Abstract
Background
Suicide is a public health concern for United States veterans and civilians. Prior research has shown neurobiological factors in suicide. However, studies of neuroimaging correlates of suicide risk have been limited. This study applied complex weighted network analyses to characterize the neural connectivity in white matter in veterans with suicide behavior.
Methods
Twenty-eight veterans without suicide behavior (NS), 29 with a history of suicidal ideation only (SI), and 23 with prior suicide attempt (SA) completed diffusion tensor brain imaging, the Columbia Suicide Severity Rating Scale and Barratt Impulsiveness Scale (BIS). Structural connectivity networks among 82 parcellated brain regions were produced using whole-brain tractography. Global and nodal metrics of network topology have been calculated.
Results
SA had shorter characteristic path length and greater global efficiency and mean weighted degree of global network metrics (p < 0.024). SA had more hub nodes than NS and SI. The left posterior cingulate cortex (PCC) showed significantly greater weighted degree in SA relative to others (p < 0.0003). Nonplanning subscale of BIS correlated with the weighted degrees of the left PCC within SA. In rich club connectivity, SA had higher local connections than others (p = 0.001).
Conclusion
Veterans with prior suicide attempt had altered connectivity networks characteristics in the white matter. These findings may be distinctive neurobiological markers for individuals with suicide attempt. Strong connectivity in the left PCC may be implicated in impulsivity in veterans with suicide attempt.
Keywords: Suicide, Veteran, Diffusion tensor imaging, White matter, Connectome, Cingulate
Highlights
-
•
Veterans with a history of suicide attempt may have altered connectivity networks in the white matter, relative to veterans with suicide ideation only, which may be neurobiological markers related to the white matter maturation changes in the earlier ages.
-
•
Hyperconnected posterior cingulate cortex in the subjects with a history of suicide attempt may be associated with impulsivity of suicide behavior.
1. Introduction
Suicide is a significant public health concern, especially for U.S. military personnel and veterans and has been identified as one of the leading causes of death in the U.S. military (Armed Forces Health Surveillance Center, 2014). Moreover, the suicide rate among U.S. veterans reportedly increased between 2001 and 2014 and risk for suicide in veterans was 21% higher than in the US civilian adults, after considering the differences in age and gender (Office of Suicide Prevention, 2016). Rates of veterans who die by suicide have increased public attention to suicide and underscored the need for research to identify risk factors and to increase suicide prevention efforts in veterans and military personnel (Ramchand et al., 2011; Department of Veterans Affairs, 2013). To date, various interventions ranging from individual to community level approaches are being scrutinized and employed for the prevention and treatment of suicide. However, a limited number of approaches in screening, prevention and treatment have been supported by empirical evidence in part due to the complexity in the psychopathology of suicide in the context of various psychiatric disorders including major depressive disorder (MDD), bipolar disorder, anxiety disorders, and substance-related disorders (Klonsky et al., 2016).
A stress-diathesis model of suicide behavior proposed by Mann and his colleagues included stressors in the lifetime and vulnerability of individuals as its components (Mann et al., 1999). In essence, when individuals with suicidal diathesis including genetic, epigenetic, and other neurobiological vulnerabilities encountered distress caused by psychiatric disorders and adverse psychosocial events, he or she would be more likely to demonstrate suicide behaviors. Suicide behaviors are defined as self-directed injurious acts with at least some intent to end one's own life (Mann, 2003). Notably, a majority of psychiatric patients do not exhibit suicide behaviors, while >90% of individuals who die by suicide may be diagnosed as having any psychiatric disorders (Bertolote and Fleischmann, 2002; Nordentoft et al., 2011). This observation was consistent with a crucial role of diathesis in the model of suicide behaviors. Moreover, it has been acknowledged that most individuals with suicidal ideation do not attempt suicide (Kessler et al., 1999; ten Have et al., 2009). The critical need for differentiating suicide attempters from suicide ideators has been identified in research on suicide (Klonsky and May, 2014) and investigating the neurobiological diathesis for suicide behavior would address this need. In fact, several structural and functional brain imaging studies on subjects with suicide ideation or history of attempt have been conducted to identify neurobiological differences along the suicide diathesis (Cox Lippard et al., 2014; van Heeringen et al., 2011).
A recent review article dealing with potential biomarkers from brain imaging for suicide attempt concluded that smaller gray matter volumes or cortical thinning mainly in prefrontal, temporal, and insular regions have been found in attempters relative to non-attempters and that there have been some inconsistent findings which included either increased or decreased white matter integrity in diffusion tensor imaging (DTI) (Sudol and Mann, 2017). Functional magnetic resonance imaging (MRI) studies with attempters have reported that altered activation of frontal lobe was observed during the emotional processing, risk-reward assessment, and decision making. Although there have been some inconsistencies which might be related to the heterogeneity of comorbid psychiatric disorders or the small sample size inherent in the brain imaging studies, other meta-analyses of functional and structural neuroimaging studies for suicide behaviors suggest that prefrontal, cingulate, and striatal area could be involved with suicide behavior (van Heeringen et al., 2014).
During the last decade, several Graph theoretical connectome studies from brain imaging data have been conducted in the field of neuroscience and have successfully detected the topological abnormalities of brain networks related to psychiatric disorders (Deco and Kringelbach, 2014; Xia and He, 2011). For example, brain structural network abnormalities in default mode network (DMN) was found in the patients with MDD and decreased efficiency in children with posttraumatic stress disorder (PTSD) using DTI (Korgaonkar et al., 2014; Suo et al., 2017). This method provides more detailed characteristic features of the brain as a network and may help us understand the pathophysiology of neurobehavioral disorders. Furthermore, the findings from the connectome could be utilized as a specific neurobiological marker for psychiatric disorders. However, to our knowledge, there have been only a few brain connectome studies investigating the neurobiology of suicide (Bijttebier et al., 2015; Myung et al., 2016).
In this study, we have conducted a connectome analysis among veterans with a history of suicidal ideation only, history of suicide attempt, and no history of suicide behaviors using DTI, to identify distinct neurobiological diatheses in veterans with a history of suicide attempt. Network characteristics of Graph theory including global and local degrees, efficiency, and clustering coefficients have been investigated and compared among the groups. Those metrics represent connection strength (degree), functional integration (efficiency) and segregation (clustering coefficient). Brain regions (nodes) which are more densely connected could be categorized as “rich club” (van den Heuvel and Sporns, 2011). Those rich club regions are more likely to have long distance connections and play a crucial role in the brain network. We also compared the connectivity among rich club and non-rich club regions in this study.
2. Methods
2.1. Subjects
Eighty-one subjects were recruited through print advertisements in the form of flyers as well as through word of mouth. Flyers were posted within the VA hospital, as well as many locations around the community, especially in areas focused on veteran services. The main inclusion criteria were that subjects be veterans between the ages of 18–55, male or female, not currently being treated as inpatients. Participants were excluded if they endorsed major sensorimotor handicaps (e.g., deafness, blindness, paralysis), had a history of claustrophobia, autism, schizophrenia, anorexia nervosa or bulimia, had a history of electroconvulsive therapy, were pregnant or lactating; an estimated IQ of <80, had previous electroconvulsive therapy, had not remained stable on their psychotropic medications for at least 90 days or had metal implanted in their body. Participants were not selected or recruited on the basis of specific psychiatric diagnoses nor the presence of suicide ideation and attempt. This study group was a self-referred, representative sample of the veteran population in and around this location.
The institutional review boards of the local University and VA Hospital approved this study. One individual was eliminated from the analyses because he was unable to complete the scanning protocol. Of the veterans recruited, 52 (65%) met criteria for current or past history of suicide behaviors. Table 1 includes demographic, clinical and diagnostic information for veteran participants. Twenty-four of 78 subjects (Clinical information of one subject in suicide ideation group was missing) who completed in clinical history taking had no comorbid disorders. While 18 subjects had single comorbid disorder, 37 had more than one comorbid disorders. Cross sectional medication data collected at time of scanning was collected; however, 28 participants were unmedicated and the distribution of antidepressant medication and other medications for the SI and SA group resulted in small sample sizes with limited power, thereby preventing a meaningful comparison. Medication types included benzodiazapines (N = 2), prazosin (N = 7), gabapentin (N = 11), serotonin-specific reuptake inhibitors (SSRI) (N = 13) and other non-SSRI antidepressants (N = 12).
Table 1.
Demographic and clinical information of participants.
| No suicide N = 28 (mean ± SD) |
Suicide ideation N = 29 (mean ± SD) |
Suicide attempt N = 23 (mean ± SD) |
p value | |
|---|---|---|---|---|
| Age | 35.9 ± 9.18 | 36.5 ± 9.73 | 38.8 ± 8.80 | p = 0.51⁎ |
| Sex (male, %) | 23 (82.1%) | 21 (72.4%) | 20 (87.0%) | p = 0.44† |
| Handedness (right-handed, %) | 23 (82.1%) | 27 (93.1%) | 21 (91.3%) | p = 0.41† |
| Education (year) | 14.9 ± 1.74 | 15.0 ± 2.31 | 14.8 ± 2.13 | p = 0.93⁎ |
| Comorbidity (N)‡ | ||||
| No comorbid disorder | 18 | 5 | 1 | |
| Mood disorder | 5 | 22 | 19 | |
| Substance dependence | 6 | 9 | 13 | |
| PTSD | 2 | 11 | 16 | |
| Total BIS‡ | 58.7 ± 8.71 | 68.9 ± 13.1 | 73.0 ± 10.4 | p < 0.001⁎ |
| Attentional | 16.3 ± 3.27 | 18.9 ± 4.15 | 20.3 ± 3.72 | p = 0.001⁎ |
| Motor | 18.4 ± 3.22 | 22.4 ± 5.12 | 23.7 ± 4.92 | p < 0.001⁎ |
| Nonplanning | 24.0 ± 5.42 | 27.6 ± 6.14 | 29.0 ± 5.25 | p = 0.008⁎ |
| HAM-D‡ | 2.11 ± 2.75 | 7.36 ± 6.46 | 14.4 ± 6.94 | p < 0.001⁎ |
Abbreviation: SD, standard deviation; PTSD, posttraumatic stress disorder; BIS, Barratt Impulsiveness Scale; HAM-D, Hamilton Depression Rating Scale.
One-way analysis of variance was used to compare the means among groups.
Fisher's exact test was used to compare the ration of categorical variables among groups.
Missing data in comorbidity, BIS, and HAM-D for one subject in Suicide ideation group and in BIS for one subject in No suicide group.
2.2. Clinical measures
All participants completed clinical measures designed to assess lifetime diagnosis and clinical state. The Structured Clinical Interview for DSM-IV Patient Version (SCID-I/P) (Spitzer et al., 1996), a widely used structured diagnostic interview designed to assess Axis I disorders in psychiatric populations, was completed by a licensed clinician with experience in working with veterans.
Additional measures administered included the Hamilton Depression Rating Scale (HAM-D), the Barratt Impulsiveness Scale (BIS), which were the parts of Columbia Suicide Severity Rating Scale (C-SSRS). HAM-D is a clinician-based assessment designed to assess symptoms of depression. It includes symptoms such as depressed mood, guilty feelings, suicide, sleep disturbances, anxiety levels and weight loss (Hamilton, 1960). BIS is a widely used self-report measure of impulsivity. The BIS-11 includes measures of attention, motor impulsivity, self-control, cognitive complexity, perseverance, and non-planning (Patton et al., 1995). C-SSRS is a well-validated measure of suicidality include emergence of suicide behaviors/number of behaviors, emergence of ideation/ideation intensity and worsening of ideation, and lethality/severity (Posner et al., 2011). Categorization of our study sample was based on responses to the C-SSRS such that participants were classified as Ideators (SI) if they reported current or history of wishing to be dead, thoughts of killing self, thoughts with methods and/or intent, and thoughts with method and intent (i.e., ideation but no attempt(s)). They were classified as Attempters (SA) if they had a history of one or more suicide attempt(s), including aborted and interrupted attempts. The third group (no suicide behaviors group, NS) consisted of veterans who denied current or historical suicidal ideation or suicide attempts. In this study suicide ideation is defined as passive thoughts of suicide such as wishing to go to sleep and not wake up, active ideation with thoughts of wanting to end your own life, intent to act on the thoughts and a well thought-out plan. In addition to having suicidal thoughts, individuals in this group were required to have never made an attempt on their life. Current suicide ideation would include the thoughts described above and would need to have occurred during the previous month. Suicide attempt is defined as a past attempt at one's own life. Behaviors associated with suicide attempts include preparatory behavior for an attempt, an actual attempt, and an interrupted attempt or a self-aborted attempt. When the self-injury was not associated with an attempt to end one's life individuals with self-injury were not included in the SA group. The participants in the SA group did report suicide ideation as well.
2.3. MR image acquisition
All imaging was performed on a 3 T Siemens (Erlangen, Germany) Vario scanner. Structural imaging data were acquired using a high-resolution, high-contrast, T1-weighted 3D MPRAGE sequence acquired axially-acquired using a 12-channel head coil with TE/TR/TI = 3.38 ms/2000 ms/1100 ms, 8° excitation flip angle, 256 × 256 acquisition matrix, 160 slices, and 1.0 mm isotropic resolution. Diffusion tensor imaging then was acquired using a DTI-MRI sequence utilizing 64 directions, 2 diffusion weightings: b = 0, 1000 s/mm2, TE/TR = 89 ms/11,800 ms, 128 × 128 acquisition matrix, 72 slices, and 2.0 mm isotropic resolution. All subjects completed a structural MR scan that was read by a neuroradiologist to examine gross structural brain changes. Original images were then transferred from the scanner in the DICOM format and coded.
2.4. Image preprocessing and network construction
Cortical and subcortical structures were parcellated using Freesurfer 5.3.0 (http://surfer.nmr.mgh.harvard.edu/) with T1-weighted images (Fischl and Dale, 2000). Region-of-interest (ROI) masks for 34 cortical and 7 subcortical structures in each hemisphere were generated based on Desikan atlas and the volumes of ROI were calculated (Desikan et al., 2006; Fischl et al., 2004). Then, each ROI mask was transformed into the space of diffusion tensor images using the matrix generated by linear registration of T1 image onto FA image. These 82 ROIs were defined for network nodes in the further analysis. Cortical surface of each subject was transformed onto the template surface provided by Freesurfer and smoothed using 10 mm Gaussian kernel for the comparison.
Diffusion weighted images were processed for Eddy Current Correction and head motion realignment using FMRIB Software Library 5.0 (Jenkinson et al., 2012). Vectors of diffusion gradient scheme were adjusted by the rotational matrix from head motion realignment. Diffusion tensor, FA, and whole brain tractography images were constructed by Diffusion Toolkit of TrackVis software using Fiber Assignment by Continuous Tracking (FACT) algorithm, with 45° of angle threshold and eight random seeds per voxel (http://trackvis.org/). The number of white matter fiber tracts in the whole brain tractography image between all pairs of 82 parcellated ROI masks were computed by UCLA multimodal connectivity package (http://ccn.ucla.edu/wiki/index.php/UCLA_Multimodal_Connectivity_Package). Fiber tracts shorter than 10 mm were disregarded since they may be spurious. The volume of tracts was calculated in each subject by taking the number of fibers and multiplying by fiber length in each connection as an anatomical network cost (van den Heuvel et al., 2012).
The connectivity network for each subject consisted of 82 ROIs as nodes and the number of fiber tracts divided by averaged volume of interconnecting two nodes as edges. Edges were defined as having a minimum of 3 streamlines between interconnecting two nodes. Finally, weighted and undirected network of each subject was constructed for the topological measurements.
2.5. Network topology metric calculation
Global and nodal metrics of network topology properties including characteristic short path length (L), clustering coefficient (C), scalar small-worldness, efficiency, and weighted degrees (strength) of each node were calculated using GRETNA in the sparsity thresholded network ranging 0.06 to 0.03 with an interval of 0.01 (Wang et al., 2015). Ranges of sparsity threshold were determined as follows: 1) from the threshold value in which the average number of connected edges of all nodes was larger than ln(82), and 2) to the value in which average nodal density (fraction of present connections to possible connections) reached the maximum level (Bullmore and Bassett, 2011; Watts and Strogatz, 1998). Normalized L (λ) and C (γ) were also calculated using 1000 randomly generated network matrices in each subject. Within the sparsity threshold ranges, all thresholded networks had γ > 2.04 and 1.09 < λ < 1.26. As a result, small-worldness scalar σ (γ/λ) within threshold range (0.06–0.30) was >1.75 in all subjects, which met the requirements for small-world network properties. Area-under-the-curve (AUC) of each global and nodal metrics within the ranges were calculated for the comparison among the groups. AUC metric has been shown to be sensitive in detecting the topological alterations in previous studies of the brain network (Uehara et al., 2014; Zhang et al., 2011). Nodes with mean high weighted degree (>mean + standard deviation across 82 nodes within group) were defined as hub nodes (Bullmore and Sporns, 2009).
The rich club coefficient Φ(k) was calculated at the degree level k in each subject and the normalized Φ(k) was obtained by mean Φ(k) from 1000 randomly generated network matrices and dividing Φ(k) for each subject (van den Heuvel and Sporns, 2011). Rich club effect was defined by mean normalized Φ(k) within group >1. At the maximum degree level of k in which rich club effect was found across the group, nodes with mean degrees within all subjects above k were selected as rich club nodes and others were defined as peripheral nodes. Then, rich club, feeder, and local connections were computed for mean connectivity between all the rich club nodes, between all the rich club and peripheral nodes, and between all the peripheral nodes, respectively.
2.6. Statistical analysis
Statistical analyses were performed with STATA software 12.1 (Stata Corporation, College Station, TX) and Matlab 2016 (Mathwork, Natick, MA). Fisher's exact test and one-way analysis of variance (ANOVA) with post hoc Bonferroni analysis were used for the comparison of categorical and continuous variables, respectively, in the demographic and clinical data among three groups. For the network metrics, One-way ANOVA was first used for the comparison among the groups and then, permutation test by 10,000 randomly re-grouped data was performed to estimate permutation corrected p value (Anderson, 2001). For the nodal degree, efficiency, and betweenness centrality across 82 nodes, Bonferroni corrected p value (0.05/82 = 0.0006) was used for the significance level to correct the multiple comparisons issue. Correlation analysis was conducted using Pearson correlation between the continuous variables.
To explore the possible confounding effects of comorbidities and clinical measures, analysis of covariance (ANCOVA) was used for the comparison of continuous variable (characteristics of connectome) between SI and SA groups.
3. Results
There were no significant differences in mean age, years of education, sex, or handedness between groups (Table 1). There were significant differences in total and subscales of BIS and HAM-D ratings across the groups (One-way ANOVA, df = 2,75 (BIS) or 76 (others), F > 5.23, p < 0.008). In post hoc analyses, there were significant pairwise differences in HAMD between groups (NS < SI < SA, p < 0.002). The results indicated that SA had greater a Total BIS score and increased attentional, motor, and nonplanning subscales compared with NS (p < 0.009), but not compared with SI (p > 0.550).
There were no significantly different volumes in 82 parcellated regions among the groups (p > 0.07). However, SA had significantly smaller estimated total intracranial volume than NS (One-way ANOVA, df = 2,77, F = 3.93, p = 0.024; post hoc Bonferroni test, NS > SA), while volume of segmented brain showed no significant difference (One-way ANOVA, df = 2,77, F = 0.91, p = 0.407). Anatomical network cost (total volume of tracts in the network matrix) among the groups did not differ significantly (One-way ANOVA, df = 2,77, F = 0.71, p = 0.493).
In examining the global metrics, it was found that SA had significantly shorter characteristic path length (L) and greater global efficiency than SI (One-way ANOVA, df = 2,77, F > 4.07, p < 0.021; post hoc Bonferroni test, SI < SA) (Table 2). There was also significantly greater mean weighted degree (connectivity strength) in SA than in SI (One-way ANOVA, df = 2,77, F = 3.90, p = 0.024; post hoc Bonferroni test, SI < SA). However, other global metrics including clustering coefficient, normalized L and C, and local efficiency were not significantly different among the groups (One-way ANOVA, df = 2,77, F < 2.61, p > 0.08). When L, global efficiency, and mean weighted degree were compared between SI and SA with controlling the comorbid PTSD, BIS, and HAMD respectively to exclude the possible confounding effects, we found L and mean weighted degree remained significant (p < 0.048, Tables S3, S4, and S5). There was significant difference in global efficiency between two groups when controlling comorbid PTSD and total BIS respectively (p < 0.38), but not when controlling HAM-D (p = 0.055, Tables S3, S4, and S5).
Table 2.
Global metrics (the area under curve of each metric with sparsity threshold ranging from 0.06 to 0.3) of network topological analysis among three groups.
| A. No suicide N = 28 (mean ± SD) |
B. Suicide ideation N = 29 (mean ± SD) |
C. Suicide attempt N = 23 (mean ± SD) |
One-way ANOVA p value |
Post hoc pairwise comparison p value† |
|||
|---|---|---|---|---|---|---|---|
| A vs B | B vs C | A vs C | |||||
| Short characteristic path length L | 17.2 ± 1.33 | 17.5 ± 1.45 | 16.4 ± 1.00 | 0.016⁎ | 1.00 | 0.013 | 0.15 |
| Normalized L, λ | 0.28 ± 0.008 | 0.29 ± 0.006 | 0.28 ± 0.008 | 0.53 | 1.00 | 0.91 | 1.00 |
| Clustering coefficient C | 0.015 ± 0.002 | 0.015 ± 0.002 | 0.014 ± 0.002 | 0.27 | 1.00 | 0.50 | 0.43 |
| Normalized C, γ | 0.76 ± 0.030 | 0.75 ± 0.037 | 0.75 ± 0.029 | 0.56 | 1.00 | 1.00 | 0.95 |
| Global efficiency (×10−3) | 3.37 ± 0.27 | 3.31 ± 0.28 | 3.52 ± 0.22 | 0.021⁎ | 1.00 | 0.02 | 0.16 |
| Local efficiency (×10−3) | 4.78 ± 0.40 | 4.70 ± 0.37 | 4.94 ± 0.36 | 0.08 | 1.00 | 0.08 | 0.40 |
| Mean weighted degree | 0.067 ± 0.005 | 0.066 ± 0.005 | 0.070 ± 0.004 | 0.024⁎ | 1.00 | 0.026 | 0.117 |
Bolded values indicate significance at p < 0.05
Permutation correction for multiple testing p < 0.05.
Post hoc Bonferroni analysis.
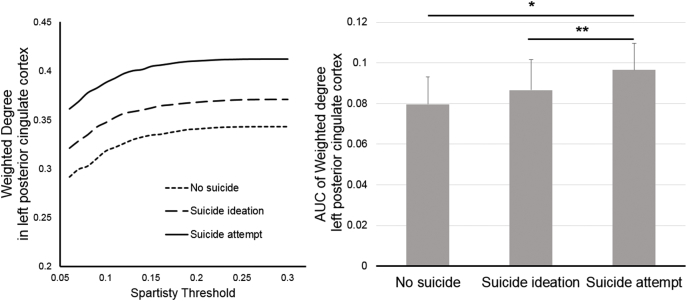
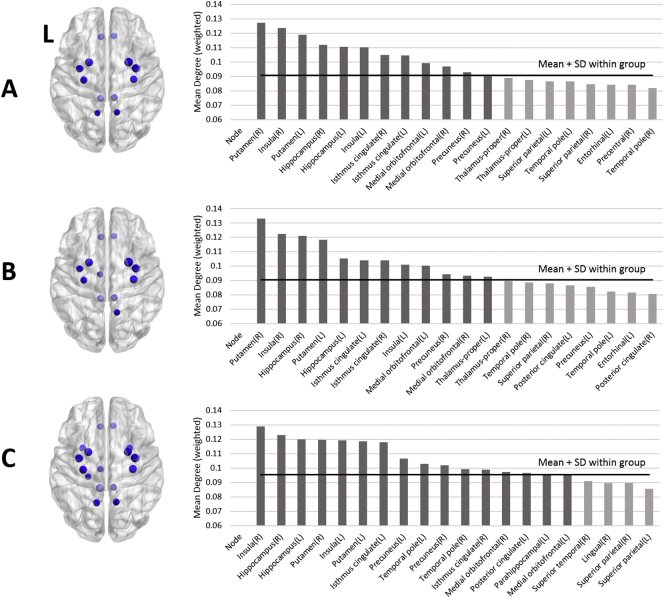
None of the 82 nodes showed significant differences in betweenness centrality and local efficiency when Bonferroni correction was applied (One-way ANOVA, df = 2,77, F < 6.56, p > 0.0023). In nodal weighted degree, left posterior cingulate cortex (PCC) in SA had significant greater degree than those in others (One-way ANOVA, df = 2,77, F = 9.14, p = 0.0003; post hoc Bonferroni test, NS < SA and SI < SA) (Fig. 1), while other nodes did not. When post hoc analyses were repeated with controlling comorbid PTSD, BIS, and HAM-D respectively, it did not show significance when it covaried by comorbid PTSD and HAM-D (p > 0.057, Tables S3 and S5), whereas it did with controlling total BIS (p = 0.035, Table S4). However, mean weighted degree in left PCC did not demonstrate a significant correlation with clinical rating scores within SI and SA. The number of hub nodes varied by group with 12 hubs in NS and SI, and 16 hubs in SA. Most of the hub nodes overlapped among the groups with the exception of left thalamus for SI and left and right temporal poles, left PCC, and left parahippocampal cortex found only in SA (Fig. 2). The left precuneus belonged to hubs in NS and SA, but was not evident in SI.
Fig. 1.
Plot of nodal weighted degree of left posterior cingulate in each group over sparsity threshold (Left) and mean (+standard deviation) comparison among groups (Right, one-way analysis of variance, df = 77,2, F = 9.14, p = 0.0003, post hoc *p < 0.001, **p < 0.039).
Fig. 2.
Hub nodes which were defined as having one standard deviation greater than mean of nodal weighted degree in each group (A: no suicide; B: suicide ideation; C: suicide attempt).
Rich club effect was observed at k = 31 of degree level in all three groups. At the level of k = 31, the bilateral superior frontal, superior parietal, precuneus, and insular cortices comprised of cortical structures and bilateral putamen, thalamus, and hippocampus comprised of subcortical structures were identified as rich club nodes. The set of rich club nodes identified in the current study are similar to these reported in the previous DTI connectome study (van den Heuvel and Sporns, 2011). Connectivity of local connection differed significantly among the groups (One-way ANOVA, df = 2,77, F = 7.52, p = 0.001; post hoc Bonferroni test, NS < SA and SI < SA), while rich club and feeder connections did not (One-way ANOVA, df = 2,77, F < 1.82, p < 0.17) (Fig. 3). Connectivity of local connection remained significant between SI and SA when covaried by comorbid PTSD, total BIS, and HAM-D respectively (p < 0.001, Tables S3, S4, and S5).
Fig. 3.
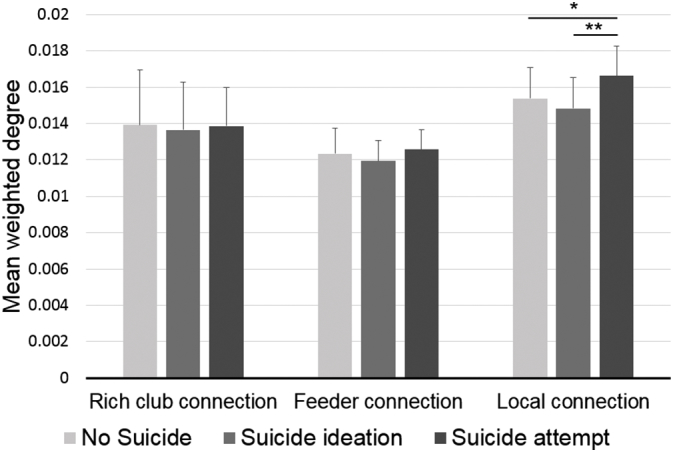
Comparisons of the rich club, feeder, and local connections among the groups. Local connection shows significant differences among the groups (one-way analysis of variance, df = 77,2, F = 7.52, p = 0.001, post hoc *p = 0.031, **p = 0.001).
In correlation analyses between network connectivity measures and clinical ratings, there was a significant correlation between nonplanning factors of BIS and weighted degrees of left PCC SA (r = 0.457, p = 0.029) (Fig. 4).
Fig. 4.
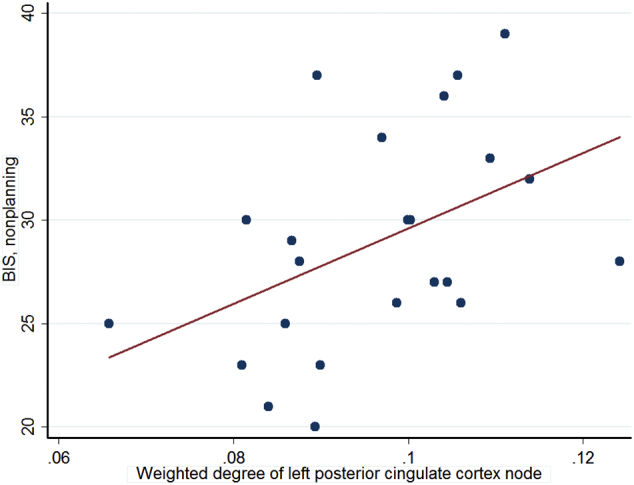
Significant correlation between weighted degree of left posterior cingulate cortex and nonplanning subscale of Barratt Impulsiveness Scale within the suicide attempt group (N = 23, r = 0.457, p = 0.029).
4. Discussion
The current study has demonstrated unique characteristics in the brain tractography connectome of veterans with a history of suicide attempt. These include decreased characteristic short path length as well as increased global efficiency and mean weighted degree in attempters, relative to ideators. The imaging data also show increased connectivity in the left PCC and local connections of veterans with prior suicide attempt relative to those seen in veterans with suicide ideation only and those who reported no suicide behaviors. To the best of our knowledge, this is the first study in veterans reporting the network properties of the brain as a neurobiological diathesis of suicide attempters compared to both suicide ideators and non-suicidal subjects.
Since global efficiency was inversely related to the path length between nodes, shorter path length (L) and greater global efficiency may share a common network characteristic. Global efficiency in the brain network is defined as “a measure of the overall capacity for parallel information transfer and integrated processing (Bullmore and Sporns, 2012).” Increased efficiency of the brain network is supposed to be relevant to increased FA of white matter as reported in previous publications with suicide attempters (Kim et al., 2015; Lee et al., 2016; Lopez-Larson et al., 2013). Local connections in rich club organization which were also found to be increased in suicide attempters, have been described as composed of more short-range fibers than the rich club and feeder connections (van den Heuvel et al., 2012). Short-range fibers may serve as local associative fibers (U-shaped), such as cortico-cortical tracts (Catani et al., 2012), which could provide shortcuts in the connections between adjacent nodes. Therefore, increased local connections in suicide attempters may contribute to increased global efficiency and connectivity strength. However, it is less likely to have association of enhanced brain structural characteristics with psychopathology or psychiatric conditions. Regarding increased global efficiency in this DTI connectome study, to our knowledge, a similar finding has been found in only one DTI connectome study and that is with heroin-dependent patients (Zhang et al., 2016a, Zhang et al., 2016b). In another functional connectome study, increased global efficiency was observed in subjects with antisocial personality disorder (Jiang et al., 2017). As suicide attempt, drug addiction, and antisocial personality share impulsivity as a common psychopathology, it suggests a possible relationship between global efficiency and BIS in this study, but it failed to show the association.
On the other hand, increased mean weighted degrees (connectivity strength) and increased global efficiency identified in the brains of suicide attempters could suggest their brain network may have an increased “wiring cost” (anatomical network cost) of Graph's network. Wiring cost in brain connectome means the burden for the building and maintaining the white matter tracts and the volume of white matter tracts in the matrix was calculated in this study (Bullmore and Sporns, 2012). However, topology of brain network might be balanced between higher efficiency and minimization of wiring cost, and higher global efficiency usually accompanies greater wiring cost. In this study, SA showed higher global efficiency without increased wiring cost. It was supposed for SA to have smaller intracranial volume which may be related to shorter physical tract length and it may lead to the conservation of the wiring cost. Besides, additional post hoc analysis suggested that increased global efficiency in SA relative to SI may be affected by their depressive symptoms. In this regard, it could not conclude the neurobiological relationship between suicide attempt and global efficiency and may suggest that the further study with larger sample to investigate if the suicide attempters may have a high-efficiency and high-cost topological network of Graph theory in white matter connectivity is warranted.
From a neurodevelopmental perspective, changes of intracranial volume, global efficiency, and connectivity in the local connections of rich club organization from the brain DTI connectome are likely to occur in the brain over the late gestation period and earlier ages of lifetime (Ball et al., 2014; Koenis et al., 2015; Pfefferbaum et al., 1994). Thus, altered findings of those metrics in subjects with suicide attempts in our study suggest that they may be associated with the changes in brain maturation from intrauterine period to young adults. For example, the preterm birth may increase local connections of rich club organization in the premature newborn human brain (Ball et al., 2014) and preterm birth is a significant risk factor for various psychiatric conditions including suicide (D'Onofrio et al., 2013). Taken together, we may speculate that white matter connectivity changes in preterm birth may predict the suicide behavior in the adulthood.
Hub nodes, which were found only in suicide attempters, belonged mostly to the regions known as DMN. The DMN is activated during passive resting state and internally-oriented mental processes, and also play roles in autobiographic memory, self-referential processing, and future thinking (Andrews-Hanna et al., 2010; Buckner et al., 2008). In a recent meta-analysis, increased functional connectivity between DMN and subgenual prefrontal cortex was found reliably in the subjects with MDD and it was often related to depressive rumination (Hamilton et al., 2015). Distorted self-referential thinking, which is mediated by cortical medial structures (Northoff and Bermpohl, 2004), is likely to be associated with MDD, PTSD, and other anxiety disorders (Batelaan et al., 2010; Evans et al., 2005; Henning et al., 2007; Kashdan et al., 2010). Furthermore, negative self-concept has been found to be linked to suicide (Bhar et al., 2008; Cox et al., 2004; Santos et al., 2009).
The PCC is a key structure of the DMN (Buckner et al., 2008) and has abundant connections with other brain regions as well as a high basal metabolism (Hagmann et al., 2008; Raichle et al., 2001). Structural and functional alterations of PCC in various psychiatric disorders have been published (Leech and Sharp, 2014). A well-established function of PCC is involvement in autobiographical memory retrieval (Maddock et al., 2001). Overgeneralization of autobiographic memory has been observed in subjects with suicide attempt and has been reported to be one of the cognitive characteristics of MDD patients (Pollock and Williams, 2001; Williams et al., 2007). In addition, preference for immediate reward has been associated with the activation of PCC area in fMRI studies (Albrecht et al., 2013; McClure et al., 2004). The PCC therefore appears to have a role in immediate gratification of reward. Considering the association between the preference of immediate reward and nonplanning subscale of BIS (De Wit et al., 2007), the preference of immediate reward in the suicide attempt group may mediate the positive correlation between nonplanning subscale and weighted degree of left PCC in this study. Therefore, impulsivity observed in suicide attempters may be associated with the hyperconnected state of left PCC.
Overall, it has been proposed that the DMN including PCC may play an important role in suicide behavior (Serafini et al., 2016). However, DMN has been scarcely reported in brain imaging studies with suicide attempters. Two studies with functional MRI have reported in the subjects with suicide attempt or ideation. One study with young depressed patients reported increased activity of the DMN in depressed patients, but decreased activity in suicide attempters using resting state functional MRI (Zhang et al., 2016a, Zhang et al., 2016b). In the other study, abnormal functional connectivity in PCC area was observed in suicide ideators with mixed composition of the subjects with or without a history of suicide attempt compared to healthy controls group (Chase et al., 2017). Role of the DMN in suicide attempt can be considered in relation with the structural abnormalities of orbitofrontal cortex in suicide attempters. Altered structure in left rectal gyrus, a part of orbitofrontal cortex has been found in suicide attempters from a meta-analysis (van Heeringen et al., 2014). Since orbitofrontal cortex has connection with PCC in DMN, this finding could provide evidence for potential involvement of PCC of DMN in suicide attempt.
One thing which should be considered in this study was that the significance of connectivity of left PCC in SA relative to in SI was mitigated when the analyses controlled for the presence of comorbid PTSD and clinical mood state (HAM-D) respectively. Since it has been proposed the DMN including PCC may be involved with MDD and PTSD (Evans et al., 2005; Kashdan et al., 2010), the connectivity differences of left PCC in SA may stem from other clinical conditions. Further studies with a range of populations who experience suicide behaviors will be necessary to clarify the potential roles of co-morbid disorders in the alterations of the DMN, including PCC, in individuals with suicide attempts.
In previous studies reporting DTI network connectivity in subjects with suicide attempt or suicide ideation, it has been shown that there was decreased connectivity strength between left olfactory cortex and anterior cingulate gyrus in euthymic suicide attempters and decreased frontal-subcortical connectivity in MDD subjects with suicide ideation (Bijttebier et al., 2015; Myung et al., 2016). However, the former did not report the topological global and nodal network metrics and the suicide attempt population was not included in the study design in the latter. In addition, the divergent findings from our study may be attributed to the difference in characteristics of study populations and methodology.
The current results raise the intriguing question as to whether the current characteristics of the DTI brain connectome could be a state or trait for suicide behavior. Since the current study was a cross-sectional design, temporal and causal relationships between suicide behaviors and the findings cannot be drawn. Even after the completion of brain development, the anatomical connectivity of the brain could not only change over a long time (more than years), but also change with relatively short training and rehabilitation (Bosnell et al., 2008; Giorgio et al., 2010; Scholz et al., 2009). Longitudinal study design will be necessary to clarify at what point the brain connectome changes emerge.
There are a few limitations in the current study that should be considered when interpreting the results. The current findings may be cautiously generalized to the general population since these findings are based on a study with veterans. Many veterans experience traumatic brain injuries during their service and it has been reported that traumatic brain injury may influence white matter tracts (Lopez-Larson et al., 2013). However, the impact of subconcussive episodes or traumatic brain injury has not been considered in the current study. Some of the white matter alterations identified in association with suicide attempts may be the result of comorbid psychiatric disorders such as PTSD and depressive symptoms. It is also possible that white matter changes are related to head injury however the current study is not powered to examine these contributions. Larger studies with well characterized participants are needed to determine the relationship between DTI changes related to TBI and suicide. However, topological properties of the brain network in PTSD patients do not seem similar to those observed in suicide attempters (Long et al., 2013). Furthermore, the impact of medications that the subjects received has not been considered. Although the potential influence of medications on DTI has been inconclusive, there are some publications that report on the effects of mood stabilizers, antipsychotics, and antidepressants on brain FA values (Minami et al., 2003; Versace et al., 2008; Yoo et al., 2007). However, regions with FA changes under medication were mostly prefrontal area and FA could be either increased or decreased by medications in those studies.
5. Conclusion
In summary, this brain connectome study demonstrated the ability to detect the subtle neurobiological difference between suicidal ideators, attempters, and non-suicidal controls. Increased efficiency contributed by enhanced cortico-cortical connections and connectivity of DMN-related regions may be pathognomonic findings for suicide attempters and may represent a biological vulnerability in individuals with suicide behavior. The current study suggests potential neuroimaging markers for suicide behavior and provides a provisional basis of therapeutic targets, which should be investigated in the future studies.
Acknowledgments
Acknowledgements
This study was based upon work supported by the Department of Veterans Affairs and the resources and the use of facilities at the VISN 19 MIRECC, but does not necessarily represent the views of the Department of Veterans Affairs or United States Government. It was funded by the Military Suicide Research Consortium (MSRC), an effort supported by the Office of the Assistant Secretary of Defense for Health Affairs under Award No. W81XWH-10-2-0178, through Merit Review 5I01CX000253-02, and the Brain Research Program (2015M3C7A1028373) supported by the National Research Foundation of Korea (NRF).
Author agreement/declaration
All authors contributed to and approved the final version of this manuscript being submitted. We warrant that the manuscript is our original work and it has not been published and is not under consideration for publication elsewhere. The authors of this study do not have any commercial associations that might pose a conflict of interest in connection with this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.04.021.
Appendix A. Supplementary data
Supplementary tables
References
- Albrecht K., Volz K.G., Sutter M., von Cramon D.Y. What do I want and when do I want it: brain correlates of decisions made for self and other. PLoS One. 2013;8 doi: 10.1371/journal.pone.0073531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001;26:32–46. [Google Scholar]
- Andrews-Hanna J.R., Reidler J.S., Sepulcre J., Poulin R., Buckner R.L. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armed Forces Health Surveillance Center Surveillance snapshot: manner and cause of death, active component, U.S. Armed Forces, 1998–2013. MSMR. 2014;21:21. [Google Scholar]
- Ball G., Aljabar P., Zebari S., Tusor N., Arichi T., Merchant N., Robinson E.C., Ogundipe E., Rueckert D., Edwards A.D., Counsell S.J. Rich-club organization of the newborn human brain. Proc. Natl. Acad. Sci. U. S. A. 2014;111:7456–7461. doi: 10.1073/pnas.1324118111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batelaan N.M., de Graaf R., Spijker J., Smit J.H., van Balkom A.J., Vollebergh W.A., Beekman A.T. The course of panic attacks in individuals with panic disorder and subthreshold panic disorder: a population-based study. J. Affect. Disord. 2010;121:30–38. doi: 10.1016/j.jad.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Bertolote J.M., Fleischmann A. Suicide and psychiatric diagnosis: a worldwide perspective. World Psychiatry. 2002;1:181–185. [PMC free article] [PubMed] [Google Scholar]
- Bhar S., Ghahramanlou-Holloway M., Brown G., Beck A.T. Self-esteem and suicide ideation in psychiatric outpatients. Suicide Life Threat. Behav. 2008;38:511–516. doi: 10.1521/suli.2008.38.5.511. [DOI] [PubMed] [Google Scholar]
- Bijttebier S., Caeyenberghs K., van den Ameele H., Achten E., Rujescu D., Titeca K., van Heeringen C. The vulnerability to suicidal behavior is associated with reduced connectivity strength. Front. Hum. Neurosci. 2015;9:632. doi: 10.3389/fnhum.2015.00632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosnell R., Giorgio A., Johansen-Berg H. Imaging white matter diffusion changes with development and recovery from brain injury. Dev. Neurorehabil. 2008;11:174–186. doi: 10.1080/17518420802289065. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bullmore E.T., Bassett D.S. Brain graphs: graphical models of the human brain connectome. Annu. Rev. Clin. Psychol. 2011;7:113–140. doi: 10.1146/annurev-clinpsy-040510-143934. [DOI] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. The economy of brain network organization. Nat. Rev. Neurosci. 2012;13:336–349. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- Catani M., Dell'acqua F., Vergani F., Malik F., Hodge H., Roy P., Valabregue R., Thiebaut de Schotten M. Short frontal lobe connections of the human brain. Cortex. 2012;48:273–291. doi: 10.1016/j.cortex.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Chase H.W., Segreti A.M., Keller T.A., Cherkassky V.L., Just M.A., Pan L.A., Brent D.A. Alterations of functional connectivity and intrinsic activity within the cingulate cortex of suicidal ideators. J. Affect. Disord. 2017;212:78–85. doi: 10.1016/j.jad.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox Lippard E.T., Johnston J.A., Blumberg H.P. Neurobiological risk factors for suicide: insights from brain imaging. Am. J. Prev. Med. 2014;47:S152–162. doi: 10.1016/j.amepre.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox B.J., Enns M.W., Clara I.P. Psychological dimensions associated with suicidal ideation and attempts in the National Comorbidity Survey. Suicide Life Threat. Behav. 2004;34:209–219. doi: 10.1521/suli.34.3.209.42781. [DOI] [PubMed] [Google Scholar]
- D'Onofrio B.M., Class Q.A., Rickert M.E., Larsson H., Långström N., Lichtenstein P. Preterm birth and mortality and morbidity: a population-based quasi-experimental study. JAMA Psychiatry. 2013;70:1231–1240. doi: 10.1001/jamapsychiatry.2013.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit H., Flory J.D., Acheson A., McCloskey M., Manuck S.B. IQ and nonplanning impulsivity are independently associated with delay discounting in middle-aged adults. Personal. Individ. Differ. 2007;42:111–121. [Google Scholar]
- Deco G., Kringelbach M.L. Great expectations: using whole-brain computational connectomics for understanding neuropsychiatric disorders. Neuron. 2014;84:892–905. doi: 10.1016/j.neuron.2014.08.034. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M.P., Sporns O. Rich-club organization of the human connectome. J. Neurosci. 2011;31:15775–15786. doi: 10.1523/JNEUROSCI.3539-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M.P., Kahn R.S., Goni J., Sporns O. High-cost, high-capacity backbone for global brain communication. Proc. Natl. Acad. Sci. U. S. A. 2012;109:11372–11377. doi: 10.1073/pnas.1203593109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J., Heron J., Lewis G., Araya R., Wolke D., ALSPAC Study Team Negative self-schemas and the onset of depression in women: longitudinal study. Br. J. Psychiatry. 2005;186:302–307. doi: 10.1192/bjp.186.4.302. [DOI] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., van der Kouwe A., Destrieux C., Halgren E., Segonne F., Salat D.H., Busa E., Seidman L.J., Goldstein J., Kennedy D., Caviness V., Makris N., Rosen B., Dale A.M. Automatically parcellating the human cerebral cortex. Cereb. Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Giorgio A., Santelli L., Tomassini V., Bosnell R., Smith S., De Stefano N., Johansen-Berg H. Age-related changes in grey and white matter structure throughout adulthood. NeuroImage. 2010;51:943–951. doi: 10.1016/j.neuroimage.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P., Cammoun L., Gigandet X., Meuli R., Honey C.J., Wedeen V.J., Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6 doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Farmer M., Fogelman P., Gotlib I.H. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol. Psychiatry. 2015;78:224–230. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heeringen C., Bijttebier S., Godfrin K. Suicidal brains: a review of functional and structural brain studies in association with suicidal behaviour. Neurosci. Biobehav. Rev. 2011;35:688–698. doi: 10.1016/j.neubiorev.2010.08.007. [DOI] [PubMed] [Google Scholar]
- van Heeringen K., Bijttebier S., Desmyter S., Vervaet M., Baeken C. Is there a neuroanatomical basis of the vulnerability to suicidal behavior? A coordinate-based meta-analysis of structural and functional MRI studies. Front. Hum. Neurosci. 2014;8:824. doi: 10.3389/fnhum.2014.00824. (eCollection 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning E.R., Turk C.L., Mennin D.S., Fresco D.M., Heimberg R.G. Impairment and quality of life in individuals with generalized anxiety disorder. Depress. Anxiety. 2007;24:342–349. doi: 10.1002/da.20249. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., Smith S.M. Fsl. NeuroImage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Jiang W., Shi F., Liao J., Liu H., Wang T., Shen C., Shen H., Hu D., Wang W., Shen D. Disrupted functional connectome in antisocial personality disorder. Brain Imaging Behav. 2017;11(4):1071–1084. doi: 10.1007/s11682-016-9572-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashdan T.B., Breen W.E., Julian T. Everyday strivings in war veterans with posttraumatic stress disorder: suffering from a hyper-focus on avoidance and emotion regulation. Behav. Ther. 2010;41:350–363. doi: 10.1016/j.beth.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Borges G., Walters E.E. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch. Gen. Psychiatry. 1999;56:617–626. doi: 10.1001/archpsyc.56.7.617. [DOI] [PubMed] [Google Scholar]
- Kim B., Oh J., Kim M.K., Lee S., Tae W.S., Kim C.M., Choi T.K., Lee S.H. White matter alterations are associated with suicide attempt in patients with panic disorder. J. Affect. Disord. 2015;175:139–146. doi: 10.1016/j.jad.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Klonsky E.D., May A.M. Differentiating suicide attempters from suicide ideators: a critical frontier for suicidology research. Suicide Life Threat. Behav. 2014;44:1–5. doi: 10.1111/sltb.12068. [DOI] [PubMed] [Google Scholar]
- Klonsky E.D., May A.M., Saffer B.Y. Suicide, suicide attempts, and suicidal ideation. Annu. Rev. Clin. Psychol. 2016;12:307–330. doi: 10.1146/annurev-clinpsy-021815-093204. [DOI] [PubMed] [Google Scholar]
- Koenis M.M., Brouwer R.M., van den Heuvel M.P., Mandl R.C., van Soelen I.L., Kahn R.S., Boomsma D.I., Hulshoff Pol H.E. Development of the brain's structural network efficiency in early adolescence: a longitudinal DTI twin study. Hum. Brain Mapp. 2015;36:4938–4953. doi: 10.1002/hbm.22988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korgaonkar M.S., Fornito A., Williams L.M., Grieve S.M. Abnormal structural networks characterize major depressive disorder: a connectome analysis. Biol. Psychiatry. 2014;76:567–574. doi: 10.1016/j.biopsych.2014.02.018. [DOI] [PubMed] [Google Scholar]
- Lee S.J., Kim B., Oh D., Kim M.K., Kim K.H., Bang S.Y., Choi T.K., Lee S.H. White matter alterations associated with suicide in patients with schizophrenia or schizophreniform disorder. Psychiatry Res. 2016;248:23–29. doi: 10.1016/j.pscychresns.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Leech R., Sharp D.J. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Z., Duan X., Xie B., Du H., Li R., Xu Q., Wei L., Zhang S.X., Wu Y., Gao Q., Chen H. Altered brain structural connectivity in post-traumatic stress disorder: a diffusion tensor imaging tractography study. J. Affect. Disord. 2013;150:798–806. doi: 10.1016/j.jad.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Lopez-Larson M., King J.B., McGlade E., Bueler E., Stoeckel A., Epstein D.J., Yurgelun-Todd D. Enlarged thalamic volumes and increased fractional anisotropy in the thalamic radiations in veterans with suicide behaviors. Front. Psych. 2013;4:83. doi: 10.3389/fpsyt.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock R.J., Garrett A.S., Buonocore M.H. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104:667–676. doi: 10.1016/s0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- Mann J.J. Neurobiology of suicidal behaviour. Nat. Rev. Neurosci. 2003;4:819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- Mann J.J., Waternaux C., Haas G.L., Malone K.M. Toward a clinical model of suicidal behavior in psychiatric patients. Am. J. Psychiatry. 1999;156:181–189. doi: 10.1176/ajp.156.2.181. [DOI] [PubMed] [Google Scholar]
- McClure S.M., Laibson D.I., Loewenstein G., Cohen J.D. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Minami T., Nobuhara K., Okugawa G., Takase K., Yoshida T., Sawada S., Ha-Kawa S., Ikeda K., Kinoshita T. Diffusion tensor magnetic resonance imaging of disruption of regional white matter in schizophrenia. Neuropsychobiology. 2003;47:141–145. doi: 10.1159/000070583. [DOI] [PubMed] [Google Scholar]
- Myung W., Han C.E., Fava M., Mischoulon D., Papakostas G.I., Heo J.Y., Kim K.W., Kim S.T., Kim D.J., Kim D.K., Seo S.W., Seong J.K., Jeon H.J. Reduced frontal-subcortical white matter connectivity in association with suicidal ideation in major depressive disorder. Transl. Psychiatry. 2016;6 doi: 10.1038/tp.2016.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Have M., de Graaf R., van Dorsselaer S., Verdurmen J., van 't Land H., Vollebergh W., Beekman A. Incidence and course of suicidal ideation and suicide attempts in the general population. Can. J. Psychiatr. 2009;54:824–833. doi: 10.1177/070674370905401205. [DOI] [PubMed] [Google Scholar]
- Nordentoft M., Mortensen P.B., Pedersen C.B. Absolute risk of suicide after first hospital contact in mental disorder. Arch. Gen. Psychiatry. 2011;68:1058–1064. doi: 10.1001/archgenpsychiatry.2011.113. [DOI] [PubMed] [Google Scholar]
- Northoff G., Bermpohl F. Cortical midline structures and the self. Trends Cogn. Sci. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Office of Suicide Prevention, V . In: Suicide Among Veterans and Other Americans 2001–2014. Affairs V., editor. Office of Suicide Prevention; 2016. [Google Scholar]
- Patton J.H., Stanford M.S., Barratt E.S. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A., Mthalon D.H., Sullivan E.V., Rawles J.M., Zipursky R.B., Lim K.O. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch. Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pollock L.R., Williams J.M. Effective problem solving in suicide attempters depends on specific autobiographical recall. Suicide Life Threat. Behav. 2001;31:386–396. doi: 10.1521/suli.31.4.386.22041. [DOI] [PubMed] [Google Scholar]
- Posner K., Brown G.K., Stanley B., Brent D.A., Yershova K.V., Oquendo M.A., Currier G.W., Melvin G.A., Greenhill L., Shen S., Mann J.J. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am. J. Psychiatry. 2011;168:1266–1277. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchand R., Acosta J., Burns R.M., Jaycox L.H., Pernin C.G. The war within: preventing suicide in the U.S. military. RAND Health Q. 2011;1:2. [PMC free article] [PubMed] [Google Scholar]
- Santos J.C., Saraiva C.B., De Sousa L. The role of expressed emotion, self-concept, coping, and depression in parasuicidal behavior: a follow-up study. Arch. Suicide Res. 2009;13:358–367. doi: 10.1080/13811110903266590. [DOI] [PubMed] [Google Scholar]
- Scholz J., Klein M.C., Behrens T.E., Johansen-Berg H. Training induces changes in white-matter architecture. Nat. Neurosci. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini G., Pardini M., Pompili M., Girardi P., Amore M. Understanding suicidal behavior: the contribution of recent resting-state fMRI techniques. Front. Psych. 2016;7:69. doi: 10.3389/fpsyt.2016.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer C., Freyberger H.J., Kessler C. Hysteria, dissociation and conversion. A review of concepts, classification and diagnostic instruments. Psychiatr. Prax. 1996;23:63–68. [PubMed] [Google Scholar]
- Sudol K., Mann J.J. Biomarkers of suicide attempt behavior: towards a biological model of risk. Curr. Psychiatry Rep. 2017;19(6):31. doi: 10.1007/s11920-017-0781-y. [DOI] [PubMed] [Google Scholar]
- Suo X., Lei D., Chen F., Wu M., Li L., Sun L., Wei X., Zhu H., Li L., Kemp G.J., Gong Q. Anatomic insights into disrupted small-world networks in pediatric posttraumatic stress disorder. Radiology. 2017;282:826–834. doi: 10.1148/radiol.2016160907. [DOI] [PubMed] [Google Scholar]
- Uehara T., Yamasaki T., Okamoto T., Koike T., Kan S., Miyauchi S., Kira J., Tobimatsu S. Efficiency of a “small-world” brain network depends on consciousness level: a resting-state FMRI study. Cereb. Cortex. 2014;24:1529–1539. doi: 10.1093/cercor/bht004. [DOI] [PubMed] [Google Scholar]
- Versace A., Almeida J.R., Hassel S., Walsh N.D., Novelli M., Klein C.R., Kupfer D.J., Phillips M.L. Elevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract-based spatial statistics. Arch. Gen. Psychiatry. 2008;65:1041–1052. doi: 10.1001/archpsyc.65.9.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Veterans Affairs . Defense, Affairs, V., Services, H.a.H., Education; 2013. National Research Action Plan: Responding to the Executive Order Improving Access to Mental Health Services for Veterans, Service Members, and Military Families. [Google Scholar]
- Wang J., Wang X., Xia M., Liao X., Evans A., He Y. GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front. Hum. Neurosci. 2015;9:386. doi: 10.3389/fnhum.2015.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts D.J., Strogatz S.H. Collective dynamics of ‘small-world’ networks. Nature. 1998;393:440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Williams J.M., Barnhofer T., Crane C., Herman D., Raes F., Watkins E., Dalgleish T. Autobiographical memory specificity and emotional disorder. Psychol. Bull. 2007;133:122–148. doi: 10.1037/0033-2909.133.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M., He Y. Magnetic resonance imaging and graph theoretical analysis of complex brain networks in neuropsychiatric disorders. Brain Connect. 2011;1:349–365. doi: 10.1089/brain.2011.0062. [DOI] [PubMed] [Google Scholar]
- Yoo S.Y., Jang J.H., Shin Y.W., Kim D.J., Park H.J., Moon W.J., Chung E.C., Lee J.M., Kim I.Y., Kim S.I., Kwon J.S. White matter abnormalities in drug-naive patients with obsessive-compulsive disorder: a diffusion tensor study before and after citalopram treatment. Acta Psychiatr. Scand. 2007;116:211–219. doi: 10.1111/j.1600-0447.2007.01046.x. [DOI] [PubMed] [Google Scholar]
- Zhang J., Wang J., Wu Q., Kuang W., Huang X., He Y., Gong Q. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol. Psychiatry. 2011;70:334–342. doi: 10.1016/j.biopsych.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Zhang S., Chen J.M., Kuang L., Cao J., Zhang H., Ai M., Wang W., Zhang S.D., Wang S.Y., Liu S.J., Fang W.D. Association between abnormal default mode network activity and suicidality in depressed adolescents. BMC Psychiatry. 2016;16:337. doi: 10.1186/s12888-016-1047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Jiang G., Tian J., Qiu Y., Wen X., Zalesky A., Li M., Ma X., Wang J., Li S., Wang T., Li C., Huang R. Abnormal white matter structural networks characterize heroin-dependent individuals: a network analysis. Addict. Biol. 2016;21:667–678. doi: 10.1111/adb.12234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables