Abstract.
To evaluate the predictive value of time to sputum culture conversion (SCC) in predicting cure and factors associated with time to SCC and cure in multidrug-resistant tuberculosis (MDR-TB) patients, a retrospective study was conducted at programmatic management unit of drug resistant tuberculosis (TB), Peshawar. A total of 428 pulmonary MDR-TB patients enrolled at the study site from January 1, 2012 to August 31, 2014 were followed until treatment outcome was recorded. Survival analysis using Cox proportional hazards model and multivariate binary logistic regression were, respectively, used to identify factors associated with time to SCC and cure. A P value < 0.05 was considered statistically significant. Overall, 90.9% patients achieved SCC, and 76.9% were cured. Previous use of second-line drugs (SLDs) (hazard ratio [HR] = 0.637; 95% confidence interval [CI] = 0.429–0.947), ofloxacin resistance (HR = 0.656; 95% CI = 0.522–0.825) and lung cavitation (HR = 0.744; 95% CI = 0.595–0.931) were significantly associated with time to SCC. In predicting cure, sensitivities of SCC at 2, 4, and 6 months were 64.1% (95% CI = 58.69–69.32), 93.0% (95% CI = 89.69–95.52), and 97.6% (95% CI = 95.27–98.94), respectively, whereas specificities were 67.7% (95% CI = 57.53–76.73), 51.5% (95% CI = 41.25–61.68), and 44.4% (95% CI = 34.45–54.78), respectively. Furthermore, patients’ age of 41–60 (odds ratio [OR] = 0.202; 95% CI = 0.067–0.605) and > 60 years (OR = 0.051; 95% CI = 0.011–0.224), body weight > 40 kg (OR = 2.950; 95% CI = 1.462–5.952), previous SLD use (OR = 0.277; 95% CI = 0.097–0.789), lung cavitation (OR = 0.196; 95% CI = 0.103–0.371) and ofloxacin resistance (OR = 0.386; 95% CI = 0.198–0.749) were significantly associated with cure. Association of SCC with cure was substantially stronger at 6 months (OR = 32.10; 95% CI = 14.34–71.85) than at 4 months (OR = 14.13; 95% CI = 7.92–25.21). However in predicting treatment outcomes, the combined sensitivity and specificity of SCC at 4 months was comparable to SCC at 6 months. Patients with risk factors for delayed SCC were also at high risk of unsuccessful outcomes.
INTRODUCTION
Multidrug-resistant tuberculosis (MDR-TB), defined as tuberculosis (TB) resistant to at least isoniazid and rifampicin, is a growing threat to public health. Sputum culture conversion (SCC) defined as “two consecutive negative cultures taken at least 30 days apart following an initial positive culture”1 is usually the first goal of MDR-TB treatment and is used for guiding therapy, shifting patients from intensive to continuation phase, and defining treatment outcomes.2 In pulmonary TB (PTB) treatment, achieving SCC is the prime and reliable indicator of noninfectiousness and effectiveness of anti-TB therapy.3,4 Based on its assumed predictive value for end-of-treatment outcomes, SCC is used as an early microbiological end point in phase II clinical trials of TB treatment.4 Few studies which have evaluated association between SCC and treatment outcomes of MDR-TB patients concluded that time to SCC was significantly associated with end-of-treatment outcomes.2,4–9 Existing evidence suggests that SCC after 2 months of treatment is an early indicator of treatment success in MDR-TB patients.2,4,7 However, the low sensitivity of SCC at 2 months of treatment in predicting MDR-TB treatment outcomes is a cause of concern and indicates that many treatments leading to long-term favorable results may not meet the criteria of SCC by 2 months.4,6
In terms of MDR-TB burden, Pakistan ranks fourth globally and first in the Eastern Mediterranean Region of the World Health Organization (WHO).10 According to the results of first National Drug Resistance Survey of Pakistan completed in September 2013, MDR-TB accounts for 3.7% of the new PTB and 18.1% of retreatment TB cases.11 Despite the clinical significance of SCC in MDR-TB treatment, our literature search found only two studies from Pakistan which have evaluated the predictors of 2 months12 and delayed SCC in MDR-TB patients,13 but none of them has evaluated association between SCC at different time points and treatment outcomes among MDR-TB patients. As the earlier detection of patient’s nonresponse to MDR-TB treatment may allow doctors to adjust therapy and, successively, avoid unsuccessful outcomes, the present study was conducted with the aim to evaluate factors associated with time to SCC and successful outcomes and sensitivity and specificity of SCC at 2, 4, and 6 months of treatment in predicting final outcomes in MDR-TB patients.
MATERIALS AND METHODS
Patient population and study design.
This was a retrospective cohort study conducted at Programmatic Management of Drug Resistant-TB (DR-TB) unit of Lady Reading Hospital, Peshawar, Pakistan. All newly diagnosed, culture-confirmed pulmonary MDR-TB patients consecutively enrolled at the study site from January 1, 2012 to August 31, 2014 irrespective of their age and comorbidity status were included in the study. Patients’ follow-up ended when a treatment outcome was reported. Patients with DR-TB other than MDR-TB (mono DR-TB, poly DR-TB, and extensively DR-TB) were excluded from the study. Furthermore, pulmonary MDR-TB patients with a previous history of MDR-TB treatment, those with negative sputum culture at the baseline visit, and those who were lost to follow-up during the treatment were also not included in the study.
Bacteriology, drug susceptibility testing (DST).
All DR-TB suspects referred to the study site were initially evaluated with two sputum samples for acid fast bacilli by direct sputum smear microscopy using Ziehl–Neelsen stain and Xpert MTB/RIF (Cepheid, Sunnyvale, CA). After positive results of smear microscopy and rapid DST, their sputum samples were sent to Aga Khan University Hospital Laboratory, Karachi, or Provincial TB reference laboratory, Peshawar for sputum culture and DST. Drug susceptibility test was carried out by using Agar proportion method on enriched Middlebrook 7H10 medium (BBL; Beckton Dickinson, Sparks, MD) at the following concentrations (WHO, 2012): rifampicin (1 μg/mL), isoniazid (0.2 μg/mL), streptomycin (2 μg/mL), ethambutol (5 μg/mL), ofloxacin (2 μg/mL), amikacin (4 μg/mL), kanamycin (5 μg/mL), capreomycin (4 μg/mL), and ethionamide (5 μg/mL). Drug susceptibility test for pyrazinamide was carried out at the concentration of 100 μg/mL by using Mycobacterial Growth Indicator Tube (Becton Dickinson, Franklin Lakes, NJ) in accordance with manufacturer’s instructions.14 Drug susceptibility test was performed at the baseline visit and repeated whenever deemed necessary, whereas acid fast bacilli culture and sputum smear was regularly carried out on a monthly basis.
Treatment protocol.
Treatment protocol of MDR-TB patients at the study site has previously been published elsewhere.15 On positive sputum smear microscopy and rapid DST, presumed MDR-TB patients underwent baseline laboratory tests including full blood count, screening for human immunodeficiency virus and hepatitis, liver, kidney, and thyroid function tests, random blood glucose, electrolytes, and urinalysis. After baseline laboratory work, treatment in presumed MDR-TB patients was initiated with empirical regimen based on the recommendations of national guidelines for the management of DR-TB. Treatment in all patients except those with a documented history of previous use of second-line drug (SLD) was initiated with amikacin/kanamycin/capreomycin + levofloxacin + ethionamide + cycloserine + pyrazinamide + vitamin B6. In those with documented history of SLD use, treatment was initiated by adding para-amino salicylic acid to the aforementioned regimen. After the availability of the DST results, patients were switched to individualized tailored regimen consisting of four effective or likely effective SLD. Effective drugs were defined as “those for which DST results had confirmed susceptibility,” whereas likely effective drugs were “those for which DST results were not available but the patients had not used it for > 1 month.” Pyrazinamide was added to the treatment regimen of all patients irrespective of DST results. Maximum recommended doses of drugs per body weight were prescribed. Patients were treated for a minimum of 18 months after SCC. Injectable SLD was continued for at least 8 months and a minimum of 6 months after SCC. All patients were treated on ambulatory basis and evaluated monthly. Treatment adherence was monitored by trained treatment supporters. For each administered dose, the treatment supporter marked the patient treatment card. On each monthly visit, the clinician also assessed patient’s adherence by inspecting the treatment card. Adherence with MDR-TB treatment was ensured by a home directly observed treatment linkage facilitator who paid home visits, linking the patients, PMDT unit, the District TB Officer, and the nearest health-care center. In addition to free treatment, patients and their treatment supporters received social support in the form of monthly food basket and conveyance allowance.
Operational definitions.
In the present study, SCC was defined as “two consecutive negative cultures taken at least 30 days apart following an initial positive culture.” Two negative culture results in sequence counted towards this definition even if there were missing or contaminated culture(s) between them.4 Initial time to SCC was defined as “the time in days from the date of initiating MDR-TB treatment to the collection date of the first of two consecutive negative sputum cultures.”1,2,4 Sputum culture reversion to positive was defined as “at least one subsequent positive culture result after initial SCC.”2,4 Treatment outcomes were assigned according to the criteria defined in the WHO guidelines for the management of MDR-TB.1 The patient who completed his/her treatment as recommended by the guidelines, had no evidence of treatment failure, and had at least five consecutive negative sputum cultures taken at least 30 days apart in the final 12 months of treatment was declared “Cured.” One positive culture was allowed if it is followed by a minimum of three consecutive negative cultures taken at least 30 days apart. Treatment outcome of “Died” was assigned to any patient who died for any reason during the course of MDR-TB treatment. The patient with two or more positive culture results of the recorded five cultures during the final 12 months of treatment, or if the treatment was terminated early because of poor clinical or radiological response or adverse event was declared “Treatment Failure.” The patient whose treatment was interrupted for two or more consecutive months for any reason other than medically approved was declared as “Loss to follow-up.” Death and treatment failure were grouped together as unsuccessful treatment outcomes.1,15 We defined sensitivity as “the proportion of patients with SCC by months 2, 4, and 6 among those with successful treatment outcome” and specificity as “the proportion of patients without SCC by months 2, 4, and 6 among those with unsuccessful outcomes.”4
Data collection.
A standardized data collection form was used to extract patients’ sociodemographic, microbiological, and clinical data from the medical records, Electronic Nominal Recording Reporting System data, and MDR-TB notification forms of the patients.
Statistical analysis.
Data were analyzed by using SPSS 17. Survival analysis using Cox proportional hazards model was conducted to identify factors associated with time to SCC. Cases were censored if their sputum cultures never converted before the last follow-up. Univariate analysis was conducted to find association between independent variables and successful treatment outcomes. Multivariate logistic regression analysis was conducted to evaluate factors associated with cure. Independent variables with a P value < 0.2 in univariate analysis were included in the final multivariate models. A P value < 0.05 was considered statistically significant. We also evaluated the sensitivity and specificity of SCC at 2, 4, and 6 months in predicting treatment outcomes. Receiver operating characteristic curves were plotted to visualize the effect of using different time points for SCC on the balance between sensitivity and specificity.
Ethical approval.
This study was approved by the Research and Ethics Committee of the Postgraduate Medical Institute Peshawar, Pakistan. Being a retrospective study, it was difficult to trace all the patients for getting consent, so the said institution granted consent waiver. The anonymity of the patients’ information was respected. All the methods were performed in compliance with the relevant guidelines and regulations.
RESULTS
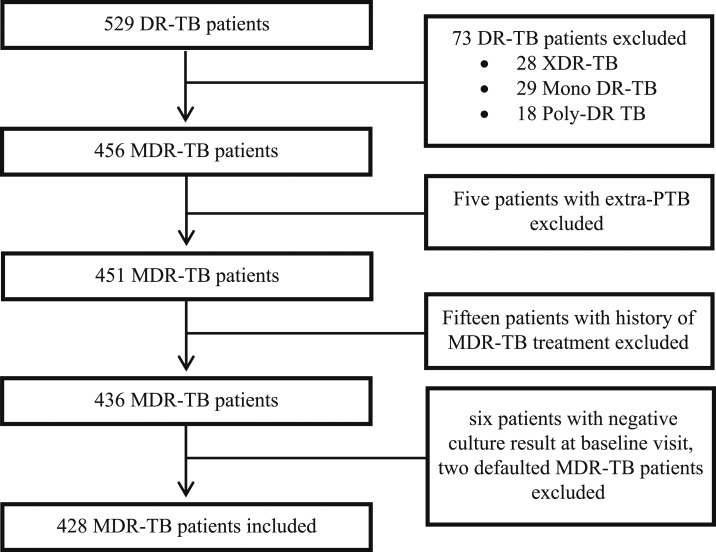
During the study period, a total of 529 DR-TB patients were enrolled at the study site. Among them, 428 met the eligibility criteria and were included in the study (Figure 1). The sociodemographic and baseline clinical characteristics of study participants are given in Table 1. A high degree of drug resistance was observed (median 5 drugs, interquartile range [IQR] 4–6 drugs); 50.7% patients were resistant to any SLD. Among SLD, resistance was highest for ofloxacin (48.1%), followed by ethionamide (5.1%).
Figure 1.
Enrollment, inclusion, and exclusion of study patients. DR-TB = drug resistant TB; MDR-TB = multidrug-resistant TB; PTB = pulmonary TB; TB = tuberculosis; XDR-TB = extensively drug-resistant TB.
Table 1.
Patients’ baseline sociodemographic and clinical characteristics
| Variable | Mean ± SD | No. (%) |
|---|---|---|
| Gender | – | |
| Female | 239 (55.8) | |
| Male | 189 (44.2) | |
| Age (years) | 30.7 ± 14.35 | |
| 10–20 | 91 (21.3) | |
| 21–40 | 238 (55.6) | |
| 41–60 | 77 (18.0) | |
| >60 | 22 (6.1) | |
| Weight (kg) | 44.9 ± 9.87 | |
| 20–40 | 150 (35.0) | |
| 41–60 | 252 (58.9) | |
| >60 | 26 (6.1) | |
| Residence | – | |
| Rural | 231 (54.0) | |
| Urban | 197 (46.0) | |
| Marital status | – | |
| Single | 198 (46.3) | |
| Married | 208 (48.6) | |
| Widow | 22 (5.1) | |
| Smoking | – | |
| Nonsmokers | 365 (85.3) | |
| Active + ex-smokers | 53 (14.7) | |
| Previous TB treatment | – | |
| No | 49 (11.4) | |
| Yes | 379 (88.6) | |
| Previous treatment regimen | – | |
| New patients | 49 (11.4) | |
| Category I* | 185 (43.2) | |
| Category II† | 162 (37.9) | |
| Unknown | 32 (7.5) | |
| History of SLD use | – | |
| No | 361 (84.3) | |
| Yes | 35 (8.2) | |
| Unknown | 32 (7.5) | |
| Sputum smear grading at baseline visit | – | |
| Negative | 32 (7.5) | |
| Scanty (1–9 AFB/100 HPF) | 12 (2.8) | |
| +1 (10–99 AFB/100 HPF) | 133 (31.1) | |
| +2 (1–9 AFB/HPF) | 127 (29.7) | |
| +3 (> 9 AFB/HPF) | 124 (29.0) | |
| Chest X-ray at baseline visit | – | |
| No cavitation | 283 (66.1) | |
| Cavitation | 145 (33.9) | |
| Comorbidity | – | |
| No | 368 (66.0) | |
| Yes | 60 (14.0) | |
| Types of comorbidities | ||
| Diabetes mellitus | 20 | |
| Hypertension | 24 | |
| Hepatitis | 6 | |
| HIV-AIDS | 1 | |
| Others | 9 |
AFB = acid fast bacilli; AIDS = acquired immune deficiency syndrome; HIV = human immunodeficiency virus; HPF = high power field; SD = standard deviation; SLD = second-line anti-TB drugs; TB = tuberculosis.
2RHZE + 4RH.
3RHZES + 5RHE.
Treatment regimen.
Treatment was empirically initiated in 388/428 patients. On the reception of DST results (median 58 days, IQR 52–78 days), treatment was modified for more than half of the patients (57.7%) who were initiated on empirical regimen. The most common modification was addition of para-amino salicylic acid (216/224). During the intensive phase of treatment, the patients received a median of six drugs (range 5–8) with a median of four effective or likely effective drugs. Twenty four (5.6%) of the study participants received suboptimal regimen (< 4 effective or likely effective drugs) during intensive phase of treatment.
Factors associated with time to SCC.
A total of 389/428 (90.9%) patients achieved SCC in a median time of 58 days (IQR 30–90 days). Of 389 patients with initial SCC, 125 (32.1%) had at least one or more subsequent positive sputum culture. Median time to sputum culture reversion was 6 months (IQR 3–10 months). Multivariate Cox proportional hazard model showed that history of SLD use (hazard ratio [HR] = 0.637; 95% confidence interval [CI] = 0.429–0.947), resistance to ofloxacin (HR = 0.656; 95% CI = 0.522–0.825) and lung cavitation (HR = 0.744; 95% CI = 0.595–0.931) had statistically significant association with time to SCC (Table 2).
Table 2.
Factors associated with time to sputum culture conversion
| Variables | No. | Univariate analysis HR (95% CI) | P value | Multivariate analysis HR (95% CI) | P value |
|---|---|---|---|---|---|
| Gender | 0.292 | – | – | ||
| Female | 239 | Reference | |||
| Male | 189 | 0.897 (0.734–1.097) | |||
| Age (years) | |||||
| 10–20 | 91 | Reference | – | – | |
| 21–40 | 238 | 1.075 (0.835–1.385) | 0.575 | – | |
| 41–60 | 77 | 1.244 (0.898–1.722) | 0.189 | – | |
| > 60 | 22 | 0.901 (0.519–1.556) | 0.712 | – | |
| Weight (kg) | 0.135 | 0.201 | |||
| < 40 | 150 | Reference | Reference | ||
| ≥ 40 | 278 | 1.176 (0.951–1.453) | 1.155 (0.926–1.441) | ||
| Residence | 0.223 | – | – | ||
| Rural | 231 | Reference | |||
| Urban | 197 | 1.132 (0.927–1.382) | |||
| Previous treatment regimen | – | ||||
| New patients | 49 | Reference | – | – | |
| Category I | 185 | 1.088 (0.776–1.526) | 0.625 | – | |
| Category II | 162 | 1.090 (0.773–1.536) | 0.622 | – | |
| Unknown | 32 | 1.083 (0.679–1.749) | 0.746 | – | |
| History of SLD use | |||||
| No | 361 | Reference | – | Reference | – |
| Yes | 35 | 0.635 (0.431–935) | 0.021 | 0.637 (0.429–0.947) | 0.026 |
| Unknown | 32 | 0.962 (0.653–1.416) | 0.843 | 0.865 (0.582–1.285) | 0.471 |
| Sputum smear grading at baseline visit | |||||
| Negative | 32 | Reference | – | – | – |
| Scanty (1–9 AFB/100 HPF) | 12 | 0.706 (0.354–1.406) | 0.322 | 0.650 (0.323–1.306) | 0.226 |
| +1 (10–99 AFB/100 HPF) | 133 | 0.679 (0.457–1.010) | 0.056 | 0.874 (0.577–1.322) | 0.523 |
| +2 (1–9 AFB/HPF) | 127 | 0.701 (0.471–1.043) | 0.080 | 0.844 (0.560–1.271) | 0.417 |
| +3 (> 9 AFB/HPF) | 124 | 0.597 (0.400–0.893) | 0.012 | 0.764 (0.503–1.162) | 0.209 |
| Comorbidity | 0.493 | – | – | ||
| No | 368 | Reference | |||
| Yes | 60 | 1.105 (0.830–1.471) | |||
| Number of resistant drugs | |||||
| < 4 | 45 | Reference | – | – | – |
| 5–6 | 370 | 0.697 (0.499–0.974) | 0.034 | 1.321 (0.719–2.427) | 0.370 |
| > 6 | 13 | 0.421 (0.209–0.847) | 0.015 | 0.984 (0.394–2.457) | 0.972 |
| Resistance to all five first lines drugs (HREZS) | |||||
| No | 180 | Reference | – | – | – |
| Yes | 248 | 0.716 (0.584–0.879) | 0.001 | 0.871 (0.526–1.443) | 0.591 |
| Resistance to streptomycin | 0.043 | 0.818 (0.506–1.322) | 0.411 | ||
| No | 129 | Reference | |||
| Yes | 299 | 0.798 (0.641–0.993) | |||
| Resistance to ethambutol | 0.027 | 0.944 (0.613–1.455) | 0.795 | ||
| No | 80 | Reference | |||
| Yes | 348 | 0.749 (0.580–0.967) | |||
| Resistance to pyrazinamide | 0.004 | 0.700 (0.425–1.153) | 0.161 | ||
| No | 45 | Reference | |||
| Yes | 383 | 0.621 (0.449–0.859) | |||
| Resistance to ofloxacin | < 0.001 | 0.656 (0.522–0.825) | < 0.001 | ||
| No | 222 | Reference | |||
| Yes | 206 | 0.625 (0.509–0.766) | |||
| Resistance to ethionamide | 0.225 | – | – | ||
| No | 406 | Reference | |||
| Yes | 22 | 0.746 (0.465–1.198) | |||
| Resistance to injectable SLDs | 0.492 | – | – | ||
| No | 424 | Reference | |||
| Yes | 4 | 0.707 (0.262–1.903) | |||
| Cavitation on baseline chest X-ray | 0.005 | 0.744 (0.595–0.931) | 0.010 | ||
| No | 283 | Reference | |||
| Yes | 145 | 0.733 (0.590–0.910) |
AFB = acid fast bacilli; CI = confidence interval; E = ethambutol; H = isoniazid; HPF = high power field; HR = hazard ratio; R = rifampicin; S = streptomycin; SLD = second-line drugs; Z = pyrazinamide.
Treatment outcomes and factors associated with cure.
Of 428 patients enrolled, 329 (76.9%) were cured, 80 (18.7%) died, and 19 (4.4%) were declared treatment failures. In multivariate analysis, patients’ age of 41–60 (odds ratio [OR] = 0.202; 95% CI = 0.067–0.605) and > 60 years (OR = 0.051; 95% CI = 0.011–0.224), body weight > 40 kg (OR = 2.950; 95% CI = 1.462–5.952), history of SLD use (OR = 0.277; 95% CI = 0.097–0.789), lung cavitation (OR = 0.196; 95% CI = 0.103–0.371), resistance to ofloxacin (OR = 0.386; 95% CI = 0.198–0.749), and SCC at four (OR = 4.580; 95% CI = 1.391–15.077) and 6 months (OR = 11.622; 95% CI = 3.188–42.371) of treatment were significantly associated with cure (Table 3).
Table 3.
Factors associated with cure
| Variable | Cure no. (%) | Univariate analysis OR (95% CI) | P value | Multivariate analysis OR (95% CI) | P value |
|---|---|---|---|---|---|
| Gender | 0.449 | – | – | ||
| Female | 187 (78.2) | Reference | |||
| Male | 142 (75.1) | 0.840 (0.535–1.319) | |||
| Age (years) | |||||
| 10–20 | 74 (81.3) | Reference | – | Reference | – |
| 21–40 | 196 (82.4) | 1.072 (0.575–2.000) | 0.827 | 0.652 (0.261–1.630) | 0.360 |
| 41–60 | 52 (67.5) | 0.478 (0.235–0.973) | 0.042 | 0.202 (0.067–0.605) | 0.004 |
| > 60 | 7 (31.8) | 0.107 (0.038–0.303) | 0.000 | 0.051 (0.011–0.224) | < 0.001 |
| Weight (kg) | < 0.001 | 0.003 | |||
| < 40 | 100 (66.7) | Reference | Reference | ||
| ≥ 40 | 229 (82.4) | 2.337 (1.477–3.697) | 2.950 (1.462–5.952) | ||
| Residence | 0.201 | – | – | ||
| Rural | 172 (74.5) | Reference | |||
| Urban | 157 (79.0) | 1.346 (0.853–2.124) | |||
| Previous treatment regimen | – | ||||
| New patients | 38 (77.6) | Reference | – | – | |
| Category I | 145 (78.4) | 1.049 (0.492–2.237) | 0.901 | – | |
| Category II | 125 (77.2) | 0.978 (0.455–2.101) | 0.954 | – | |
| Unknown | 21 (65.6) | 0.553 (0.205–1.489) | 0.241 | – | |
| History of SLD use | |||||
| No | 288 (79.8) | Reference | – | Reference | – |
| Yes | 20 (57.1) | 0.338 (0.165–0.692) | 0.003 | 0.277 (0.097–0.789) | 0.016 |
| Unknown | 21 (65.6) | 0.484 (0.223–1.049) | 0.066 | 0.367 (0.103–1.071) | 0.063 |
| Comorbidity | 0.304 | – | – | ||
| No | 286 (77.7) | Reference | |||
| Yes | 43 (71.1) | 0.725 (0.393–1.339) | |||
| Baseline chest X-ray | < 0.001 | < 0.001 | |||
| No cavitation | 246 (86.9) | Reference | Reference | ||
| Cavitation | 83 (57.2) | 0.201 (0.125–0.324) | 0.196 (0.103–0.371) | ||
| Baseline smear grading | – | ||||
| Negative | 27 (84.4) | Reference | – | – | |
| Scanty (1–9 AFB/100 HPF) | 11 (91.7) | 2.037 (0.213–19.494) | 0.537 | – | |
| +1 (10–99 AFB/100 HPF) | 101 (75.9) | 0.584 (0.208–1.643) | 0.309 | – | |
| +2 (1–9 AFB/HPF) | 99 (78.0) | 0.655 (0.231–1.857) | 0.426 | – | |
| +3 (> 9 AFB/HPF) | 91 (73.4) | 0.511 (0.182–1.436) | 0.203 | – | |
| Number of resistant drugs | – | ||||
| < 4 | 35 (77.8) | Reference | – | – | |
| 5–6 | 286 (77.3) | 0.973 (0.462–2.046) | 0.942 | – | |
| > 6 | 8 (61.5) | 0.457 (0.122–1.711) | 0.245 | – | |
| Resistance to all five first line drugs (HREZS) | 0.933 | – | – | ||
| No | 138 (76.7) | Reference | |||
| Yes | 191 (77.0) | 1.020 (0.647–1.607) | |||
| Resistance to streptomycin | 0.430 | – | – | ||
| No | 96 (74.4) | Reference | |||
| Yes | 233 (77.9) | 1.214 (0.750–1.963) | |||
| Resistance to ethambutol | 0.882 | – | – | ||
| No | 62 (77.5) | Reference | |||
| Yes | 267 (76.7) | 0.957 (0.535–1.710) | |||
| Resistance to pyrazinamide | 0.370 | – | – | ||
| No | 37 (82.2) | Reference | |||
| Yes | 292 (76.2) | 0.694 (0.312–1.543) | |||
| Resistance to ofloxacin | < 0.001 | 0.005 | |||
| No | 191 (86.0) | Reference | Reference | ||
| Yes | 138 (67.0) | 0.329 (0.204–0.531) | 0.386 (0.198–0.749) | ||
| Resistance to ethionamide | 0.637 | – | – | ||
| No | 313 (77.1) | Reference | |||
| Yes | 16 (72.7) | 0.792 (0.301–2.083) | |||
| Resistance to injectable SLD | 0.227 | – | – | ||
| No | 327 (77.1) | Reference | |||
| Yes | 2 (50.0) | 0.297 (0.041–2.134) | |||
| SCC at month 2 | < 0.001 | 0.232 | |||
| No | 118 (63.8) | Reference | Reference | ||
| Yes | 211 (86.8) | 3.744 (2.322–6.036) | 0.608 (0.269–1.374) | ||
| SCC at month 4 | < 0.001 | 0.012 | |||
| No | 23 (31.1) | Reference | Reference | ||
| Yes | 306 (86.4) | 14.136 (7.925–25.215) | 4.580 (1.391–15.077) | ||
| SCC at month 6 | < 0.001 | < 0.001 | |||
| No | 8 (15.4) | Reference | Reference | ||
| Yes | 321 (85.4) | 32.100 (14.340–71.855) | 11.622 (3.188–42.371) |
AFB = acid fast bacilli; CI = confidence interval; E = ethambutol; H = isoniazid; HPF = high power field; OR = odds ratio; R = rifampicin; S = streptomycin; SCC = sputum culture conversion; SLD = second-line drug; Z = pyrazinamide.
Diagnostic performance of 2-, 4-, and 6-month SCC in predicting cure.
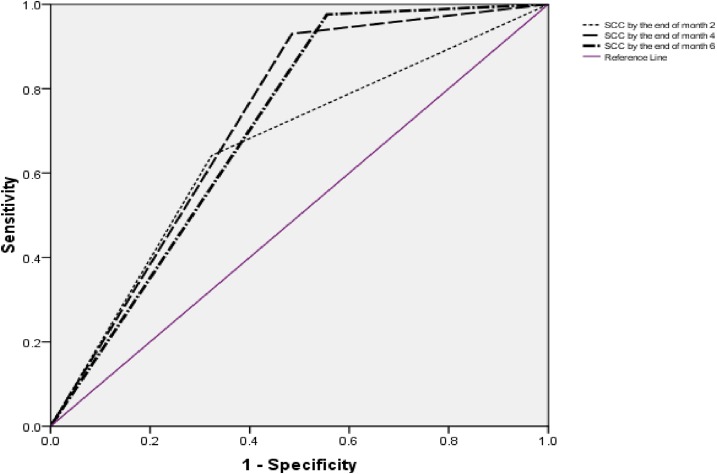
While comparing cure with death and treatment failure, sensitivities of SCC by the end of 2, 4, and 6 months were 64.1% (95 % CI = 58.69–69.32), 93.0% (95% CI = 89.69–95.52), and 97.6% (95% CI = 95.27–98.94), whereas specificities were 67.7% (95% CI = 57.53–76.73), 51.5% (95% CI = 41.25–61.68), and 44.4% (95% CI = 34.45–54.78), respectively (Table 4). Receiver operating characteristic curve shows the effect of using different time points of SCC on the balance between sensitivity and specificity (Figure 2).
Table 4.
Association of sputum culture conversion status with treatment outcomes
| Month of treatment | Treatment outcome death + failure cure | Odds ratio (95% CI) | P value* | Sensitivity (95% CI) | Specificity (95% CI) | |
|---|---|---|---|---|---|---|
| 2-month | < 0.001 | 64.1 (58.69–69.32) | 67.7 (57.53–76.73) | |||
| Did not convert | 67 (67.7) | 118 (35.9) | Reference | |||
| Converted | 32 (32.3) | 211 (64.1) | 3.74 (2.32–6.03) | |||
| 4-month | < 0.001 | 93.0 (89.69–95.52) | 51.5 (41.25–61.68) | |||
| Did not convert | 51 (51.5) | 23 (7.0) | Reference | |||
| Converted | 48 (48.5) | 306 (93.0) | 14.13 (7.92–25.21) | |||
| 6-month | < 0.001 | 97.6 (95.27–98.94) | 44.4 (34.45–54.78) | |||
| Did not convert | 44 (44.4) | 8 (2.4) | Reference | |||
| Converted | 55 (55.6) | 321 (97.6) | 32.10 (14.34–71.85) | |||
CI = confidence interval.
Univariate binary logistic regression analysis.
Figure 2.
Receiver operating characteristic curve for diagnostic performance of timing of initial sputum culture conversion in predicting treatment outcomes. This figure appears in color at www.ajtmh.org.
DISCUSSION
This study included 428 newly diagnosed culture-confirmed pulmonary MDR-TB patients. It investigated the predicted value of SCC at different time points in predicting treatment outcomes. It also evaluated factors associated with time to SCC and cure. Overall, the findings revealed that 90.9% patients achieved initial SCC in a median time of 58 days (IQR 30–90 days), and 76.9% were cured. Time to SCC was significantly associated with treatment outcomes. In univariate analysis, achieving SCC at 2, 4, and 6 months of treatment had statistically significant positive association with cure. However, in multivariate analysis, SCC at 2 months did not reach the level significance, and association of SCC with cure was substantially stronger at 6 months (OR = 11.62) than at 4 months (OR = 4.58) (Table 3). Stronger association between SCC at 6 months and successful outcomes has been reported by studies conducted elsewhere.4,6
In our study, for predicting cure compared with death and treatment failure, SCC at 2 months had a sensitivity of 64.1% and specificity of 67.7%. Low sensitivity of SCC at 2 months of treatment (64.1%) demonstrates that, if it is used as a proxy marker for final outcomes, 35.9% of patients with ultimate cure would be misclassified as treatment failures. Such misclassification carries the risk of underrating overall therapeutic efficacy of a regimen, replacing effective drugs, terminating potentially effective regimen,4,6 and adding unnecessary drugs. In the current cohort, high sensitivity of SCC at 6 months (97.6%) indicates that treatment which failed to produce SCC at this time point was unlikely to produce cure. However, its low specificity (44.4%) suggests that 55.6% patients with eventual unsuccessful outcomes would be misclassified as effectively treated, thus overrating the effectiveness of the regimen. Our findings suggest that, although achieving SCC at 2 months gives some assurance about regimen efficacy, but because of its low sensitivity, lack of SCC at 2 months may be too early to decide about effectiveness of the regimen and its modification, unless the patient’s condition is worsening. On the other hand, as previously described by Kurbatova et al.,4 6 months may be too long to wait for SCC, again, depending on the overall clinical picture. In most cases, clinicians would not wait for a long time of 6 months before reevaluating the patient and changing the regimen. In the current cohort, sensitivity and specificity of SCC at 4 months were 93.0% and 51.5%, respectively. Our literature search did not identify a single study which has evaluated sensitivity and specificity of SCC at 4 months in predicting treatment outcomes of MDR-TB. As the combined sensitivity and specificity of SCC at 4 months (93.0% and 51.5%, respectively) was comparable with that of SCC at 6 months (97.6% and 44.4%, respectively), using SCC at month 4 as a proxy marker in predicting final outcomes can reduce the physicians’ waiting period to decide about the effectiveness of the regimen.
In the present study, all the three factors associated with delayed SCC, that is, resistance to ofloxacin, lung cavitation at baseline visit, and history of SLD use were common in patients with unsuccessful treatment outcomes. We observed significantly delayed SCC and high rate of death and treatment failure among those patients who were resistant to ofloxacin. Fluoroquinolones’ use with susceptibility has widely been related to cure15–20 and early SCC among MDR-TB patients.12,21 Because these agents have favorable therapeutic characteristics of peak drug concentration, good tissue penetration particularly into lung parenchyma and fast bactericidal and sterilizing effects,22 finding a positive association between resistance to fluoroquinolones, and both delayed SCC and unsuccessful outcomes is not an astonishing one.
In the current cohort, lung cavitation also had statistically significant positive association with both delayed SCC and unsuccessful outcomes. Cavitary lung disease has previously been reported as a risk factor for delayed SCC12,23–25 and unsuccessful outcomes among MDR-TB patients.8,15,18 Patients with lung cavitation at baseline visit often have more severe disease and longer delay before obtaining medical care. As the presence of lung cavities owing to parenchymal damage decreases the penetration of antibacterial drugs and hence their efficacy,22 the finding of significantly delayed SCC and higher odds of unsuccessful treatment outcomes in this group of patients was justifiable. In the present analysis, documented history of SLD use was another common risk factor for both delayed SCC and death and treatment failure. This variable has previously been reported as a risk factor for unsuccessful outcomes in MDR-TB.18,26 Amplification of drug resistance due to faulty TB treatment, exposure to SLD, and patients’ poor adherence with therapy could be the possible reason for delayed SCC and poor outcomes in patients with a history of SLD use.18
In multivariate analysis, patients’ baseline body weight of < 40 kg and age > 40 years also emerged as risk factors for unsuccessful outcomes. Similar positive association between lower body weight and unsuccessful treatment outcomes among MDR-TB patients has been reported by other studies conducted elsewhere.2,15,18,26 Along with other several mechanisms, poor nutritional status of patients may be a contributing factor for subtherapeutic serum drug levels in TB patients.27 Malnourishment results in decrease in the plasma drug concentration time curve, and increase in renal clearance of free drug, thus leading to subtherapeutic serum drug concentration which may lead to increased morbidity, mortality, and acquired drug resistance.20 In addition, insufficient dosing of anti-TB drugs in underweight patients might be another contributing factor for high rate of death or treatment failure.27 High risk of death and treatment failure in patients with age > 40 years of current cohort was in line with findings of other studies conducted elsewhere.15,18,28
CONCLUSION
The overall association of SCC with cure was substantially stronger at 6 months than at 4 and 2 months of treatment. However, low sensitivity of SCC at 2 months and specificity at 4 and 6 months of treatment suggest that none of these prognostic markers appear to be perfect in predicting treatment outcomes in MDR-TB patients. The missed opportunities to replace a more effective treatment regimen for patients with eventual unsuccessful treatment outcomes were common. As the combined sensitivity and specificity of SCC at 4 months was comparable with that of SCC at 6 months, using SCC at month 4 as a proxy marker in predicting final outcomes can reduce the physicians’ waiting period to decide about regimen efficacy. In the current cohort, risk factors for delayed SCC were common in patients with unsuccessful treatment outcomes. These factors are generally identifiable before diagnosis of MDR-TB or early in the course of treatment, providing enhanced clinical management and special attention to these patients may improve treatment outcomes further.
Large sample size and patients with different disease severity and degree of drug resistance were the strengths of the present study. The study site had a wide catchment area, where MDR-TB patients from a widely distributed geographical area were referred for treatment,29 nevertheless, being a study from a single center, its results cannot be generalized. Because of lack of information on cause of death, we could not ascertain that all deaths were TB related. Because of retrospective design, we did not evaluate the effects of incidence of adverse events and regimen modification on SCC and treatment outcomes. A multicenter study with prospective design is suggested to confirm the current findings.
Acknowledgments:
We thank all patients and record-keeping staff at the study site for their support in conducting this study. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
REFERENCES
- 1.World Health Organization , 2016, Companion Handbook to the WHO Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis (WHO/HTM/TB/2014.11). Geneva, Switzerland: WHO. Available at: http://apps.who.int/iris/bitstream/10665/130918/1/9789241548809_eng.pdf. Accessed November 22, 2017. [PubMed]
- 2.Holtz TH, Sternberg M, Kammerer S, Kammerer S, Laserson KF, Riekstina V, Zarovska E, Skripconoka V, Wells CD, Leimane V, 2006. Time to sputum culture conversion in multidrug-resistant tuberculosis: predictors and relationship to treatment outcome. Ann Intern Med 144: 650–659. [DOI] [PubMed] [Google Scholar]
- 3.Fortún J, Martín-Dávila P, Molina A, Navas E, Hermida JM, Cobo J, Gómez-Mampaso E, Moreno S, 2007. Sputum conversion among patients with pulmonary tuberculosis: are there implications for removal of respiratory isolation? J Antimicrob Chemother 59: 794–798. [DOI] [PubMed] [Google Scholar]
- 4.Kurbatova EV, et al. 2015. Sputum culture conversion as a prognostic marker for end-of-treatment outcome in patients with multidrug-resistant tuberculosis: a secondary analysis of data from two observational cohort studies. Lancet Respir Med 3: 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joseph P, Desai VBR, Mohan NS, Fredrick JS, Ramachandran R, Raman B, Wares F, Ramachandran R, Thomas A, 2011. Outcome of standardized treatment for patients with MDR-TB from Tamil Nadu, India. Indian J Med Res 133: 529–534. [PMC free article] [PubMed] [Google Scholar]
- 6.Lu P, Liu Q, Martinez L, Yang H, Lu W, Ding X, Zhu L, 2017. Time to sputum culture conversion and treatment outcome of patients with multidrug-resistant tuberculosis: a prospective cohort study from urban China. Eur Respir J 49: 1601558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ige O, Akindele Y, Oladokun R, Adebiyi O, 2014. Sputum culture conversion among the first cohorts of MDR-TB patients managed in Nigeria at a tertiary care hospital. Eur Respir J 44: 2625. [Google Scholar]
- 8.Rodriguez M, et al. 2013. Successful management of multidrug-resistant tuberculosis under programme conditions in the Dominican Republic. Int J Tuberc Lung Dis 17: 520–525. [DOI] [PubMed] [Google Scholar]
- 9.Wells CD, Gupta R, Hittel N, Geiter LJ, 2015. Long-term mortality assessment of multidrug-resistant tuberculosis patients treated with delamanid. Eur Respir J 45: 1498–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization , 2016. Global Tuberculosis Report 2016 Geneva, Switzerland: WHO. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed November 23, 2017.
- 11.Tahseen S, Qadeer E, Khanzada FM, Rizvi AH, Dean A, Van Deun A, Zignol M, 2016. Use of Xpert (R) MTB/RIF assay in the first national anti-tuberculosis drug resistance survey in Pakistan. Int J Tuberc Lung Dis 20: 448–455. [DOI] [PubMed] [Google Scholar]
- 12.Basit A, et al. 2014. Predictors of two months culture conversion in multidrug-resistant tuberculosis: findings from a retrospective cohort study. PLoS One 9: e93206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qazi F, Khan U, Khowaja S, Javaid M, Ahmed A, Salahuddin N, Hussain H, Becerra MC, Golub JE, Khan AJ, 2011. Predictors of delayed culture conversion in patients treated for multidrug-resistant tuberculosis in Pakistan. Int J Tuberc Lung Dis 15: 1556–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Javaid A, Ahmad N, Khan AH, Shaheen Z, 2017. Applicability of the World Health Organization recommended new shorter regimen in a multidrug-resistant tuberculosis high burden country. Eur Respir J 3: 1601967. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad N, Javaid A, Basit A, Afridi A, Khan M, Ahmad I, Sulaiman SA, Khan AH, 2015. Management and treatment outcomes of MDR-TB: results from a setting with high rates of drug resistance. Int J Tuberc Lung Dis 19: 1109–1114. [DOI] [PubMed] [Google Scholar]
- 16.Falzon D, et al. 2012. Resistance to fluoroquinolones and second-line injectable drugs: impact on MDR-TB outcomes. Eur Respir J 42: 156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM, 2009. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLoS One 4: e6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurbatova EV, et al. 2012. Predictors of poor outcomes among patients treated for multidrug-resistant tuberculosis at DOTS-plus projects. Tuberculosis (Edinb) 92: 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Migliori G, et al. 2008. Resistance to second-line injectables and treatment outcomes in multidrug-resistant and extensively drug-resistant tuberculosis cases. Eur Respir J 31: 1155–1159. [DOI] [PubMed] [Google Scholar]
- 20.Javaid A, Shaheen Z, Shafqat M, Khan AH, Ahmad N, 2017. Risk factors for high death and loss-to-follow-up rates among patients with multidrug-resistant tuberculosis at a programmatic management unit. Am J Infect Control 45: 190–193. [DOI] [PubMed] [Google Scholar]
- 21.Hovhannesyan A, Breeze E, 2012. Time to sputum conversion in multidrug-resistant tuberculosis patients in Armenia: retrospective cohort study. Global J Med Pub Health 1: 24–28. [Google Scholar]
- 22.Yew WW, Chan CK, Chau CH, Tam CM, Leung CC, Wong PC, Lee J, 2000. Outcomes of patients with multidrug-resistant pulmonary tuberculosis treated with ofloxacin/levofloxacin-containing regimens. Chest 117: 744–751. [DOI] [PubMed] [Google Scholar]
- 23.Su W, Feng J, Chiu Y, Huang S, Lee Y, 2011. Role of 2-month sputum smears in predicting culture conversion in pulmonary tuberculosis. Eur Respir J 37: 376–383. [DOI] [PubMed] [Google Scholar]
- 24.Gammino V, et al. 2011. Bacteriologic monitoring of multidrug-resistant tuberculosis patients in five DOTS-Plus pilot projects. Int J Tuberc Lung Dis 15: 1315–1322. [DOI] [PubMed] [Google Scholar]
- 25.Tierney DB, et al. 2014. Time to culture conversion and regimen composition in multidrug-resistant tuberculosis treatment. PLoS One 9: e108035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leimane V, Riekstina V, Holtz TH, Zarovska E, Skripconoka V, Thorpe LE, Laserson KF, Wells CD, 2005. Clinical outcome of individualised treatment of multidrug-resistant tuberculosis in Latvia: a retrospective cohort study. Lancet 365: 318–326. [DOI] [PubMed] [Google Scholar]
- 27.Byrd RP, Mehta JB, Roy TM, 2002. Malnutrition and pulmonary tuberculosis. Clin Infect Dis 35: 634–635. [DOI] [PubMed] [Google Scholar]
- 28.Tahaoğlu K, Törün T, Sevim T, Ataç G, Kir A, Karasulu L, Ozmen I, Kapakli N, 2001. The treatment of multidrug-resistant tuberculosis in Turkey. N Engl J Med 345: 170–174. [DOI] [PubMed] [Google Scholar]
- 29.Ahmad N, Javaid A, Sulaiman SAS, Basit A, Afridi AK, Jaber AAS, Khan AH, 2016. Effects of multidrug resistant tuberculosis treatment on patients’ health related quality of life: results from a follow up study. PLoS One 11: e0159560. [DOI] [PMC free article] [PubMed] [Google Scholar]