Abstract.
Infection of the brain with Taenia solium larvae (neurocysticercosis) is a leading cause of preventable epilepsy worldwide. Effective and sustainable strategies to control parasite transmission in rural endemic communities are needed to prevent the disease. Surveillance and targeted intervention around infected pigs (ring control strategy) have been shown to be effective when carried out by research teams. However, this strategy has not been implemented or tested as a community-based program. In this small trial in northern Peru, eight villages were randomly assigned to community-led surveillance and treatment (five villages, 997 residents) or control (three villages, 1,192 residents). In intervention villages, community-led surveillance and reporting were promoted by community health workers, radio advertisement, and school and household education. Each suspected pig infection was verified, with confirmed cases resulting in treatment with niclosamide for taeniasis and oxfendazole for pigs in clusters of homes nearby. No incentives beyond human and pig treatment were offered. Control villages received basic disease education but no treatment intervention in response to reports. Despite 14 case reports, community-based replication of ring control strategy did not replicate prior results. After 12 months, there was no change in seroincidence in intervention villages between the baseline and study end, and no difference compared with control villages. There was no difference in prevalence of taeniasis or porcine cysticercosis at study end. Community members described lack of knowledge as the main reason for not reporting infected pigs. Further exploration of methods to transfer ring strategy and other control interventions for cysticercosis to the community is needed.
INTRODUCTION
Pork tapeworm (Taenia solium) infection is a leading cause of preventable epilepsy in low- and middle-income countries.1 Humans are the definitive host of this zoonosis, harboring the adult tapeworm in the small intestine, a condition known as taeniasis. Both humans and pigs can develop cysticercosis, a soft tissue infection with larval stage T. solium. When T. solium infects the human brain, it is known as neurocysticercosis (NCC), a condition that can manifest in seizures, headache, and other neurologic syndromes.2 In Latin America alone, an estimated 400,000–1.35 million people have seizure disorders attributable to NCC.3,4
Control interventions aimed at interrupting T. solium transmission include mass screening and/or treatment of humans for taeniasis,5–8 combined mass treatment of humans for taeniasis and pigs for cysticercosis,9,10 targeted treatment of humans,11 vaccination of pigs,12–14 improved sanitation,15 meat inspection,16 and health education.17–22 However, there is still little understanding of how these interventions can be implemented effectively and sustainably in impoverished rural areas where T. solium is endemic. In a recent study, we tested an approach based on case detection and targeted intervention (ring control strategy) as an alternative to mass treatment, resulting in a > 40% reduction in transmission over 1 year when research teams carried out the intervention.11 It is unclear whether this approach will be effective if implemented as a community-based program in which communities themselves assume the role for surveillance and treatment response.
The objective of this study was to pilot a community-based program in which the surveillance and response activities were passed to the communities. Under this program, villagers screened for and reported infected pigs to the local health post, which in turn provided screening and treatment of human taeniasis and porcine cysticercosis in homes within 100 m of the reported pig as the response. We conducted a health education campaign to encourage community-generated surveillance and reporting, trained community health workers to collect reports, and worked with the local health posts to establish protocols for screening and treatment response. Because of the low number of reports, a secondary objective of this study was added to define and describe community-perceived barriers to reporting.
MATERIALS AND METHODS
Study design.
This was a small prospective trial in which eight villages were randomly selected to receive the intervention (N = 5) or control (N = 3) for 12 months, with a 4-month lead in time to measure baseline incidence. Household- and school-based education about the parasite life cycle and methods to prevent infection were offered in all villages. A local surveillance and response system was established in intervention villages along with a campaign to promote reporting of infected pigs. No surveillance system was established or promoted in the control villages. The primary study outcome was porcine seroincidence measured every 4 months; secondary outcomes included the prevalence of porcine cysticercosis and human taeniasis at study end. A survey to identify barriers to report was applied 8 months into the study after suboptimal reporting was observed.
Study site and participants.
The study was conducted in Piura, a province in northern Peru, where T. solium remains endemic. The region is arid much of the year with the exception of a 3-month rainy season. Villagers commonly raise small number of pigs as a source of income and as a highly desired source of meat. Pigs are commonly allowed to roam outside of corrals throughout the village to forage. Latrine coverage across study villages at baseline was 67.5%. The average education level among adults is sixth grade. We selected eight eligible study villages in the same region based on their similarity of size, terrain, and pig-rearing practices. The eight villages were randomly assigned to the intervention or control group using a computer algorithm. The algorithm was repeated until the human population in both groups was approximately equal (within 10% of the total study population divided by 2). The closest distance between any two villages with opposing assignment was 5 km, a distance deemed sufficient to adequately limit any risk of contamination. One village had a health post in the community, whereas the remainder had a health post within 3–15 km. Each village had one or more community health workers who were involved and trained to assist in reporting cases to the health post. None of the villages had existing strategies for T. solium control in place. The interest of village leaders and other stakeholders was also considered in village selection. No previous cysticercosis control studies had been conducted in the study area. All residents of the study communities were invited to participate in the study. Children younger than 2 years of age were included in the census but excluded from the screening and treatment activities.
Enrollment, census, and mapping.
Enrollment of participants was accomplished by visits to all households in study communities. Household characteristics including occupancy, infrastructure (latrines, electricity, and water), and animal-rearing practices were collected using a structured questionnaire, as were individual-level characteristics including age, sex, and education-level. Latitude and longitude coordinates of each house were collected using global positioning system receivers (GeoExplorer II; Trimble, Sunnyvale, CA) and postprocessed using differential correction for submeter accuracy. ArcMAP 10 GIS software (ESRI, Redlands, CA) was used to map each village, and 100-m buffers were created for each household to define neighboring households that would be included in any subsequent interventions resulting from disease reports.
Education activities in all study villages.
On enrollment, all study households, regardless of study arm, were provided with a 15- to 20-minute basic educational talk about the T. solium life cycle and prevention methods. Cartoon representations of life cycle, pig tongue examination, and prevention methods were developed with the help of community members and used as visual aids during the educational sessions (see Supplemental Figure). School-based education directed at all elementary through high school students was also provided in two 60-minute lessons which focused on modes of transmission and methods of prevention using games, homework, stories, and lectures. Additional information regarding surveillance and reporting, detailed in the following paragraphs, was provided in schools in the intervention villages only.
Establishment and promotion of surveillance system in the intervention villages.
In intervention communities only, community leaders, key actors (political and religious authorities, community health workers, pig farmers, and vendors), and the Ministry of Health were given detailed information about the study in two educational sessions. The surveillance system was setup within the existing health infrastructure and organization through consultation with these stakeholders. The study team collaborated with health posts and community health workers throughout the intervention.
Surveillance for infected pigs was carried out by village residents using two techniques: 1) pig tongue inspection during intermittent checks by owner or at the time of purchase of a live pig and 2) careful inspection of meat after slaughter for visible cysts. Typically, pig traders carry out tongue inspection with pig owners at the point of sale. Traders within the community were identified and visited in their homes to explain the intervention, including how to report a case to health post or community health workers. Community health workers from each village were trained to receive reports of pig infection or contaminated pork, to document photographic evidence of the infection whenever possible, and to communicate case reports to the local health posts. When photographic confirmation was not possible, the health post worker spoke with the reporting individual or community health worker to verify that the community-identified tissue lesion matched the description of T. solium cysts. The study team was present to support health post staff in verifying case reports. Professionals at the health posts were trained to investigate and report confirmed cases to the regional Ministry of Health Office of Epidemiology, which would then trigger the treatment response. The regional Office of Epidemiology added taeniasis/cysticercosis to their monthly communicable disease reports.
Promotion of villager participation in surveillance and reporting was carried out through door-to-door visits, initially by study staff and subsequently by community health workers. Tongue examination (palpation of the pig’s tongue for cysts) was reviewed and recommended as a preferred way to detect heavily infected pigs before the time of slaughter, as treatment of these pigs would be provided in the response which could potentially preserve the value of the animal. Inspection of meat after slaughter was encouraged as an additional form of surveillance, although it was stressed that the value of the meat at this point could not be recuperated. Villagers were instructed on how to report porcine cysticercosis cases to the community health workers or directly to the local health post.
Community training workshops on surveillance methods (inspecting pig tongues for cysts; identifying infected meat) were held at the beginning of the study. Ongoing promotion and diffusion of the intervention were carried out with community-wide distribution of informative material (calendars, posters, brochures, and key chains) and radio spots explaining how to identify and report infected pigs (See Supplemental Figure).
Response to confirmed reports.
The local health post was responsible for carrying out the treatment response for all reported cases of porcine cysticercosis and for providing final reports to the Office of Epidemiology. Given staffing shortage and turnover of personnel, the local health posts requested the study staff to assist with providing treatment. The treatment response involved identifying all households located within 100 m (rings) of the house where the reported pig was raised, and provision of presumptive treatment of taeniasis to all residents of these homes, as well as treatment of porcine cysticercosis for all pigs raised by these households. Treatment of taeniasis was offered twice (days 1 and 14) at a standard dose of oral niclosamide by weight (1 g for 1–34 kg; 1.5 g for 35–50 kg; and 2 g for > 50 kg, per dose). Two doses of niclosamide were administered to decrease treatment failure among participants treated during targeted response within a ring.23,24 All pigs within the radius were treated with oxfendazole (30 mg/kg, live weight) in a single oral drench dose, and residents were instructed not to slaughter these animals for at least 3 weeks after treatment. Adverse effects of niclosamide and oxfendazole were monitored through door-to-door follow-up 24 hours after administration.
In control communities, spontaneous case reports were met with the standard-of-care recommendation to eliminate infected meat by incineration or burial to prevent human consumption. No treatment was offered for pigs or humans. No additional education was provided.
Periodic serosurveys to measure porcine seroincidence.
The primary outcome measure was seroincidence of antibodies against T. solium cysticercosis among pigs born during the intervention. Study veterinary teams went door-to-door every 4 months to capture all pigs in both intervention and control villages, to place an ear tag with unique identifier, and to collect a blood sample. Pigs aged 2–4 months were entered into a cohort and seronegative animals or those becoming seronegative (assumedly by loss of maternal antibodies) were followed serologically using lentil-lectin glycoprotein electroimmunotransfer blot (LLGP EITB) until they seroconverted to positive or were lost to follow-up. Seropositivity was defined as reactivity to any of the glycoprotein antigens present in the LLGP EITB (GP42, GP24, GP21, GP18, GP14, and GP13) with the exception of the larger molecular weight GP50 band. The GP50 band was not considered, given recent studies suggesting cross-reactivity with Taenia hydatigena, which is endemic to this region.25
Mass screening for taeniasis at study end.
The prevalence of taeniasis at the end of the study was a secondary outcome. Study staff went door-to-door to offer mass treatment with a single oral dose of niclosamide to all residents aged 2 years or older and instructed them in hygienic collection of the first posttreatment stool. We left a 500-mL plastic container with a secure lid with each participant and returned within 24 hours to collect the sample. We visually examined whole stools for intact worms or segments and collected 10 mL aliquots of stool in 40 mL of 5% formol-phosphate–buffered saline. These samples were processed by enzyme-linked immunosorbent assay (ELISA) to detect Taenia sp. coproantigens as previously described.26 Samples with an optical density (OD) ratio of ≥ 20, defined as the OD of the sample divided by the OD of a strong positive T. solium control, were considered to be positive. In positive samples only, we also evaluated the sample for the presence of Taenia sp. eggs using rapid sedimentation and light microscopy. All participants who tested positive for taeniasis were followed by repeat stool screening and treatment as needed until cured.23
Culling and necropsy of seropositive pigs at study end.
The prevalence of porcine cysticercosis was also determined at the end of the study as an additional outcome measure. Using the result of the final serosurvey, we returned to all villages and offered to purchase seropositive pigs regardless of cohort and treatment status. Purchased pigs were transported to the animal facilities at the Center for Global Health, Tumbes, where they were anesthetized and humanely euthanized, then dissected in entirety using fine slices of < 3 mm. Further details of the necropsy methods can be found elsewhere.27
Barriers to report survey.
After 8 months of intervention, in response to few reports of infected pigs, a 27-question survey was administered to a random sample of 30% of households (N = 166/568) from both intervention and control communities in an attempt to better understand perceived barriers to reporting. Detailed demographic characteristics including family makeup and wealth measures, along with assessment of risk factors (open-defecation practices) and porcine cysticercosis surveillance practices (tongue examination and other techniques) were collected. The final portion of the survey inquired about surveillance practices and observed cases of the disease over the intervention period and perceived barriers to surveillance and report of detected cases.
Statistical methods.
We analyzed all data in STATA SE14 (StataCorp, College Station, TX). We used χ2 tests to compare distributions of proportions between pairs of categorical measures. We used t tests to compare means of continuous variables. All tests were two-sided with significance set at 0.05. We used Poisson family Generalized Estimating Equations with a log link and robust sandwich-type errors to estimate population-averaged seroincidence among pigs while accounting for the effect of intra-household clustering. We used quasi-likelihood information criteria to select the working correlation structure and the variables to include in the final model.28,29 The outcome variable was the count of pig seroconversions to positive aggregated by house and sampling period, and stratified by covariates including intervention group, pig age in months, and presence of latrine. The offset variable for analysis was the log of the total observed pig-months per strata. We report population-averaged seroincidence as the number of new seroconversions per 100 pigs per month during each 4-month sampling interval. We report unadjusted prevalence of human taeniasis and porcine cysticercosis at study end.
Ethics statement.
This study was reviewed and approved by the Institutional Review Boards at the Universidad Peruana Cayetano Heredia (UPCH) and at Oregon Health & Science University (OHSU). All adult participants provided written informed consent. Written informed consent from a parent or guardian was required for participation of minors. The study was reviewed and approved by the Institutional Ethics Committee for the Use of Animals at UPCH and the Institutional Animal Use and Care Committee at OHSU. Treatment of animals adhered to the Council for International Organizations of Medical Sciences International Guiding Principles for Biomedical Research Involving Animals.
RESULTS
Village characteristics and census data.
The study population included 2,189 people, with 997 (45.6%) in five intervention communities and 1,192 (54.4%) in three control communities. Thirty-seven children were ineligible for taeniasis screening based on age criteria (younger than 2 years). Population characteristics were similar between intervention and control villages with the exception of a greater average number of pigs raised by pig owners (4.5 versus 3.5) and fewer homes with electricity (80.6% versus 93.6%) in intervention communities (Table 1).
Table 1.
Characteristics of intervention and control villages, Piura, Peru
| Intervention | Control | |
|---|---|---|
| Villages, no. | 5 | 3 |
| Residents, no. | 997 | 1,192 |
| Households, no. | 266 | 302 |
| Single family dwelling, no. (%) | 223 (86.4) | 249 (88.6) |
| Houses with latrines, no. (%) | 167 (64.7) | 164 (58.4) |
| Houses with treated water, no. (%) | 166 (63.9) | 183 (60.8) |
| Houses with electricity, no. (%)* | 208 (80.6) | 263 (93.6) |
| Houses raising pigs, no. (%) | 176 (66.2) | 185 (61.3) |
| Corral present on property, n (%) | 87 (49.3) | 95 (51.4) |
| Mean no. of pigs raised (SD)* | 4.4 (4.4) | 3.5 (3.4) |
| Mean no. of residents per house (SD) | 3.7 (2.0) | 3.9 (1.9) |
| Mean no. of rooms per house (SD) | 3.5 (1.5) | 3.4 (1.6) |
SD = standard deviation.
P < 0.05.
Reports of infected meat and treatment response.
During the 12-month study period, intervention community members reported 14 cases of infected meat to the health post. By comparison, control community members reported five cases of infected meat over the same time. Of the 19 reports, 16 were prompted by pig tongue inspection; six by pig traders, six by pig owners, and four by other residents who intended to purchase the pigs. Two reports were made by pig owners after identification of infected meat after slaughter and one was made by the owner of a local slaughter house. In control communities, most of the case reports occurred at the study beginning compared with intervention communities where reports were evenly distributed throughout the duration of the study.
In the study, 376 humans (80.1% of all eligible individuals; 33.3% of the entire population in intervention villages) received at least one dose of niclosamide and 238 pigs (72.1% of eligible animals; 38% of all pigs) received treatment with oxfendazole. No humans or pigs received treatment in the control village (Table 2).
Table 2.
Coverage of human and pig treatment within 100-m rings around reported pigs
| Eligible houses (no.) | Eligible humans (no.) | Received one dose no. (%) | Received two doses no. (%) | Eligible pigs (no.) | Received one dose no. (%) |
|---|---|---|---|---|---|
| 8 | 31 | 24 (77.4) | 22 (71.0) | 33 | 24 (72.7) |
| 13 | 47 | 41 (87.2) | 37 (76.6) | 43 | 37 (86.0) |
| 11 | 43 | 35 (81.4) | 34 (79.1) | 24 | 18 (75.0) |
| 27 | 91 | 71 (75.0) | 62 (68.1) | 49 | 37 (75.5) |
| 18 | 32 | 28 (87.5) | 22 (69.0) | 27 | 19 (70.3) |
| 3 | 14 | 11 (78.6) | 8 (57.1) | 9 | 7 (77.6) |
| 5 | 18 | 17 (94.4) | 11 (61.1) | 26 | 18 (69.2) |
| 6 | 27 | 18 (66.7) | 16 (59.3) | 12 | 7 (58.3) |
| 8 | 26 | 22 (84.6) | 18 (60.2) | 27 | 21 (77.8) |
| 12 | 45 | 33 (68.8) | 27 (56.3) | 22 | 20 (90.9) |
| 7 | 24 | 15 (62.5) | 15 (62.3) | 9 | 7 (77.8) |
| 2 | 7 | 7 (100) | 7 (100) | 18 | 11 (61.1) |
| 8 | 42 | 36 (85.7) | 36 (85.7) | 24 | 7 (29.2) |
| 5 | 19 | 18 (94.7) | 18 (94.7) | 7 | 5 (71.4) |
| Total | 469 | 376 (80.1) | 332 (70.1) | 330 | 238 (72.1) |
Seroincidence of T. solium antibodies in pigs.
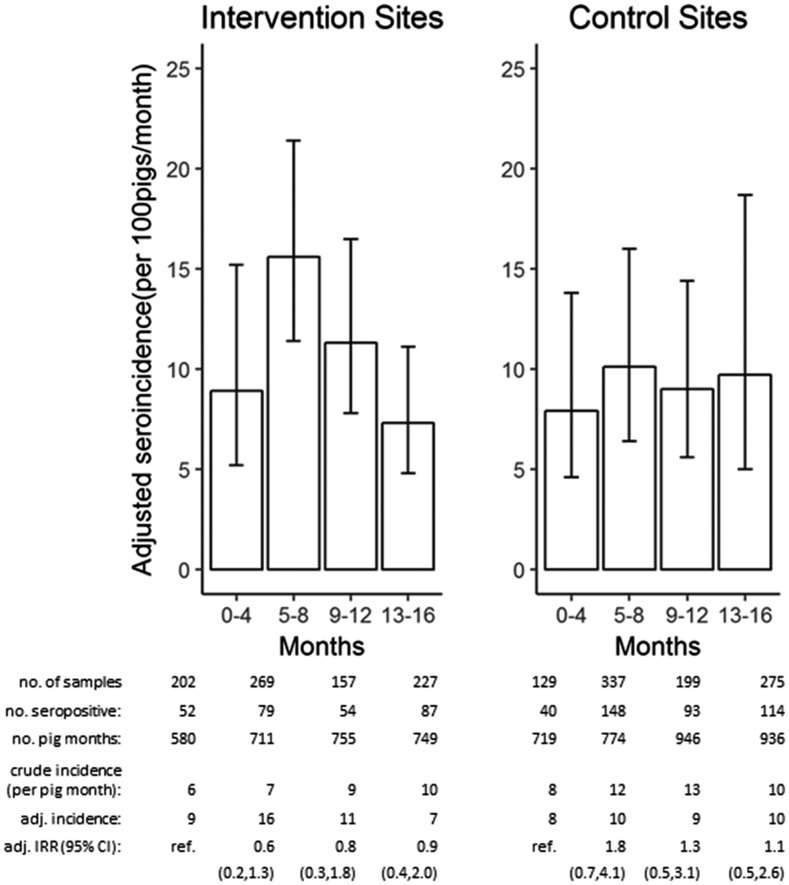
There were 1,250 pigs in the cohort including 615 (49.2%) in the intervention villages and 635 (50.8%) in the control. These pigs generated a total of 1,795 serum samples over 12 months; 788 (63.0%) pigs had one sample, 379 (30.3%) had two samples, and 83 (6.6%) had three samples. One hundred and sixty-two samples were censored based on the pig previously seroconverting to positive, leaving a total of 1,633 samples for incidence calculations. The population-averaged seroincidence at the baseline of the study sample was 8.9% per month (95% confidence interval [CI]: 5.2–15.2) in intervention communities and 7.9% (95% CI: 4.6–13.8) in control communities. There was no difference in the seroincidence over time in the intervention versus control villages (Figure 1).
Figure 1.
Population-averaged seroincidence among 100 unexposed (at risk) pigs per month in intervention villages compared with control villages. Error bars represent 95% confidence interval (CI). Accompanying table shows number of total samples and seropositive samples per round, total number of pig-months contributed, crude and population-averaged incidence, and the incidence rate ratio (IRR) and 95% CI for each time point relative to baseline in both intervention and control groups.
Porcine cysticercosis and human taeniasis at study end.
There was no significant difference in the prevalence of porcine cysticercosis or human taeniasis between control and intervention groups at study end. Villagers were reluctant to sell their pigs for necropsy based on serologic results; only 158/433 (36%) total seropositive pigs were purchased for necropsy including 80 (50.6%) from the intervention villages, of which 22/80 (27.5%) were infected with live, viable cysts, and 78 (49.4%) from the control, of which 27/78 (34.6%) were infected (P = 0.3). Detailed results of the necropsy, including cyst burden and type, have been reported elsewhere.27 Posttreatment stool samples were collected from a total of 1,509/1,948 (77.5%) participants over 2 years of age, with equal participation in both intervention (678/875, 77.4%) and control (831/1,073, 77.4%) villages. There were 15/831 (1.8%) cases of taeniasis in the intervention villages and 19/678 (2.8%) in the control (P = 0.2). Of the 34 total cases of taeniasis identified by coproantigen ELISA, 21 (61.8%) had Taenia sp. eggs present in the stool.
Barrier analysis.
The barrier analysis survey administered in month eight of the intervention was collected from 166 randomly selected households across all communities. Most (125/166, 75%) respondents were female. Most (116/166, 70%) households raised pigs and 41/116 (35%) of surveyed houses with pigs reported having a pig with cysts that they did not report during the study period, with a significant difference (P = 0.001) in the proportion of those who lived in intervention communities (9/41, 22%) and those who lived in control communities (32/41, 78%). Among those who did not report a known infected pig, the most commonly stated barrier was lack of knowledge of how to report a case (N = 5; 55% intervention; N = 11; 34% control), followed by general lack of knowledge of the intervention (N = 2; 22% intervention; N = 7; 22% control). Less commonly stated barriers included perceived lack of importance of reporting a case (N = 1; 11% intervention; N = 4; 12.5%; control) and fear of reporting (N = 1; 11% intervention; N = 4; 12.5% control). Poor access to health posts was also stated once (N = 2; 6% control). Men more commonly stated lack of technical knowledge (pig tongue examination) as a barrier to report, whereas women more commonly stated lack of motivation as a barrier across all communities.
Among those surveyed in all communities, pig owners who did not check their pig’s tongues as a form of active disease surveillance (N = 50) most commonly stated a lack of knowledge of how to inspect tongues (N = 18; 36%) as a barrier to surveillance. In addition, many respondents stated more generally that they “didn’t know” (N = 23; 46%). Lack of motivation (N = 7; 14%) and fear (N = 2; 4%) were also stated barriers.
When asked what should be done to increase the number of reports in the community, 74% (N = 123) of respondents stated that they believed more education would improve reporting outcomes. The remaining respondents suggested that municipal ordinances mandating report (N = 40; 24%) and replacement of infected pigs with uninfected pigs (N = 3; 2%) would help motivate report at the community level.
DISCUSSION
In this pilot study, community-led surveillance and reporting did not reduce transmission of T. solium in endemic communities in contrast to previous results from a research team–led intervention study.11 Despite more reports of positive pigs, there was no change in the seroincidence of cysticercosis among pigs over 1 year in the intervention communities and no difference in seroincidence between intervention and control groups. Furthermore, there was also no significant difference in the final prevalence of taeniasis or porcine cysticercosis between study groups. This outcome suggests that transfer of ring strategy to the community will require different approaches to education and participation, as well as interventions that simultaneously address community-identified barriers to reporting. The barrier analysis applied during the study sheds light on numerous factors which resulted in suboptimal community report. Collectively, these findings can inform the development of future approaches to improve the likelihood of resident participation in surveillance and reporting.
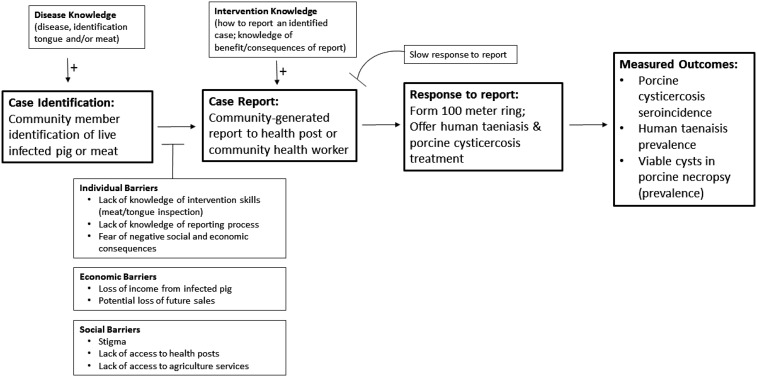
A comparable study that investigated the effectiveness of ring strategy where case detection was carried out by research teams that actively surveyed the live pig population every 4 months found 34 cases (30 tongue positive; four slaughter positive) over a 1-year period in a demographically comparable population to the intervention communities. In that study, subsequent screening and treatment of taeniasis in neighboring homes resulted in the diagnosis and treatment of 35 people with taeniasis. Overall, the result was a 41% reduction in seroincidence and significantly lower prevalence of taeniasis in intervention communities compared with control communities after 1 year. The null effect in the community-led intervention reported here is likely attributable to a lack of reporting (only 14 reports), which resulted in insufficient application of antiparasitic treatment to noticeably affect overall transmission. The barrier analysis conducted late in the study indicated that there were infected pigs detected in the community but not reported, suggesting that a number of barriers between detection and reporting could have diminished the level of disease control (Figure 2). In addition, relatively low participation of both humans and pigs eligible to receive treatment during formed rings could have diminished the effectiveness of ring strategy in this setting.
Figure 2.
Conceptual model for steps leading to report, including community-described barriers to report and participation, based on community responses to barrier analysis and study evaluation.
The key community-described barriers to surveillance and case report, lack of knowledge and the social and economic disincentives to case report, are outlined in a conceptual model generated based on community responses shared in the barrier analysis survey (Figure 2). Most of the adults stated lack of knowledge of the intervention, which suggests that the chosen educational activities were ineffective or poorly targeted. Disproportionate focus on school-based strategies may have incorrectly assumed that child-to-parent diffusion of knowledge would be sufficient to result in adult behavior change of surveillance and reporting practices. More attention to direct adult education and surveillance skills building (tongue and meat inspection) is likely necessary to increase both surveillance and subsequent reporting. Moreover, clearer consistent messaging around how to report a case and the treatment benefits of reporting was needed.
The negative economic consequences and accompanying fear and stigma associated with identifying an infected pig were additional factors that likely limited reporting. In the study region, people raise free-roaming pigs to save money for unanticipated costs. The loss of a pig to cysticercosis infection causes substantial immediate economic losses to the owner (up to 75% of the monthly family income) in the precise moment the income is most needed (Brian Garvey, Personal communication). Moreover, reporting infected meat poses the potential risk of future loss of pig sales because of decreased interest from pig buyers, who are also negatively impacted by purchasing pigs that turn out to be infected. Many individuals either elect to sell the pig at reduced prices to recoup a portion of lost revenue, consume the meat within the household, or discretely dispose the meat to minimize loss of future sale opportunities. Although the intervention sought to minimize disease stigma through educational activities and to normalize reporting, the intervention may not have balanced or mitigated the various economic disincentives of pig report.
This pilot study also revealed aspects of institutional-level coordination that are necessary for effective, sustainable implementation of the ring strategy at the community level. The timely response to a report of an infected pig requires parallel action by different institutions: human taeniasis treatment (led by the Ministry of Health) and porcine cysticercosis treatment (led by the Ministry of Agriculture). Particularly, in the setting of limited human and financial resources with high turnover of personnel, achieving a timely dual-institutional response was a consistent challenge and an area of frustration for local residents. A timely response is critical: when achieved, it acts as a positive reinforcement for community report, given all the disincentives to report; when not achieved, it acts to foster and perpetuate distrust in institutions, creating disincentives to future reporting. Thus, well-coordinated and consistent institutional responses to report are essential for future development and implementation of sustainable community-led strategies.
There are a number of important limitations of this study which should be considered in interpreting and generalizing results. First, this was a small pilot study conducted in a single region of rural Peru. The cultural context and community structure are likely to play an important role in participation and acceptance of this community-level intervention, so we cannot rule out a different outcome had we conducted the study elsewhere. Second, the study did not incorporate knowledge, attitude, or practice metrics or other measures of cognitive behavioral change; thus, there was no measurement of baseline or intermediate educational effects.30 The study was only designed to detect changes in disease transmission and did not detect intermediate changes that may have allowed better direction of the education during the intervention. Finally, it is possible that a limited follow-up period of 12 months was not sufficient to accurately capture changes in disease transmission or intermediate processes of behavioral change.
Current evidence suggests that taeniasis/cysticercosis caused by T. solium is potentially eliminable24 and widespread control interventions are unlikely to be sustainable without active community participation. The data here presented suggest that transferring intervention responsibilities to the population is not easy and is subject to multiple barriers. As such, there is a need for further exploration of methods of transferring ring strategy and other control interventions for cysticercosis to the community. Adult engagement through education and capacity building should be explored as methods to mitigate the barriers to knowledge, low participation, stigma of report, and perceived lack of capacity that were encountered in this trial. Furthermore, a deeper analysis and exploration of how to balance the significant but unquantified economic disincentives of report are needed in light of the role they likely play in reducing reports of detected pig infection. We are currently undertaking a second pilot intervention of community-led ring surveillance and control to investigate the effects of incorporating participatory research methodologies that focus on the democratization of disease-related evidence and more direct involvement of community members.30–32 This study incorporates measures that capture intermediate measures of behavior change including knowledge, attitudes, and practices, and address the role economic incentives may play in stimulating community-wide report.
Supplementary Material
Acknowledgments:
We thank the residents of the study communities for their participation, as well as the Ministries of Health and Agriculture.
Note: Supplemental figures appear at www.ajtmh.org.
REFERENCES
- 1.Ndimubanzi PC, Carabin H, Budke CM, Nguyen H, Qian Y-J, Rainwater E, Dickey M, Reynolds S, Stoner JA, 2010. A systematic review of the frequency of neurocysticercosis with a focus on people with epilepsy. PLoS Negl Trop Dis 4: e870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia HH, Nash TE, Del Brutto OH, 2014. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol 13: 1202–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coyle CM, et al. 2012. Neurocysticercosis: neglected but not forgotten. PLoS Negl Trop Dis 6: e1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bern C, Garcia HH, Evans C, Gonzalez AE, Verastegui M, Tsang VCW, Gilman RH, 1999. Magnitude of the disease burden from neurocysticercosis in a developing country. Clin Infect Dis 29: 1203–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruz M, Davis A, Dixon H, Pawlowski ZS, Proano J, 1989. Operational studies on the control of Taenia solium taeniasis/cysticercosis in Ecuador. Bull World Health Organ 67: 401–407. [PMC free article] [PubMed] [Google Scholar]
- 6.Sarti E, Schantz PM, Avila G, Ambrosio J, Medina-Santillán R, Flisser A, 2000. Mass treatment against human taeniasis for the control of cysticercosis: a population-based intervention study. Trans R Soc Trop Med Hyg 94: 85–89. [DOI] [PubMed] [Google Scholar]
- 7.Keilbach NM, de Aluja AS, Sarti-Gutierrez E, 1989. A programme to control taeniasis-cysticercosis (T. solium): experiences in a Mexican village. Acta Leiden 57: 181–189. [PubMed] [Google Scholar]
- 8.Allan JC, Velasquez-Tohom M, Fletes C, Torres-Alvarez R, Lopez-Virula G, Yurrita P, Soto de Alfaro H, Rivera A, Garcia-Noval J, 1997. Mass chemotherapy for intestinal Taenia solium infection: effect on prevalence in humans and pigs. Trans R Soc Trop Med Hyg 91: 595–598. [DOI] [PubMed] [Google Scholar]
- 9.Garcia HH, Gonzalez AE, Gilman RH, Moulton LH, Verastegui M, Rodriguez S, Gavidia C, Tsang VCW, 2006. Combined human and porcine mass chemotherapy for the control of T. solium. Am J Trop Med Hyg 74: 850–855. [PubMed] [Google Scholar]
- 10.Okello AL, et al. 2016. Assessing the impact of a joint human-porcine intervention package for Taenia solium control: results of a pilot study from northern Lao PDR. Acta Trop 159: 185–191. [DOI] [PubMed] [Google Scholar]
- 11.O’Neal SE, Moyano LM, Ayvar V, Rodriguez S, Gavidia C, Wilkins PP, Gilman RH, Garcia HH, Gonzalez AE, Cysticercosis Working Group in Peru , 2014. Ring-screening to control endemic transmission of Taenia solium. PLoS Negl Trop Dis 8: e3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molinari JL, Rodríguez D, Tato P, Soto R, Arechavaleta F, Solano S, 1997. Field trial for reducing porcine Taenia solium cysticercosis in Mexico by systematic vaccination of pigs. Vet Parasitol 69: 55–63. [DOI] [PubMed] [Google Scholar]
- 13.Assana E, Kyngdon CT, Gauci CG, Geerts S, Dorny P, De Deken R, Anderson GA, Zoli AP, Lightowlers MW, 2010. Elimination of Taenia solium transmission to pigs in a field trial of the TSOL18 vaccine in Cameroon. Int J Parasitol 40: 515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayashi CM, Kyngdon CT, Gauci CG, Gonzalez AE, Lightowlers MW, 2012. Successful immunization of naturally reared pigs against porcine cysticercosis with a recombinant oncosphere antigen vaccine. Vet Parasitol 188: 261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bulaya C, Mwape KE, Michelo C, Sikasunge CS, Makungu C, Gabriel S, Gabriel S, Dorny P, Phiri IK, 2015. Preliminary evaluation of community-led total sanitation for the control of Taenia solium cysticercosis in Katete district of Zambia. Vet Parasitol 207: 241–248. [DOI] [PubMed] [Google Scholar]
- 16.Joshi DD, Maharjan M, Johansen MV, Willingham AL, Sharma M, 2003. Improving meat inspection and control in resource-poor communities: the Nepal example. Acta Trop 87: 119–127. [DOI] [PubMed] [Google Scholar]
- 17.Sarti E, et al. 1997. Development and evaluation of a health education intervention against Taenia solium in a rural community in Mexico. Am J Trop Med Hyg 56: 127–132. [DOI] [PubMed] [Google Scholar]
- 18.Ngowi HA, Mlangwa JED, Mlozi MR, Tolma EL, Kassuku AA, Carabin H, Willingham AL, 2009. Implementation and evaluation of a health-promotion strategy for control of Taenia solium infections in northern Tanzania. Int J Health Promot Educ 47: 24–34. [Google Scholar]
- 19.Ngowi HA, Carabin H, Kassuku AA, Mlozi MRS, Mlangwa JED, Willingham AL, 2008. A health-education intervention trial to reduce porcine cysticercosis in Mbulu District, Tanzania. Prev Vet Med 85: 52–67. [DOI] [PubMed] [Google Scholar]
- 20.Wohlgemut J, Dewey C, Levy M, Mutua F, 2010. Evaluating the efficacy of teaching methods regarding prevention of human epilepsy caused by Taenia solium neurocysticercosis in western Kenya. Am J Trop Med Hyg 82: 634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Aluja AS, Suárez-Marín R, Sciutto-Conde E, Morales-Soto J, Martínez-Maya JJ, Villalobos N, 2014. Evaluation of the impact of a control program against taeniasis-cysticercosis (Taenia solium). Salud Publica Mex 56: 259–265. [PubMed] [Google Scholar]
- 22.Mwidunda SA, Carabin H, Matuja WBM, Winkler AS, Ngowi HA, 2015. A school based cluster randomised health education intervention trial for improving knowledge and attitudes related to Taenia solium cysticercosis and taeniasis in Mbulu district, northern Tanzania. PLoS One 10: e0118541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bustos JA, et al. 2012. Detection of Taenia solium taeniasis coproantigen is an early indicator of treatment failure for taeniasis. Clin Vaccine Immunol 19: 570–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia HH, et al. 2016. Elimination of Taenia solium transmission in northern Peru. N Engl J Med 374: 2335–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muro C, et al. 2017. Porcine cysticercosis: possible cross-reactivity of Taenia hydatigena to GP50 antigen in the enzyme-linked immunoelectrotransfer blot assay. Am J Trop Med Hyg 97: 1830–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guezala M-C, Rodriguez S, Zamora H, Garcia HH, Gonzalez AE, Tembo A, Allan JC, Craig PS, 2009. Development of a species-specific coproantigen ELISA for human Taenia solium taeniasis. Am J Trop Med Hyg 81: 433–437. [PubMed] [Google Scholar]
- 27.Flecker RH, Pray IW, Santivaňez SJ, Ayvar V, Gamboa R, Muro C, Moyano LM, Benavides V, Garcia HH, O’Neal SE, 2017. Assessing ultrasonography as a diagnostic tool for porcine cysticercosis. PLoS Negl Trop Dis 11: e0005282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan W, 2001. Akaike’s information criterion in generalized estimating equations. Biometrics 57: 120–125. [DOI] [PubMed] [Google Scholar]
- 29.Cui J, Qian G, 2007. Selection of working correlation structure and best model in GEE analyses of longitudinal data. Commun Stat Simul Comput 36: 987–996. [Google Scholar]
- 30.Bardosh K, Inthavong P, Xayaheuang S, Okello AL, 2014. Controlling parasites, understanding practices: the biosocial complexity of a one health intervention for neglected zoonotic helminths in northern Lao PDR. Soc Sci Med 120: 215–223. [DOI] [PubMed] [Google Scholar]
- 31.Carabin H, Traoré AA, 2014. Taenia solium taeniasis and cysticercosis control and elimination through community-based interventions. Curr Trop Med Rep 1: 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.FAO , 2011. Challenges of Animal Health Information Systems and Surveillance for Animal Diseases and Zoonoses. Proceedings of the International Workshop Organized by FAO, November 23–26, 2010, Rome, Italy. FAO Animal Production and Health Proceedings, No. 14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.