panel provide examples of cell somas
In an effort to visualize pre- and postsynaptic localization of the CB1 and trkB receptor at excitatory synapses in layer 5 via immunohistochemical analysis, we performed triple-labeling with antibodies against CB1, trkB, and the presynaptic glutamatergic marker, vGlut1 (Figure 1c, top). In addition, we used the neuronal marker, Map2, which labels dendritic processes of cortical pyramidal neurons, as an indicator of postsynaptic localization (Figure 1c, bottom). Visualization of antibody staining suggests that both CB1 and trkB receptors are closely associated with vGlut1-positive puncta and Map2-positive neuronal processes (Figure 1c). In accordance with our previous observations using vGlut1 as an excitatory presynaptic marker in layer 5, we observed CB1 receptor-positive puncta adjacent to Map2-positive neuronal processes, providing further evidence of presynaptic CB1 receptors at neocortical synapses (Figure 1c bottom, white arrows). Additionally, trkB staining visualized along dendritic processes appeared to colocalize with Map2-positive processes within layer 5 (Figure 1c bottom). There was also evidence of trkB present at sites juxtaposing Map2-positive dendrites, where they colocalized with CB1 receptor-positive puncta, suggesting that the trkB receptor may be present at presynaptic synapses as well as on postsynaptic dendrites (Figure 1c bottom). Both CB1-positive and trkB-positive puncta along dendritic processes suggest that these receptors may be present at the same excitatory synapses in layer 5 of mouse somatosensory cortex. CB1 receptor expression was also observed around the soma of putative pyramidal neurons situated in layer 5 (Figure 1c). We believe that these CB1 receptors are expressed in presynaptic inhibitory terminals, which contact the soma of pyramidal neurons, consistent with previous anatomical and physiological studies (Bodor et al., 2005; Egertova, Cravatt, & Elphick, 2003; Fortin & Levine, 2007; Katona, Sperlagh, et al., 1999; Katona, Urban, et al., 2006; Pertwee, 2008; Trettel, Fortin, & Levine, 2004; Trettel & Levine, 2002; Zhao & Levine, 2014; Zhao et al., 2015). However, we cannot rule out the potential expression of CB1 receptors on the soma of pyramidal neurons as well.
3.2 |. Effect of BDNF on spontaneous release probability at excitatory synapses
We first examined the effect of exogenous BDNF (20 ng/ml, 0.8 nM) on isolated AMPA-mediated mEPSCs in layer 5 of mouse somatosensory cortex. As illustrated by sample traces and group data in Figure 2a, bath application of 20 ng/ml BDNF (0.8 nM) had no significant effect on mEPSC frequency. The amplitude of mEPSCs was also not affected by bath-applied BDNF in any cells sampled (Figure 2a bottom). A higher concentration of BDNF (50 ng/ml, 2.0 nM) also failed to alter mEPSC frequency or amplitude in layer 5 of somatosensory cortex (Figure 2b).
FIGURE 2.
BDNF has region-specific effects at cortical excitatory synapses. (a) Group time courses showing lack of effect of 0.8 nM BDNF on mEPSC frequency (top) and amplitude (bottom) in layer 5 pyramidal neurons of somatosensory cortex (n = 14 cells; 5 animals). Insets, in this and following panels, show sample traces during baseline and in the presence of BDNF from a representative experiment. Scale bars in all sample traces are 10 pA, 100 ms. (b) Similar lack of effect of 2.0 nM BDNF on mEPSC frequency (top) and amplitude (bottom) in layer 5 of somatosensory cortex (n = 12 cells; 3 animals). (c) Effect of BDNF (0.8 nM) on mEPSC frequency and amplitude in CA1 hippocampal pyramidal cells (n = 7 cells; 2 animals—1 CD1, 1 C57). Data from individual experiments compare mEPSC frequency during minutes 3–5 of BDNF exposure to baseline. Bottom graph, effect of BDNF on mEPSC frequency is blocked in the presence of the trkB receptor antagonist, ANA12 (10 mM; n 5 4 cells; 1 CD1, 1 C57). (d) Effect of BDNF (0.8 nM) on mEPSC frequency and amplitude in layer 5 of visual cortex (n = 6 cells; 1 CD1, 1 C57). Data from individual experiments compare mEPSC frequency during minutes 3–5 of BDNF exposure to baseline. Bottom graph, effect of BDNF on mEPSC frequency is blocked in the presence of the trkB receptor antagonist, ANA12 (10 μM; n = 4 cells; 1 CD1, 1 C57). *p < .05
In light of the negative results obtained with BDNF in layer 5 of somatosensory cortex, we also examined the effect of BDNF in areas where it had been previously shown to increase mESPC frequency, including layer 5 of visual cortex (Madara & Levine, 2008), and hippo-campus (Alder et al., 2005; Gibon, Barker, & Seguela, 2016; Leal, Afonso, Salazar, & Duarte, 2015). In contrast to the lack of effect in layer 5 of somatosensory cortex, BDNF (0.8 nM) increased mEPSC frequency in hippocampal CA1 PNs (ANOVA (F)14,74 = 2.75, p < .05; Baseline, 6.63 ± 1.83 Hz; BDNF, 7.87 ± 2.01, n = 7 cells, 2 animals) (Figure 2c) and in layer 5 PNs of visual cortex (ANOVA (F)14,73 = 1.86, p < .05; Baseline, 2.05 ± 0.88 Hz; BDNF, 2.54 ± 0.83, n = 6 cells, 2 animals) (Figure 2d). As expected, BDNF did not enhance the amplitude of AMPA-mediated mEPSCs in these areas (Figure 2c,d). Lastly, we confirmed the role of BDNF/trkB signaling in mediating the increase in mEPSC frequency in both CA1 of hippocampus and layer 5 of visual cortex by bath-applying BDNF in the presence of the trkB receptor-specific antagonist, ANA-12 (10 μM) (Figure 2c,d). We did not observe any effect of ANA-12 alone on mEPSC frequency or amplitude. Furthermore, ANA-12 blocked the increase in mEPSC frequency elicited by exogenous BDNF (2.0 nM) application in the CA1 of hippocampus and layer 5 of visual cortex (Figure 2c,d). The effect of BDNF and its block by ANA-12 were seen in both CD1 and C57 mice, thus data from the two strains were pooled for the above analysis.
3.3 |. Endocannabinoid signaling opposes BDNF-mediated potentiation of excitatory neurotransmission
We hypothesized that the inability of BDNF to produce a net increase in mEPSC frequency in layer 5 somatosensory cortical neurons may be the result of BDNF-trkB induced release of eCBs that have an opposing effect on presynaptic release probability. We first confirmed the effects of CB1 receptor activation at these excitatory synapses with the CB1 receptor agonist WIN 55,212–2 (WIN; 5 μm). Bath application of WIN decreased mEPSC frequency (Baseline 1.82 6 0.45 Hz; WIN 1.03 ± 0.25 Hz; Student’s t test; n 5 8, 4 animals) (Figure 3d) with no change in mEPSC amplitude. Because CB1 receptor signaling typically attenuates presynaptic release via inhibition of voltage-gated calcium channels (VGCCs) (Kreitzer & Regehr, 2001; Lozovaya, Min, Tsintsadze, & Burnashev, 2009; Szabo et al., 2014), we examined the effect of WIN in the presence of cadmium (Cd+2; 100 μM) to block VGCCs. As shown in Figure 3d, bath application of Cd+2 alone reduced mEPSC frequency (Baseline 2.75 ± 0.97 Hz; Cd+2 1.18 ± 0.48 Hz; Student’s t test, n = 4, 1 animal). Subsequent addition of WIN in the presence of Cd+2 had no further effect on mEPSC frequency (Figure 3d). Taken together, these data indicate that VGCCs contribute to spontaneous glutamatergic release at these synapses, and that cannabinoid-mediated suppression of release requires VGCC activity, similar to what was reported for layer 2/3 inhibitory synapses (Madara & Levine, 2008; Trettel & Levine, 2002).
FIGURE 3.
Blocking CB1 receptors unmasks BDNF-induced potentiation in layer 5 somatosensory cortex pyramidal cells. (a) Sample sweeps of mEPSCs during baseline, in the presence of SR141716A (SR; 10 μM) alone, and in the presence of SR plus BDNF (0.8 nM). Scale bar 10 pA, 100 ms. (b) Group time course showing the effect of bath-applied SR (10 μM) on mEPSC frequency, followed by BDNF (0.8 nM) in the presence of SR. n = 10 cells, 2 animals. (c) Group time course for the same cells as (c) showing no effect of SR alone or BDNF plus SR on mEPSC amplitude. *p < .05 compared to SR alone. (d) Left, Cannabinoid agonist WIN55,212–2 (WIN; 5 mm) decreased mEPSC frequency (n = 8 cells; 4 animals). Right, Blocking voltage-gated calcium channels with cadmium (Cd+2; 100 μM) decreased mEPSC frequency, and prevented the effect of WIN (n = 4 cells, 1 animal). *p < .05
To test whether BDNF-induced eCB release was mitigating the direct effect of BDNF, we examined the effect of BDNF (0.8 nM) in the presence of the CB1 receptor-specific inverse agonist, SR141716A (SR; 10 μM) (Figure 3). In this experiment, SR was bath-applied for 10 minutes, followed by a 10 min perfusion of BDNF (0.8 nM) in the continued presence of SR. Representative traces from a single experiment are shown in Figure 3a. Application of SR resulted in a small but nonsignificant and transient increase in the frequency of mEPSCs in the majority of cells tested, as shown in the group data presented in Figure 3b. The amplitude of mEPSCs was not affected by SR alone (Figure 3c). As shown in the group data presented in Figure 3b, exogenous BDNF (0.8 nM) application in the presence of SR resulted in a statistically significant increase in mEPSC frequency. mEPSC frequency was increased to 159.4% ± 13.5% at 5 min of BDNF exposure in the presence of SR (ANOVA (F)14,131 = 1.76, p < .05; SR 1.36 Hz ± 0.27; SR 1 BDNF 1.97 Hz ± 0.36; n 5 15, 5 animals) (Figure 3b). These results support our hypothesis that BDNF/trkB signaling causes the release of eCBs at excitatory synapses, which directly opposes direct BDNF-mediated potentiation of glutamatergic release probability.
3.4 |. BDNF triggers the release of eCBs from postsynaptic terminals in layer 5 of somatosensory cortex
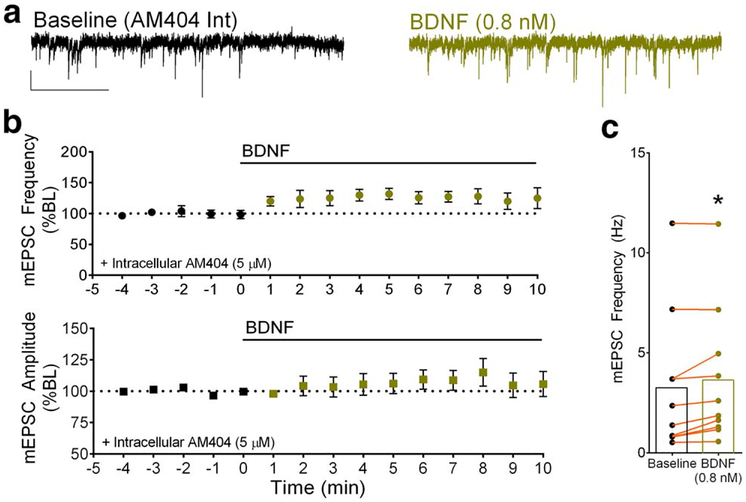
Next, we employed the eCB transport inhibitor, AM404 (Beltramo et al., 1997; Di Marzo et al., 1994; Fegley et al., 2004; Hillard, Edgemond, Jarrahian, & Campbell, 1997) in the patch pipette to provide further evidence for postsynaptic BDNF-induced eCB release at layer 5 excitatory synapses. Intracellular loading of AM404 has been shown to disrupt the release of eCBs during LTD (Ronesi, Gerdeman, & Lovinger, 2004; Zhao et al., 2015). Sample traces from a single experiment are shown in Figure 4a during baseline (top) and during bath-application of BDNF (0.8 nM) in the presence of intracellular AM404 (5 μM). We found that bath-application of BDNF (0.8 nM) in the presence of intracellular AM404 caused a statistically significant increase in mEPSC frequency compared to baseline (ANOVA, (F14,131 = 1.21), 131.7% ± 9.92, p < .05) (Figure 4b top, C). Comparisons of minutes 3–5 of BDNF (0.8 nM) wash-in for individual experiments are illustrated in Figure 4c, indicating a statistically significant increase in mEPSC frequency during exogenous BDNF exposure in the presence of intracellular AM404 (5 μM) (Baseline 3.48 ± 1.48 Hz; AM404 (Int) + BDNF 4.20 Hz ± 1.63; p < .03; n = 10, 2 animals). There was no effect of BDNF on mEPSC amplitude under these conditions (Figure 4b, bottom). In a set of parallel experiments, we added the DMSO vehicle (final concentration 0.1%) to the intracellular recording solution and found that it had no effect on the frequency or amplitude of mEPSCs (data not shown).
FIGURE 4.
Blocking eCB transport unmasks BDNF-induced potentiation in somatosensory cortex. (a) Representative traces of mEPSCs during baseline in the presence of intracellular AM404 (5 μM) and during bath-applied BDNF (0.8 nM) in the presence of intracellular AM404. Scale bar 10 pA, 100 ms. (b) Group time courses showing the effect of bath-applied BDNF (0.8 nM) in the presence of intracellular AM404 on mEPSC frequency (top) and amplitude (bottom). (c) Data from individual experiments showing a statistically significant increase in mEPSC frequency during minutes 3–5 of BDNF exposure in the presence of internal AM404. *p < .03 compared to baseline
Taken together, these results suggest that BDNF induces eCB release at layer 5 excitatory synapses, and BDNF and eCB signaling have opposing direct effects on presynaptic release probability. BDNF by itself had no net effect at these synapses, but the direct effect of BDNF could be unmasked by either blocking CB1 receptor activation or inhibiting postsynaptic eCB release from pyramidal neurons.
4 |. DISCUSSION
We recently showed that BDNF induces release of eCBs from postsynaptic pyramidal cells in layers 2/3 of somatosensory cortex (Lemtiri-Chlieh & Levine, 2010). This effect of BDNF is initiated by postsynaptic trkB signaling, requires downstream phospholipase-Cƴ (PLCƴ) signaling and is independent of metabotropic glutamate receptor (mGluR) activation (Zhao & Levine, 2014). Furthermore, we established a role for endogenous BDNF in eCB-mediated plasticity at cortical inhibitory synapses, where strong theta burst stimulation (TBS) in layers 2/3 of somatosensory cortex can induce iLTD that requires BDNF-trkB and diacylglycerol (DAG) signaling (Zhao et al., 2015). To complement these studies of BDNF and eCB interactions at cortical inhibitory synapses, we asked whether BDNF also triggers the release of eCBs at cortical excitatory synapses. We have shown that BDNF and eCBs exert opposing effects at cortical excitatory synapses (Fortin & Levine, 2007; Madara & Levine, 2008). However, whether these two distinct signaling systems interact and the net functional outcome of these influences at excitatory synapses remains unexplored. We examined this issue in layer 5 of somatosensory cortex, since previous reports suggest that effects of eCBs on excitatory synapses are prominent in this region of the neocortex.
First, we employed immunohistochemistry on fixed, cryosectioned mouse somatosensory cortex with antibodies against the CB1 receptor and the trkB receptor. Our observations confirm previous studies exploring the synaptic localization of the CB1 (Egertova et al., 2003; Marsicano & Lutz, 1999; Matsuda, Bonner, & Lolait, 1993; Tsou, Brown, Sanudo-Pena, Mackie, & Walker, 1998) and trkB (Cabelli et al., 1996; Fryer et al., 1996; Miller & Pitts, 2000) receptors in the neocortex. Moreover, our immunohistochemical analysis provides an important first step in fully elucidating the synaptic topography of CB1 and trkB receptors at excitatory terminals. Scanning electron microscopy or super resolution microscopy may be required to obtain more precise visualization of receptor localization at the synapse. The use of high resolution microscopy may also aid in characterizing excitatory terminals with respect to receptor composition, that is, CB1 receptor-positive, trkB receptor-positive, or both.
We then examined the synaptic effects of eCB-CB1 receptor signaling and BDNF-trkB receptor signaling on spontaneous transmission at excitatory terminals within layer 5 of the somatosensory cortex. Surprisingly, we found that BDNF by itself did not increase mEPSC frequency (or amplitude) at these synapses. To confirm previous reports of BDNF effects on mEPSC frequency in other brain regions (Carmignoto et al., 1997; Li et al., 1998; Schinder, Berninger, & Poo, 2000; Tyler & Pozzo-Miller, 2001), we examined the effect of BDNF in pyramidal neurons in the hippocampal CA1 region and layer 5 of visual cortex. We found that BDNF significantly increased mEPSC frequency in these regions, these effects were blocked by a trkB receptor antagonist, and these effects were consistent across two different mouse strains.
We next determined whether a BDNF-induced release of eCBs at excitatory synapses in layer 5 of somatosensory cortex might offset the direct presynaptic effects of BDNF. Blocking eCB signaling with a CB1 receptor antagonist unmasked a BDNF-induced increase in mEPSC frequency. Blocking postsynaptic release of eCBs had a similar effect. These results suggest that BDNF-trkB signaling induces the release of eCBs at excitatory synapses. While our group and others have previously described an increase in presynaptic release probability as a result of acute BDNF exposure in other brain regions, this is the first study examining synaptic effects of BDNF in layer 5 of mouse somatosensory cortex. In this region, we find that BDNF activates pre-synaptic trkB receptors to enhance spontaneous release probability, while simultaneously triggering the postsynaptic release of eCBs that act to decrease release via presynaptic CB1 receptors.
The presynaptic effects of BDNF on spontaneous glutamate transmission may have functional consequences of their own (Carmignoto et al., 1997; Lessmann & Heumann, 1998; Li et al., 1998; Schinder et al., 2000; Tyler & Pozzo-Miller, 2001) and may also translate to changes in evoked AP-dependent release. However, there is a lack of clear evidence for effects of BDNF on evoked glutamate release. This may in fact reflect the offsetting effect of BDNF-induced eCB release, and/or the effects of BDNF on evoked release may depend on specific AP activity patterns. It is also important to recognize that evoked (action potential-dependent) and spontaneous (action potential-independent) neurotransmitter release may utilize distinct vesicle pools that can be differentially regulated (Huntwork & Littleton, 2007; Littleton, Stern, Schulze, Perin, & Bellen, 1993; Maximov et al., 2007; Pang et al., 2011; Yoshihara et al., 1999). Furthermore, nonoverlapping populations of postsynaptic NMDA receptors have been implicated in differentiating between spontaneous and evoked release (Atasoy et al., 2008; Melom, Akbergenova, Gavornik, & Littleton, 2013).
In summary, our study contributes to uncovering region-specific roles of the eCB-CB1 receptor and BDNF-trkB receptor signaling pathways at cortical excitatory synapses. Moreover, we provide evidence that BDNF-trkB signaling may play an important role in triggering eCB release at cortical excitatory synapses. These two signaling systems have been implicated in various neurological disease states, including autism spectrum disorders, schizophrenia and epilepsy. There is, at present, a continuously growing body of evidence that the eCB system and BDNF system interact at various levels across multiple fields, including synaptic plasticity (De Chiara, Angelucci, et al., 2010; De Chiara, Motta, et al., 2013; Maison et al., 2009), neuropathic pain (Luongo, Maione, & Di Marzo, 2014), cortical development (Berghuis et al., 2005; Galve-Roperh et al., 2013) and neuroprotection in depression and epileptic seizures (Aguado et al., 2007; Aso et al., 2008; Khaspekov et al., 2004; Marsicano et al., 2003; Vinod et al., 2012). Careful examination and elucidation of these interactions at excitatory and inhibitory synapses will be essential to the development and implementation of novel and effective therapeutic strategies.
Acknowledgments
Funding Information
This work was supported by NIH/NIMH R01 MH094896.
REFERENCES
- Aguado T, Romero E, Monory K, Palazuelos J, Sendtner M, Marsicano G, … Galve-Roperh I (2007). The CB1 cannabinoid receptor mediates excitotoxicity-induced neural progenitor proliferation and neurogenesis. The Journal of Biological Chemistry, 282(33), 23892–23898. [DOI] [PubMed] [Google Scholar]
- Alder J, Thakker-Varia S, Crozier RA, Shaheen A, Plummer MR, & Black IB (2005). Early presynaptic and late postsynaptic components contribute independently to brain-derived neurotrophic factor-induced synaptic plasticity. The Journal of Neuroscience, 25(12), 3080–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger BE (2012). Endocannabinoids at the synapse a decade after the dies mirabilis (29 March 2001): what we still do not know. The Journal of Physiology, 590(Pt 10), 2203–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso E, Ozaita A, Valdizan EM, Ledent C, Pazos A, Maldonado R, & Valverde O (2008). BDNF impairment in the hippocampus is related to enhanced despair behavior in CB1 knockout mice. Journal of Neurochemistry, 105(2), 565–572. [DOI] [PubMed] [Google Scholar]
- Atasoy D, Ertunc M, Moulder KL, Blackwell J, Chung C, Su J, & Kavalali ET (2008). Spontaneous and evoked glutamate release activates two populations of NMDA receptors with limited overlap. The Journal of Neuroscience, 28(40), 10151–10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, & Piomelli D (1997). Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science, 277(5329), 1094–1097. [DOI] [PubMed] [Google Scholar]
- Berghuis P, Dobszay MB, Wang X, Spano S, Ledda F, Sousa KM, … Harkany T (2005). Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proceedings of the National Academy of Sciences of the United States of America, 102(52), 19115–19120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berninger B, & Poo M (1996). Fast actions of neurotrophic factors. Current Opinion in Neurobiology, 6(3), 324–330. [DOI] [PubMed] [Google Scholar]
- Bodor AL, Katona I, Nyiri G, Mackie K, Ledent C, Hajos N, & Freund TF (2005). Endocannabinoid signaling in rat somatosensory cortex: Laminar differences and involvement of specific interneuron types. The Journal of Neuroscience, 25(29), 6845–6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabelli RJ, Allendoerfer KL, Radeke MJ, Welcher AA, Feinstein SC, & Shatz CJ (1996). Changing patterns of expression and sub-cellular localization of TrkB in the developing visual system. The Journal of Neuroscience, 16(24), 7965–7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignoto G, Pizzorusso T, Tia S, & Vicini S (1997). Brain-derived neurotrophic factor and nerve growth factor potentiate excitatory synaptic transmission in the rat visual cortex. The Journal of Physiology, 498 (Pt 1), 153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Younts TJ, Chavez AE, & Hashimotodani Y (2012). Endocannabinoid signaling and synaptic function. Neuron, 76(1), 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, & Castillo PE (2006). Endocannabinoid-mediated synaptic plasticity in the CNS. Annual Review of Neuroscience, 29, 37–76. [DOI] [PubMed] [Google Scholar]
- Crozier RA, Black IB, & Plummer MR (1999). Blockade of NR2B-containing NMDA receptors prevents BDNF enhancement of glutamatergic transmission in hippocampal neurons. Learning Memory, 6(3), 257–266. [PMC free article] [PubMed] [Google Scholar]
- De Chiara V, Angelucci F, Rossi S, Musella A, Cavasinni F, Cantarella C, … Centonze D (2010). Brain-derived neurotrophic factor controls cannabinoid CB1 receptor function in the striatum. The Journal of Neuroscience, 30(24), 8127–8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Chiara V, Motta C, Rossi S, Studer V, Barbieri F, Lauro D, … Centonze D (2013). Interleukin-1beta alters the sensitivity of cannabinoid CB1 receptors controlling glutamate transmission in the striatum. Neuroscience, 250, 232–239. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, & Piomelli D (1994). Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature, 372(6507), 686–691. [DOI] [PubMed] [Google Scholar]
- Egertova M, Cravatt BF, & Elphick MR (2003). Comparative analysis of fatty acid amide hydrolase and cb(1) cannabinoid receptor expression in the mouse brain: evidence of a widespread role for fatty acid amide hydrolase in regulation of endocannabinoid signaling. Neuroscience, 119(2), 481–496. [DOI] [PubMed] [Google Scholar]
- Fegley D, Kathuria S, Mercier R, Li C, Goutopoulos A, Makriyannis A, & Piomelli D (2004). Anandamide transport is independent of fatty-acid amide hydrolase activity and is blocked by the hydrolysis-resistant inhibitor AM1172. Proceedings of the National Academy of Sciences of the United States of America, 101(23), 8756–8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin DA, & Levine ES (2007). Differential effects of endocannabinoids on glutamatergic and GABAergic inputs to layer 5 pyramidal neurons. Cerebral Cortex, 17(1), 163–174. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, & Piomelli D (2003). Role of endogenous cannabinoids in synaptic signaling. Physiological Reviews, 83(3), 1017–1066. [DOI] [PubMed] [Google Scholar]
- Fryer RH, Kaplan DR, Feinstein SC, Radeke MJ, Grayson DR, & Kromer LF (1996). Developmental and mature expression of full-length and truncated TrkB receptors in the rat forebrain. The Journal of Comparative Neurology, 374(1), 21–40. [DOI] [PubMed] [Google Scholar]
- Galve-Roperh I, Chiurchiu V, Diaz-Alonso J, Bari M, Guzman M, & Maccarrone M (2013). Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Progress in Lipid Research, 52(4), 633–650. [DOI] [PubMed] [Google Scholar]
- Gibon J, Barker PA, & Seguela P (2016). Opposing presynaptic roles of BDNF and ProBDNF in the regulation of persistent activity in the entorhinal cortex. Molecular Brain, 9, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, & Kano M (2007). Presynaptic monoacylglycerol lipase activity determines basal endocannabinoid tone and terminates retrograde endocannabinoid signaling in the hippocampus. The Journal of Neuroscience, 27(5), 1211–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, Edgemond WS, Jarrahian A, & Campbell WB (1997). Accumulation of N-arachidonoylethanolamine (anandamide) into cerebellar granule cells occurs via facilitated diffusion. Journal of Neuro-chemistry, 69(2), 631–638. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Weinlander KM, & Stuhr KL (2012). Contributions of endocannabinoid signaling to psychiatric disorders in humans: Genetic and biochemical evidence. Neuroscience, 204, 207–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Yasuda H, Sarihi A, & Tsumoto T (2008). Roles of endocannabinoids in heterosynaptic long-term depression of excitatory synaptic transmission in visual cortex of young mice. The Journal of Neuroscience, 28(28), 7074–7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntwork S, & Littleton JT (2007). A complexin fusion clamp regulates spontaneous neurotransmitter release and synaptic growth. Nature Neuroscience, 10(10), 1235–1237. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, & Watanabe M (2009). Endocannabinoid-mediated control of synaptic transmission. Physiological Reviews, 89(1), 309–380. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, & Freund TF (1999). Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippo-campal interneurons. The Journal of Neuroscience, 19(11), 4544–4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, … Freund TF (2006). Molecular composition of the endocannabinoid system at glutamatergic synapses. The Journal of Neuroscience, 26(21), 5628–5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, & Shatz CJ (1996). Synaptic activity and the construction of cortical circuits. Science, 274(5290), 1133–1138. [DOI] [PubMed] [Google Scholar]
- Khaspekov LG, Brenz Verca MS, Frumkina LE, Hermann H, Marsicano G, & Lutz B (2004). Involvement of brain-derived neurotrophic factor in cannabinoid receptor-dependent protection against excitotoxicity. The European Journal of Neuroscience, 19(7), 1691–1698. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, & Regehr WG (2001). Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron, 29(3), 717–727. [DOI] [PubMed] [Google Scholar]
- Leal G, Afonso PM, Salazar IL, & Duarte CB (2015). Regulation of hippocampal synaptic plasticity by BDNF. Brain Research, 1621, 82–101. [DOI] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, & Levine ES (2010). BDNF evokes release of endogenous cannabinoids at layer 2/3 inhibitory synapses in the neocortex. Journal of Neurophysiology, 104(4), 1923–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessmann V, & Heumann R (1998). Modulation of unitary glutamatergic synapses by neurotrophin-4/5 or brain-derived neurotrophic factor in hippocampal microcultures: Presynaptic enhancement depends on pre-established paired-pulse facilitation. Neuroscience, 86(2), 399–413. [DOI] [PubMed] [Google Scholar]
- Levine ES, Crozier RA, Black IB, & Plummer MR (1998). Brain-derived neurotrophic factor modulates hippocampal synaptic transmission by increasing N-methyl-D-aspartic acid receptor activity. Proceedings of the National Academy of Sciences of the United States of America, 95(17), 10235–10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YX, Zhang Y, Lester HA, Schuman EM, & Davidson N (1998). Enhancement of neurotransmitter release induced by brain-derived neurotrophic factor in cultured hippocampal neurons. The Journal of Neuroscience, 18(24), 10231–10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Wu K, Levine ES, Mount HT, Suen PC, & Black IB (1998). BDNF acutely increases tyrosine phosphorylation of the NMDA receptor subunit 2B in cortical and hippocampal postsynaptic densities. Brain Research Molecular Brain Research, 55(1), 20–27. [DOI] [PubMed] [Google Scholar]
- Littleton JT, Stern M, Schulze K, Perin M, & Bellen HJ (1993). Mutational analysis of Drosophila synaptotagmin demonstrates its essential role in Ca(21)-activated neurotransmitter release. Cell, 74(6), 1125–1134. [DOI] [PubMed] [Google Scholar]
- Lozovaya N, Min R, Tsintsadze V, & Burnashev N (2009). Dual modulation of CNS voltage-gated calcium channels by cannabinoids: Focus on CB1 receptor-independent effects. Cell Calcium, 46(3), 154–162. [DOI] [PubMed] [Google Scholar]
- Luongo L, Maione S, & Di Marzo V (2014). Endocannabinoids and neuropathic pain: Focus on neuron-glia and endocannabinoidneurotrophin interactions. The European Journal of Neuroscience, 39(3), 401–408. [DOI] [PubMed] [Google Scholar]
- Madara JC, & Levine ES (2008). Presynaptic and postsynaptic NMDA receptors mediate distinct effects of brain-derived neurotrophic factor on synaptic transmission. Journal of Neurophysiology, 100(6), 3175–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison P, Walker DJ, Walsh FS, Williams G, & Doherty P (2009). BDNF regulates neuronal sensitivity to endocannabinoids. Neuro-science Letters, 467(2), 90–94. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, … Lutz B (2003). CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science, 302(5642), 84–88. [DOI] [PubMed] [Google Scholar]
- Marsicano G, & Lutz B (1999). Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. The European Journal of Neuroscience, 11(12), 4213–4225. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Bonner TI, & Lolait SJ (1993). Localization of cannabinoid receptor mRNA in rat brain. The Journal of Comparative Neurology, 327(4), 535–550. [DOI] [PubMed] [Google Scholar]
- Maximov A, Shin OH, Liu X, & Sudhof TC (2007). Synaptotagmin-12, a synaptic vesicle phosphoprotein that modulates spontaneous neurotransmitter release. The Journal of Cell Biology, 176(1), 113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, & Parker LA (2013). The endocannabinoid system and the brain. Annual Review of Psychology, 64, 21–47. [DOI] [PubMed] [Google Scholar]
- Melom JE, Akbergenova Y, Gavornik JP, & Littleton JT (2013). Spontaneous and evoked release are independently regulated at individual active zones. The Journal of Neuroscience, 33(44), 17253–17263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, & Pitts FA (2000). Neurotrophin receptors in the somatosensory cortex of the mature rat: Co-localization of p75, trk, iso-forms and c-neu. Brain Research, 852(2), 355–366. [DOI] [PubMed] [Google Scholar]
- Minichiello L (2009). TrkB signalling pathways in LTP and learning.Nature Reviews Neuroscience, 10(12), 850–860. [DOI] [PubMed] [Google Scholar]
- Pang ZP, Bacaj T, Yang X, Zhou P, Xu W, & Sudhof TC (2011). Doc2 supports spontaneous synaptic transmission by a Ca(21)-independent mechanism. Neuron, 70(2), 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, & Poo MM (2013). Neurotrophin regulation of neural circuit development and function. Nature Reviews Neuroscience, 14(1), 7–23. [DOI] [PubMed] [Google Scholar]
- Pertwee RG (2008). The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. British Journal of Pharmacology, 153(2), 199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roloff AM, Anderson GR, Martemyanov KA, & Thayer SA (2010). Homer 1a gates the induction mechanism for endocannabinoid-mediated synaptic plasticity. The Journal of Neuroscience, 30(8), 3072–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi J, Gerdeman GL, & Lovinger DM (2004). Disruption of endocannabinoid release and striatal long-term depression by postsynaptic blockade of endocannabinoid membrane transport. The Journal of Neuroscience, 24(7), 1673–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinder AF, Berninger B, & Poo M (2000). Postsynaptic target specificity of neurotrophin-induced presynaptic potentiation. Neuron, 25(1), 151–163. [DOI] [PubMed] [Google Scholar]
- Szabo GG, Lenkey N, Holderith N, Andrasi T, Nusser Z, & Hajos N (2014). Presynaptic calcium channel inhibition underlies CB(1) can nabinoid receptor-mediated suppression of GABA release. The Journal of Neuroscience, 34(23), 7958–7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trettel J, Fortin DA, & Levine ES (2004). Endocannabinoid signalling selectively targets perisomatic inhibitory inputs to pyramidal neurones in juvenile mouse neocortex. The Journal of Physiology, 556(Pt 1), 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trettel J, & Levine ES (2002). Cannabinoids depress inhibitory synaptic inputs received by layer 2/3 pyramidal neurons of the neocortex. Journal of Neurophysiology, 88(1), 534–539. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, & Walker JM (1998). Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience, 83(2), 393–411. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, & Pozzo-Miller LD (2001). BDNF enhances quantal neuro-transmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. The Journal of Neuroscience, 21(12), 4249–4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod KY, Xie S, Psychoyos D, Hungund BL, Cooper TB, & Tejani-Butt SM (2012). Dysfunction in fatty acid amide hydrolase is associated with depressive-like behavior in Wistar Kyoto rats. PLoS One, 7(5), e36743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh ML, Gonda Y, Mommersteeg MT, Barber M, Ypsilanti AR, Hanashima C, … Andrews WD (2014). Robo1 modulates proliferation and neurogenesis in the developing neocortex. The Journal of Neuroscience, 34(16), 5717–5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara M, Ueda A, Zhang D, Deitcher DL, Schwarz TL, & Kidokoro Y (1999). Selective effects of neuronal-synaptobrevin mutations on transmitter release evoked by sustained versus transient Ca21 increases and by cAMP. The Journal of Neuroscience, 19(7), 2432–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, & Levine ES (2014). BDNF-endocannabinoid interactions at neocortical inhibitory synapses require phospholipase C signaling. Journal of Neurophysiology, 111(5), 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Yeh ML, & Levine ES (2015). Role for endogenous BDNF in endocannabinoid-mediated long-term depression at neocortical inhibitory synapses. eNeuro, 2(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong P, Liu Y, Hu Y, Wang T, Zhao YP, & Liu QS (2015). BDNF interacts with endocannabinoids to regulate cocaine-induced synaptic plasticity in mouse midbrain dopamine neurons. The Journal of Neuroscience, 35(10), 4469–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]