Abstract.
Although Plasmodium vivax infections in Malaysia are usually imported, a significant autochthonous outbreak of vivax malaria was detected in a remote indigenous (Orang Asli) settlement located in northern peninsular Malaysia. Between November 2016 and April 2017, 164 cases of P. vivax infection were detected. Although 83.5% of the vivax cases were identified through passive case detection and contact screening during the first 7 weeks, subsequent mass blood screening (combination of rapid diagnostic tests, blood films, and polymerase chain reaction [PCR]) of the entire settlement (N = 3,757) revealed another 27 P. vivax infections, 19 of which were asymptomatic. The mapped data from this active case detection program was used to direct control efforts resulting in the successful control of the outbreak in this region. This report highlights the importance of proactive case surveillance and timely management of malaria control in Malaysia as it nears malaria elimination.
Malaysia is in the elimination phase of malaria control and has stated a commitment to achieve malaria elimination by 2020.1 Although the recent focus on malaria elimination in Malaysia is zoonotic (Plasmodium knowlesi); the continued reintroduction of Plasmodium vivax by foreign or local workers returning from malaria-endemic areas provides a significant cause for concern. This short report describes a P. vivax outbreak (November 16, 2016–December 28, 2016) and the cases thereafter (up till April 20, 2017) detected through proactive case surveillance, at the Orang Asli (Indigenous) Settlement of Pos Kemar, Gerik, Perak, Malaysia (Figure 1). It highlights the successful control of a malaria outbreak through proactive surveillance, swift multi-sectorial cooperation, and use of sensitive molecular diagnostic methods. Of the 164 P. vivax cases reported here, 137 were diagnosed during the outbreak with one isolated Plasmodium falciparum infection. Ethical approval for this study was granted by the Medical Research and Ethics Committee of the Malaysian Ministry of Health (NMRR-16-2840-33769).
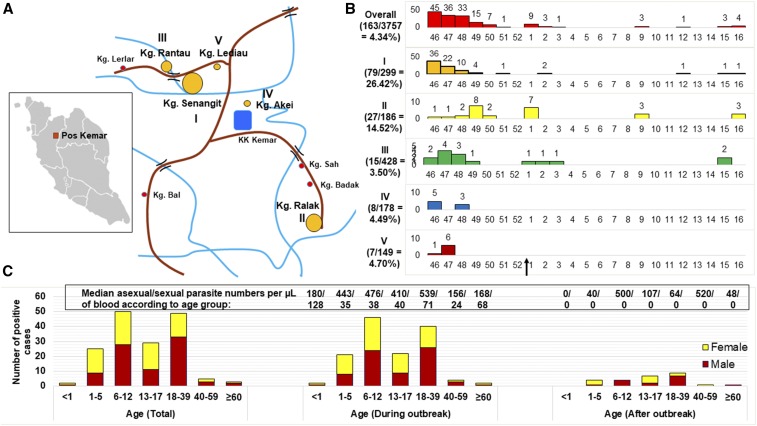
Figure 1.
Demographic data of the outbreak. (A) Map of the Pos Kemar Orang Asli settlement showing the location of the five villages (I–V) with the highest number of cases. The size of the orange circles (big to small) next to the roman numerals denotes the relative number of cases (descending order). (B) Graphs showing the number of cases diagnosed in each week of the year from November 16, 2016 to April 20, 2017 in the five villages (I–V). The attack rate in percentage (number of cases/number of population) is shown on the left of each graph. The arrow on the x-axis at the bottom denotes the end of the outbreak. (C) Breakdown of the cases into age and gender. The bar chart on the left shows the total number of cases from November 16, 2016 to April 20, 2017, whereas the remaining graphs show the total number of cases during and after the outbreak period until April 20, 2017, respectively. The numbers above the bars denote the median asexual parasite number/sexual parasite number per microliters of blood. I: Kampung (Kg.) Senangit; II: Kg. Ralak; III: Kg. Rantau; IV: Kg. Akei; and V: Kg. Lediau. This figure appears in color at www.ajtmh.org.
Since the initiation of the national malaria control program in year 2000 in Malaysia, the number of malaria cases in Hulu Perak, Perak has declined except for localized outbreaks in the Kenering sub-district in 2007 (127 cases).2 Thereafter, only 110 cases were reported from 2008 to 2015. The outbreak site reported here, Pos Kemar is located 85 km from Gerik town, accessible only by four-wheel drives via logging roads from the Siput River or by boat from the Banding Jetty. The livelihood of the indigenous villagers depends mainly on fishery, rubber-tapping, and forest foraging. There are about 18 villages in Pos Kemar, with a total population of 3,757. There is one health clinic in the settlement, whereas the nearest hospital Hospital Gerik is 1 hour 15 minutes away. There were only two previous malaria cases from Pos Kemar, a P. vivax and P. knowlesi infection in 2009 and 2011, respectively.
The malaria outbreak operation was initiated on November 17, 2016 after three confirmed P. vivax cases were detected during an active case detection (screening of febrile individuals at areas with high risk of malaria) by the Hulu Perak district health office on 16 November in Kampung Senangit, Pos Kemar. After that, contact screening (reactive case detection) within 2 km radius from the suspected site of infection was carried out. During the outbreak, positive cases were identified through contact screening and passive case detection (detection of malaria in patients that seek medical care), whereas most cases detected after the outbreak came from mass blood survey (MBS) also known as proactive case detection (screening of all individuals in an area). Malaria parasite infections were diagnosed using a combination of rapid diagnostic tests, blood films, and nested PCR.3 Of the 18 villages, villages with the most number of cases (in descending order) were Kampung (Kg.) Senangit (I), Kg. Ralak (II), Kg. Rantau (III), Kg. Akei (IV), Kg. Lediau (V), and the Kemar Islands, whereas the remaining villages had 0–3 cases (Figure 1A and B). The overall attack rate was 4.34%. Male and female constituted the cases in similar numbers, whereas more than half of the cases occurred among people aged between 6–12 years and 18–39 years, both in similar numbers (Figure 1C).
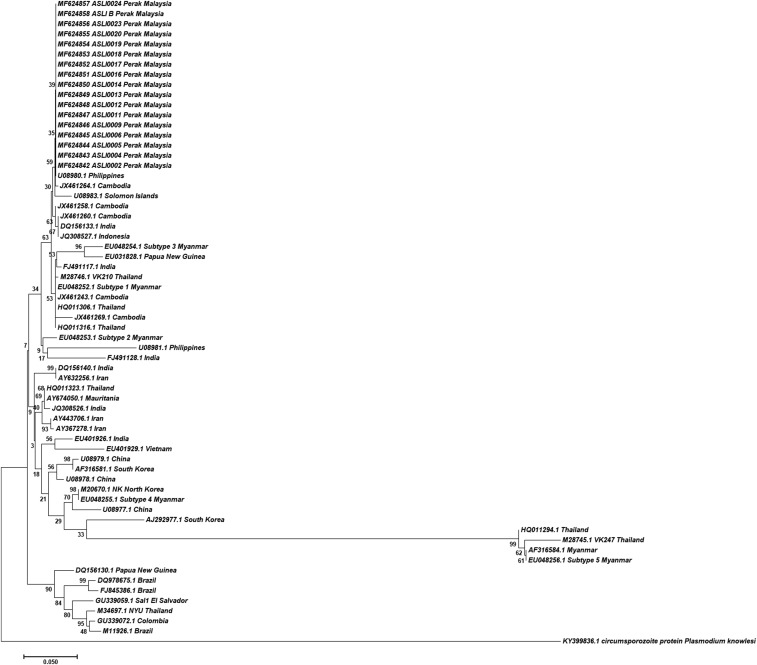
Diagnostic PCR by amplification of the Plasmodium small-subunit rRNA gene4–6 were performed on a number of microscopy-positive P. vivax samples: 25 samples from cases detected on November 19, 2016; eight samples from January 4, 2017, two samples from March 11, 2017, one sample each from March 13, 19, and 23, 2017 (Asli B) and May 7, 2017 (Asli J). Phylogenetic analyses were also performed using the P. vivax circumsporozoite protein7 and Duffy-binding protein genes that were successfully PCR-amplified and sequenced from 17 to 18 samples. These P. vivax isolates are clustered together and are closely related to isolates of Southeast Asian origin (Figures 2 and 3). Eighty-six bloodspots from villagers and 37 from foreigners during a MBS on March 20, 2017 were also received. Four of the 123 samples were PCR-positive for P. vivax. These individuals, who were asymptomatic and microscopy-negative, were subsequently treated.
Figure 2.
Phylogenetic tree of P. vivax circumsporozoite protein genes. The neighbor-joining trees were constructed using nucleotide sequences of the protein, using the Jukes–Cantor model (bootstrap value = 1,000).
Figure 3.
Phylogenetic tree of P. vivax Duffy-binding protein genes. The neighbor-joining trees were constructed using nucleotide sequences of the protein, using the Jukes–Cantor model (bootstrap value = 1,000).
The outbreak ended on December 28, 2016. The directly observed therapy of Riamet (artemether/lumefantrine) and primaquine3 was administered to all malaria-positive individuals. Most cases identified during contact screening and passive case detection were treated within 1–3 days after the collection of thin blood smears. Whereas, the turnaround time from blood smear collection to treatment during MBS was 1–3 weeks. All individuals were treated by April 20, 2017 except for one who was treated a few weeks later because of uncooperativeness. Overall, 89.0% of the villagers in Pos Kemar were screened during two rounds of MBS/active case detection (November 17, 2016–December 20, 2016; December 27, 2016–December 30, 2016), followed by weekly screening in January and subsequent monthly screening. The health authorities have taken continuous measures to screen (active case detection and MBS) and follow up on cases and relapses in Pos Kemar with the hope of detecting the next possible case and breaking the transmission cycle. Preemptive measures have also been put in place to screen nearby settlements but as of now, the cases are confined to Pos Kemar itself.
Multiple modalities of vector control, that is, residual spraying, larvaciding, and distribution of insecticide-treated nets (ITNs) and insect repellants were implemented during the outbreak.8 Although larvae of Anopheles maculatus, Anopheles barbirostris, Anopheles hyrcanus, and Anopheles aitkenii were identified, no adult mosquito was successfully caught in Kg. Senangit and Kg. Badak. Entomological surveys were hampered by a lack of manpower and unfavorable weather. However, in June 2017, using an animal-baited trap, an adult Anopheles umbrosus was caught near Kg. Bal and was positive for P. knowlesi in the midgut and P. vivax in the salivary glands. Ninety-eight percent of the households were protected with ITNs with good compliance.
Hitherto, the sources of the infections were not successfully identified. Nonetheless, based on the phylogeny constructed in this study and the abundant indigenous vectors in this forested area, the cases were believed to be autochthonous.9 However, the possibility that foreign workers in a nearby construction site or villagers with travel histories reintroduced this outbreak cannot be ruled out.10 The first three individuals positive for vivax malaria at the beginning of the outbreak had traveled to Kg. Podek, in the neighboring state, Kelantan. At the same time, the villagers had travel histories from village to village within Pos Kemar. It should be noted that, although efforts to screen the foreign workers using microscopy failed to detect infections,11 the use of PCR detected P. vivax in one migrant worker from Myanmar.
The rapid containment of the outbreak is largely attributed to the efficient multi-sectorial effort involving the Malaysian health authorities, the army, the public health laboratories, research institutions, Department of Orang Asli Development, and many others. The identification of the outbreak is due in large part to the continual vigilance by the Malaysian authorities through proactive screening and monitoring of population and places at higher risks of malaria.12 Indeed, it has been reported that an increase in cases occurs after improved quality of malaria control and detection, which paradoxically points to progress toward the elimination of malaria.13 However, it appears that there is still an underappreciated burden exacted by P. vivax, despite the well-founded concern for zoonotic P. knowlesi, the major cause of malaria in Malaysia.14 Vivax infection is a relapsing infection, largely asymptomatic, and sub-patent, with a considerable submicroscopic reservoir which remains undetected. Thus, the more sensitive molecular techniques for diagnosis of malaria have been proven to be valuable in cases such as this. Moreover, it has been reported that rapid diagnostic tests and microscopy failed to detect three-quarter of participants with malaria parasites.15 The use of large volume blood DNA extraction (> 1 mL) has been extremely effective in uncovering hidden reservoirs of sub-patent, asymptomatic P. vivax infections in other areas of southeast Asia.16 Certainly, these tests should ideally be rapid to deliver on-the-spot diagnosis, especially in remote settings such as the one reported here. Hence, it is of utmost importance that the development of high-throughput, ultrasensitive, point-of-care molecular diagnostic techniques underpin continued efforts to eliminate malaria from Malaysia.
Acknowledgments:
We sincerely thank all the staff from the state and district health offices for their commitment to the continuous effort to control the outbreak.
REFERENCES
- 1.World Health Organization , 2016. World Malaria Report 2016. Geneva, Swizterland: WHO. [Google Scholar]
- 2.Yoep N, Hasim H, Yusoff UN, Yusoff M, Mahpot NR, 2015. Spatio-temporal distribution of malaria in Perak, Malaysia. Adv Infect Dis 5: 154–161. [Google Scholar]
- 3.Ministry of Health Malaysia , 2013. Management Guidelines of Malaria in Malaysia. Kuala Lumpur, Malaysia: Vector Borne Disease Sector, Disease Control Division. [Google Scholar]
- 4.Snounou G, Singh B, 2002. Nested PCR analysis of Plasmodium parasites. Methods Mol Med 72: 189–203. [DOI] [PubMed] [Google Scholar]
- 5.Imwong M, Tanomsing N, Pukrittayakamee S, Day NP, White NJ, Snounou G, 2009. Spurious amplification of a Plasmodium vivax small-subunit RNA gene by use of primers currently used to detect P. knowlesi. J Clin Microbiol 47: 4173–4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuehrer HP, Stadler MT, Buczolich K, Bloeschl I, Noedl H, 2012. Two techniques for simultaneous identification of Plasmodium ovale curtisi and Plasmodium ovale wallikeri by use of the small-subunit rRNA gene. J Clin Microbiol 50: 4100–4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alves RT, Povoa MM, Goldman IF, Cavasini CE, Rossit AR, Machado RL, 2007. A new polymerase chain reaction/restriction fragment length polymorphism protocol for Plasmodium vivax circumsporozoite protein genotype (VK210, VK247, and P. vivax-like) determination. Diagn Microbiol Infect Dis 59: 415–419. [DOI] [PubMed] [Google Scholar]
- 8.Abdullah NH, 2016. Kejadian Wabak malaria di Pos Kemar, Hulu Perak. Kuala Lumpur, Malaysia: Director-General of Health Malaysia, Ministry of Health Malaysia. [Google Scholar]
- 9.Romi R, Boccolini D, Menegon M, Rezza G, 2012. Probable autochthonous introduced malaria cases in Italy in 2009–2011 and the risk of local vector-borne transmission. Euro Surveill 17: 20325. [PubMed] [Google Scholar]
- 10.The Star Online , 2017. Mah: Outsiders May Have Caused Malaria Outbreak Available at: https://www.thestar.com.my/news/nation/2017/01/04/mah-outsiders-may-have-caused-malaria-outbreak/. Accessed July 24, 2017.
- 11.Khoo VJ, 2016. New Malaria Outbreak in Perak Caused by Different Strain Available at: https://today.mims.com/topic/new-malaria-outbreak-in-perak-caused-by-different-strain. Accessed July 24, 2017.
- 12.Wen S, et al. 2016. Targeting populations at higher risk for malaria: a survey of national malaria elimination programmes in the Asia Pacific. Malar J 15: 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen R, Cardona JS, Navarro ES, Padilla N, Reyes L, Villar RJP, Masuoka P, Bernart C, Peruski LF, Bryan JP, 2017. Outbreak investigation of Plasmodium vivax malaria in a region of Guatemala targeted for malaria elimination. Am J Trop Med Hyg 96: 819–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barber BE, Rajahram GS, Grigg MJ, William T, Anstey NM, 2017. World malaria report: time to acknowledge Plasmodium knowlesi malaria. Malar J 16: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imwong M, et al. 2015. The epidemiology of subclinical malaria infections in south-east Asia: findings from cross-sectional surveys in Thailand–Myanmar border areas, Cambodia, and Vietnam. Malar J 14: 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imwong M, Hanchana S, Malleret B, Renia L, Day NP, Dondorp A, Nosten F, Snounou G, White NJ, 2014. High-throughput ultrasensitive molecular techniques for quantifying low-density malaria parasitemias. J Clin Microbiol 52: 3303–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]