Abstract.
Members of the genera Alphavirus (family Togaviridae) and Flavivirus (family Flaviridae) are important zoonotic human and equine etiologic agents of neurologic diseases in the New World. In 2010, an outbreak of Madariaga virus (MADV; formerly eastern equine encephalitis virus) and Venezuelan equine encephalitis virus (VEEV) infections was reported in eastern Panamá. We further characterized the epidemiology of the outbreak by studying household contacts of confirmed human cases and of equine cases with neurological disease signs. Serum samples were screened using a hemagglutination inhibition test, and human results were confirmed using plaque reduction neutralization tests. A generalized linear model was used to evaluate the human MADV and VEEV seroprevalence ratios by age (in tercile) and gender. Overall, antibody prevalence for human MADV infection was 19.4%, VEEV 33.3%, and Mayaro virus 1.4%. In comparison with individuals aged 2–20 years, people from older age groups (21–41 and > 41 years) were five times more likely to have antibodies against VEEV, whereas the MADV prevalence ratio was independent of age. The overall seroprevalence of MADV in equids was 26.3%, VEEV 29.4%, West Nile virus (WNV) 2.6%, and St. Louis encephalitis virus (SLEV) was 63.0%. Taken together, our results suggest that multiple arboviruses are circulating in human and equine populations in Panamá. Our findings of a lack of increase in the seroprevalence ratio with age support the hypothesis of recent MADV exposure to people living in the affected region.
INTRODUCTION
Zoonotic human and equine neurotropic arboviral diseases in the New World are principally associated with infections by members of the genera Alphavirus (family Togaviridae) and Flavivirus (family Flaviviridae).1–3 Among the most important alphaviruses for public health are members of the eastern equine encephalitis (EEE) and Venezuelan equine encephalitis (VEE) antigenic complexes, as well as Mayaro virus (MAYV) in the Semliki Forest complex.1,4,5 Recently, EEE viruses (EEEVs) have been reclassified into two species: EEEV (in North America and the Caribbean during past outbreaks) and Madariaga virus (MADV, in Central and South America), based primarily on differences in their genetic, antigenic, and virulence profiles.6 The association of MADV with an outbreak of neurologic human disease was first reported in 2010 during an equine epizootic in the eastern province of Darién in Panamá. Before 2010, surveillance and research efforts in Latin America did not demonstrate any association of MADV with human disease outbreaks, even during major equine epizootics.7–10
Human infection with VEE virus (VEEV) was first described in Panamá in a fatal case during 1961.11 Since then, human infections have been recognized throughout the country with a case fatality rate of approximately 10%.12 In Panamá, VEEV causes human disease during spillover infections from an enzootic cycle that involves sylvatic rodents and Culex (Melanoconion) spp. mosquitoes. During the 2010 encephalitis outbreak in Panamá, MADV and VEEV were co-circulating and both caused severe human neurologic disease.7
Previous studies have suggested a gender, age, and occupational bias during MADV and VEEV outbreaks.13 In addition, an age-related difference in seroprevalence may suggest a difference in the activity level of the viruses over time and a recent human MADV emergence in Panamá.13 To better understand the epidemiologic features of the 2010 human and equine MADV outbreak, we performed a human cross-sectional serosurvey for MADV and VEEV in 72 household contacts of the 2010 outbreak, and we analyzed the serologic results and geographical distribution of equine disease. We also aimed to determine the age and gender variation of MADV and VEEV exposure in household contacts of the 2010 human cases.
METHODS
Study site.
The 2010 encephalitic outbreak was reported principally in Darién Province (7°38′0″N to 76°57′0″W), in eastern Panamá, close to the border with Colombia. There are two major wetland habitats in these regions. The first, Bayano Lake, covers an area of 350 km2 and is located in the region of Choco– Darién. The vegetation of the region is classified as moist tropical forest with 85% relative humidity, 2,300 mm annual rainfall, and a mean temperature of 26°C. The second, Matusagarati Lagoon, has an area of 140 km2, and is located near the communities of Aguas Calientes and Yaviza in the Chepigana area of Darién Province. The area around Matusagarati Lagoon is also considered a moist tropical forest, with a mean average temperature of 25°C and annual rainfall of 2,000 mm. The rainy season at both sites lasts for 8–9 months (May–December) and the dry period usually occurs for 3–4 months (January–April).14
Human survey.
Household contacts of confirmed human and equine cases were surveyed. Contacts were defined as persons who lived with a MADV- or VEEV-confirmed human case and persons who lived on a farm where a confirmed equine case was reported. The survey was conducted between June 4 and September 15, 2010. Participants aged 2 years or older were eligible for inclusion. Each participant was interviewed using a standardized epidemiological form to collect clinical and demographic information. Trained phlebotomists collected 10 mL of blood (3 mL for children 2–8 years old) by peripheral venipuncture using standard aseptic technique. The samples were processed on-site within 12 hours by centrifugation to separate serum, then stored in liquid nitrogen, and transported to the Gorgas Memorial Institute or University of Texas Medical Branch for testing. Samples were collected during a public health response, so an institutional review board approval was not required. However, all identifying information of participants was removed and confidentiality was strictly respected.
Equine infections.
From early May to September, 2010, 194 horses with evidence of neurologic disease were detected in the provinces of Darién, Panamá, Colón, and Coclé. Detailed information of the epizootic and associated disease was reported previously.7 After owners reported evidence of equine disease to the Ministry of Agriculture, veterinarians and epidemiologists collected blood samples and recorded the geographical coordinates where the diseases horses were located. Of 194 horses sampled, 180 had geographic coordinates recorded, and these were used for spatial analysis.
Laboratory testing.
Antibodies against MADV, VEEV, and MAYV were tested using an ELISA-IgM15 in household contacts. Sera of equines with evidence of neurologic disease were screened by hemagglutination inhibition (HI) against MADV and VEEV, and also St. Louis encephalitis virus (SLEV) known to circulate in Panamá8 and West nile virus (WNV), at the beginning of the outbreak. For HI, sera were tested at a 1:20 dilution and at further 2-fold dilutions to the endpoint of 1:2,560. A probable past infection was indicated by an HI titer 4-fold or higher than that obtained for related viruses. Hemagglutination inhibition titers in general were moderate and ranged from 1:20 to 1:640.16–18 After secondary infections, there is typically an immediate anamnestic response with titers increasing during the first few days of illness, often reaching 1:640 to 10,240 or higher.16,18 Plaque reduction neutralization tests (PRNT) were used to confirm HI results as described previously.13,19 Vero cells monolayers (African green monkey, ATCC CCL-81; American Type Culture Collection, Manassas, VA) on 6-well plates were inoculated with serial dilutions of heat-inactivated sera mixed with 800 pfu/mL of a given virus and stained with crystal violet 10% after approximately 2 days of incubation at 37°C. A positive sample was reported as the reciprocal of the highest serum dilution that reduced the plaque count by ≥ 80% (PRNT80), a standard endpoint used for alphaviruses. Strains used for the PRNT were the chimeric Sindbis/MADV,19 Sindbis/VEEV_475,20 and MAYV wild-type strain CH1.21
Statistical analysis.
Humans.
Descriptive statistics for age (mean and range) and gender (frequencies) of participants were recorded. Crude seroprevalences for human MADV, VEEV, and MAYV were calculated. The association between seroprevalence for each virus with age (divided into terciles: 2−20, 21−41, and > 41 years old) and gender were expressed as prevalence ratios (PRs) using generalized linear models with a binomial distribution and log link. Bivariate and multivariate PRs were calculated with 95% confidence intervals (CIs) using robust standard errors to account for household clustering.22
Equids.
Crude HI antibody prevalence and 95% CIs for VEEV, MADV, SLEV, and WNV equine were calculated. Results were grouped into infections at the level of the genera alphavirus or flavivirus and mapped using the ArcGIS 10.2.2. (ESRI, Redlands, CA) to evaluate spatial clustering of equine infection. All human and equine analyses were performed using the statistical package Stata v14 (StataCorp, College Station, TX). P values with alpha < 0.05 were considered significant.
RESULTS
Human antibody prevalence.
A total of 72 household contacts of confirmed human MADV or VEEV infections was surveyed during the 2010 outbreak investigation, with a mean age of 31.8 years (range 2−96), and of which 63.9% (46/72) were male. Antibody prevalence for MADV and VEEV in surveyed contacts was 19.4% (14/72) and 33.3% (24/72), respectively. Of these, 11 participants (15.3%) were positive for both MADV and VEEV antibodies. Only one participant was positive for MAYV antibodies (1.4%). The prevalence of MADV by age group was 20.8% in the 2–20 years of age class, 20.0% in the 21–41 years of age class, and 17.4% in the ≥ 42 years of age class. Seroprevalence for VEEV by age was 8.3% in the 2–20 year group, 48.0% in the 21–41 year group, and 43.5% in the ≥ 42 year group.
Human age-based PR.
Seroprevalence for VEEV was statistically associated with age, with older classes having significantly higher prevalence rates. Compared with the reference age category (2–20 years of age), antibody prevalence for VEEV was five times higher in participants 21–41 years of age and in participants ≥ 42 years of age; these estimates were not affected by gender in the model. By contrast, the prevalence rate for MADV was independent of age, as there were no differences in estimators according to age categories. Human MADV exposure was also independent of gender (Table 1).
Table 1.
Characteristics of the human population studied and results of the multivariate generalized linear model of MADV and VEEV seroprevalence (PR; N = 72)
| Demographic characteristics | n (%) | Crude VEEV PR (95% CI) | P value | Adjusted VEEV* PR (95% CI) | P value | Crude MADV PR (95% CI) | P value | Adjusted MADV PR (95% CI) | P value |
|---|---|---|---|---|---|---|---|---|---|
| Age (terciles of age) | |||||||||
| 2–20 | 24 (33.3) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 21–41 | 25 (34.7) | 5.76 (1.42–23.3) | 0.014 | 5.77 (1.42–22.5) | 0.014 | 0.96 (0.32–2.92) | 0.943 | 0.91 (0.31–2.68) | 0.867 |
| > 41 | 23 (31.9) | 5.22 (1.27–21.5) | 0.022 | 5.16 (1.29–21.2) | 0.023 | 0.83 (0.25–2.75) | 0.767 | 0.88 (0.26–2.97) | 0.831 |
| Gender | |||||||||
| Male | 16/46 (34.8) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Female | 8/26 (30.8) | 0.88 (0.44–1.79) | 0.773 | 0.91 (0.46–1.80) | 0.783 | 1.77 (0.69–4.52) | 0.233 | 1.76 (0.70–4.41) | 0.225 |
CI = confidence interval; MADV = Madariaga virus; n = sample size per group; PR = prevalence ratio; VEEV = Venezuelan equine encephalitis virus.
Adjusted PR by age (terciles) and gender.
Equine infections.
A total of 194 equids with evidence of neurologic disease was tested, with seroprevalence estimated at 26.3% for MADV, 29.4% for VEEV, 2.6% for WNV, and 63.0% for SLEV (Table 2).
Table 2.
Regional (by province) and overall seroprevalence for MADV, VEEV, SLEV, and WNV in horses with neurologic signs
| Characteristics | N (%) | MADV (%) | CI 95%* | VEEV | CI 95%* | WNV | CI 95%* | SLEV | CI 95%* |
|---|---|---|---|---|---|---|---|---|---|
| Provinces | |||||||||
| Darién | 163 (84) | 43/120 (26.4) | 19.5–33.2 | 46/117 (28.2) | 21.2–35.2 | 0/163 | 0 | 68/95 (71.5) | 34.1–49.43 |
| Coclé | 3 (1.6) | 0/3 | 0 | 1/3 (33.3) | 11.0–76.0 | 2/3 (66.7) | 7.6–210 | 0/3 | – |
| Colón | 2 (1) | 0/2 | 0 | 2/2 (100) | 1 | 0/2 | – | 2/2 (100) | 1 |
| Panamá | 26 (13.4) | 8/18 (31.0) | 11.7–49.8 | 8/18 (30.8) | 11.7–49.8 | 3/23 (11.5) | 1.6–24.7 | 5/21 (23.8) | 3.0–35.5 |
| Total | 194 (100) | 51/143 (26.3) | 20.0–32.5 | 57/137 (29.4) | 23.1–36.3 | 5/189 (2.6) | 0.33–4.82 | 75/119 (63.0) | 31.7–45.6 |
CI = confidence interval; HI = hemagglutination inhibition; MADV = Madariaga virus; SLEV = St. Louis encephalitis virus; VEEV = Venezuelan equine encephalitis virus; WNV = West nile virus. These results are based on HI testing.
5% CI.
Geographic distribution of equine cases.
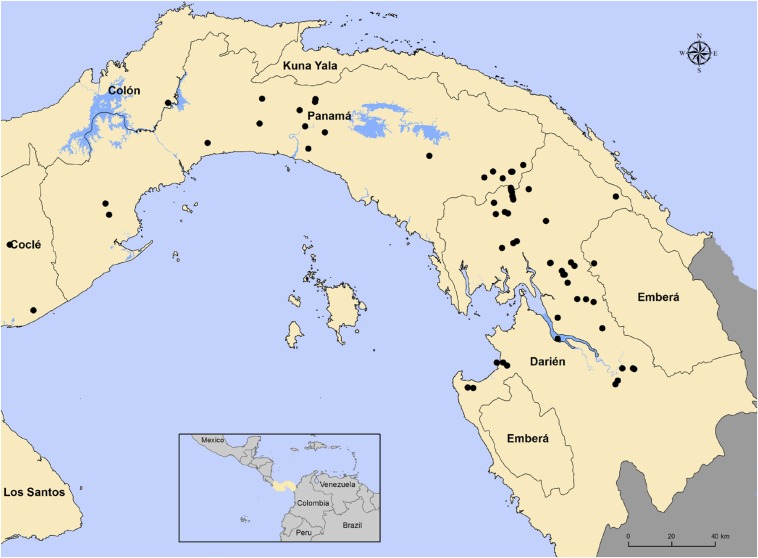
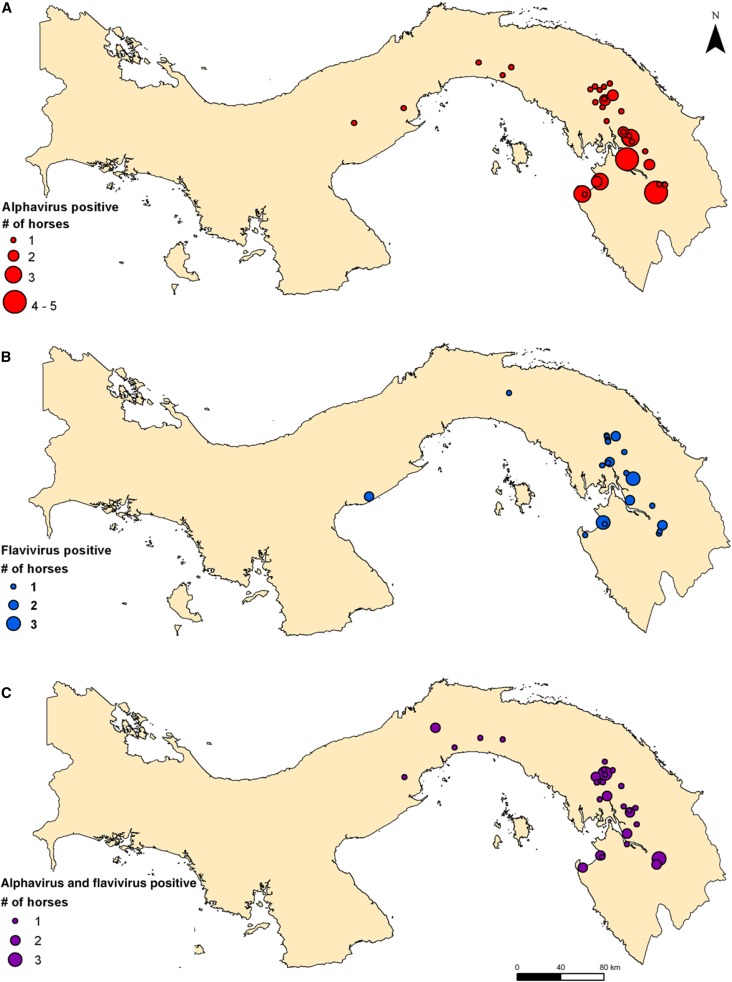
Equine cases with neurological signs were principally reported in the province of Darién (N = 163), with additional cases in the provinces of Coclé (N = 3), Colón (N = 2), and Panamá (N = 26) (Table 2, Figure 1). Most cases were located in the communities of El Real de Santa Maria, Aruza, and Tamarindo in Darién Province (Figure 2). Of the horses with location data, the majority apparently had a primary alphavirus antibody response: 53 were antibody-positive for only an alphavirus (see Figure 2A for distribution), 32 were antibody-positive for only a flavivirus (see Figure 2B for distribution), whereas 41 horses were antibody-positive for both virus groups (see Figure 2C). Of the 194 equids, 68 were negative for both alphaviruses and flaviviruses. Horses with neurologic signs were largely concentrated in the northwest of Darién Province, with animals positive only for an alphavirus having the highest numbers of cases per site (Figure 2A–C).
Figure 1.
Map showing locations of 180 horses with neurological signs sampled. This figure appears in color at www.ajtmh.org.
Figure 2.
Geographic distribution of horses with evidence of neurologic disease that had an (A) alphavirus, (B) flavivirus, and (C) alphavirus and flavivirus antibodies. This figure appears in color at www.ajtmh.org.
DISCUSSION
Mechanisms responsible for the 2010 MADV/VEEV emergences remain unknown. Human and equine neurologic cases during 2010 began in early May, at the beginning of the rainy season,7 a pattern also observed during previous epizootics in Panamá.8,23 In addition, additional equine cases were reported outside of the main outbreak area, a pattern shared with the 1973 and 1986 MADV epizootics.8,23 This timing could be related to bird migration and arrival in the wetlands,24,25 which could serve as active transmission foci due to the availability of resources for both vertebrates and mosquitoes. However, phylogenetic analyses suggest that MADV is stably enzootic in Panamá.7 Thus, migration of birds could mask the more important role of local, nonmigratory species because wetlands may provide resources for the birds of both types.14,24 Further studies should explore avian MADV seroprevalence to assess whether resident and/or migratory birds are amplifying hosts, in addition to rodents as suggested earlier.13
Here, we sought to better characterize the epidemiology of the 2010 equine and human encephalitis outbreak in Panamá by analyzing antibody prevalence data from household contacts of neurologic human cases and of horses with neurologic signs. Adjusted human MADV and VEEV seroprevalence rates demonstrated an increase of VEEV exposure with age (Table 1). This trend was not observed for MADV seroprevalence. The lack of increase in MADV exposure with age, as observed with VEEV, supports the hypothesis of only recent MADV human exposure in Panamá.7,13
The overall human MADV seroprevalence was 19.4%, and VEEV was 33.3%. Our estimate for MADV was higher than the 4.8% value reported in our 2012 study that enrolled around 770 participants.13 However, our current sample selection from household contacts may overestimate the overall population exposure. Subclinical infections with MADV are probable, supported by the high prevalence of neutralizing antibodies in household contacts without evidence of febrile or neurologic disease during the 2010 outbreak. High rates of subclinical infection were reported after a 1959 EEEV epizootic in New Jersey.26 However, our findings of antibodies against MADV in healthy persons during the 2010 Panamá outbreak contrast sharply with the lack of antibody detection in 1,700 humans tested during the 1973 Panamanian equine epizootic,8 a finding that also supports the hypothesis of recent change in human exposure or MADV infectivity in Darién, Panamá. No cases of MADV infection have been identified in other provinces of Panamá, although equine epizootics and MADV isolation have been documented in central and west Panamá during the past.8,27
We also detected 1.4% antibody prevalence for MAYV based on a single seropositive person. Circulation of MAYV has been previously detected in field-collected mosquitos from Panamá.28 However, human infection has not been detected in Panamá despite surveillance and epidemiologic investigations for alphavirus infections.8,28,29 Our results highlight the need for further investigation of MAYV epidemiology in Panamá to assess its potential to cause outbreaks of severe, chronic arthralgia, as observed in South America.4 A recent report of MAYV infection in Haiti suggests the possibility of recent spread.30
A total of 194 cases of equine disease was reported during the 2010 Panamanian outbreak including from the provinces of Darién, Coclé, Colón, and Panamá. Equids with evidence of neurologic disease and HI antibodies against alphaviruses appeared to be concentrated in Darién Province (Figure 2). Livestock farming is increasing in the Darién 31 and may facilitate equine alphaviral infection. Although 29.4% of equids were VEEV-seropositive, it is probable that little, if any, equine disease was related to VEEV infection because past studies demonstrated the equine avirulence of Panamanian strains.32 However inapparent equine VEEV infections are immunogenic,8,32 consistent with the antibody prevalence we observed. Overall, our results are consistent with MADV being the principal etiologic agent of the 2010 Panamanian equine epizootic.8,12,23,29
Our results suggest that WNV also circulates in Panamá, with seropositive horses in Coclé. A high prevalence of HI antibodies against SLEV was also detected (63.0%), consistent with the co-circulation of SLEV reported during previous MADV epizootics in Panamá.8 Evidence of high SLEV seroprevalence has been found in Argentina and Brazil.33–35 However, it is unclear if SLEV in Latin America causes neurologic disease in equids.36 This high level of SLEV exposure could explain, in part, the lack of fatal equine cases in Latin America due to WNV infection; cross-protective immunity among flavivirus infections has been hypothesized as a major contributor of the lack of WNV cases in Latin America.37
There are several important limitations of our study. First, there was an inherent sampling bias, as we targeted only household contacts of human cases and equine cases with neurological signs, which may have resulted in an overestimate of overall regional exposure. However, our aim was to explore the exposure of human contacts and equine cases to better understand factors leading to risk for alphavirus and flavivirus infection during this outbreak. The equine diagnoses were based only on HI assays, which can cross-react after a secondary infection. However, most equine cases were detected during acute infection and our HI titers of 1:20–1:640 suggest primary infections.16,18 Despite these limitations, HI has been an important technique for screening a family of viruses during outbreaks of unknown etiology, as occurred during the 2010 MADV outbreak.7 Although we found some relationships between MADV and VEEV infections and age, there may be other risk factors such as gender that we were not able to assess because our human sample size was too small. Further studies should increase sample sizes and explore land use analysis to increase statistical power and test the hypothesis of different sites of transmission.38,39
In conclusion, our results of age-stratified MADV but not VEEV seroprevalence suggest only recent exposure of people to the former virus, in contrast to VEEV where long-term endemic exposure is suggested. Our data also suggest the circulation of multiple neurotropic arboviruses in Panamá, including VEEV, MADV, SLEV, and WNV. In addition, evidence of MAYV infection in humans was found, emphasizing the need for further surveillance to detect and characterize other emerging mosquito-borne infections in Panamá. The temporal pattern of human and equine cases indicates seasonal transmission that starts during the early rainy season. Future studies should focus on identifying the enzootic hosts and mosquito vectors of MADV, as well as the environmental conditions that facilitate arboviral encephalitis outbreaks in Panamá.
Supplementary Material
Acknowledgments:
We thank the personnel of the Ministry of Health and Ministry of Agriculture Development involved in the 2010 outbreak response.
Note: Supplemental table appears at www.ajtmh.org.
Disclaimer: The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the Gorgas Memorial Institute of Health Studies or the Panamanian Government, and the institutions with which the authors are affiliated.
REFERENCES
- 1.Smith DW, Mackenzie JS, Weaver SC, 2009. Alphaviruses. Richman DD, Whitley RJ, Hayden FG, eds. Clinical Virology. Washington, DC: ASM Press, 1241–1274. [Google Scholar]
- 2.Weaver SC, Winegar R, Manger ID, Forrester NL, 2012. Alphaviruses: population genetics and determinants of emergence. Antiviral Res 94: 242–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindenbach BD, Rice CM, 2007. Flaviviridae: the viruses and their replication. Fields Virol 2: 1101–1151. [Google Scholar]
- 4.Tesh RB, et al. 1999. Mayaro virus disease: an emerging mosquito-borne zoonosis in tropical South America. Clin Infect Dis 28: 67–73. [DOI] [PubMed] [Google Scholar]
- 5.Aguilar PV, Estrada-Franco JG, Navarro-Lopez R, Ferro C, Haddow AD, Weaver SC, 2011. Endemic Venezuelan equine encephalitis in the Americas: hidden under the dengue umbrella. Future Virol 6: 721–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arrigo NC, Adams AP, Weaver SC, 2010. Evolutionary patterns of eastern equine encephalitis virus in North versus South America suggest ecological differences and taxonomic revision. J Virol 84: 1014–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrera J-P, et al. 2013. Eastern equine encephalitis in Latin America. N Engl J Med 369: 732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietz WH, Galindo P, Johnson KM, 1980. Eastern equine encephalomyelitis in Panama: the epidemiology of the 1973 epizootic. Am J Trop Med Hyg 29: 133–140. [DOI] [PubMed] [Google Scholar]
- 9.Sabattini MS, Daffner JF, Monath TP, Bianchi TI, Cropp CB, Mitchell CJ, Aviles G, 1991. Localized eastern equine encephalitis in Santiago del Estero Province, Argentina, without human infection. Medicina (B Aires) 51: 3–8. [PubMed] [Google Scholar]
- 10.Aguilar PV, et al. 2007. Endemic eastern equine encephalitis in the Amazon region of Peru. Am J Trop Med Hyg 76: 293–298. [PubMed] [Google Scholar]
- 11.Johnson KM, Shelokov A, Peralta PH, Dammin GJ, Young NA, 1968. Recovery of Venezuelan equine encephalomyelitis virus in Panama. A fatal case in man. Am J Trop Med Hyg 17: 432–440. [DOI] [PubMed] [Google Scholar]
- 12.Quiroz E, Aguilar PV, Cisneros J, Tesh RB, Weaver SC, 2009. Venezuelan equine encephalitis in Panama: fatal endemic disease and genetic diversity of etiologic viral strains. PLoS Negl Trop Dis 3: e472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vittor AY, et al. 2016. Epidemiology of emergent Madariaga encephalitis in a region with endemic Venezuelan equine encephalitis: initial host studies and human cross-sectional study in Darien, Panama. PLoS Negl Trop Dis 10: e0004554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holdridge LRDG, 1956. Report on an ecological survey of the republic of Panama. Caribb For 17: 92–110. [Google Scholar]
- 15.Beaty BJ, Calisher CH, Shope RE, 1989. Arboviruses. Schmidt NJ, Emmons RW, eds. Diagnostic Procedures for Viral, Rickettsial and Chlamydial Infections. 6th edition. Washington, DC: American Public Health Association, 797–855. [Google Scholar]
- 16.Clarke DH, Casals J, 1958. Techniques for hemagglutinatination-inhibition with arthropod borned viruses. Am J Trop Med Hyg 7: 561–573. [DOI] [PubMed] [Google Scholar]
- 17.Calisher CH, Karabatsos N, Dalrymple JM, Shope RE, Porterfield JS, Westaway EG, Brandt WE, 1989. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J Gen Virol 70: 37–43. [DOI] [PubMed] [Google Scholar]
- 18.Shope RE, 1963. The use of a micro-hemaglutination inhibition test to follow antibodie response after arthropod-borne virus infection in a community of forest animals. An Microbiol 11: 167–169 (Rio J). [Google Scholar]
- 19.Johnson BW, Kosoy O, Wang E, Delorey M, Russell B, Bowen RA, Weaver SC, 2011. Use of sindbis/eastern equine encephalitis chimeric viruses in plaque reduction neutralization tests for arboviral disease diagnostics. Clin Vaccin Immunol 18: 1486–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ni H, Yun NE, Zacks MA, Weaver SC, Tesh RB, Travassos Da Rosa AP, Powers AM, Frolov I, Paessler S, 2007. Recombinant alphaviruses are safe and useful serological diagnostic tools. Am J Trop Med Hyg 76: 774–781. [PubMed] [Google Scholar]
- 21.Weise WJ, Hermance ME, Forrester N, Adams AP, Langsjoen R, Gorchakov R, Wang E, Alcorn MDH, Tsetsarkin K, Weaver SC, 2014. A novel live-attenuated vaccine candidate for Mayaro fever. PLoS Negl Trop Dis 8: e2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barros AJD, Hirakata VN, 2003. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol 3: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obaldía Nicanor, et al. 1991. Encefalomielitis Equina del Este, Epizootia de 1986 en Panamá. Notas Vetrinarias 1: 4–7. [Google Scholar]
- 24.Bayly NJ, Gómez C, Cárdenas LC, 2011. Darién—Migration Monitoring during 2011 in the Tacarcuna Natural Reserve Bogotá. Available at: https://avesmigratoriascolombia.wordpress.com/. Accessed April 23, 2018.
- 25.Bayly NJ, Ortiz LC, Rubio M, Gómez C, 2014. Migration of raptors, swallows and other diurnal migratory birds through the Darien of Colombia. Ornitol Neotrop 25: 63–71. [Google Scholar]
- 26.Goldfield M, Welsh JN, Taylor BF, 1968. The 1959 outbreak of eastern encephalitis in New Jersey. 5. The inapparent infection:disease ratio. Am J Epidemiol 87: 32–33. [DOI] [PubMed] [Google Scholar]
- 27.Sunthorn S, Galindo P, 1967. The isolation of eastern equine encephalitis from Culex (Melanoconion) taeniopus Dyar and Knab in Panama. Mosq News 27: 74–76. [Google Scholar]
- 28.Galindo P, Srihongse S, De Rodaniche E, Grayson MA, 1966. An ecological survey for arboviruses in Almirante, Panama, 1959–1962. Am J Trop Med Hyg 15: 385–400. [DOI] [PubMed] [Google Scholar]
- 29.Carrera J-P, et al. 2017. Unusual pattern of chikungunya virus epidemic in the Americas, the Panamanian experience. PLoS Negl Trop Dis 11: e0005338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lednicky J, et al. 2016. Mayaro virus in child with acute febrile illness, Haiti, 2015. Emerg Infect Dis 22: 2000–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson GC, Harris V, Stone SW, 2001. Deforestation, land use, and property rights: empirical evidence from Darien, Panama. Land Econ 77: 187–205. [Google Scholar]
- 32.Walton TE, Alvarez O, Jr., Buckwalter RM, Johnson KM, 1973. Experimental infection of horses with enzootic and epizootic strains of Venezuelan equine encephalomyelitis virus. J Infect Dis 128: 271–282. [DOI] [PubMed] [Google Scholar]
- 33.Monath TP, Sabattini MS, Pauli R, Daffner JF, Mitchell CJ, Bowen GS, Cropp CB, 1985. Arbovirus investigations in Argentina, 1977–1980. IV. Serologic surveys and sentinel equine program. Am J Trop Med Hyg 34: 966–975. [PubMed] [Google Scholar]
- 34.Pauvolid-Correa A, Tavares FN, Costa EV, Burlandy FM, Murta M, Pellegrin AO, Nogueira MF, Silva EE, 2010. Serologic evidence of the recent circulation of Saint Louis encephalitis virus and high prevalence of equine encephalitis viruses in horses in the Nhecolandia sub-region in south Pantanal, central-west Brazil. Mem Inst Oswaldo Cruz 105: 829–833. [DOI] [PubMed] [Google Scholar]
- 35.Rodrigues SG, Oliva, O P, Francisco AAA, Martins LC, Chiang JO, Henriques DF, Pinto da Silva EV, Daniela SGR, Assis do, SCP, 2010. Epidemiology of Saint Louis encephalitis virus in the Brazilian Amazon region and in the State of Mato Grosso do Sul, Brazil: elevated prevalence of antibodies in horses. Rev Pan-Amazônica Saúde 1: 81–86. [Google Scholar]
- 36.Komar N, Clark GG, 2006. West Nile virus activity in Latin America and the Caribbean. Rev Panam Salud Publica 19: 112–117. [DOI] [PubMed] [Google Scholar]
- 37.Tesh RB, Travassos da Rosa APA, Guzman H, Araujo TP, Xiao SY, 2002. Immunization with heterologous flaviviruses protective against fatal West Nile encephalitis. Emerg Infect Dis 8: 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fleiss JL, 1986. Letters to the editor: confidence intervals vs significance tests: quantitative interpretation. Am J Public Health 76: 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foxman B, Frerichs RR, 1986. Letters to the editor: response from Drs. Foxman and Frerichs. Am J Public Health 76: 587.3963291 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.