Abstract.
In September 2014, an increase in the number of Cryptosporidium spp. gastrointestinal tract infections was reported over a 6-month period among children living in a remote area along the Maroni River in French Guiana. Children presented gastroenteritis symptoms with Cryptosporidium-positive stools. Questionnaires were administered and stool examinations were controlled 3 months after the onset of symptoms. Data collection included demographics, food consumption, river behavior, symptoms, and outcome. Stool specimens were tested using microscopy and polymerase chain reaction. Samples from the water systems were examined for turbidity and culture for bacteria. Data from the reference laboratory were analyzed to calculate the median cryptosporidiosis incidence among immunocompetent patients from 2008 to 2015. Data on gastroenteritis cases reported by the Delocalized Center for Prevention and Care in the area were also collected. We report a cluster of 14 cases. All cases were children, aged between 4.5 and 38 months. Seven reported moderate to severe dehydration and required hospitalization. Speciation and microbiological typing revealed the cluster strain was Cryptosporidium hominis subtype IbA10G2 but C. hominis IbA9G2 and IbA15G1 strains were also identified. The median incidence in French Guiana was 5.8 cases per year before the outbreak. The first cases of the cluster appeared in the dry season. We describe the clinical features, epidemiology, and state of current investigations for the largest documented outbreak of cryptosporidiosis in French Guiana.
BACKGROUND
Human cryptosporidiosis is caused by Cryptosporidium spp., a protozoan parasite that can infect humans and animals. Cases are currently reported worldwide in all age groups, but particularly in children aged less than 2 years who appear to be affected more frequently and more severely.1–4 In South America, community-based studies have established that cryptosporidiosis was common in immunocompetent children and may present as multiple symptomatic episodes.5 Cryptosporidiosis usually causes profuse self-limiting diarrhea in immunocompetent individuals, but may result in chronic or systemic symptoms in immunodeficient patients.2 Treatment is usually symptomatic but nitazoxanide has shown activity against Cryptosporidium and is an effective treatment.6,7 The human disease is predominantly caused by the zoonotic Cryptosporidium parvum which infects both humans and ruminants, and Cryptosporidium hominis, which is almost exclusively restricted to humans.6,8
Cryptosporidium spp. oocysts represent the infective form, which survives outside hosts and remain infective for several months, particularly in moist and cool environments.9 Transmission usually occurs through the fecal–oral route, by ingestion of oocysts from contaminated food or water, direct contact with animals, or person-to-person contact.6 Waterborne Cryptosporidium spp. which may disseminate either directly (manures/feces) or through oocysts transfer in hydric environments have been shown to cause cryptosporidiosis outbreaks worldwide in relation to contaminated drinking/recreational waters.10 The presence of viable, infective oocysts in water sources and their presence in waters used for human consumption and agricultural uses is facilitated by environmental factors such as abundant livestock, soil vulnerability (chalk aquifers), heavy rainfalls,11,12 and resistance to disinfection procedures (chlorine) commonly used in water treatment plants.13
In September 2014, an unprecedented increase in the number of Cryptosporidium spp. infections was detected by analyses of fecal samples from children with gastrointestinal complaints in an autochthonous community (Amerindian and Maroon population) living along the Maroni river in a remote area of the Amazonian forest in French Guiana. This study describes microbiological and epidemiological investigations conducted to establish the presence of a potential epidemic context, determine the etiology, evaluate risk factors, and contribute to elaboration of preventive procedures.
METHODS
Annual incidence rate of Cryptosporidium spp. positive stools in children from French Guiana (2008–2013).
The number of pediatric cryptosporidiosis cases recorded from January 2008 to December 2013 in immunocompetent children from French Guiana was obtained from the database of routine Cryptosporidium spp.–positive stool of French Guiana reference parasitology laboratory to retrospectively evaluate the annual incidence rate before the 2014 outbreak.
Clustered cryptosporidiosis cases on the Upper Maroni study site (January 2014–June 2015).
The study area consisted of remote villages on the Upper Maroni River in French Guiana, a French overseas territory located on the northeastern part of South America (Figure 1). This river runs through primary Amazonian rain forest and delineates a natural border with Surinam. The climate is divided into four seasons: a short rainy season (November to January), a short dry season (February and March), followed by a long rainy season (mid-March to mid-July), and finally a dry season (August to November). The autochthonous populations, mainly Maroons, Amerindians (Wayana and Teko communities), and Brazilian communities with low socioeconomic status, have no access to quality construction materials nor electricity, and lack safe drinking water and sewage facilities, which results in high rates of gastrointestinal infections especially among children (personal data from delocalized health care centers information system).
Figure 1.
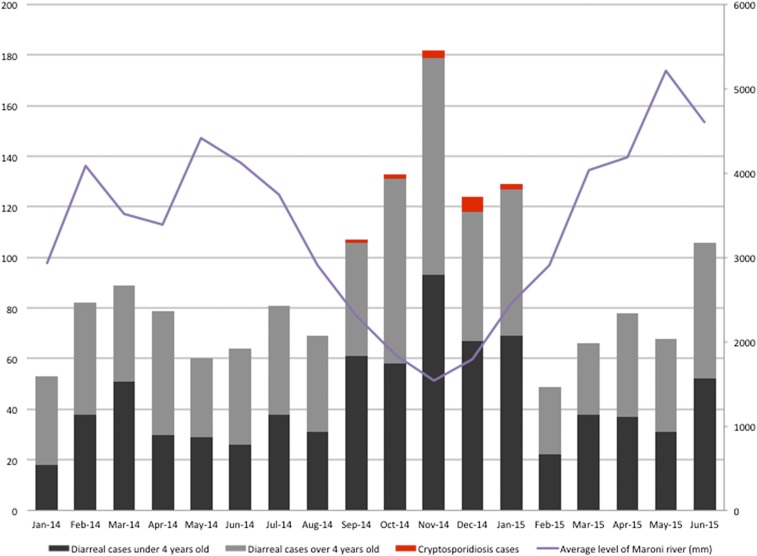
Month of gastroenteritis by age (< and > 4 years old), cryptosporidiosis cases and average level of Maroni River (mm), upper Maroni River area, French Guiana, January 2014–June 2015 (n cases of gastroenteritis = 1,619, n cases of cryptosporidiosis = 14). In this article, we used datasets and laboratory findings to represent the important part of the diarrheal disease in this area. However, other studies are needed to estimate the real part and the possible correlation of cryptosporidiosis in diarrheal disease. This figure appears in color at www.ajtmh.org.
Case definition, clinical and epidemiological investigations.
We defined a case as a resident living along the Maroni River presenting gastroenteritis signs (nausea and/or vomiting and diarrhea) or a recent medical history of diarrhea with a Cryptosporidium-positive stool test from September 2014 to June 2015.
Immunocompetent patients were defined by the absence of medical history of known immunosuppression predisposing to infection.
Children were examined at the Upper Maroni outpatient health center (Delocalized Center for Prevention and Care [CDPS]) serving the villages of Maripasoula, Antecume pata, Taluen, and Papaïchton. They were included in a comprehensive study from September 2014 to June 2015 when they presented 1) gastroenteritis/recent diarrheic episode (defined above); 2) Cryptosporidium spp.–positive stools; and 3) absence of medical history consistent with immunosupression. Their parents/caregivers were interviewed using a structured questionnaire to assess medical history, especially recent illnesses, underlying conditions, and potential exposure to infection (water consumption, recreational use of water, breastfeeding, contact with animals, distance between house and river, household structure, and river activities).
The study protocol was approved by the ethical committee of Cayenne hospital. Parents and/or caregivers of children provided written informed consent.
Detection of Cryptosporidium spp. oocysts, intestinal parasites, bacteria, and viruses in stool samples.
Fecal samples were screened for intestinal parasites using a centrifuge-sedimentation technique and Kato-Katz technique. For Cryptosporidium oocysts identification, a modified Ziehl–Neelsen stain was performed on sediments obtained from the Ritchie’s concentration.14 To account the possibility of other infectious etiologies, stool specimens were also tested by bacterial culture and by rapid stool test screening for adenovirus and rotavirus.
Cryptosporidium spp. genotyping.
All stool samples were stored at +4°C in tubes containing 2.5% potassium dichromate and sent to two reference laboratories for genotyping (Laboratoire de Parasitologie, CHU Dijon and Laboratoire de parasitology, CHU Rouen) using a real-time polymerase chain reaction to determine Cryptosporidium species.15
All C. hominis isolates were subtyped by sequencing the gp60 gene reference to identify if the increase of cases was due to a particular subtype.16
Environmental investigations in the Upper Maroni study site.
Bacterial culture and turbidity analysis of water distribution sources of the area (“tapping points” of village of the Upper Maroni River) performed by the French health department (Agence Régionale de la Santé de la Guyane) between January 2014 and December 2015 were reported. The average Maroni River flow rate monitored by the French environmental department (Direction de l’Environnement, de l’Aménagement et du Logement, de la Guyane) in Maripasoula area was also reported and correlated with the occurrence of diarrhea and crypstosporidiosis cases, respectively.
Incidence of gastroenteritis in the study area (January 2014–June 2015).
To take into account the burden of diarrheal disease during the study period, we reported the total number of visits for diarrhea. We collected data for all medical consultations in the Upper Maroni CDPS site (Maripasoula, Papaïchton, Taluen, and Antecume Pata area) between January 2014 and June 2015. Data were extracted from the information system using ICD 10 coding (International Classification of Diseases, 10th version). We examined the A09 code “infectious gastroenteritis” as principal or associated diagnosis during that period.
RESULTS
Retrospective (2008–2013) evaluation of the prevalence of pediatric cryptosporidiosis cases in the Upper Maroni study site.
From January 2008 to December 2013, a total of 30 cases of Cryptosporidium-positive stool tests in immunocompetent children were reported. Scattered cases appeared in the Wayana Amerindian and Maroon villages along the Maroni river from the database of the reference laboratory. The average age was 18.7 months [13.6–24.01].
There was an average of five cases (range 1–8) by year. This was a seasonal pattern with a greater number of positive stools occurring in January and February (peak of the rainy season) representing 33% of total cases (N = 10/30).
Prevalence, clinical characteristics, and isolate genotypes of clustered Cryptosporidium spp. gastroenteritis cases.
From January 2014 to June 2015, 1,619 gastroenteritis cases were reported (1,062 pediatric cases younger than 19 years 560 of whom [53%] were younger than 4 years and 557 cases in adult subjects, respectively) in villages of the study site (Maripasoula, Papaïchton, Taluen, and Antecume Pata area). Increases in the number of diarrhea cases corresponded to the beginning of the dry seasons and low Maroni River levels, i.e., in February 2014 (“short dry season”) and August–September 2015 (long dry season) (Figure 1) regardless of age, a peak of cases was observed in November 2014.
On the same period, Cryptosporidiosis was diagnosed in 14 pediatric gastroenteritis/diarrhea cases (Figure 2). There were nine males and five females, with a median age of 18 months (range: 4.5–38 months). Three children were 1 year old or younger. The most common symptoms were diarrhea (N = 13/14) and vomiting (N = 9/14). In two cases, fever was reported or present on initial examination. Most cases (13/14) exhibited weight loss due to dehydration ranging from moderate (< 5%) to intermediate (5–10%) and severe (> 10% with sunken eyes and dry mucous membranes) in seven and six cases, respectively. Half of the cases required hospitalization. No co-infection with other parasites or bacteria was identified in stools, and in one case, a rotavirus stool test was positive. All children were considered as immunocompetent, but two of them presented a medical history of asthma or sickle cell disease. Two children had growth retardation.
Figure 2.
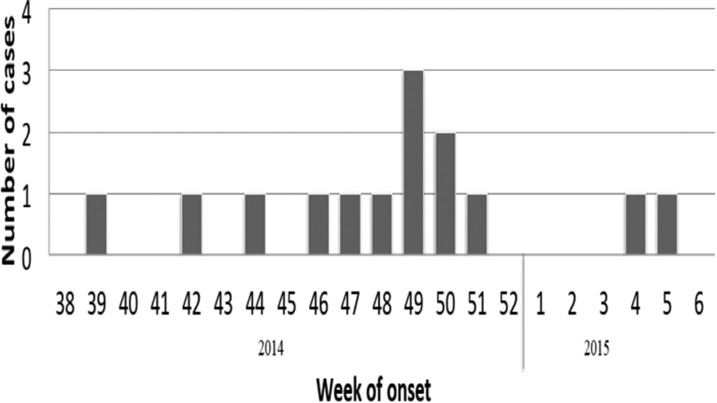
Weeks of onset of cryptosporidiosis cases, French Guiana, September 2014–February 2015 (N = 14).
All patients recovered after oral or intravenous rehydration. The average median recovery duration was 7.8 days (range 1–19 days). Stools from all patients which were examined 3–6 months later were negative for Cryptosporidium spp. oocysts (N = 12/14). In the stool samples from 9/14 patients, genotyping revealed C. hominis with subtypes IbA10G2, IbA15G1, and IbA9G2 in four, one, and one samples, respectively. For the samples of the other patients, genotyping was unsuccessful because of unreadable gp60 sequences.
Epidemiological characteristics of clustered Cryptosporidium spp. gastroenteritis cases.
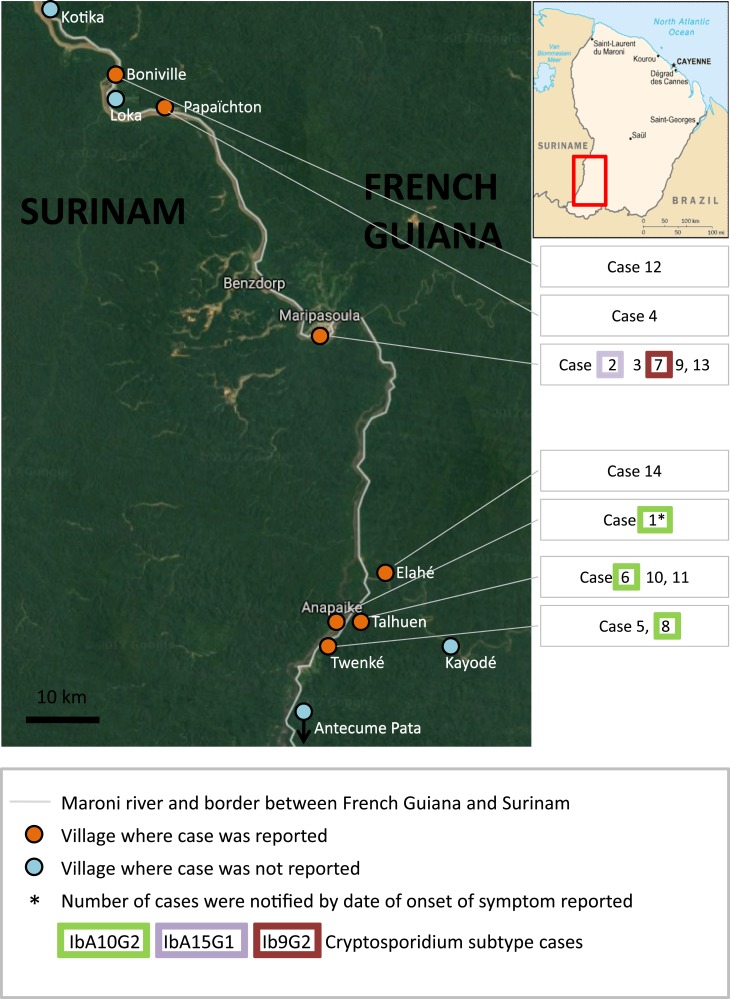
Cryptosporidiosis cases were distributed among villages of Amerindian (Wayana), Maroon (Aluku), and Brazilian communities (seven, six, and one case, respectively) (Figure 3). After the occurrence of a first case in September 2014, the number of cryptosporidiosis patients increased during the dry season to a peak in December at the beginning of the rainy season and decreased afterward (Figure 1).
Figure 3.
Mapping of cryptosporidiosis cases by local villages of Maripasoula and Papaïchton town of High Maroni River area, French Guiana, September 2014–February 2015 (N = 14). This figure appears in color at www.ajtmh.org.
From parents/caregivers interviews, the following risk factors were reported: swimming (9/14) and urinating/defecating (7/14) in the river, contacts with animals such as dogs, monkeys, chicken, or peccaries (Tayassupecari) (6/14), recent person-to-person contact with someone with cryptosporidium-positive stool before the first symptoms (8/14). Most of the contact cases (probable secondary cases) belonged to the same family or were neighbors. The average distance of case’s houses from the river was 226 m (range 30–850 m).
All the families of children with cryptosporidiosis used water from tapping points maintained by local village authorities. Tap water–turbidity levels were > 5 formazine nephelometric unit in all villages of the Maripasoula area site (Maripasoula, Papaïchton, Taluen, and Antecume Pata area). A tap water–bacterial contamination (> 1 n/100 mL of Enterococcus, Echerichia coli, and/or coliforme bacteria) was detected in Elahé, Taluen, Twenke, and Loka village.
DISCUSSION
In this study, the first and largest recognized cryptosporidiosis case cluster was identified from September 2014 to February 2015, in an autochthonous children population from a remote area. An epidemic context (outbreak) was first suggested by the occurrence of 14 cases over a 6-month period, which represented a peak which contrasted with the previous lower incidence of sporadic cases in the study area as well as in French Guiana as a whole in the eight preceding years. The limited number of previously recorded cases in French Guiana or at the study site precludes any comparison with previous estimations in children. Everywhere in the world, cryptosporidiosis cases are recognized as underdiagnosed, and in the present study, the true size of the outbreak was probably larger as many parents/caregivers probably did not consult nor undergo laboratory testing for Cryptosporidium spp.
An epidemic context was also consistent with the spatiotemporal characteristics of cases, which were restricted to adjacent villages along the Upper Maroni River and during the dry periods, which differed from previous observations of sporadic cases at the site as well as in the rest of French Guiana and from other observations worldwide showing transmission of the waterborne parasite during rainy seasons.17
The fact that C. hominis was the only species in 9/14 patients was suggestive of direct/waterborne human contacts. Although concerning a limited number of cases, the prevalence of subtype IbA10G2 was also consistent with an epidemic transmission.18 The temporal association of several cases with different subtypes could also reflect environmental contamination. Interviews reporting contacts between cases with the same subtype also suggested person-to-person transmission of isolates of environmental origin.
Evidence for waterborne exposure was provided by the epidemiological characteristics of human activities in the Maroni river area, notably the numerous beach playgrounds where families wash clothes and themselves, cook and defecate. It seems likely that children contracted the infection when playing, bathing, and eating there. Investigations of water supplies revealed high turbidity levels in most tapping sites and enteropathogen bacterial contamination was found in four of them, consistent with fecal contamination.
Although the practical limitations of the study did not allow the identification of the origin of all clustered Crytosporidium-positive cases, the present observational findings corroborate previous reports which highlight Cryptosporidium spp. infection as a clear signal of an increased risk of children contracting severe infectious diseases.2,19
Likewise, we observed a significant increase in the number of cryptosporidiosis cases in children during the outbreak, the incidence of this infection was underestimated because of the difficulty to perform parasitological examination in remote area in all diarrhea cases. Because of the difficulty to promptly perform research in the context of an outbreak (particularly in remote areas) and of the retrospective nature of our study, we were unable to compare acute diarrhea cases due to cryptosporidiosis and other agents. Therefore, we are planning to organize a case–control study recruiting a larger sample size in the future to better clarify potential causes and effects of cryptosporidiosis in immunocompetent children. However, from a public health perspective, present data prompt to plan specific interventions aimed at reducing waterborne infections in high-risk groups of children.
Detection and removal of Cryptosporidium are essential but challenging for supplying drinking water particularly in developing and isolated areas.
This outbreak reflects the multifactorial dynamics of waterborne disease in tropical areas, including human behavior (particularly water consumption) and probable immunity in precarious conditions of the exposed children population. Nevertheless, early detection, investigation, and appropriate control of outbreaks can reduce their impact.
Acknowledgments:
We thank the families who participated in this study. We also thank Jean-Philippe Pavy of the Direction de l’Environnement, de l’Aménagement et du Logement de la Guyane, for his environmental data.
REFERENCES
- 1.Bera P, Das S, Saha R, Ramachandran VG, Shah D, 2014. Cryptosporidium in children with diarrhea: a hospital-based study. Indian Pediatr 51: 906–908. [DOI] [PubMed] [Google Scholar]
- 2.Checkley W, et al. , 2015. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. Lancet Infect Dis 15: 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotloff KL, et al. , 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382: 209–222. [DOI] [PubMed] [Google Scholar]
- 4.Sow SO, et al. , 2016. The burden of Cryptosporidium diarrheal disease among children < 24 months of age in moderate/high mortality regions of sub-Saharan Africa and South Asia, utilizing data from the Global Enteric Multicenter Study (GEMS). PLoS Negl Trop Dis 10: e0004729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bern C, Ortega Y, Checkley W, Roberts JM, Lescano AG, Cabrera L, Verastegui M, Black RE, Sterling C, Gilman RH, 2002. Epidemiologic differences between cyclosporiasis and cryptosporidiosis in Peruvian children. Emerg Infect Dis 8: 581–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casemore DP, 1990. Epidemiological aspects of human cryptosporidiosis. Epidemiol Infect 104: 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossignol JF, Ayoub A, Ayers MS, 2001. Treatment of diarrhoea caused by Cryptosporidium parvum: a prospective randomized, double-blind, placebo-controlled study of Nitazoxanide. J Infect Dis 184: 103–106. [DOI] [PubMed] [Google Scholar]
- 8.Giles M, Chalmers R, Pritchard G, Elwin K, Mueller-Doblies D, Clifton-Hadley F, 2009. Cryptosporidium hominis in a goat and a sheep in the UK. Vet Rec 164: 24–25. [DOI] [PubMed] [Google Scholar]
- 9.O’Donoghue PJ, 1995. Cryptosporidium and cryptosporidiosis in man and animals. J Parasitol 25: 139–195. [DOI] [PubMed] [Google Scholar]
- 10.Guzman-Herrador B, et al. , 1998. Waterborne outbreaks in the Nordic countries, 1998 to 2012. Euro Surveill 18: 20. [DOI] [PubMed] [Google Scholar]
- 11.Lake I, Bentham G, Kovats RS, Nichols GL, 2005. Effects of weather and river flow on cryptosporidiosis. J Water Health 3: 469–474. [DOI] [PubMed] [Google Scholar]
- 12.Gertler M, et al. , 2015. Outbreak of Cryptosporidium hominis following river flooding in the city of Halle (Saale), Germany, August 2013. BMC Infect Dis 15: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nasser AM, 2016. Removal of Cryptosporidium by wastewater treatment processes: a review. J Water Health 14: 1–13. [DOI] [PubMed] [Google Scholar]
- 14.Henriksen SA, Pohlenz JF, 1981. Staining of cryptosporidia by a modified Ziehl-Neelsen technique. Acta Vet Scand 22: 594–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalle F, et al. , 2003. Molecular characterization of isolates of waterborne Cryptosporidium spp. collected during an outbreak of gastroenteritis in South Burgundy, France. J Clin Microbiol 41: 2690–2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sulaiman IM, Hira PR, Zhou L, Al-Ali FM, Al-Shelahi FA, Shweiki HM, Iqbal J, Khalid N, Xiao L, 2005. Unique endemicity of cryptosporidiosis in children in Kuwait. J Clin Microbiol 43: 2805–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morse TD, Nichols RA, Grimason AM, Campbell BM, Tembo KC, Smith HV, 2007. Incidence of cryptosporidiosis species in paediatric patients in Malawi. Epidemiol Infect 135: 1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li N, Xiao L, Cama VA, Ortega Y, Gilman RH, Guo M, Feng Y, 2013. Genetic recombination and Cryptosporidium hominis virulent subtype IbA10G2. Emerg Infect Dis 19: 1573–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tallant C, Huddleston P, Alshanberi A, Misra S, 2016. Acute, severe Cryptosporidiosis in an immunocompetent pediatric patient. Clin Pract 6: 837. [DOI] [PMC free article] [PubMed] [Google Scholar]