Abstract
The risks and benefits of structured treatment interruption (STI) in HIV-1-infected subjects are not fully understood. A pilot study was performed to compare STI with continuous highly active antiretroviral therapy (HAART) in chronic HIV-1-infected subjects with HIV-1 plasma RNA levels (VL) <400 copies per ml and CD4+ T cells >400 per μl. CD4+ T cells, VL, HIV-1-specific neutralizing antibodies, and IFN-γ-producing HIV-1-specific CD8+ and CD4+ T cells were measured in all subjects. STIs of 1-month duration separated by 1 month of HAART, before a final 3-month STI, resulted in augmented CD8+ T cell responses in all eight STI subjects (P = 0.003), maintained while on HAART up to 22 weeks after STI, and augmented neutralization titers to autologous HIV-1 isolate in one of eight subjects. However, significant decline of CD4+ T cell count from pre-STI level, and VL rebound to pre-HAART baseline, occurred during STI (P = 0.001 and 0.34, respectively). CD4+ T cell counts were regained on return to HAART. Control subjects (n = 4) maintained VL <400 copies per ml and stable CD4+ T cell counts, and showed no enhancement of antiviral CD8+ T cell responses. Despite increases in antiviral immunity, no control of VL was observed. Future studies of STI should proceed with caution.
Keywords: CD8+ T cells‖neutralizing antibodies‖CD4+ T cells
The HIV-1 plasma RNA level (VL) measured after acute HIV-1 infection has been correlated with rate of subsequent disease progression (1, 2). Cell-mediated immunity, in particular, has been shown to be important for suppressing HIV-1 viral replication (3, 4). Indeed, inverse relationships exist between antiviral CD8+ T cell responses and VL as well as CD4+ T helper responses and VL in some subjects (5–7). These observations suggest that there is a balance between virus and host immune responses, and that augmented immunity may lead to decreased VL.
Patients on highly active antiretroviral therapy (HAART) have decreases in the magnitude, activity, and activation level of HIV-1-specific cellular immune responses (8–13). Thus, it is not surprising that the majority of subjects who interrupt HAART experience viral load rebound (14–19). However, anecdotal case reports describe HIV-1-infected subjects who received early treatment but were intermittently adherent to HAART. In these patients, antiviral CD8+ T cell responses and neutralizing antibody titers increased during HAART, and VLs remained low when HAART was interrupted (20, 21). These cases opened the possibility of using cycles of structured treatment interruptions (STI) to augment the anti-HIV-1 immunological response, reduce VL, and reduce antiretroviral drug exposure and toxicities (22–25).
The first STIs were recently assessed in a group of HIV-1-infected patients identified and treated with HAART early after infection (26). In these subjects, HIV-1-specific T helper responses were preserved, and HIV-1-specific CD8+ T cell responses were augmented, by periods of STI. When compared with historical controls from the MultiCenter AIDS Cohort Study, the effects on VL after final treatment interruption were dramatic. Six of thirteen patients (≈50%) maintained VL <500 copies per ml for a median of 6.5 months without HAART (26).
The majority of HIV-1-infected patients start HAART during the chronic stages of disease. Reports of asymptomatic, chronically HIV-1-infected subjects who are intermittently adherent to HAART suggest that STI may increase HIV-1-specific CD8+ and CD4+ T cell responses in these subjects (27–29). Here, we report on a pilot study of the immunologic and virologic effects of STI in asymptomatic patients with chronic HIV-1 infection.
Materials and Methods
Patients.
Twelve asymptomatic, chronically HIV-1-infected patients were recruited from the Duke University Adult Infectious Disease Clinic between June 1999 and February 2000. The eligibility criteria were: (i) documented HIV-1 infection; (ii) CD4+ T cell count ≥400 cells per μl for at least 6 months before entry; (iii) VL <400 copies per ml for at least 3 months before entry; and (iv) renal, hepatic, and hematopoietic function tests within defined limits. While on HAART, these subjects are representative of early chronic HIV-1 disease. The study protocol was approved by the Duke University Institutional Review Board, and all patients gave written informed consent.
Study Design.
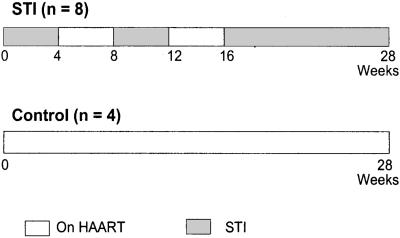
Eight patients were randomized to undergo STIs (STI subjects) and four patients to continuous HAART (control subjects) through a computer-generated randomization model. The subjects then followed the treatment schedules outlined in Fig. 1. STI subjects underwent two cycles, each consisting of 1 month of STI followed by 1 month on HAART, based on a previous study showing high viral rebound during a month-long treatment interruption followed by efficient resuppression of virus 1 month after returning to HAART (15). After the second cycle, the safety and immunological effects of STI were studied during a 3-month period without HAART. The control subjects received continuous HAART for the entire 7-month study period. The study protocol mandated treatment resumption if the VL was >100,000 copies per ml during the final 12-week STI, if the CD4+ T cell count decreased to <200 cells per μl at any point during the study, or if a patient withdrew consent. Peripheral blood samples for laboratory measurements were obtained weekly during STI, and every other week during HAART.
Figure 1.
Schematic presenting schedule of structured antiretroviral treatment interruptions (STI). STI subjects underwent two 4-week-long STIs and one 12-week-long STI. Control subjects remained on HAART. Gray rectangles, STI periods; White rectangles, on HAART periods.
Viral Load and CD4+ T Cell Counts.
Plasma samples were assayed for HIV-1 RNA with a reverse transcription (RT)-PCR assay (Roche Amplicor, Branchburg, NJ; lower limit of detection 400 copies per ml). CD4+ T cell counts in samples of peripheral blood mononuclear cells (PBMC) were quantified with standard flow cytometric analyses using fluorescent monoclonal antibodies (Becton Dickinson).
Measurement of HIV-1-Specific CD8+ and CD4+ T Cell Responses.
HIV-1-specific CD8+ T cell responses were measured from cryopreserved PBMC samples by cytokine flow cytometry with recombinant vaccinia virus constructs expressing HIVIIIB Env-gp160, Gag-p55, RT-Pol, and Nef (Therion Biologics, Cambridge, MA) as described (30–32). HIV-1-specific CD4+ T cell responses in cryopreserved PBMC samples were also measured by the same method except with p24 whole-protein stimulation at 5 μg/ml (Protein Sciences, Meriden, CT; refs. 29 and 30). Background control frequencies were low (mean = 0.14% for control TK− vaccinia virus construct and 0.09% for baculovirus extract control). Antigen-specific frequencies were obtained by subtracting background response and were expressed as percent of CD3, CD8 or CD3, CD4 double-positive cells, respectively. A response was considered positive if it was greater than one standard deviation above the distribution of control conditions (standard deviation for TK construct was 0.14% and for baculovirus extracts was 0.08%). The total HIV-1-specific CD8+ T cell response was calculated by summing the positive responses specific for HIVIIIB Env-gp160, Gag-p55, RT-Pol, and Nef. The breadth of the HIV-1-specific CD8+ T cell response was obtained by summing the number of different antigen-specific positive responses.
Virus Isolation and Characterization.
Viruses of STI subjects were isolated by coculturing PBMCs with phytohemagglutinin-stimulated PBMCs (PHA-PBMCs) from a healthy, uninfected donor as described (33). Viral stocks were propagated in PHA-PBMCs, and the coreceptor use was determined by p24 production on established cell lines (34).
Neutralizing Antibody Titers.
Viral isolates were incubated with sequential autologous serum samples and neutralization was monitored in PHA-PBMC. The neutralization titer measured was defined as the reciprocal of the serum dilution that caused an 80% reduction in p24-Gag antigen synthesis as described (33, 35). Neutralizing antibodies were also measured with the T cell line adapted (TCLA) strains HIV-1IIIB, HIV-1MN, and HIV-1SF2 in MT-2 cells (34). Titers in the MT-2 assay were defined as the reciprocal of the serum dilution at which 50% of cells were protected from virus-induced killing as measured by Neutral Red uptake. Stocks of the TCLA strains were prepared in H9 cells as described (34).
Statistical Analysis.
Statistical analyses were performed with SIGMASTAT 2.03 software (SPSS, Chicago). Tests were matched to data type and distribution. Statistical analyses included the Wilcoxon signed-rank test, paired t test, and general descriptive statistics. P values ≤ 0.05 were considered significant if the power coefficient was greater than 0.80 with alpha coefficient of 0.05.
Results
Pre-STI Characteristics of Study Subjects.
Twelve patients with chronic HIV-1 infection were enrolled, and the demographics of these subjects are shown in Table 1. After completion of the continuous HAART schedule, subject 15 asked to undergo the STI schedule as a nonrandomized subject. Before initiation of HAART, the median VL was 42,529 copies per ml (minimum = 700 copies per ml; maximum = 760,000 copies per ml; mean = 180,000 copies per ml; 95% confidence interval (CI) = 309,168 copies per ml) and the median CD4+ T cell count was 414 cells/μl (minimum = 21 cells per μl; maximum = 576 cells per μl; mean = 374 cells per μl; CI = 142 cells per μl) in STI subjects (Table 1). These subjects received HAART for at least 1.6 years (median 2.7 years) before enrollment in this study. During HAART, the median CD4+ T cell count increased from pre-HAART level to 598 cells per μl (P < 0.001; power = 0.998; paired t test). The VL was suppressed to <400 copies per ml for a minimum of 1.1 years before initiating STI (median 2.0 years; Table 1).
Table 1.
The demographic, immunologic, and virologic profiles of subjects
| Patient no. | Study arm | Age | Ethnicity, sex | Pre-HAART
|
Pre-STI
|
End of STI
|
Post-STI¶
|
Coreceptor usage | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4 count* | Viral load (logs)* | CD4 count* | Viral load (logs)* | CD4 count* | Viral load (logs)* | CD4 count* | Viral load (logs)* | |||||
| 1 | STI | 56 | Wh, M | 463 | 4.28 | 641 | <2.60 | 590 | 4.68 | 705 | <2.60 | R5 |
| 2 | STI | 49 | NA, M | 450 | 2.84 | 478 | <2.60 | 341 | 3.62 | 529 | <2.60 | R5 |
| 3 | STI | 37 | Bl, M | 271 | 4.71 | 425 | <2.60 | 250‡ | 4.98‡ | 428 | <2.60 | R5 |
| 6 | STI | 44 | Wh, M | 487 | 5.34 | 728 | <2.60 | 539 | 5.07 | 793 | 3.05 | R5 |
| 7 | STI | 56 | Wh, M | 379 | na | 779 | <2.60 | 543 | 4.44 | 801 | <2.60 | R5 |
| 10 | STI | 44 | Wh, M | 352 | 4.53 | 556 | <2.60 | 345 | 4.48 | 333§ | 4.77§ | R5/X4 |
| 12 | STI | 49 | Wh, M | 21 | 5.88 | 363 | <2.60 | 224‡ | 5.02‡ | 333 | 3.40 | R5/X4 |
| 11† | STI | 44 | Bl, F | na | na | nd | <2.60 | nd† | nd† | nd† | nd† | nd† |
| 15 | Cntrl, STI | 54 | Wh, M | 576 | na | 851 | <2.60 | 730 | 3.96 | 723 | 3.31 | R5 |
| 4 | Cntrl | 45 | Wh, M | 329 | na | |||||||
| 8 | Cntrl | 54 | Bl, M | 326 | 5.27 | |||||||
| 9 | Cntrl | 38 | Wh, M | na | na | |||||||
Wh = caucasian, NA = Native American, Bl = Black, M = male, F = female, na = not available, nd = not tested.
CD4+ T cell counts are measured in units of cells per μl, and viral loads are in units of log10 (RNA copies per ml).
Subject withdrew consent at week 6 and could not be included in analysis.
Subjects did not complete final 12-week STI. Parameters were measured at last time point before returning to HAART.
Subject remains off HAART for 30 weeks after the end of the final scheduled STI, and values represent most recent measurements off HAART.
Post-STI values represent measurements taken between 38 and 64 weeks from enrollment.
Study Intervention and Adverse Events.
Three STI subjects did not complete the full 28-week STI schedule. Subject 11 had rash with hives upon resuming her antiretroviral drug regimen (lamivudine, stavudine, and nevirapine) at week 6 and withdrew from the study. Subject 3 chose to resume HAART at week 20 while presenting with respiratory symptoms, lymphadenopathy, and an enlarged left tonsil with exudate and mild erythema. At this time, his CD4+ T cell count had decreased to 250 cells per μl and his VL had increased to 95,611 copies per ml (Table 1). Subject 12 had increased fatigue, sinus congestion, headache, and myalgias at week 22. At this time, he met the criterion for HAART resumption with VL of 103,641 copies per ml. Thus, a total of eight STI subjects (seven randomized and one nonrandomized) completed at least 20 weeks of the study and were used in the analysis of STI.
Viral Loads and CD4+ T Cell Counts.
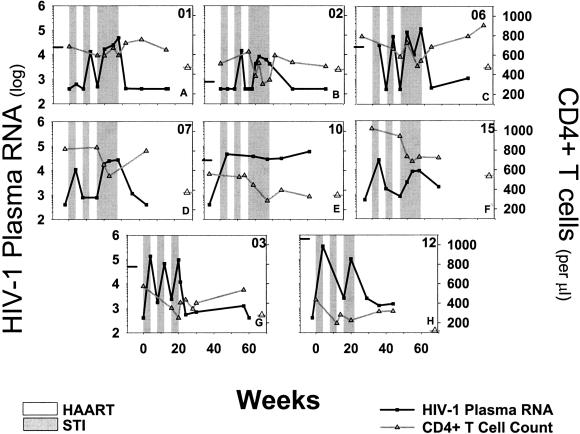
Historical HIV-1 RNA determinations and CD4+ T cell counts garnered from clinical records were used to establish pre-HAART baselines (Fig. 2, Table 1). RNA determinations were not available for subjects 7 and 15. During the first STI, seven of eight subjects experienced VL rebound. Subjects 3, 6, 7, 10, 12, and 15 had VL rebound greater than 10,000 copies per ml during the first STI. Subjects 2, 3, 6, 10, and 12 had a VL peak measured between study weeks 19–24 during the last STI. As a group with some individual variation, VL returned to pre-HAART baseline levels by the end of the last STI (P = 0.35; power = 0.054; paired t test). VL was resuppressed to a low level after return to HAART post-STI in all subjects.
Figure 2.
Longitudinal follow-up of HIV-1 plasma RNA levels and CD4+ T cell levels in eight chronically HIV-1-infected individuals undergoing STI. Time of follow-up was in weeks after initiation of first treatment interruption. HIV-1 plasma RNA, and CD4+ T cell counts are presented along the left and right y axes, respectively. If measurements were available, tabs on the left and right y axes represent pre-HAART baseline for HIV-1 plasma RNA level and CD4+ T cell count, respectively. Gray rectangles, STI periods; White rectangles, on HAART periods.
During STI, the CD4+ T cell counts decreased. Subjects 1, 3, 6, 12, and 15 had CD4+ T cell counts decrease after the first two STIs. By the end of the last STI, all subjects had declines in CD4+ T cell counts. Importantly, in no case did the levels fall consistently below pre-HAART baseline, and the CD4+ T cell count never fell consistently below 200 cells per μl (Fig. 2, Table 1). In subjects 3 and 12, the CD4+ T cell percentage decreased >50% from pre-STI level (data not shown). In all eight of the STI subjects the CD4+ T cell levels returned to pre-STI levels after resumption of HAART (P = 0.94; power = 0.050; paired t test).
Effect of STI on HIV-1-Specific Cellular Immune Responses.
STI had little effect on HIV-1-specific CD4+ T cell responses in this cohort. In all subjects, CD4+ T cell responses were below detection before STI and remained so over the vast majority of time points during STI (data not shown). Of note, the HIV-1-specific CD4+ T cell responses were measured from cryopreserved PBMC samples, and may not have been optimal for measuring lower-level CD4+ T helper responses expected to be found in chronically HIV-1-infected subjects. The effect of STI on the HIV-1-specific CD8+ T cell responses is shown in Table 2. Before STI, HIV-1-specific CD8+ T cell responses were generally low or undetectable. The mean total HIV-1-specific CD8+ T cell percentage was 0.30% of CD8+ T cells (CI = 0.34%). The breadth of the response was also low with a mean breadth of 1.13 antigens (CI = 1.13 antigens; data not shown). These results are consistent with the decrease of HIV-1-specific CD8+ T cell responses to a low level on HAART (8–11, 27).
Table 2.
Effect of STI on HIV-1-specific neutralizing antibodies and CD8+ T cells
| Patient no. | Study week | Nab titer to virus*
|
Study week | CD8+ T cells
|
|||
|---|---|---|---|---|---|---|---|
| Autologous | HIV-1IIIB | HIV-1MN | HIV-1SP2 | Total response, % | |||
| 1 | 4 | 25 | 285 | 763 | 858 | 0 | 0.47 |
| 12 | 110 | 351 | 1,002 | 444 | 6 | 0.84 | |
| 20 | 105 | 264 | 753 | 532 | 16 | 0.75 | |
| 24 | >256 | 259 | 1,150 | 970 | 28 | 2.58 | |
| 28 | 100 | 455 | 1,762 | 1,217 | 41 | 1.96 | |
| 2 | 4 | 7 | 398 | 2,077 | 1,441 | 0 | 0.00 |
| 9 | — | 478 | 1,189 | 847 | 6 | 0.47 | |
| 12 | 150 | 1,019 | 3,095 | 3,837 | 16 | 3.03 | |
| 20 | 18 | 406 | 3,859 | 2,313 | 27 | 2.48 | |
| 24 | 10 | 667 | 7,632 | 5,426 | 31 | 1.06 | |
| 28 | 7 | 799 | 6,662 | 8,564 | |||
| 3 | 4 | 7 | 271 | 728 | 805 | 1 | 0.00 |
| 12 | 50 | 1,258 | 1,485 | 1,504 | 16 | 0.38 | |
| 20 | 19 | 465 | 377 | 788 | 20 | — | |
| 24 | 44 | 325 | 777 | 710 | 43 | 0.50 | |
| 28 | — | — | — | — | |||
| 6 | 4 | <4 | 273 | 1,985 | 1,430 | −6 | 0.25 |
| 12 | 5 | 664 | 2,307 | 680 | 16 | — | |
| 20 | 4 | 316 | 2,024 | 900 | 28 | 1.01 | |
| 22 | 6 | 602 | 16,594 | 598 | 41 | 0.67 | |
| 24 | 18 | 919 | 2,876 | 1,000 | |||
| 28 | 20 | 797 | 2,434 | 712 | |||
| 7 | 4 | <4 | 115 | 432 | 1,126 | 0 | 0.28 |
| 12 | 18 | 220 | 861 | 3,978 | 8 | 0.00 | |
| 20 | — | 177 | 811 | 2,353 | 16 | 0.63 | |
| 22 | — | 263 | 668 | 2,162 | 27 | 1.23 | |
| 24 | 7 | 260 | 433 | 3,175 | 33 | 0.70 | |
| 28 | 17 | 199 | 1,070 | 1,247 | |||
| 10 | 4 | 38 | 282 | 390 | 1,040 | 0 | 1.23 |
| 12 | 6 | 359 | 1,456 | 841 | 8 | 2.52 | |
| 20 | <4 | 119 | 1,076 | 1,209 | 16 | 2.89 | |
| 22 | — | 31 | 897 | 1,994 | 28 | — | |
| 24 | <4 | 180 | 822 | — | |||
| 28 | 30 | — | — | — | |||
| 12 | 4 | <4 | 109 | 391 | 472 | 0 | 0.00 |
| 12 | <4 | 378 | 630 | 1,835 | 6 | 1.22 | |
| 20 | <4 | 102 | 1,006 | 2,862 | 16 | 2.68 | |
| 22 | 0.46 | ||||||
| 15 | 4 | 256 | 83 | 2,507 | 452 | 0 | 0.14 |
| 12 | 122 | 130 | 2,080 | 997 | 8 | 2.66 | |
| 20 | — | 75 | 1,204 | 4,899 | 16 | 2.34 | |
| 24 | 210 | — | — | — | 27 | 2.70 | |
Neutralizing antibodies were measured in either PHA-PBMC (autologous primary isolates) or MT-2 cells (HIV-1IIIB, HIV-1MN, and HIV-1SF2). Dashes represent time points not tested.
During STI, the percentage of HIV-1-specific CD8+ T cells increased markedly to a maximum of greater than 2.5% of CD8+ T cells in five subjects. The total HIV-1-specific CD8+ T cell frequency was lower (<0.50% of CD8+ T cells) in subjects 3 and 12 at time of withdrawing from STI and returning to HAART. The mean total HIV-1-specific CD8+ T cell percentage and breadth by the end of the last STI were 1.77% (CI = 1.04%) and 3.33 antigens (CI = 0.86 antigens). Compared with pre-STI level, both the percentage and breadth of the total HIV-1-specific CD8+ T cell response increased significantly by end of the last STI (P = 0.003 and 0.02, respectively; paired t tests).
Augmented HIV-1-specific CD8+ T cell responses were maintained (mean magnitude, 0.98% of CD8+ T cells; mean breadth, 2.80 antigens; CIs of 0.72% and 1.04 antigens, respectively) up to 22 weeks after return to HAART in the five subjects for whom follow-up was available. The increases were significant compared with pre-STI levels (P < 0.03; paired t tests). No significant increases in HIV-1-specific CD8+ or CD4+ T cell responses were measured in control subjects during follow-up (data not shown).
Effect of STI on HIV-1-Specific Neutralizing Antibody Titers.
Neutralizing antibody titers against T cell line adapted (TCLA) strains and autologous HIV-1 isolate were measured longitudinally during follow-up (Table 2). The assessments were made with sera collected during STI to avoid possible artifacts due to residual amounts of antiretroviral drugs in the samples. In general, high titers were observed against all three TCLA strains with no change in titer during STI. Autologous neutralizing titers were assessed with virus that was isolated between 3 and 7 weeks after the initiation of the last STI. Unexpectedly, the isolates were sensitive to neutralization by earlier autologous serum samples in seven of eight cases. A spectrum of potencies was observed. Subjects 2, 6, 7, and 10 had neutralizing antibody titers that were sporadically detectable, whereas subject 12 had no detectable neutralizing antibody titers against autologous virus. Subject 15 had high neutralizing antibody titers, and subject 3 had moderate neutralizing titers, against autologous virus at the first STI, which were maintained in both subjects for the entire study period. Subject 1 had low autologous neutralizing antibody titers initially, but these increased to high levels by the last STI (Table 2).
Discussion
Assessing the risks and benefits of STI is the key for determining whether it can be applied to patients with chronic HIV-1 infection. This randomized, controlled pilot study of cyclic STIs in chronically HIV-1-infected subjects showed that all subjects had VL rebound and CD4+ T cell counts decline during STI. HIV-1-specific CD8+ T cell immunity was significantly augmented during STI and maintained up to 22 weeks after resumption of HAART, whereas HIV-1-specific neutralizing antibody titers increased in one. However, the boosted antiviral immunity was not associated with an effect on VL rebound, during STI, and no changes in HIV-1-specific CD4+ T cell frequency were observed. VL decreased to a low level and CD4+ T cell counts returned to pre-STI levels after resumption of HAART. No clear therapeutic benefits of STI were observed in this study, although the procedure was relatively safe and resulted in increased HIV-1-specific immune responses and temporary periods off continuous HAART dosing. Although other studies have shown an effect on HIV-1 rebound after cycles of treatment interruption in chronically infected subjects, closer analysis must be performed to determine the optimal schedule for reproducing the effects observed in such cases (28).
Safety is the primary concern in performing STIs. Previous studies of HIV-1-infected subjects intermittently adherent to HAART or undergoing STI have shown little risk for selecting drug-resistant viral strains during interruption periods (15–17, 28, 36). Resumption of HAART after month-long STI results in VL suppression kinetics similar to those in drug-naive individuals (15–17, 36). However, VL rebounds back to pre-HAART baseline levels during therapy interruption in the majority of subjects, suggesting that a single STI may have no effect on the level of VL rebound (14–17, 19, 37). In the current study, VL rebound to pre-HAART levels and efficient resuppression of VL to a low level on resumption of HAART after last STI was observed, consistent with previous findings (15–17, 28, 36). The effect of this VL rebound on long-term clinical outcome is unknown.
Previous studies of asymptomatic, chronically HIV-1-infected subjects report small change in CD4+ T cell levels during short treatment interruptions lasting 7 to 55 days (17, 28, 36). In the current study, CD4+ T cell levels did not drop after two cycles of month-long STIs in three of eight subjects. Subjects 3 and 12 had progressive drops during the first two STIs and >50% declines from pre-STI CD4+ T cell baselines during the last STI. By the end of the final STI, CD4+ T cell counts had fallen to some degree in all subjects. Importantly, on HAART resumption, CD4+ T cell counts returned to pre-STI levels in all subjects. The mechanism of these temporary drops in CD4+ T cell levels and the effect on disease progression need further analysis. Direct HIV-1-mediated toxicity, activation, and redistribution of these cell subsets into lymphoid tissues, impaired T cell production, and increased T cell turnover may all play roles in CD4+ T cell declines.
STIs can reverse some of the toxicities associated with prolonged HAART treatment (25), but are not free of adverse events. In addition to CD4+ T cell declines, symptoms of primary HIV-1 infection may recur (38). Two subjects in this study had physical symptoms during STI. One had mild left cervical lymphadenopathy with tenderness and an enlarged tonsil with exudate and mild erythema. A second experienced increased fatigue, sinus congestion, headache, and myalgias. Both of these subjects had VL rebound to levels near 105 copies per ml and CD4+ T cell declines. A third subject reported rash and hives, commonly associated with nevirapine-use in females (39), and decided to return to HAART. Future studies should take careful note to watch for adverse physical and psychological events and to determine whether or not they are associated with the extent of VL rebound, CD4+ T cell decline, HAART regimen, and/or STI-based anxiety.
There may be some immunological benefits to performing STI. Preliminary, supportive information has been gained from chronically HIV-1-infected patients who interrupted treatment either to reduce adverse events or for treatment holiday (28, 29). In these subjects, HIV-1-specific CD4+ and CD8+ T cell responses increase significantly (28, 29). In prospective studies of chronically HIV-1 infected subjects undergoing month-long STI, not all subjects had increases in antiviral CD8+ T cell responses (27, 36). Similarly, after the first STI in our study two of eight subjects had definitive increases in HIV-1-specific CD8+ T cell responses. After three cycles of STI, CD8+ T cell responses had increased significantly in all eight subjects. The magnitude of these increases varied from subject to subject. The augmented CD8+ T cell responses were maintained for up to 22 weeks after STI, but as yet there are no predictive indicators of which subjects are most likely to mount stronger CD8+ T cell responses after STI.
We did not observe increases in HIV-1-specific CD4+ T cell responses in this cohort. The absence of detectable increases in anti-HIV-1 CD4+ T cell cytokine responses from cryopreserved samples suggests that the CD4+ T cell responses were either weak or undetectable in this cohort. Although some subjects are able to augment HIV-1-specific CD4+ T cell responses, not all chronically infected subjects have sustained CD4+ T cell responses after STI (28, 36). Additionally, the lack of complete viral load suppression to below detection between month-long STI periods in some study subjects may have also contributed to the lack of observed increases in HIV-1-specific CD4+ T cell responses, which have been noted to rise upon efficient suppression of VL (28).
Interestingly, STI may also affect the HIV-1-specific humoral response. Neutralization titers against autologous HIV-1 isolates increase in some early-treated subjects interrupting HAART, but the effect of STI on humoral responses of chronically infected subjects is poorly understood (20). Seven of our subjects had detectable neutralization titers, suggesting that the neutralizing antibody response continues to mature in chronically infected subjects during HAART, whereas the neutralization determinants of the virus remain relatively constant because of the suppression of virus replication. Two subjects did maintain moderate to high neutralization titers throughout longitudinal follow-up against viral isolates obtained from the end of the third STI period, strongly suggesting that there is little risk of selecting neutralization escape variants in an STI regimen. In fact, just the opposite may occur, as one chronically infected subject mounted a humoral response with increased neutralization potential against autologous HIV-1 strains. Therefore, a minority of chronically HIV-1-infected subjects may augment neutralization titers in response to STI, whereas the majority of subjects seem to have little or no changes. It is not possible to rule out that if humoral immunity could be augmented, the outcome of STI may be different.
In the current study, we did not observe an effect on VL rebound after three STIs, perhaps because of the lack of detectable increase in antiviral CD4+ T cell responses required for CD8+ T cell function, or the lack of increase in neutralizing antibody titers in the majority of subjects, or due to a STI schedule that may not have been optimal for observing immune-mediated control of viral replication after STI. Whether an effect on VL would have been observed had the patients remained off therapy for a longer duration, upon additional STIs, or after longer on-HAART “rest” periods cannot be determined. Predictive indicators of which chronically infected subjects are most likely to benefit from STI are still needed.
In conclusion, we observed a significant augmentation in HIV-1 immunity after three cycles of STI. The magnitude of the augmentation of antiviral CD8+ T cell responses and neutralization titers varied, and CD4+ T cell counts and VL did not seem to be a predictor. STI, however, came at a cost. VL rebound occurred and CD4+ T cell levels declined during STI, but were reversible upon HAART resumption. Despite augmented anti-HIV-1 immunity, there was no blunting of VL rebound during STI. These results suggest that month-long cycles of STI and HAART were relatively safe but may not impact VL rebound during prolonged HAART interruption.
Acknowledgments
We thank the patients for participation in this study and Marie Senegal for technical assistance. We thank Mike McCune, Jeff Harris, and Steve Deeks for helpful comments, and Stephen Ordway and Gary Howard for editorial assistance. This work was supported by National Institutes of Health Grants R01 AI44595 (to D.F.N.) and AI40237 (to D.M.), and K24 Grant AI01744 (to J.A.B.). We are grateful for financial assistance for plasma virus load determinations from Agouron Pharmaceuticals. G.M.O. is a student in the Tri-Institutional M.D./Ph.D. Program supported by the National Institutes of Health Medical Scientists Training Program Grant GM0773 and Minority Predoctoral Fellowship F31 GM20068. D.F.N. is an Elizabeth Glaser Scientist of the Elizabeth Glaser Pediatric AIDS Foundation.
Abbreviations
- STI
structured treatment interruption
- HAART
highly active antiretroviral drug therapy
- VL
HIV-1 plasma RNA level
- PBMC
peripheral blood mononuclear cells
- CI
95% confidence interval
References
- 1.Mellors J W, Rinaldo C R, Jr, Gupta P, White R M, Todd J A, Kingsley L A. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien T R, Blattner W A, Waters D, Eyster E, Hilgartner M W, Cohen A R, Luban N, Hatzakis A, Aledort L M, Rosenberg P S, et al. J Am Med Assoc. 1996;276:105–110. [PubMed] [Google Scholar]
- 3.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. J Virol. 1994;68:4650–4665. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalams S A, Buchbinder S P, Rosenberg E S, Billingsley J M, Colbert D S, Jones N G, Shea A K, Trocha A K, Walker B D. J Virol. 1999;73:6715–6720. doi: 10.1128/jvi.73.8.6715-6720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, et al. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg E, Billingsley J, Caliendo A, Boswell S, Sax P, Kalams S, Walker B. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 8.Ogg G S, Jin X, Bonhoeffer S, Moss P, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Hurley A, et al. J Virol. 1999;73:797–800. doi: 10.1128/jvi.73.1.797-800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalams S A, Goulder P J, Shea A K, Jones N G, Trocha A K, Ogg G S, Walker B D. J Virol. 1999;73:6721–6728. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spiegel H M, DeFalcon E, Ogg G S, Larsson M, Beadle T J, Tao P, McMichael A J, Bhardwaj N, O'Callaghan C, Cox W I, et al. J Infect Dis. 1999;180:359–368. doi: 10.1086/314867. [DOI] [PubMed] [Google Scholar]
- 11.Rinaldo C R, Jr, Huang X L, Fan Z, Margolick J B, Borowski L, Hoji A, Kalinyak C, McMahon D K, Riddler S A, Hildebrand W H, et al. J Virol. 2000;74:4127–4138. doi: 10.1128/jvi.74.9.4127-4138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson J D, Ogg G S, Allen R L, Davis C, Shaunak S, Downie J, Dyer W, Workman C, Sullivan S, McMichael A J, Rowland-Jones S L. AIDS. 2000;14:225–233. doi: 10.1097/00002030-200002180-00003. [DOI] [PubMed] [Google Scholar]
- 13.Stranford S A, Ong J C, Martinez-Marino B, Busch M, Hecht F M, Kahn J, Levy J A. Proc Natl Acad Sci USA. 2001;98:597–602. doi: 10.1073/pnas.021550598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staszewski S, Miller V, Sabin C, Berger A, Hill A, Phillips A. AIDS. 1998;12:2360. [PubMed] [Google Scholar]
- 15.Neumann A U, Tubiana R, Calvez V, Robert C, Li T S, Agut H, Autran B, Katlama C. AIDS. 1999;13:677–683. doi: 10.1097/00002030-199904160-00008. [DOI] [PubMed] [Google Scholar]
- 16.Garcia F, Plana M, Vidal C, Cruceta A, O'Brien W A, Pantaleo G, Pumarola T, Gallart T, Miro J M, Gatell J M. AIDS. 1999;13:F79–F86. doi: 10.1097/00002030-199907300-00002. [DOI] [PubMed] [Google Scholar]
- 17.Ruiz L, Martinez-Picado J, Romeu J, Paredes R, Zayat M K, Marfil S, Negredo E, Sirera G, Tural C, Clotet B. AIDS. 2000;14:397–403. doi: 10.1097/00002030-200003100-00013. [DOI] [PubMed] [Google Scholar]
- 18.Jubault V, Burgard M, Le Corfec E, Costagliola D, Rouzioux C, Viard J P. AIDS. 1998;12:2358–2359. [PubMed] [Google Scholar]
- 19.Davey R T, Jr, Bhat N, Yoder C, Chun T W, Metcalf J A, Dewar R, Natarajan V, Lempicki R A, Adelsberger J W, Miller K D, et al. Proc Natl Acad Sci USA. 1999;96:15109–15114. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ortiz G M, Nixon D F, Trkola A, Binley J, Jin X, Bonhoeffer S, Kuebler P J, Donahoe S M, Demoitie M A, Kakimoto W M, et al. J Clin Invest. 1999;104:R13–R18. doi: 10.1172/JCI7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lisziewicz J, Rosenberg E, Lieberman J, Jessen H, Lopalco L, Siliciano R, Walker B, Lori F. N Engl J Med. 1999;340:1683–1684. doi: 10.1056/NEJM199905273402114. [DOI] [PubMed] [Google Scholar]
- 22.Mulligan K, Grunfeld C, Tai V W, Algren H, Pang M, Chernoff D N, Lo J C, Schambelan M. J Acquired Immune Defic Syndr. 2000;23:35–43. doi: 10.1097/00126334-200001010-00005. [DOI] [PubMed] [Google Scholar]
- 23.Brinkman K, Smeitink J A, Romijn J A, Reiss P. Lancet. 1999;354:1112–1115. doi: 10.1016/S0140-6736(99)06102-4. [DOI] [PubMed] [Google Scholar]
- 24.Powderly W G. J Neurovirol. 2000;6, Suppl. 1:S8–S13. [PubMed] [Google Scholar]
- 25.Hatano H, Miller K D, Yoder C P, Yanovski J A, Sebring N G, Jones E C, Davey R T., Jr AIDS. 2000;14:1935–1942. doi: 10.1097/00002030-200009080-00008. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg E S, Altfeld M, Poon S H, Phillips M N, Wilkes B M, Eldridge R L, Robbins G K, D'Aquila R T, Goulder P J, Walker B D. Nature (London) 2000;407:523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 27.Mollet L, Li T S, Samri A, Tournay C, Tubiana R, Calvez V, Debre P, Katlama C, Autran B. J Immunol. 2000;165:1692–1704. doi: 10.4049/jimmunol.165.3.1692. [DOI] [PubMed] [Google Scholar]
- 28.Papasavvas E, Ortiz G M, Gross R, Sun J, Moore E C, Heymann J J, Moonis M, Sandberg J K, Drohan L A, Gallagher B, et al. J Infect Dis. 2000;182:766–775. doi: 10.1086/315748. [DOI] [PubMed] [Google Scholar]
- 29.Haslett P A, Nixon D F, Shen Z, Larsson M, Cox W I, Manandhar R, Donahoe S M, Kaplan G. J Infect Dis. 2000;181:1264–1272. doi: 10.1086/315381. [DOI] [PubMed] [Google Scholar]
- 30.Pitcher C J, Quittner C, Peterson D M, Connors M, Koup R A, Maino V C, Picker L J. Nat Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 31.Betts M R, Casazza J P, Patterson B A, Waldrop S, Trigona W, Fu T M, Kern F, Picker L J, Koup R A. J Virol. 2000;74:9144–9151. doi: 10.1128/jvi.74.19.9144-9151.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moretto W J, Drohan L A, Nixon D F. AIDS. 2000;14:2625–2627. doi: 10.1097/00002030-200011100-00034. [DOI] [PubMed] [Google Scholar]
- 33.Pilgrim A K, Pantaleo G, Cohen O J, Fink L M, Zhou J Y, Zhou J T, Bolognesi D P, Fauci A S, Montefiori D C. J Infect Dis. 1997;176:924–932. doi: 10.1086/516508. [DOI] [PubMed] [Google Scholar]
- 34.Montefiori D C, Pantaleo G, Fink L M, Zhou J T, Bilska M, Miralles G D, Fauci A S. J Inf Dis. 1996;173:60–67. doi: 10.1093/infdis/173.1.60. [DOI] [PubMed] [Google Scholar]
- 35.Zhou J Y, Montefiori D C. J Virol. 1997;71:2512–2517. doi: 10.1128/jvi.71.3.2512-2517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carcelain G, Tubiana R, Samri A, Calvez V, Delaugerre C, Agut H, Katlama C, Autran B. J Virol. 2001;75:234–241. doi: 10.1128/JVI.75.1.234-241.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hatano H, Vogel S, Yoder C, Metcalf J A, Dewar R, Davey R T, Jr, Polis M A. AIDS. 2000;14:1357–1363. doi: 10.1097/00002030-200007070-00008. [DOI] [PubMed] [Google Scholar]
- 38.Kilby J M, Goepfert P A, Miller A P, Gnann J W, Jr, Sillers M, Saag M S, Bucy R P. Ann Intern Med. 2000;133:435–438. doi: 10.7326/0003-4819-133-6-200009190-00011. [DOI] [PubMed] [Google Scholar]
- 39.Bersoff-Matcha S J, Miller W C, Aberg J A, van Der Horst C, Hamrick Jr, Powderly W G, Mundy L M. Clin Infect Dis. 2001;32:124–129. doi: 10.1086/317536. [DOI] [PubMed] [Google Scholar]