Abstract.
Controlled Human Malaria Infection (CHMI) has become an increasingly important tool for the evaluation of drugs and vaccines. Controlled Human Malaria Infection has been demonstrated to be a reproducible model; however, there is some variability in time to onset of parasitemia between volunteers and studies. At our center, mosquitoes infected with Plasmodium falciparum by membrane feeding have variable and high salivary gland sporozoite load (mean 78,415; range 26,500–160,500). To determine whether this load influences parasitemia after CHMI, we analyzed data from 13 studies. We found no correlation between the sporozoite load of a mosquito batch and time to parasitemia or parasite density of first-wave parasitemia. These findings support the use of infected mosquito bite as a reproducible means of inducing P. falciparum infection and suggest that within this range, salivary gland sporozoite load does not influence the stringency of a CHMI.
Controlled human malaria infection (CHMI) by the bites of Plasmodium falciparum–infected, laboratory-reared Anopheles mosquitoes have been used to study the infection since the 1980s. In recent years, CHMI has become highly standardized and is used to assess the efficacy of antimalarial drugs and vaccines before large-scale field trials. This has been possible in part because of the high reproducibility of CHMI studies within and between centers.1 Nevertheless, there is variability in time to detectable parasitemia between studies and between centers.2,3 As in any biological system, there is significant variability between batches of infected mosquitoes used for CHMI, most notably in the number of salivary gland sporozoites. Recently, CHMI centers have increasingly started using intravenous injection of cryopreserved P. falciparum sporozoites to initiate infection,4,5 in part, because it is easier to standardize dosage. However, using the natural route of infection via the mosquito still has advantages over intravenous injection. Most importantly, it includes the immune response in the skin, which likely plays a role in both vaccine-induced protection6 and the response to primary infection.7
To test whether differences in salivary gland sporozoite load between mosquito batches influenced the outcomes of CHMI studies at our center, we collected data from all past clinical trials since 2007 in which malaria-naive volunteers were challenged with bites from five NF54 strain P. falciparum–infected Anopheles mosquitoes, as described previously.3 In these studies, mosquito batch percent infectivity and sporozoite load were quantified 1 day before CHMI by dissection of salivary glands from a sample of 10 mosquitoes per batch. Dissected salivary glands were pooled and homogenized using a glass grinder; sporozoites were quantified in a counting chamber using a phase-contrast microscope. The mean salivary gland sporozoite number per mosquito was calculated. After the malaria infection, volunteers were followed up once to three times daily from day 6 after infection until 3 days after the treatment of parasitemia. Quantitative real-time polymerase chain reaction (qPCR) was performed on blood samples either prospectively as the primary diagnostic test or retrospectively if thick blood smears were used as the primary diagnostic test.8,9
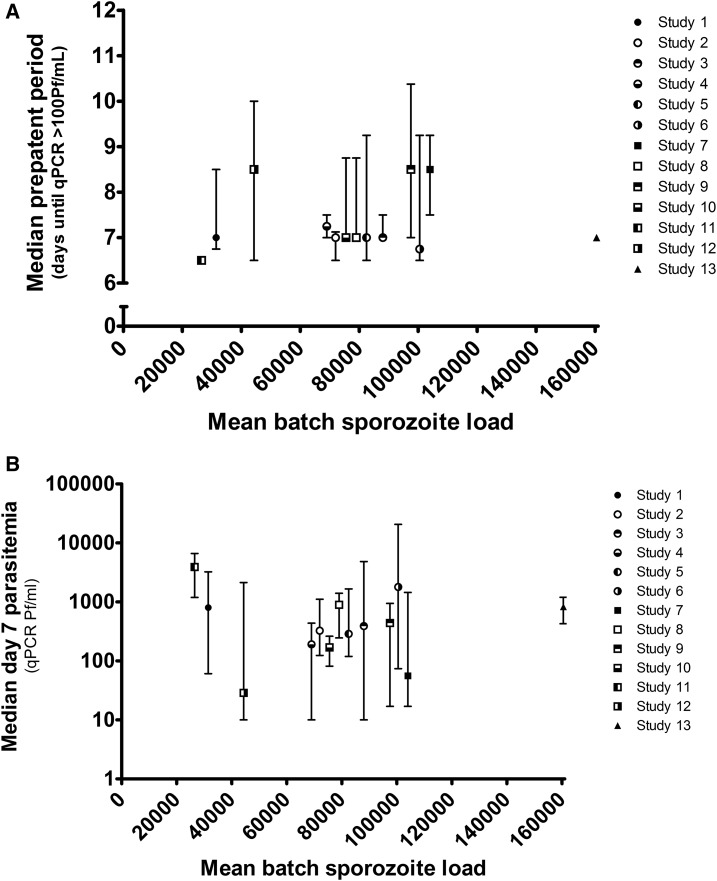
We analyzed data from 13 CHMIs taking place between 2007 and 2016, involving 75 malaria-naive volunteers (Table 1). The mean sporozoite load of the mosquito batches was variable, between 26,500 and 160,500 (mean 78,415; 95% confidence interval: 59,627–97,204). We found no correlation between the mean sporozoite load of the batch used and the time to parasitemia detectable by qPCR (Spearman r = 0.10), Figure 1A. Day 7 parasitemia can be used as a reliable proxy measurement for liver parasite burden: parasite density on day 7, after the challenge, correlates strongly with the mean parasitemia of the first wave of parasites to emerge from the liver (N = 50; Spearman r = 0.90; P < 0.0001), when antimalarial treatment is initiated after the first peak. There was no correlation between mosquito sporozoite load and parasitemia on day 7 after CHMI (Spearman r = 0.05), Figure 1B. Forty-nine volunteers (65%) were exposed to exactly five mosquitoes, the rest required a second exposure because either a mosquito was unfed or a fed mosquito was uninfected. As unfed mosquitoes may still have probed, possibly transmitting sporozoites, we performed a second analysis adjusting the total infected mosquito exposure. Here, there was also no correlation with time to parasitemia (Spearman r = 0.17) or day 7 parasitemia (Spearman r = 0.06).
Table 1.
Controlled human malaria infection studies used in this analysis
| Year | Trial registration number | Number of volunteers | Sporozoite prevalence (% mosquitoes infected) | Sporozoite intensity (mean number of sporozoites per mosquito) | |
|---|---|---|---|---|---|
| Study 1 | 2007 | NCT00442377 | 5 | 80 | 31,500 |
| Study 2 | 2008 | NCT00509158 | 18 | 100 | 72,800 |
| Study 3 | 2009 | NCT00757887 | 5 | 96.5 | 88,000 |
| Study 4 | 2010 | NCT01002833 | 4 | 100 | 69,000 |
| Study 5 | 2011 | NCT01218893 | 5 | 100 | 79,500 |
| Study 6 | 2012 | NCT01422954 | 4 | 100 | 98,250 |
| Study 7 | 2012 | NCT01627951 | 5 | 100 | 101,250 |
| Study 8 | 2012 | NCT01728701 | 5 | 93.8 | 75,800 |
| Study 9 | 2012 | NCT01728701 | 4 | 100 | 98,000 |
| Study 10 | 2015 | NCT02080026 | 5 | 100 | 74,000 |
| Study 11 | 2015 | NCT02098590 | 2 | 90 | 26,500 |
| Study 12 | 2015 | NCT02098590 | 3 | 100 | 44,300 |
| Study 13 | 2016 | NCT02692963 | 10 | 100 | 160,500 |
| Total | 75 |
Figure 1.
Correlation between Plasmodium falciparum mosquito infection and infectivity to humans. The mean salivary gland sporozoite load determined by the dissection of a sample of 10 mosquitoes compared with (A) the time to parasitemia detectable by PCR (> 100 parasites per milliliter) and (B) the height of parasitemia on day 7 postinfection. Points and error bars show the median and interquartile range.
In CHMIs where the number of injected sporozoites is precisely controlled, as in intravenous injection studies, increasing the sporozoite number has an effect on prepatent period; injection of 50 or 3,200 sporozoites resulted in a prepatent of 13.3 and 11.2 days, respectively.4 However, this analysis shows that between our CHMI trials, there is no difference in infectivity to volunteers depending on salivary gland sporozoite load of the mosquito batch used. In contrast, a recent study found that the probability of malaria transmission to humans decreased when mosquitoes had sporozoite loads of less than 1,000.10 However, only mosquitoes with 1–10, 11–100, 101–1,000, and > 1,000 sporozoites were compared, a much lower load than the mosquitoes used in our analysis. Taken together, these observations suggest that above a certain threshold, more sporozoites in the salivary glands no longer increase the number of sporozoites transmitted.
In the past, findings on the association between the mosquito salivary gland load of P. falciparum and the number of sporozoites injected by a salivating mosquito have been contradictory, with some studies finding a correlation11 and others not.12,13 In these studies, mosquitoes were typically induced to salivate onto glass cover slips or into capillary tubes. The number of sporozoites released during salivation was shown to be between 1 and nearly 1,000 sporozoites per mosquito, even when a much greater number was present in the salivary glands.13,14 More recently, studies with live imaging of fluorescent parasites have confirmed that only a small number of sporozoites are present in the mosquito salivary ducts and that each mosquito injects tens to hundreds of sporozoites during a feed.15,16 However, these types of studies have always been confined to rodent malaria models. Modeling of parasitemia after CHMI has calculated that an infected mosquito transmits an average of 21 sporozoites that successfully infect the liver,17 but studies directly linking the mosquito sporozoite load and liver parasite burden of P. falciparum are lacking.
The current data support the idea that the number of sporozoites injected by a feeding mosquito is independent of the number in the salivary glands, at high infection intensities. It is important to emphasize that all mosquito batches used in these studies had salivary gland sporozoite loads far exceeding that found in the field (usually less than 10,000).18 Other CHMI centers either have mosquito salivary gland sporozoite loads that are much lower or in the same range as the studies presented here.10,19,20 Irrespective of mosquito sporozoite load, most of these studies have prepatent periods similar to those at our center.9,19,20
A weakness of this analysis is that it used only pooled mean mosquito batch sporozoite counts. In future studies, qPCR can be applied to analyze individual mosquito sporozoite loads in relation to volunteer prepatent periods. In such a study, it would also be interesting to generate mosquitoes with more variable sporozoite loads by titrating gametocyte concentration in their blood meal.
In conclusion, we demonstrate that the infectivity of mosquitoes to humans in CHMI studies at our center is independent of the salivary gland sporozoite load of the mosquito batch used. This finding supports the use of infected mosquito bite as a reproducible means of inducing P. falciparum infection and suggests that at high levels of infectivity, increased salivary gland sporozoite load does not increase the stringency of a mosquito challenge.
Acknowledgments:
We would like to thank all the staff from the Radboud University Medical Center, Leiden University Medical Center, Harbour Hospital, and the Erasmus Medical Center in Rotterdam who worked on these studies. Especially, we would like to thank Marga van de Vegte-Bolmer and Rianne Siebelink-Stoter for their work in generating the malaria parasites used in the CHMIs. Finally, we want to thank all the volunteers who participated in these trials.
REFERENCES
- 1.Sauerwein RW, Roestenberg M, Moorthy VS, 2011. Experimental human challenge infections can accelerate clinical malaria vaccine development. Nat Rev Immunol 11: 57–64. [DOI] [PubMed] [Google Scholar]
- 2.Roestenberg M, O’Hara GA, Duncan CJ, Epstein JE, Edwards NJ, Scholzen A, van der Ven AJ, Hermsen CC, Hill AV, Sauerwein RW, 2012. Comparison of clinical and parasitological data from controlled human malaria infection trials. PLoS One 7: e38434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verhage DF, Telgt DS, Bousema JT, Hermsen CC, van Gemert GJ, van der Meer JW, Sauerwein RW, 2005. Clinical outcome of experimental human malaria induced by Plasmodium falciparum-infected mosquitoes. Neth J Med 63: 52–58. [PubMed] [Google Scholar]
- 4.Mordmuller B, et al. 2015. Direct venous inoculation of Plasmodium falciparum sporozoites for controlled human malaria infection: a dose-finding trial in two centres. Malar J 14: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roestenberg M, et al. 2013. Controlled human malaria infections by intradermal injection of cryopreserved Plasmodium falciparum sporozoites. Am J Trop Med Hyg 88: 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keitany GJ, et al. 2014. Immunization of mice with live-attenuated late liver stage-arresting Plasmodium yoelii parasites generates protective antibody responses to preerythrocytic stages of malaria. Infect Immun 82: 5143–5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mac-Daniel L, Buckwalter MR, Berthet M, Virk Y, Yui K, Albert ML, Gueirard P, Menard R, 2014. Local immune response to injection of Plasmodium sporozoites into the skin. J Immunol 193: 1246–1257. [DOI] [PubMed] [Google Scholar]
- 8.Hermsen CC, de Vlas SJ, van Gemert GJ, Telgt DS, Verhage DF, Sauerwein RW, 2004. Testing vaccines in human experimental malaria: statistical analysis of parasitemia measured by a quantitative real-time polymerase chain reaction. Am J Trop Med Hyg 71: 196–201. [PubMed] [Google Scholar]
- 9.Walk J, Schats R, Langenberg MC, Reuling IJ, Teelen K, Roestenberg M, Hermsen CC, Visser LG, Sauerwein RW, 2016. Diagnosis and treatment based on quantitative PCR after controlled human malaria infection. Malar J 15: 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Churcher TS, et al. 2017. Probability of transmission of malaria from mosquito to human is regulated by mosquito parasite density in naive and vaccinated hosts. PLoS Pathog 13: e1006108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg R, Wirtz RA, Schneider I, Burge R, 1990. An estimation of the number of malaria sporozoites ejected by a feeding mosquito. Trans R Soc Trop Med Hyg 84: 209–212. [DOI] [PubMed] [Google Scholar]
- 12.Ponnudurai T, Lensen AH, van Gemert GJ, Bolmer MG, Meuwissen JH, 1991. Feeding behaviour and sporozoite ejection by infected Anopheles stephensi. Trans R Soc Trop Med Hyg 85: 175–180. [DOI] [PubMed] [Google Scholar]
- 13.Beier JC, Onyango FK, Koros JK, Ramadhan M, Ogwang R, Wirtz RA, Koech DK, Roberts CR, 1991. Quantitation of malaria sporozoites transmitted in vitro during salivation by wild Afrotropical Anopheles. Med Vet Entomol 5: 71–79. [DOI] [PubMed] [Google Scholar]
- 14.Habluetzel A, Merzagora L, Jenni L, Betschart B, Rotigliano G, Esposito F, 1992. Detecting malaria sporozoites in live, field-collected mosquitoes. Trans R Soc Trop Med Hyg 86: 138–140. [DOI] [PubMed] [Google Scholar]
- 15.Frischknecht F, Baldacci P, Martin B, Zimmer C, Thiberge S, Olivo-Marin JC, Shorte SL, Menard R, 2004. Imaging movement of malaria parasites during transmission by Anopheles mosquitoes. Cell Microbiol 6: 687–694. [DOI] [PubMed] [Google Scholar]
- 16.Amino R, Thiberge S, Martin B, Celli S, Shorte S, Frischknecht F, Menard R, 2006. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat Med 12: 220–224. [DOI] [PubMed] [Google Scholar]
- 17.Coffeng LE, Hermsen CC, Sauerwein RW, de Vlas SJ, 2017. The power of malaria vaccine trials using controlled human malaria infection. PLoS Comput Biol 13: e1005255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pringle G, 1966. A quantitative study of naturally-acquired malaria infections in Anopheles gambiae and Anopheles funestus in a highly malarious area of East Africa. Trans R Soc Trop Med Hyg 60: 626–632. [DOI] [PubMed] [Google Scholar]
- 19.Lyke KE, et al. 2010. Plasmodium falciparum malaria challenge by the bite of aseptic Anopheles stephensi mosquitoes: results of a randomized infectivity trial. PLoS One 5: e13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talley AK, et al. 2014. Safety and comparability of controlled human Plasmodium falciparum infection by mosquito bite in malaria-naive subjects at a new facility for sporozoite challenge. PLoS One 9: e109654. [DOI] [PMC free article] [PubMed] [Google Scholar]