Abstract
Microcystins have been the subject of increasingly alarming popular and scientific articles, which have taken as their unquestionable foundation the provisional Guideline of 1 μg/L established by the WHO Panel on microcystins levels in water, and mechanically translated by the Oregon government as 1 μg/g of Klamath Aphanizomenon flos aquae microalgae. This article underlines the significant limitations and ultimately scientific untenability of the WHO Guideline on microcystins in water, for being based on testing methodologies which may lead to a significant overestimation of the toxicity of microcystins. I propose criteria for the realization of new experimental studies on the toxicity of microcystins, based on the essential understanding that drinking water is contaminated by whole cyanobacterial microalgae rather than purified microcystins, while it is important to differentiate between water and cyanobacterial supplements. It is indeed a mistake to automatically apply standards that are proper for water to cyanobacterial supplements, as they have different concentrations of the antioxidant substances that inactivate or significantly reduce the toxicity of microcystins, a fact that also require that each cyanobacterial supplement be tested individually and through realistic testing methodologies.
Keywords: Microcystins, Cyanobacteria, Klamath algae, Aphanizomenon flos aquae, Spirulina, Chlorella, Water safety
1. Introduction
Microcystins are cyclic non-ribosomal peptides produced by some cyanobacteria such as Microcystis aeruginosa, the main producer of these cyanotoxins. They are considered powerful hepato-toxins, and are treated as very dangerous, even though on the ground of rather questionable scientific data. Yet, every so often appears an article that raises concerns about the possible toxicity of microalgae, and particularly of the cyanobacterium Aphanizomenon flos aquae (AFA) from Klamath Lake, Oregon, USA, due to microcystins contamination. In 2012, for example, 2 articles were published, one from researchers of the Italian Istituto Superiore di Sanità [1], and a second one from a German University team [2]. Both of them report that some Klamath algae products tested were above the limit of 1 μg/g of microcystins, the safety limit established by the Government of Oregon for the cyanobacterial supplements, and this is supposed to constitute some kind of danger, even though the authors never tested the actual toxicity of such cyanobacterial supplements, and only took for granted the 1 μg/L of the WHO Guideline, and its automatic application to microalgae at 1 μg/g. Is this alarm and concern about Klamath AFA, as well as other microalgae such as Spirulina or Chlorella, warranted? And more widely, is the danger of microcystins as high as it has been purported to be?
Often, the special focus on Klamath AFA rests on the claim that Spirulina and Chlorella are not affected by cyanobacterial toxins. However, this is clearly not true. For instance, in 2001 an Italian group of researchers from the Istituto Superiore di Sanità (ISS) tested Spirulina products sold in shops in Rome and found them contaminated by anatoxins [3]. A study on Spirulina products sold in China found a large number of them contaminated by microcystins [4]. More recently, a study where the authors tested the three microalgae Spirulina, Chlorella and Klamath, found that among the 3 main edible microalgae the most microcystins-contaminated was Chlorella, followed by Spirulina, with Klamath AFA being actually the least contaminated. When considering all the possible contaminants, and not just microcystins, the researchers ([5], p. 10) concluded that “…the most contaminated products were those containing Spirulina”.
However, all three microalgae have been widely distributed worldwide for decades, and no proven toxicity cases have ever been reported. This applies not only to Spirulina and Chlorella but also to the Aphanizomenon flos aquae Ralphs ex Born. & Flah. Var. flos aqua strain from Upper Klamath Lake, OR, USA, a strain that has been established as a non-toxic [6], and only potentially subjected to microcystin contamination. Could this be due to the fact that microcystins are not as dangerous as generally thought? Could it be that the limits established by some governments, based on the WHO's 1998 Guideline of 1 μg/L of water, which the Government of Oregon has translated automatically as 1 μg/g of Klamath AFA, are not only excessively stringent, but also not supported by convincing scientific data?
2. Microcystins: how dangerous are they?
The recommended maximum level of 1 μg of microcystins per liter of water was established by a special Panel of the WHO, only in relation to water, and only as a guideline [7]. The panel of the WHO established the guideline by taking into account only 3 animal studies on the liver toxicity of microcystins:
1) In the first study [8], an extract of Microcystis aeruginosa containing microcystins ranging from 750 μg/kg/bw to 12,000 μg/kg/bw, were administered dietarily (not by gavage) to mice for 1 year, a period that for mice corresponds to chronic exposure. While at the higher levels there was significant liver damage, the level of 750 μg/kg/bw did not differ, in terms of mortality or histopathological liver changes, from the control. Thus, even though the study did not reach any conclusion about a possible NOAEL, the authors concluded that: “…the oral consumption of toxic M. aeruginosa by mice has relatively little effect at low doses” (p. 304), where the low dose was the one of 750 μg/kg/bw. This study is interesting for different reasons: a) the toxin was administered dietarily; b) it was administered for a whole year; c) it was administered as a natural component of the cyanobacterial microalgae. Transferred to a man of 60 kg, the 750 μg/kg/bw dose would mean that 45,000 μg (or 45 mg) of microcystins taken daily as natural component of whole cyanobacterial algae, would not generate any harm. True, as Falconer et al. point out, both the control group and lower dose group developed some minor liver alterations with increasing age; but the fact that there was no difference between the control group, which did not ingest any algae, and the lower dose group, clearly indicates that at the lower dose of 750 μg/kg/bw no harm was caused by the algae, and the only harm found was the same as the one, probably due to age and environmental conditions, that afflicted also the control group mice. In this case, there was no need for a Chronic Exposure Uncertainty Factor, given that 1 year of administration for mice is equal to chronic exposure. Therefore, the UF reduction standard should have been 100, which would mean that a 60 kg human being could consume 450 μg /day of microcystins for life without any harm, clearly assuming that such microcystins were ingested as natural component of whole cyanobacterial algae. The administration of the toxin as the natural component of the cyanobacterial microalgae, which was the methodology used by Falconer et al. [8], may be considered the closest to the actual human ingestion of microcystins, given that in real life water is not contaminated by pure, laboratory produced microcystins, but by the whole Microcystis aeruginosa with its natural content of microcystins. Even if applying a UF reduction of 1000, the chronic daily safe intake, for a 60 kg human, would be 45 μg.
2) In another study [9], different amounts of microcystins were administered to pigs, even in this case as components of the Microcystis aeruginosa cyanobacterial scum that was frozen, then thawed, then administered to the pigs. Here too the administration was dietary and without gavage, and so most similar to normal human consumption; pig is considered, among the animals, the one physiologically most similar to humans; and finally the study tested again the whole cyanobacterial microalgae. The lower dose of 280 μg/kg/bw day was found to be the safe NOAEL (No Adverse Effect Limit). The authors still applied a safety or uncertainty reduction factor of 1000 to the 280 μg/kg/bw level, concluding thus (p. 138):
“This then provides a guideline safe intake for humans of 0.28 μg/kg/ day, which should result in no adverse effect as seen by direct liver injury. To apply this to a 60 kg adult drinking 2 L water/day, a consumption of water containing 8.4 μg toxins/L should be safe…”.
Given the physiological similarity of pigs to humans, possibly the UF reduction for inter- species variability could have been lower than ×10: with a ×6 UF, the safe guideline would have been 0.46 μg/kg/day, which for a 60 kg human would mean a safe intake of 28 μg/day, or 14 μg/L of water.
3) The third study considered was the one by Fawell et al. [10], whose data were published again in Fawell et al. [11] (this is the article we will analyze). This was the article that in the end was chosen by the WHO Panel, led by the same Prof. Fawell, for the determination of the Guideline. With mice used as subjects of the study, the final NOAEL was established at 40 μg/kg/bw. In this case, the transposition to humans would give the dose of 2400 μg as a safe daily dose, which, once reduced by the Uncertainty Factor of 1000, roughly corresponds to 1 μg/L of water (assuming a daily consumption of up to 2 L of water as the 80% supplier of microcystins for human beings). The question to ask is: what was in Fawell's study that made it preferable to the two other studies by Falconer?
The WHO's Panel document does not offer any explanation for that, probably because there was none, except the need to reach as strict a guideline as possible. In fact, the Fawell's study was flawed under many respects. One thing that nobody seems to have noticed is the fact that Fawell et al. [11] administered the “purified microcystins…prepared in distilled water” (p. 163). Here we have two full “unnatural” and unrealistic factors: a) no one drinks purified microcystins, except in the toxicologist’s lab; b) no one drinks, normally, distilled water, which is deprived of any substance, such as the minerals present in drinkable water, that can play a buffering role (even if minimal) relative to the toxin, in addition to the protective molecules already present in the whole Microcystis aeruginosa (of which we shall talk later).
Moreover, the fact that Fawell et al. had chosen mice as their subjects constituted another weakness of the study, which is indirectly recognized by the authors themselves when they write that, between mouse and rat, “…the mouse was the more sensitive of the two species to the toxin.” (p. 164). Finally, Fawell's method of administration was gavage, which is clearly not the normal way humans or animals drink water.
In spite of all these unnatural and artificial shortcomings, the WHO Panel chose Fawell’s study: as a consequence, the WHO Guideline regulated drinking water, which is normally contaminated by Microcystis aeruginosa with its natural content of microcystins, by first discarding the two studies that did actually reproduce natural and normal drinking water conditions, and then selecting the only study that had adopted an unrealistic methodology by using purified microcystins in purified (distilled) water administered through gavage, all conditions that are never to be found in real drinking water situations.
Besides its methodological flaws, there is another critical passage in Fawell et al. [11] study, the jump from a possible NOAEL of 200 μg/kg/bw to a NOAEL of 40 μg/kg/bw, a jump that allowed Fawell et al. to reach a safe value of 2500 μg of daily consumption of microcystins, which was then subjected to the rather high Uncertainty Factor of 1000. The uncertainty factors normally used are 100 for a NOAEL from an animal study, and 10 from a study of human volunteers (http://archive.food.gov.uk/committees/evm/papers/evm0105.pdf). To understand how we rarely deviate from this parameter, one needs only look to the treatment of one of the most dangerous agricultural insecticides, Chlorpyrifos, so toxic that its use in private homes has been altogether banned. The NOAEL for this very harmful insecticide was established by applying a reduction factor of 100 in the acute, and 160 in the chronic. If we had applied the same criterion for microcystins, we would have obtained, even starting with the lowest result of 2500 μg by Fawell et al., a safe daily consumption of 25 μg for the acute and of about 16 μg for the chronic. At these levels, microcystins would be a minor problem, relevant only to extreme situations. Instead, by applying the UF of ×1000, the final value set by the WHO’s Panel was of 2.5 μg/day, and thus of 1 μg/L of water.
Maybe due also to the weaknesses of the WHO panel’s decision process, the value was established only as a Guideline, subsequently to be verified and tested more deeply. Yet, almost 20 years have passed, no reevaluation of the Guideline have been performed, and the guideline has become a de facto mandatory standard for many health authorities, though not for all of them ([12], pp. 53–55), and a reference standard for many toxicologists.
This consideration acquires particular weight if we consider that the WHO Panel on microcystins was so intensely driven by a super-precautionary desire to establish the lowest possible safety standard, as to completely overlook the flaws of Fawell et al.’s study, including the very questionable jump from a possible NOAEL at 200 μg/kg/bw to the 40 μg/kg/bw NOAEL. To check for possible liver damages, they tested the mice's liver for enzymes (Table 1).
Table 1.
Table 2 of Fawell et al. ([11], p. 165).
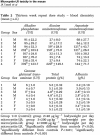 |
As we can see, in the 200 μg/kg/bw group, or Group 3, there was actually a stability in the male group and a reduction in the female group of alkaline phosphatase (ALP); and reduction, for both male and female groups, of gamma glutamyl transaminase (GGT) levels, in relation to the control group, a finding that actually supports the notion that the liver’s metabolism of the mice had actually improved relative to control; as to alanine aminotransferase (ALT) and aspartate aminotransferase (AST), there was an increase of these enzymes in the male group, which was not confirmed, though, in the female group, where there was a reduction. Overall, the findings of the liver enzymes analysis did not support the idea that the 200 μg/Kg/bw was not a safe level. Yet, the authors ([11], p. 165) state that:
“…the significance of these findings remains unclear. Results, however, which were both clear and in agreement with other work done, were produced by histopathological examination of the liver. Here the lesions observed were generally slight and occurred predominantly in the top dose group.”
After having liquidated as “unclear” hepato-chemical data which did not match the original toxicity level hypothesis, the authors focused on the histopathological lesions, which they themselves admit were “generally slight” and mostly “in the top dose group”, that is in the mice that took the dose of 1000 μg/kg/bw. Again, not much of a proof that the 200 μg/kg/bw was not the proper NOAEL.
The supposed proof that the only true safe level was 40 μg/kg/bw came from microscopic examination of the liver tissue of the mice. Here, the damaging changes are described as “…multifocal minimal/slight chronic inflammation with deposits of hemosiderin and multifocal single hepatocyte degeneration.” So, the liver damage was verified through two forms of degeneration: the chronic inflammation with hemosiderin deposit which was minimal; and a multifocal “single hepatocyte degeneration”. Even this does not seem to be very significant, as it involves only a few single hepatocytes.
Moreover, these light damages “…were predominantly found in animals from the top dose group…”, again not in the 200 μg/kg/bw group. But the authors add that there were “… similar but less marked lesions occurring in a smaller number of a mid dose group”: such small number is just 1 male and 1 female out of 30 mice.
Moreover, the authors state that the lesions, that is the “single hepatocyte degeneration” was “less marked” than those of the top dose group. Given that “single hepatocyte degeneration” is already a very “slight” damage, a lesion less marked than that seems indeed a very, very slight lesion, one that it would take only a very powerful interpretive lens to appreciate. It is true that the authors also define the “single hepatocyte degeneration” as “multi-focal”, but this could just mean a few foci. Certainly not enough to rush into discarding the 200 μg/kg/bw, especially in light of the fact, underlined by the authors themselves, that mice is much more sensitive to toxins than rats or pigs (or humans), and that the administration method was gavage, which is known to highly increase the toxicity of the toxins ingested ([13], pp. 102–3):
“Indeed, gavage corresponds to a bolus dose, resulting in tissue concentrations higher than those attained after the more gradual introduction of a dietary treatment, giving time to the detoxification/excretion systems to be efficient.”
In comparison, Funari et al. ([13], p. 103) continue:
“When mice were subchronically administered with MC-LR-containing extracts through the diet, a regimen more similar to human exposure, the NOAEL value was higher (333 μg/kg/bw/day)”.
Given the extremely tenuous data to discard the 200 μg/kg/bw as the safe limit, we can safely assume that if the microcystins had been administered through a more physiological dietary route, even if through unnatural and unrealistic factors such as distilled water and purified microcystins, the 200 μg/kg/bw would have likely been the actual NOAEL, one slightly lower than the 280 μg/kg bw NOAEL previously established by Falconer et al. [9], or the 333 μg/kg bw NOAEL set by Schaeffer et al. [14], through fully natural means (dietary administration, microcystins as natural component of Microcystins aeruginosa, regular drinking water).
And again, even if the 200 μg/kg/bw was not considered safe enough, given the extremely tenuous toxicity data at that level, why not test lower NOAELs at 180, 150 or 100 μg/kg/bw? For example, a NOAEL of 150 μg/kg/bw. would have produced, even by applying the high 1000x uncertainty factor, a safe daily exposure level, for a 60 kg individual, of 9 μg, and thus an acceptable content of 4.5 μg/L of water (at 2 L of consumption per day). This level would have significantly reduced the problem of microcystins, at least in normal water conditions.
When analyzing the way in which the WHO Panel's Guideline was set, one cannot avoid the sensation that the logical order of research was reversed: rather than doing tests and deriving a limit, it looks like the limit to be achieved was established first, and then the tests were accustomed to that requirement. This is made even more evident by the strange twist that one finds at the end of the 1994 study by Falconer et al., which first and throughout proposed a NOAEL of 280 μg/kg/bw, and then in the last paragraphs (p. 138), as to adjust to an external, last-minute requirement, in order to settle to the required limit of 1 μg/L, chooses to apply an almost unheard uncertainty reduction factor of 10,000!
It is interesting to analyze briefly such twist. In the 1994 study, Falconer et al. lowered the previous NOAEL of 45 μg/day to one of 16.8 μg/day for a 60 kg human. In this study, there was no chronic exposure, but the subjects of the study were pigs, thus with a possible lesser UF for inter-species variability. Yet, apparently this very precautionary approach did not seem enough, because all of a sudden, at the very end of the same article, without ever erasing the claim of the 16.8 mcg/day NOAEL as the natural outcome of the study, Falconer et al. [9] have a second thought, and argue that the 1000 UF may not be enough, because we also need to consider the fact that microcystins may play a role in tumor promotion, so that a further 10 uncertainty factor needs to be applied, bringing the total UF to an unprecedented 10,000, and only thus the result in line with the guideline of 1 μg/L water.
Apart from the fact that the shift is clearly contradictory with the immediately previous and strongly worded establishment of the NOAEL at 8.4 μg/L, the logic itself of the shift does not seem to hold: cancer promotion would be an indirect effect of microcystins toxicity, not a direct result of it, and at most would be a component of chronic exposure. If we were to apply a 10x factor for any type of damage produced by a toxin, the uncertainty factor would easily grow into the millions, as any new damage discovery, such as cardiovascular, respiratory, or urinary, would require a further 10 UF. Besides, the statement by ([9], p.138) that “…tumor promotion by these toxins…is now thoroughly established” was a very perfunctory conclusion, one that was inconclusive then and is most inconclusive now [15]. In sum, the bad timing and faulty logic of Falconer et al.'s shift only reveals a too eager availability of many toxicologists to want to join the WHO Panel in erring on the super-precautionary side.
In fact, that Fawell et al. [10,11] guideline decision was seriously faulted is beginning to be recognized by other toxicologists ([13], p. 110):
“In the case of MC-LR, WHO [7] selected the already mentioned subchronic NO(A)EL of 40 μg/kg/bw/day [10]. The choice of this NO(A)EL represents an example of the application of a conservative approach, indeed: it has been obtained in a study on mice, which are more sensitive to acute effects of MC-LR than rats; furthermore, in the study the space among doses is large, the effects at LO(A)EL are slight and involve a limited number of animals, and finally the route of exposure is gavage rather than dietary [10].”
The awareness that Fawell’s approach was faulted in multiple ways seems to be quite common, and the only virtue of this otherwise faulted study seems to be its “conservatism”. But was it conservatism? Or wasn’t it instead an unwarranted revolution, which radically altered a previous situation, whereby microcystins were an unknown contaminant that never seems to have created much of a problem, except in very extreme situation such as dialysis patients, or animals gorging on blue green toxic blooms, or poor countries with very poor sanitation and water controls? The truth, indeed, would have been truly “conservative”: microcystins is a relatively non threatening toxin in normal conditions, ordinarily found below any toxicity risk thresholds in relation to normal dietary exposure and as natural component of Microcystis aeruginosa (or other microcystin-producing cyanobacteria). Sure, it would still need to be monitored and controlled, but without turning it into a ferocious potential killer to be handled with a sledgehammer rather than with a chisel.
The fact that within the toxicological community the discussion has been reopened, and not all toxicologists accept the 1 μg/L of water Guideline, manifests a deserving honesty of thought. However, there are still toxicologists who seem bent towards always raising at all costs the alarm bar on natural toxins, indifferent to the social and financial costs of such a war. That is why governments would be wise to listen to such toxicologists with a grain of salt, refusing to jump into extreme conclusions. The “king” should know that for the militant toxicologist an error that sets the standard of public alarm higher is but a “noble lie” to establish his or her radical worldview, and should thus refuse to easily raise public alarm and expenditure.
It would be time to reconsider the results of the tests that were done with natural and realistic methods, all of which, as we have seen, even by applying a strong UF of ×1,000, generated a chronic between 16.8 μg/day and 45 μg/day for the usual 60 kg human. At this level, microcystins would have remained a small toxicological problem, with a significantly reduced alarm and public expenditure in this area, as the current level of microcystins in water was found to be “…from below 1.0 μg MC-LR equiv./L to more than 8.0 μg/L in raw water…” [16]. Instead, still today there are those who push towards standards even lower than 1 μg/L, while proposing new and more expensive microcystins tests [17,2].
Unfortunately, there seems to be a strong tradition among too many toxicologists who prefer non physiological, not realistic methods of toxins administration. In a recent article by Buratti et al. [12], two tables summarize the studies that have been done over the years to establish a reference value for MC-LR. Concerning the studies done on acute toxicity on various types of microcystins, of the 14 studies reported none was done through dietary intake, and all of them used either gavage, intra-peritoneal or intra-venous methods of administration. As to the subacute/subchronic toxicity, of a total of 18 studies reported, 9 used an intra-peritoneal injection, 2 used inhalation, and of the remaining 7 based on oral administration, only 4 were done through dietary intake, while 3 were done through gavage [12]. When we add to this list the most important of all studies, the one by Fawell et al. [10,11] used to set the WHO's Guideline, we get an overall picture of only 4 studies out of 19 done through ordinary dietary routes of administration, and 15 done with non physiological methods, such as i.p or gavage. Moreover, of the 4 studies that used dietary consumption of drinking water as the method of administration, 3 were essentially irrelevant, because they did not even test for hepatic damage, and used only very low quantities of MC-LR, between 1 μg/L–40 μg/L, that is quantities that are anyway below the 40 μg/L used to set the WHO’s Guideline, so with the only plausible intention of possibly lowering the standard of the WHO’s Guideline [[18], [19], [20]].
In sum, out of 33 studies (14 on acute and 19 on subacute/subchronic toxicity) only 1 was done through normal dietary means and with normal drinking water. It would be about time that any future decision on toxins in food or drinking water be based on studies using realistic and physiological methods, discarding any test that is done through toxins purified from the matrix they are normally ingested with, and through methods of administration such as intra-peritoneal, intra-venous or gavage. I find it sorrily indicative, that “…there have been no pharmacokinetic studies with orally administered microcystins” (https://pubchem.ncbi.nlm.nih.gov/compound/Microcystin_LR#section=OtherPreventative-Measures, p. 13).
As a matter of fact, not all authorities follow unquestionably the WHO Guideline. The US EPA, for instance, sets a limit of 3 μg/L of water for school-aged children and adults, while Health Canada has a limit of 1.5 μg/L ([12], Table 10). Thus, there seems to be a move towards less excessively stringent standards for microcystins in water. Let's take the following statement ([13], p. 111):
“For an adult with a 60-kg bw, the acute no-effect total dose is 150 μg/ person…At this level of exposure to total MCs, no acute effect is expected, also considering that the evaluation has been based on data on MC-LR, which is among the most toxic variants.”
If we were to apply to this standard a UF for chronic exposure of 6, as for Chlorpyrifos, we would have a standard of 25 μg/day; and even by applying a UF of 10, we would still have a 15 μg/day. These are the results obtained when the route of administration considered is dietary.
Any future study on microcystin toxicity should be characterized by the following elements: a) the subjects of the studies should be preferably rats, or even better pigs; and if mice is used, it must be done with the awareness of their heightened sensitivity to the toxin; b) they should be preferably performed for periods that incorporate the chronic exposure, thus reducing the overall UF to 100; c) the studies should be realistic, also in terms of including not only MCLR, but also for the other variants (such as MCLA). However, the only realistic and scientifically sound way of doing the test on all variants of MCs is to get them as natural components of the whole Microcystis aeruginosa, as it is the whole cyanobacterium that contaminates water, not any purified toxin from it; d) the animals should be fed through a normal dietary route (not through gavage or injections); e) finally, even though water is contaminated by the whole cyanobacterium, not all cyanobacteria are the same, and thus every single cyanobacterium should be tested independently, and in the form in which it is ingested, as water if in water, or as supplement if a supplement.
3. Microcystins: microalgae vs. water
Paradoxically, when the Oregon’s health authorities, without doing any specific tests on the toxicity of the AFA microalgae from Upper Klamath Lake, took the 1 μg/L WHO’s Guideline and applied it automatically to AFA algae as 1 μg/g, logically they did not make a serious transferring mistake, as water is indeed contaminated by whole cyanobacteria. But they made a big factual mistake, as they applied to AFA microalgae a WHO’s Guideline which was established by testing unrealistically purified microcystins through gavage, a way of administration that certainly has nothing to do with the normal intake of AFA microalgae.
However, the mistake by the Oregon government was made even worse by the fact that they regulated whole cyanobacterial microalgae, not water, and so the need to consider the role that other cyanobacterial molecules play in relation to microcystins toxicity should have emerged even more clearly. Even if, say, the standard chosen had been based on the Falconer et al. [8] study, where the test was done on water contaminated by the microalgae rather than the toxin, my argument is that it would still be necessary to define the level of toxicity of cyanobacterial supplements on their own, by testing specifically their individual level of toxicity. To begin with, it is clear that one cannot transfer automatically a value that is established for a liquid product onto a dried supplement. In fact, while in a dry product you can refer the value to a gram of dried supplement, you do not know how much Microcystis aeruginosa is present in the water to produce 1 μg/L, because the concentration of the toxin in the cyanobacterium is variable. In other words, the Microcystis aeruginosa in drinking water may contain 10 μg/g, so that the liter of water needs only to be contaminated by just 100 mg. of the microalgae to achieve the limit of 1 μg/L. Why is this relevant? Because there is a big difference between 1 μg of toxin contained in 100 mg and 1 μg contained in 1 g of the whole cyanobacterium. Microcystins perform their toxic activity by destroying essential hepatic enzymes (PP1 and PP2A) via oxidation. They are thus oxidating toxins. However, there have been numerous studies, some of them available since 1991, proving that pretty much all anti-oxidant substances counteract the toxicity of microcystins, substances such as: vitamin E [21]; polyphenols [22]; carotenes [23]; selenium [24]; GSH or glutathione [25], sylimarin [26], as well as microalgal polysaccharides [27]. All these substances either inactivate or greatly reduce microcystins’s toxicity. The interesting thing is that all microalgae, in different measures, contain most of these antioxidant substances, and many of them in relevant quantity. For instance, in a recent study which our research group has authored [28], we have proved the ability of an extract of AFA Klamath to increase, up to more than 40%, the plasma concentration in human subjects of carotenes, tocopherols (vitamin E) and very powerful xantophylilc carotenoids such as lutein, astaxanthin and lycopene. To this it must be added that both Klamath algae and Spirulina are rich in the specific cyanobacterial pigment phycocyanin. Recently we have proved [29] that the specific phycocyanin from AFA Klamath microalgae has the highest ORAC, i.e. the official standard of anti-oxidant capacity, among all purified molecules.
Here, we need to go back to Falconer et al. [8] study, because we may get an idea of why it had the highest NOAEL of all the studies that were looked at on microcystins. We already saw that in that study, as opposed to the purified microcystins used by Fawell et al. (and most other toxicologists), the actual Microcystis aeruginosa with its natural content of microcystins was used. However, in reality the authors explained ([8], p. 292) that they made an extract of the cyanobacterial microalgae:
“Bulk cyanobacterial bloom was concentrated, frozen at −10 °C, thawed, mixed with an equal concentration of water, and left overnight at 4 °C. The suspension was then centrifuged at approximately 1500 g for 30 min to remove cell debris and the clear blue supernatant was collected for use.”
It is then clear that these authors used an extract that concentrated at the same time microcystins and phycocyanins, the powerful antioxidant blue pigment that turned the supernatant blue. Given the high ORAC of such phycocyanins, this is the reason why the factual NOAEL of Falconer et al. [8] study was so much higher (750 μg/kg/bw/day) than the NOAEL of the Falconer et al. [9] study (280 μg/kg/bw/day), where the whole Microcystis rather than extract was used; which in turn was so much higher than the NOAEL of the Fawell et al. [10,11] study with purified microcystins (40 μg/kg/bw/day). This is a further confirmation of the need to test realistically with the whole Microcystis contaminated water when talking of water; with the whole microcystins-contaminated cyanobacterial supplement when testing for supplements; and with the actual cyanobacterial extract concentrating one or more of its antioxidant molecules when testing for an extract based supplement.
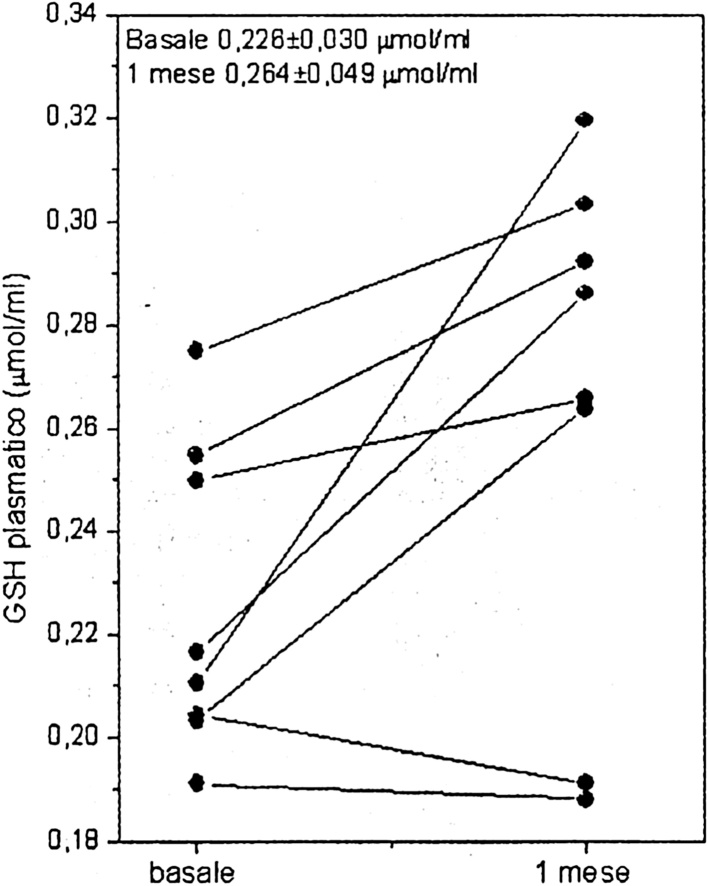
Another particularly relevant way of mycrocystins detoxification has been recently underlined ([12], p. 20): “The role of GSH and its concentration are crucial factors for MC detoxification”. One of the keys to counteract, and in fact detoxify microcystins, is to increase the levels of endogenous GSH (glutathione peroxidase). This is exactly what happened when 10 healthy subjects were administered a Klamath AFA algae product, in the first study on AFA performed by our research group at the University of Urbino (Fig. 1 – [30], p. 69).
Fig. 1.
Increase in the levels of plasma GSH (+16.8%) after 1 month of supplementation with an AFA algae product.
We must also consider the fact that microcystins’s toxicity is “a multi-pathways process…and it has been proposed that it is the result of ‘cross-talking’ and cooperative effects between different pathways, responsible for cytoskeleton alterations, lipid peroxidation, oxidative stress and apoptosis …” ([12], p. 24). Now, we have already seen how AFA Klamath algae is able to counteract oxidative stress and supports the level and action of endogenous antioxidants. But Klamath algae, or extracts thereof, have shown to be able to greatly reduce lipid peroxidation, on average by 34–37% in 1–2 months, as measured by malonildialdehyde (MDA) plasma concentration, both in animal and human studies [28,31,32]. Moreover, a study performed at the University of Salamanca, Spain, showed the ability of a Klamath algae extract, even just at the nanomolar level, to completely prevent the increase in apoptosis of human neuronal cells [33]. Finally, as it is well known that the target organ for microcystins's toxicity is the liver, it is important to underline the hepato-protective activity of cyanobacterial phycocyanins [34], and how an extract of Aphanizomenon flos aquae was able to greatly reduce the damage to the liver by paracetamol intoxication [35].
Before closing, I would like to stress the fact that recently the distinction between the toxic effect of purified microcystins, and the whole microalgae containing it, has been available not just indirectly from a more detailed analysis of the two Falconer et al. studies (10988, [9]), or of Schaeffer et al. study, but through a straight comparison of the toxicity of those two substances [36,37]. For instance, in a study on the toxic effect on bivalves of purified or concentrated microcystins on the one hand, and of the whole Microcystis aeruginosa naturally containing the same amount of microcystins on the other, it was found that, while the purified microcystins did create some liver challenge with a parallel significant increase in endogenous GSH, the whole algae with the same amount of microcystins did not ([36], p. 742):
“Intact M. aeruginosa cells did not induce any significant response from the mussels, showing that these animals are quite resistant to the cyanobacteria if they are intact”.
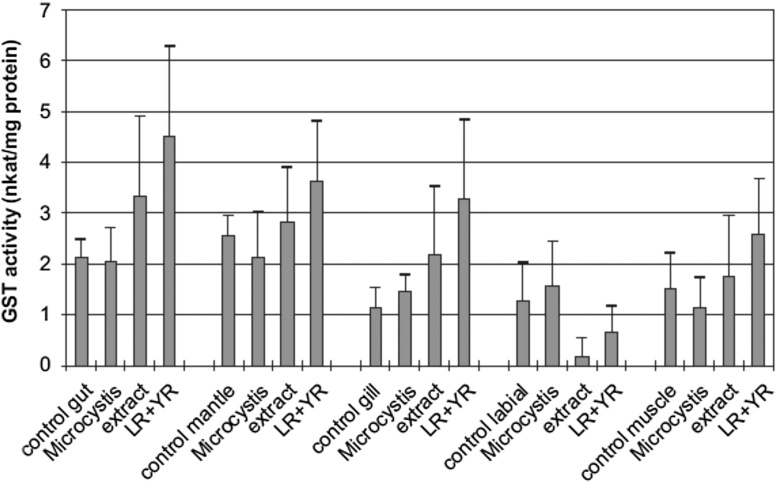
To understand why, we need to look at the chart reporting the GST increases and decreases in the various organs of the bivalve (Fig. 2). As we can see, the GST increase caused by the concentrated or purified microcystin's toxicity is pretty significant in all organs, with the whole Microcystis aeruginosa being always close to control. In fact, it is interesting to notice how the whole Microcystis aeruginosa, in spite of having the same amount of toxin as the microcystin extract and as the purified microcystins, in 3 out of 5 organs generates indeed a small decrease of GST relative to control, which may be significant as we are about to see. There is, though, an anomaly: in the labial pulps, the extract and the purified toxins caused an opposite effect, namely a radical decrease of GST. The authors thus explain the anomaly (p. 744):
“Both the pure toxins and, even more, the Microcystis extract caused a sharp decrease in GST activity. This suppression may be an indication that these organs may be responsible for the control of the MC intake and by that reduce the amount of toxin that enters the mussels”.
Fig. 2.
Cytosolic GST activity (nkat/mg protein) of mussel organs exposed to Microcystis cells, extracts and pure toxins (LR+YR) compared with control animals. (36, p.743).
Thus, the labial pulps, by blocking the entering microcystins, generate such an accumulation as to force the expenditure of most of the available GST, which is thus radically reduced compared to control. In light of this, it emerges as particularly relevant the fact that the whole microalgae the GST, instead of being reduced as with the microcystins extract or purified, actually increased relative to control, in spite of the fact that the level of toxins in the whole algae is the same as in the extract and the purified microcystins. This can only mean one thing: the algae as a whole contains enough of its own antioxidants as to inactivate the microcystins, and possibly to promote the formation of GST itself in an area where the control of toxins is especially relevant.
This is the same reason why, as noted above, in 3 out of 5 organs, where the microcystin challenge is lower than in the labial pulps, the whole microalgae not only did not increase the GST production as both microcystins extract and purified toxins did, but in fact it actually decrease the GST level even relative to control: this can only mean, again, that the microcystins content of the whole microalgae is completely offset by its content of antioxidant molecules, to the point that it actually reduces the need for the endogenous antioxidant protection by endogenous GST.
Those who stress the risk of MC toxicity in microalgae, instead of just mechanically transposing the unrealistic water standard of 1 μg/L–1 g of microalgae, should therefore adopt a proper scientific approach, testing specifically each cyanobacterial supplement as such, be it whole microalgae such as Klamath AFA algae, or Spirulina or Chlorella, or specific extracts thereof, on mice or other animals. Indeed, when that was done, results clearly showed the untenability of crying out loud only for the fact that the MC level is just slightly above the mythical 1 μg/mg guideline. In the already quoted study by Schaeffer et al. [14], mice who had been dietarily fed for 6 months AFA Klamath microalgae providing 333 μg/kg/bw/day of microcystins, at the end of the 6 months period were in excellent health, with a liver in perfect condition. What is to be noticed is also the fact that the 333 μg/kg/bw/day was actually the only level of microcystins tested, which means that if higher levels of microcystins as contaminants of Klamath algae had been tested, the NOAEL could have been similarly higher. The authors of the study concluded (p. 73) that:
“…the safe level of MCLR as a contaminant of A. flos aquae products is calculated to be 10.0 μg MCLR/g”.
Of course, everyone is entitled to disagree with this result, as verification and falsification are the fundamental principles of scientific research. But in order to prove this result wrong, one must repeat the experiment by using the whole cyanobacterial microalgae, with the same or variable quantities of contaminating microcystins, administered through proper dietary means. Any other way would not be proper experimental science, but an a priori application of merely theoretical standards.
In 1999, the Italian Istituto Superiore di Sanità, requested by the public prosecutors of the Court of Rome first, and finally of the Court of Urbino, Italy, tested microcystins-containing Klamath AFA algae on mice for almost one year in different concentrations, and established, as officially reported in the final decision, that the product is safe and suited for human consumption (Ufficio del G.I.P. di Urbino, Decreto di Archiviazione del procedimento, 29 Ottobre 1999).
Not all toxicologists insist on adopting abstract standards, established by testing with purified microcystins in purified water, to be then transposed automatically to microalgae. During the meetings of the Government of Oregon panel, which eventually established the only existing standard on microcystins in microalgae by applying to AFA Klamath supplements the 1 μg/g rule derived from the WHO water Guideline, there were differing positions. Proposals were presented, by such renown toxicologists such a Prof. Carmichael and Dr. Gary Flamm, for microcystins limits such as 5 μg/g up to 10 μg/g (documentation available at the Oregon Department of Agriculture). It is time to reevaluate the wisdom of such positions, putting them to the test of proper experimental science. It is my intention, as a follow-up to this review article, to perform a further experimental animal study to assess, along the lines of scientific research proposed here, the actual toxicity of microalgal and cyanobacterial supplements.
Conflict of interest
Dr. Stefano Scoglio is R&D Director of a company distributing also cyanobacterial nutritional supplements.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2018.07.002.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Vichi S. Contamination by Microcystis and microcystins of blue–green algae food supplements (BGAS) on the Italian market and possible risk for the exposed population. Food Chem. Toxicol. 2012;50:4493–4499. doi: 10.1016/j.fct.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 2.Heussner A.H. Toxin content and cytotoxicity of algal dietary supplement. Toxicol. Appl. Pharmacol. 2012;265(December (2)):263–271. doi: 10.1016/j.taap.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Draisci R. Identification of anatoxins in blue-green algae food supplements using liquid chromatography-tandem mass spectrometry. Food Addit. Contam. 2001;18(June (6)):525–531. doi: 10.1080/02652030118558. [DOI] [PubMed] [Google Scholar]
- 4.Jiang Y. Detection of the hepatotoxic microcystins in 36.660 kinds of cyanobacteria Spirulina food products in China. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008;25(7) doi: 10.1080/02652030701822045. [DOI] [PubMed] [Google Scholar]
- 5.Gallo P. 2012. Contaminazione da biotossine in prodotti ittici e integratori alimentari; pp. 6–11. Ingredienti Alimentari. XI, Ottobre. [Google Scholar]
- 6.Carmichael W. Harvesting of Aphanizomenon Flos Aquae Ralfs ex Born. & Flah. Var. flos aquae (Cyanobacteria) from Klamath Lake for human dietary use. J. Appl. Phycol. 2000;12:585–595. [Google Scholar]
- 7.WHO . Addendum to Vol. 2. Health Criteria and Other Supporting Information. 2nd ed. World Health Organization; Geneva: 2003. Cyanobacterial toxins: microcystin-LR in drinking-water, originally published in guidelines for drinking-water quality. 1998. [Google Scholar]
- 8.Falconer I.R. Oral toxicity of a bloom of the Cyanobacterium Microcystis Aeruginosa administered to mice over periods up to 1 year. J. Toxicol. Environ. Health. 1988;24(3):291–305. doi: 10.1080/15287398809531163. [DOI] [PubMed] [Google Scholar]
- 9.Falconer I.R. Toxicity of the blue-green alga (cyanobacterium) Microcystis Aeruginosa in drinking water to growing pigs, as an animal model for Human injury and Risk Assessment. Environ. Toxic. Water Qual. 1994;9:131–139. [Google Scholar]
- 10.Fawell J.K., James C.P., James H.A. Foundation of Water Research; Marlow, UK: 1994. Toxins from Blue-Green Algae: Toxicological Assessment of microcystin-LR and a Method for Its Determination in Water. Report No. FR 0359/2/ DoE 3358/2; pp. 1–46. [Google Scholar]
- 11.Fawell J.K. The toxicity of cyanobacterial toxins in the mouse: I Microcystin-LR. Hum. Exp. Toxicol. 1999;18:162–167. doi: 10.1177/096032719901800305. [DOI] [PubMed] [Google Scholar]
- 12.Buratti F.M. Cyanotoxins: producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017;91(3):1049–1130. doi: 10.1007/s00204-016-1913-6. Mar. [DOI] [PubMed] [Google Scholar]
- 13.Funari E., Testai E. Human health risk assessment related to cyanotoxins exposure. Crit. Rev. Toxicol. 2008;38:97–125. doi: 10.1080/10408440701749454. [DOI] [PubMed] [Google Scholar]
- 14.Schaeffer D.J. Risk assessment of microcystins in dietary aphanizomenon flos aquae. Ecotoxicol. Environ. Saf. 1999;44:73–80. doi: 10.1006/eesa.1999.1816. [DOI] [PubMed] [Google Scholar]
- 15.IARC . vol. 94. 2010. Ingested nitrate and nitrite, and cyanobacterial peptide toxins; pp. 1–412. (IARC Working Group on the Evaluation of Carcinogenic Risks to Humans). v-vii. [PMC free article] [PubMed] [Google Scholar]
- 16.Hoeger S.J. Occurrence and elimination of cyanobacterial toxins in drinking water treatment plants. Toxicol. Appl. Pharmacol. 2005;203:231–242. doi: 10.1016/j.taap.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida T., Ueno Y. Acute Oral Toxicity of microcystin-LR, a cyanobacterial hepatoxin, in mice. Nat. Toxins. 1997;5(3):91–95. doi: 10.1002/1522-7189(1997)5:3<91::AID-NT1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 18.Wang C. The toxic effects of microcystin-LR on mouse lungs and alveolar type II epithelial cells. Toxicon. 2016;115:81–88. doi: 10.1016/j.toxicon.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X. Chronic exposure to microcystin-LR affected mitochondrial DNA maintenance and caused pathological changes of lung tissue in mice. Environ Pollut. 2016;210:48–56. doi: 10.1016/j.envpol.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Ueno Y. No chronic oral toxicity of a low dose of microcystin-LR, a cyanobacterial hepatotoxin, in female BALB/c mice. Environ. Toxicol. 1999;14(1):45–55. [Google Scholar]
- 21.Gehringer M.M. An investigation of the role of vitamin E in the protection of mice against microcystin toxicity. Environ. Toxicol. 2003;18(April (2)):142–148. doi: 10.1002/tox.10110. [DOI] [PubMed] [Google Scholar]
- 22.Jayaraj R. Hepatoprotective efficacy of certain flavonoids against microcystin induced toxicity in mice. Environ. Toxicol. 2007;22(October (5)):472–479. doi: 10.1002/tox.20283. [DOI] [PubMed] [Google Scholar]
- 23.Matsushima-Nishiwaki R. Suppression by carotenoids of microcystin-induced morphological changes in mouse hepatocytes. Lipids. 1995;30(November (11)):1029–1034. doi: 10.1007/BF02536288. [DOI] [PubMed] [Google Scholar]
- 24.Gehringer M.M. An investigation into the effect of selenium supplementation on microcystin hepatotoxicity. Toxicon. Mar. 2003;41(4):451–458. doi: 10.1016/s0041-0101(02)00362-8. [DOI] [PubMed] [Google Scholar]
- 25.Hermansky S.J. Evaluation of potential chemoprotectants against microcystin-LR hepatotoxicity in mice. J. Appl. Toxicol. 1991;11(February (1)):65–73. doi: 10.1002/jat.2550110112. [DOI] [PubMed] [Google Scholar]
- 26.Mereish K.A. Protection against microcystin-LR-induced hepatotoxicity by Silymarin: biochemistry, histopathology, and lethality. Pharm. Res. 1991;8(February (2)):273–277. doi: 10.1023/a:1015868809990. [DOI] [PubMed] [Google Scholar]
- 27.Mohamed Z.A. Polysaccharides as a protective response against microcystin-induced oxidative stress in Chlorella vulgaris and Scenedesmus quadricauda and their possible significance in the aquatic ecosystem. Ecotoxicology. 2008;17(August (6)):504–516. doi: 10.1007/s10646-008-0204-2. [DOI] [PubMed] [Google Scholar]
- 28.Scoglio S. Effect of a two-months treatment with Klamin®, a Klamath algae extract, on the general well-being, antioxidant profile and oxidative status of postmenopausal women. Gynecol. Endocrinol. 2009;25(4):235–240. doi: 10.1080/09513590802632506. [DOI] [PubMed] [Google Scholar]
- 29.Benedetti S. Oxygen radical absorbance capacity of phycocyanin and phycocyanobilin from the food supplement Aphanizomenon flos-aquae. J. Med. Food. 2010;13(February (1)):223–227. doi: 10.1089/jmf.2008.0257. [DOI] [PubMed] [Google Scholar]
- 30.Benedetti S. Stato antiossidante e perossidazione lipidica in risposta alla supplementazione con alga Klamath. Medicina Naturale. 2003;(Novembre):67–72. [Google Scholar]
- 31.Sedriep S. Beneficial nutraceutical modulation of cerebral erythropoietin expression and oxidative stress: an experimental study. J. Biol. Regul. Homeost. Agents. 2011;25(April–June (2)):187–194. [PubMed] [Google Scholar]
- 32.Kuriakose G.C., Kurup M.G. Evaluation of renoprotective effect of Aphanizomenon flos-aquae on cisplatin-induced renal dysfunction in rats. Ren. Fail. 2008;30(7):717–725. doi: 10.1080/08860220802134730. [DOI] [PubMed] [Google Scholar]
- 33.Benedetti Y. Università di Urbino, Facoltà di Scienze, Dottorato in Metodologie Biochimiche e Farmacologiche; 2012. Neuromodulating and Neuroprotective Activity of Microalga Aphanizomenon flos Aquae. [Google Scholar]
- 34.Ou Y. Protective effect of C-phycocyanin against carbon tetrachloride-induced hepatocyte damage in vitro and in vivo. Chem. Biol. Interact. 2010;185(April (2)):94–100. doi: 10.1016/j.cbi.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Kuriakose G.C., Kurup M.G. Antioxidant and hepatoprotective activity of Aphanizomenon flos-aquae Linn against paracetamol intoxication in rats. Indian J. Exp. Biol. Nov. 2010;48(11):1123–1130. [PubMed] [Google Scholar]
- 36.Vasconcelos V.M. Dynamics of glutathione-S-transferases in Mytilus galloprovincialis exposed to toxic Microcystis aeruginosa cells, extracts and pure toxins. Toxicon. 2007;50(November (6)):740–745. doi: 10.1016/j.toxicon.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Li X.Y. Oral exposure to Microcystis increases activity-augmented antioxidant enzymes in the liver of loach (Misgurnus mizolepis) and has no effect on lipid peroxidation. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005;141(July (3)):292–296. doi: 10.1016/j.cca.2005.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.