Abstract.
The differential diagnosis of dengue virus (DENV) and yellow fever virus (YFV) infections in endemic areas is complicated by nonspecific early clinical manifestations. In this study, we describe an internally controlled, multiplex real-time reverse transcription polymerase chain reaction (rRT-PCR) for the detection of DENV and YFV. The DENV–YFV assay demonstrated specific detection and had a dynamic range of 2.0–8.0 log10 copies/μL of eluate for each DENV serotype and YFV. Clinical performance was similar to a published pan-DENV assay: 48/48 acute-phase samples from dengue cases were detected in both assays. For YFV detection, mock samples were prepared with nine geographically diverse YFV isolates over a range of concentrations. The DENV–YFV assay detected 62/65 replicates, whereas 54/65 were detected using a reference YFV rRT-PCR. Given the reemergence of DENV and YFV in areas around the world, the DENV–YFV assay should be a useful tool to narrow the differential diagnosis and provide early case detection.
Yellow fever (YF) is a zoonotic flaviviral disease caused by YF virus (YFV) and is endemic and/or epidemic in regions of South America and Africa.1 In South America, six countries reported suspected and confirmed YF cases in 2017, and in Brazil, an outbreak that was originally detected in December 2016 resulted in 3,240 reported cases, of which 792 (24.4%) were confirmed.2 In Africa, an outbreak occurred in Angola in 2016–2017 resulting to 3,818 suspected cases, of which 879 were confirmed (23%), before spreading to the Democratic Republic of the Congo and to Uganda.3 YF may present with signs and symptoms that are similar to dengue, which is caused by four related serotypes of dengue virus (DENV-1–4), a related flavivirus endemic to all regions with YFV transmission.4,5 An outbreak of YF may go undetected if cases are initially attributed to DENV, or another cause of similar symptoms, and specific YFV testing is not performed. Reported cases will then underestimate the true number of YFV infections.1
The laboratory diagnosis of YFV and DENV infections is typically accomplished using serological and/or molecular methods.6–8 Serological testing for these infections has well-documented limitations: antiviral immunoglobulin M may not be detectable early in the course of infection, a rise in immunoglobulin G between acute and convalescent samples can only provide a retrospective diagnosis, and anti-flavivirus antibodies may cross-react with one another.7,8 Molecular assays have been developed for YFV that can provide accurate detection within the first 5–7 days since symptom onset, and testing other specimen types may prolong YFV detection.4,6,7,9–11 The use of molecular testing and assay performance characteristics for DENV detection are well established.8,12 Previously, our group developed a pan-DENV real-time reverse transcription polymerase chain reaction (PCR) (rRT-PCR) for use in monoplex or for inclusion in multiplex assays targeting other pathogens.12–14 This assay has proven more sensitive than other molecular comparators for DENV; however, it uses probes modified with proprietary technology, which increases cost and may limit availability.12
The goal of the present study was to develop an internally controlled rRT-PCR for DENV and YFV. In addition, we sought to 1) redesign the pan-DENV assay for use with fewer primers and a single, unmodified hydrolysis probe and 2) design a new assay for YFV that was compatible with existing laboratory protocols for the detection of related pathogens. The clinical performance of the resulting DENV–YFV assay was compared with reference assays using clinical samples for DENV and mock samples for YFV, which were prepared with a diverse set of reference isolates.
The primers and probes included in the DENV–YFV assay and concentrations in the final reaction are shown in Table 1. Primers and probes were initially evaluated and optimized in monoplex reactions for DENV and YFV using nucleic acids from viral isolates and synthesized oligonucleotides (Ultramer ssDNA; Integrated DNA Technologies, Coralville, IA,) that contained the consensus target sequence for each DENV serotype and YFV. Assays were then combined and optimized in multiplex with a published assay for RNase P detection.12,13 Additional methodological details are provided in Supplemental Text.
Table 1.
Primer and probe sequences for the DENV–YFV assay
| Name | Sequence (5′ → 3′) | Concentration (nM)* | Location† |
|---|---|---|---|
| DENV primers and probe | |||
| DENV-1, 2, 3 forward | AGATYTCTGATGAAYAACCAACG | 300 | 87–109 |
| DENV-4 forward | GGAAGCTTGCTTAACACAGTTCT | 300 | 34–56 |
| DENV-1, 3 reverse | GAATCTCTTCGCCAACTGTGA | 300 | 174–194 |
| DENV-2 reverse | TGCAGCATTCCAAGTGAGAATCT | 300 | 190–212 |
| DENV-4 reverse | GAGAATCTCTTCACCAACCCTTG | 300 | 169–191 |
| DENV probe‡ | CAATATGCTGAAACGCGHGAGAAACCG | 300 | 134–160 |
| RNase P primers and probe | |||
| RNase P forward | AGATTTGGACCTGCGAGCG | 50 | NA |
| RNase P reverse | GAGCGGCTGTCTCCACAAGT | 50 | NA |
| RNase P probe‡ | TTCTGACCTGAAGGCTCTGCGCG | 50 | NA |
| YFV primers and probe | |||
| YFV forward | AGGTGCATTGGTCTGCAAAT | 300 | 13–32 |
| YFV reverse | TCTCTGCTAATCGCTCAACG | 300 | 77–96 |
| YFV reverse G → T | TCTCTGCTAATCGCTCAAAG | 300 | 77–96 |
| YFV probe‡ | GTTGCTAGGCAATAAACACATTTGGA | 200 | 36–61 |
DENV = dengue virus; YFV = yellow fever virus.
* Concentration of each oligonucleotide in the final reaction mixture is provided.
† Genomic locations for viral primers and probes are provided based on the following reference sequences: DENV-1 US/Hawaii/1944 (GenBank: EU848545.1), DENV-2 New Guinea C Strain (GenBank: AF038403.1), DENV-4 strain H-241 (GenBank: AY947539.1), and YFV Asibi strain, complete genome (Genbank: KF769016.1). For sequences present in multiple serotypes, locations are shown for DENV-1.
5′ fluor and 3′ quencher pairs were the following: DENV, FAM and BHQ-1; RNase P, Cal Fluor 560 and BHQ-1; YFV, Quasar 670 and BHQ-2.
The DENV–YFV assay was performed using 5 μL of nucleic acid template in 25 μL-reactions of the SuperScript III Platinum One-Step qRT-PCR Kit (Thermo Fisher Scientific, Waltham, MA) on a Rotor-Gene Q instrument (Qiagen, Germantown, MD) using the following cycling conditions: 52°C for 15 minutes; 94°C for 2 minutes; and 45 cycles of 94°C for 15 seconds, 55°C for 40 seconds (signal acquired), and 68°C for 20 seconds.12–14 For DENV and YFV, all samples with cycle threshold (Ct) values ≤ 38.5 and those with reproducible Ct values > 38.5 were considered positive. For RNase P, samples that had Ct values > 40 and negative results in the other channels were considered internal-control failures.
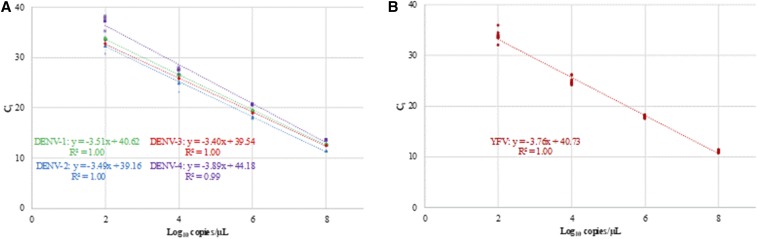
Analytical characterization was performed as previously described.13–15 To replicate clinical samples during the analytical evaluation, pooled nucleic acid eluate from negative serum samples was added to the master mix (5 μL per reaction). The dynamic range and the lower limit of 95% detection (95% LLOD) were established using quantitated ssDNA. The dynamic range of the DENV–YFV assay extended from 2.0 to 8.0 log10 copies/μL of eluate for each DENV serotype and YFV (Figure 1A and B). The 95% LLOD for each target, calculated by probit analysis (SPSS software, IBM, Armonk, NY) and expressed in copies/μL of eluate, was the following: DENV-1, 17.6; DENV-2, 9.4; DENV-3, 3.8; DENV-4, 150.1; and YFV, 11.3.
Figure 1.
Linear range of the DENV–YFV assay for (A) each DENV serotype and (B) YFV. Linear range was established using quantitated ssDNA containing the respective target sequences. Points represent each of four replicates at 2.0, 4.0, 6.0, and 8.0 log10 copies/μL for DENV-1 (green circles), DENV-2 (blue triangles), DENV-3 (red diamonds), and DENV-4 (purple squares). Points represent each of 10 replicates for YFV. DENV = dengue virus; YFV = yellow fever virus. This figure appears in color at www.ajtmh.org.
In an evaluation of assay exclusivity, no amplification was observed in the DENV–YFV assay when tested using genomic RNA from the following arboviruses: chikungunya virus, St. Louis encephalitis virus, West Nile virus, Zika virus, tick-borne encephalitis virus, Japanese encephalitis virus, Semliki Forest virus, Mayaro virus, Ross River virus, Getah virus, Barmah Forest virus, and Una virus. No cross-reaction was observed when genomic RNA from strains of DENV serotypes 1–4 and YFV were tested. Finally, 88 serum samples from patients at Emory Hospital with hepatitis B virus infections were tested; all samples had positive signals for RNase P but tested negative for DENV and YFV.
To evaluate clinical performance for DENV, acute-phase serum samples from 48 confirmed dengue cases were tested side by side using the DENV–YFV assay and the pan-DENV assay, as previously described (Table 2).12 Samples had been collected as part of the Pediatric Dengue Cohort Study in Managua, Nicaragua.16 Serotyping and quantitation were performed using a DENV multiplex assay17; samples included infections with DENV-1 (N = 12), DENV-2 (12), and DENV-3 (24). The concentration of DENV RNA in these samples is shown in Table 2. All samples were detected in both the DENV–YFV and pan-DENV assays. In addition, two isolates of DENV-4, strain H-241 (BEI resources and Zeptometrix), were tested and detected in both assays, with lower Ct values in the DENV–YFV assay.
Table 2.
DENV serotypes and YFV isolates tested in side by side comparisons of the DENV–YFV assay and comparator rRT-PCRs for each virus
| Virus | Detected | ||
|---|---|---|---|
| Concentration (cp/μL)* | DENV–YFV† | Comparator† | |
| DENV serotype | |||
| DENV-1 | 60–355,700 | 12/12 | 12/12 |
| DENV-2 | 1,800–381,700 | 12/12 | 12/12 |
| DENV-3 | 400–186,300 | 24/24 | 24/24 |
| YFV isolate‡ | |||
| INHRR 10a-10–Venezuela 2010 | 10–10,800 | 5/5 | 5/5 |
| Couma–Ethiopia 1961 | 5–499,500 | 9/9 | 8/9 |
| MIS 1034–Peru 2011 | 6–5,800 | 5/5 | 4/5 |
| FMD 1240–Peru 2007 | 4–35,300 | 7/7 | 7/7 |
| FVB 0196–Bolivia 2006 | 2–23,100 | 7/7 | 6/7 |
| CAREC M2-09–Trinidad 2009 | 8–8,400 | 6/7 | 3/7 |
| SH 281586, Angola 2016, human serum | 4–299,800 | 9/9 | 8/9 |
| DakArA MT7, Cote d’Ivoire 1973 | 5–51,600 | 7/9 | 8/9 |
| ArD 114896, Senegal 1965 | 3–28,100 | 7/7 | 5/7 |
DENV = dengue virus; rRT-PCR = real-time reverse transcription PCR; YFV = yellow fever virus.
Concentration expressed as copies per microliter of eluate for comparison with the lower limit of 95% detection .
Number of replicates detected/number tested for each assay over the range of concentrations shown in the table.
The listed concentrations include all dilutions that tested positive in one or both YFV assays. Replicates with negative results were the lowest concentration tested for each isolate; for CAREC M2-09, the YFV comparator tested negative with replicates at 8 and 80 cp/μL.
Yellow fever virus detection was evaluated using 19 YFV strains, including 17 viral isolates and two serum samples from acute human cases (Supplemental Table 1). We selected nine isolates for additional testing (Table 2). Six serial 10-fold dilutions were prepared for each isolate and tested side by side in the DENV–YFV assay and a comparator YFV rRT-PCR.4 RNA concentration was calculated from a 4-point standard curve included on each DENV–YFV run. The lowest concentration dilutions were tested in duplicate. The DENV–YFV assay detected YFV RNA in 42/54 dilutions, compared with 41/54 in the comparator rRT-PCR. However, of the dilutions that tested positive in one or both assays, 62/65 replicates were detected in the DENV–YFV assay versus 54/65 in the comparator (Table 2).
In conclusion, we describe the development of an internally controlled, multiplex rRT-PCR for DENV and YFV. During the analytical evaluation, the DENV–YFV assay demonstrated sensitive and specific detection of both viruses, and the assay performed similarly to the original pan-DENV assay when evaluated using samples from confirmed acute dengue cases with DENV-1, DENV-2, and DENV-3. Dengue virus-4 was not prevalent in Nicaragua during the period of the study from which these samples were obtained. However, the DENV-4 H-241 strain was tested from two sources, and these samples yielded lower Ct values in the DENV–YFV assay compared with the pan-DENV assay. A full clinical evaluation for DENV-4 is warranted, but these data suggest that the multiplex assay will perform similarly to the original pan-DENV assay.
Relative to a published comparator for YFV detection,4 the DENV–YFV assay detected a similar number of mock sample dilutions prepared with a diverse set of viral isolates, and the DENV–YFV assay detected more replicates from those dilutions than the comparator. Similarities in performance between the DENV–YFV assay and comparator rRT-PCR are consistent with the designs of these tests, which both target the same region of the YFV genome.4 For the present study, however, the comparator assay was modified to use 5 μL of eluate in each reaction rather than 2 μL as originally described. This was performed to standardize the eluate volumes used in each test.
The clinical performance of the YFV assay will need to be established using more samples from naturally infected patients, which requires implementation in endemic regions. The present study, however, provides a rigorous evaluation of the assay using mock samples prepared from virus isolated in South America, and West and Central Africa. These samples contained greater strain diversity than would occur in a given outbreak and demonstrate the generalizability of this test for use in different regions. Finally, although quantitated viral loads are rarely reported in YF cases, a broad range of viral loads are expected at the time of presentation, including low values such as that observed in the clinical sample SH 281788 (Supplemental Table 1).9,18 Increased YFV detection in mock samples with low viral loads using the DENV–YFV assay is, therefore, expected to be clinically significant.
Despite control efforts, the number of DENV cases worldwide has continued to increase. Yellow fever virus has also reemerged in certain areas, and recent estimates indicate that 43–52% of the population in YFV-endemic regions still require vaccination.19 Endemic countries are urged to continue efforts aimed at the timely confirmation and treatment of YF cases.5,20 For these reasons, the DENV–YFV assay described here should be a useful tool to narrow the differential diagnosis and provide early case detection.
Supplementary Material
Acknowledgments:
We thank the staff at the National Center for Emerging and Zoonotic Infectious Diseases of the Centers for Disease Control and Prevention, who kindly provided genomic RNA from YFV isolates in the Arbovirus Reference Collection. We thank members of the study team based at the Centro de Salud Sócrates Flores Vivas, the National Virology Laboratory in the Centro Nacional de Diagnóstico y Referencia, and the Sustainable Sciences Institute in Nicaragua for their dedication and high-quality work, and we are grateful to the study participants and their families.
Note: Supplemental text and table appears at www.ajtmh.org.
REFERENCES
- 1.Monath TP, Vasconcelos PF, 2015. Yellow fever. J Clin Virol 64: 160–173. [DOI] [PubMed] [Google Scholar]
- 2.Dexheimer Paploski IA, et al. 2017. Epizootic outbreak of yellow fever virus and risk for human disease in Salvador, Brazil. Ann Intern Med 168: 301–302. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization , 2017. Yellow Fever Outbreak Angola, Democratic Republic of the Congo and Uganda 2016–2017 Geneva, Switzerland: World Health Organization. Available at: http://www.who.int/emergencies/yellow-fever/en/. Accessed January 8, 2018.
- 4.Domingo C, Patel P, Yillah J, Weidmann M, Méndez JA, Nakouné ER, Niedrig M, 2012. Advanced yellow fever virus genome detection in point-of-care facilities and reference laboratories. J Clin Microbiol 50: 4054–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization , 2016. Yellow Fever Geneva, Switzerland: World Health Organization. Available at: http://www.who.int/mediacentre/factsheets/fs100/en/. Accessed April 4, 2017.
- 6.Domingo C, Escadafal C, Rumer L, Mendez JA, Garcia P, Sall AA, Teichmann A, Donoso-Mantke O, Niedrig M, 2012. First international external quality assessment study on molecular and serological methods for yellow fever diagnosis. PLoS One 7: e36291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan American Health Organization , 2017. Laboratory Diagnosis of Yellow Fever Virus Infection. Washington, DC: Pan American Health Organization. [Google Scholar]
- 8.World Health Organization , 2009. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva, Switzerland: WHO Press. [PubMed] [Google Scholar]
- 9.Bae HG, Nitsche A, Teichmann A, Biel SS, Niedrig M, 2003. Detection of yellow fever virus: a comparison of quantitative real-time PCR and plaque assay. J Virol Methods 110: 185–191. [DOI] [PubMed] [Google Scholar]
- 10.Nunes MR, Palacios G, Nunes KN, Casseb SM, Martins LC, Quaresma JA, Savji N, Lipkin WI, Vasconcelos PF, 2011. Evaluation of two molecular methods for the detection of yellow fever virus genome. J Virol Methods 174: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reusken CBEM, Knoester M, GeurtsvanKessel C, Koopmans M, Knapen DG, Bierman WFW, Pas S, 2017. Urine as sample type for molecular diagnosis of natural yellow fever virus infections. J Clin Microbiol 55: 3294–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waggoner JJ, et al. 2013. Development of an internally controlled real-time reverse transcriptase PCR assay for pan-dengue virus detection and comparison of four molecular dengue virus detection assays. J Clin Microbiol 51: 2172–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waggoner JJ, et al. 2014. Multiplex nucleic acid amplification test for diagnosis of dengue fever, malaria, and leptospirosis. J Clin Microbiol 52: 2011–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waggoner JJ, Gresh L, Mohamed-Hadley A, Ballesteros G, Davila MJ, Tellez Y, Sahoo MK, Balmaseda A, Harris E, Pinsky BA, 2016. Single-reaction multiplex reverse transcription PCR for detection of zika, chikungunya, and dengue viruses. Emerg Infect Dis 22: 1295–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burd EM, 2010. Validation of laboratory-developed molecular assays for infectious diseases. Clin Microbiol Rev 23: 550–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuan G, Gordon A, Aviles W, Ortega O, Hammond SN, Elizondo D, Nunez A, Coloma J, Balmaseda A, Harris E, 2009. The Nicaraguan pediatric dengue cohort study: study design, methods, use of information technology, and extension to other infectious diseases. Am J Epidemiol 170: 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waggoner JJ, et al. 2013. Comparison of the FDA-approved CDC DENV-1–4 real-time reverse transcription-PCR with a laboratory-developed assay for dengue virus detection and serotyping. J Clin Microbiol 51: 3418–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colebunders R, et al. 2002. A Belgian traveler who acquired yellow fever in the Gambia. Clin Infect Dis 35: e113–e116. [DOI] [PubMed] [Google Scholar]
- 19.Shearer FM, et al. 2017. Global yellow fever vaccination coverage from 1970 to 2016: an adjusted retrospective analysis. Lancet Infect Dis 17: 1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan American Health Organization/World Health Organization , 2017. Epidemiological Update: Yellow Fever. Washington, DC: PAHO/WHO. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.