Abstract
With the growing importance of optical techniques in medical diagnosis and treatment, there exists a pressing need to develop and optimize materials platform for biophotonic applications. Particularly, the design of biocompatible and biodegradable materials with desired optical, mechanical, chemical, and biological properties is required to enable clinically relevant biophotonic devices for translating in vitro optical techniques into in situ and in vivo use. This technological trend propels the development of natural and synthetic polymeric biomaterials to replace traditional brittle, nondegradable silica glass based optical materials. In this review, we present an overview of the advances in polymeric optical material development, optical device design and fabrication techniques, and the accompanying applications to imaging, sensing and phototherapy.
Keywords: Biophotonics, Biomaterials, Imaging, Sensing, Phototherapy
Graphical abstract

Highlights
-
•
Recent progress on the development of natural and synthetic polymeric optical biomaterials were reviewed.
-
•
Advanced design and fabrication techniques for polymeric biomaterial-based biophotonic devices were discussed.
-
•
Applications of bioimaging, biosensing, and phototherapy using polymeric biomaterial-based biophotonics were summarized.
1. Introduction
The swelling demand for higher quality healthcare and disease treatments has drawn considerable attention to biophotonics, which integrates optical techniques into the fields of biology and medicine to enable new imaging, sensing, and therapeutic strategies. The development of biophotonic technology promotes the progress of early detection and diagnosis of diseases, new modalities of light-guided and light-activated therapies, as well as increased understanding of biological systems [1]. Specifically, optical bioimaging, which forms a major thrust of biophotonics, provides non-invasive, high-sensitivity and high-resolution macroscopic information of a wide range of biological specimens (from cells to tissues), realizing effective and affordable diagnosis of various diseases including cervical cancer, oral cancer, and epithelial cancer [[2], [3], [4], [5]]. Optical biosensing is another widely studied biophotonic application for chemical identification and disease diagnosis. Optical biosensors utilize optical responses (intensity, wavelength, or polarization variations) created by chemical or biological changes to indicate a physiological disorder or problem. The sensitivity and selectivity of the optical responses are strongly dependent on the functionalization of biorecognition elements on an optical device, such as an optical waveguide or an interface supporting surface plasmonic resonance [2,6]. With the development of optical biosensors, real-time, remote, and multiplexed sensing can be achieved within a single device. In fact, many optical biosensors have been involved in applications for detecting biomarkers of various cancers including breast cancer, lung cancer, melanoma cancer and lymphoma cancer [[7], [8], [9]], as well as diseases such as celiac disease and Alzheimer's disease [[10], [11], [12]]. Additionally, biophotonics promotes the development of light-activated therapy that has emerged as a promising treatment of many medical problems. For example, photodynamic therapy (PDT) [2,13], which utilizes photosensitizing agents along with light to kill tumor tissues, bacteria, fungi and viruses, has been applied as a non-invasive treatment for diseases such as endodontic disease [14], and cancers such as skin cancer [15], breast cancer [16], leukemia cancer [17], as well as head and neck cancer [18]. Indeed, biophotonics has been so effective in its aims that it is being hailed as one of the most promising technologies in biomedical fields.
To achieve the full potential of biophotonic technology, the availability of suitable optical materials is of crucial importance, as their optical properties, mechanical properties, chemical structures, and biological functionalities significantly affect the device performance [19,20]. A fundamental requirement for suitable optical materials is the capability to achieve high-efficiency light delivery with low loss. To date, silica glass remains the mainstream optical material platform. Silica-based materials possess excellent optical properties, including high transparency over a broad spectral range from the visible to the near infrared (NIR) wavelengths, allowing them to be adopted in a wide variety of applications including optical sensors [21]. However, silica glass is a poor match for many biological applications as the material's mechanical fragility and brittleness are an injury risk to biological tissues. Additionally, biological applications require materials with improved biocompatibility and biological functionalities, which are challenging to realize with traditional silica material. Nondegradability is another significant hindrance for in vivo usage of silica-based optical devices, as implantable medical devices ideally should be degraded and cleared following their use, leading to a pressing need for biodegradable optical materials to replace silica. Therefore, the development of novel biocompatible polymeric materials with flexible mechanics, high optical efficiency, adjustable degradation, and design versatility are required to realize the full potential of biophotonics and create the next generation of optical sensing, imaging, and treatment technologies [[22], [23], [24], [25]].
Benefiting from the recent progress of polymeric biomaterials, many biophotonic devices with desired properties and functionalities for various medical applications have been developed with advanced polymeric biomaterials. Nature-inspired optical systems based on biological components such as bacterias [[26], [27], [28]], have been demonstrated; however, these systems suffer from limited sources, restrictive designability, and inherent variability. Such limitations have led to the development of natural [[29], [30], [31], [32], [33], [34], [35]] or synthetic polymer [25,36,37] based optical materials, which have more design flexibility to enable versatile physical, chemical, or biological properties and functionalities. In this review, we discuss the various families of biomaterials, including bacteria cell-based biomaterials, naturally derived biomaterials, and synthetic biomaterials, in the field of biophotonics (Table 1). Their material properties, fabrication strategies, functionalities and applications (in particular, optical probing, diagnostics, and light-activated therapies) are described. We aim to provide systematic understanding of the development of optical biomaterials, their critical requirements, and an outlook on future directions.
Table 1.
Summary of polymeric biomaterials for biophotonic applications.
| Type | Examples | Pros | Cons | Applications | |
|---|---|---|---|---|---|
| Living biomaterials |
E. coli, Cyanobacteria, |
Superior biocompatiblity | Small size, Limited modification methods, Strict process condition |
Waveguide [[26], [27], [28]] | |
| Naturally derived polymers | DNA, Silk, Chitosan, Cellulose, Agarose |
Biocompatibility, Biodegradability |
Property variability, Limited sources, Poor designability |
Waveguide [31,34,36,53], Optical fiber [35,42], Biosensor [44,[46], [47], [48], [49], [50], [51], [52]] |
|
| Synthetic polymers | Non-degradable polymers | Polyethylene glycol (PEG), Polyacrylamide (PAM), Polydimethylsiloxane (PDMS) |
Flexible designability | Non-degradability | Waveguide [57], Optical fiber [37], Biosensor [55,56,58,59], Optogenetic therapy [25] |
| Biodegradable polymers | Poly (lactic acid) (PLA), Poly (lactic-co-glycolic acid) (PLGA), Citrate-based Biomaterials |
Flexible designability, Biodegradability |
Waveguide [65], Optical fiber [24], Biosensor [67], Microneedle array [66], Biomaterial laser [67,68], Phototherapy [65,66] |
||
| Combinations of Materials | PEG/alginate, Alginate/PAM, P (AM-co-PEGDA)/alginate |
Flexible designability | Waveguide, Optical fiber [79], Biosensor [80,81], Phototherapy [79], Light amplification [79] |
||
2. Properties of polymeric optical biomaterials
For the applications of biophotonics including optical imaging, optical sensing, and light activated therapy, optical materials are required to fabricate optical elements such as waveguides (a physical structure that guides electromagnetic waves in the optical spectrum) and lenses (optical components designed to focus or diverge light) to transmit, detect and transform light. Optical materials can be defined as materials with the function to control or alter electromagnetic radiation in the ultraviolet (UV), visible, and infrared (IR) spectral regions. For biophotonics applications, materials need to meet certain optical, mechanical, chemical, and biological properties [38].
In the choice of an optical material, the most important properties are often the degree of transparency and the refractive index, along with their spectral dependency [39]. Materials with high transparency have relatively low reflection, absorption, and scattering of light, which together result in low optical loss. For silica fibers, atomic structural imperfections can cause light scattering (Rayleigh scattering) [40], which decreases with increasing wavelength. With technical advances in glass purification, glass manufacturing, and application of long-wavelength light around 1550 nm, silica fibers with low optical loss (∼0.2 dB/km) are widely applied and popular for long-haul fiber-optic communication. Polymeric biomaterials, which refer to polymers that have been engineered to interact with biological systems for therapeutic or diagnostic purposes, usually have inhomogeneity arise from a partially disordered network of polymer chains and macromolecules. The sizes of the inhomogeneity extend to tens of nanometers to micron scales rather than the atomic scale. Mie-type light scattering [41], which can be several orders of magnitude stronger than Rayleigh scattering, occurs when the inhomogeneity size scale is greater than about one tenth of optical wavelength. In addition, organic molecules usually have strong absorption at short wavelengths below 350–400 nm, while some conjugated structures can have absorption peak in the visible spectrum, so generally conjugated polymeric biomaterials are not considered for optical device applications. Commonly developed transparent polymeric biomaterials have an optical loss in the range of 0.3–1 dB/cm, which is much higher than that of silica glass. Despite the relatively higher optical loss, polymeric optical biomaterials can still meet the distance of interest (usually less than 1 m) in most biomedical applications. The refractive index of a material, defined as the ratio of speed of light in vacuum to the phase velocity of light in a material or a medium, also affects material optical loss. For single-material waveguides, the refractive index of the material should be higher than that of the surrounding tissue (1.38–1.41 at visible wavelengths) in order for total internal reflection to occur. In addition to optical transparency and refractive index, other factors including material surface roughness and bending degrees can also affect the optical loss of light guiding materials.
Besides optical properties, the mechanical, chemical, and biological properties of polymeric biomaterials are also crucial for biophotonic applications. While the conventional silica glasses have favorable optical properties, they are usually toxic and not biocompatible for medical uses, and moreover, their mechanical fragility and brittleness pose an injury risk to surrounding living tissues. Optically transparent polymeric biomaterials are emerging as key optical materials, as they often have advantages over inorganic silica optical materials to be designed and synthesized with soft mechanical property, biocompatibility, biodegradability, as well as desired chemical and biological functionalities. Furthermore, polymeric optical biomaterials can be easily manufactured into complex structures with high optical efficiency. The advantages mentioned above have motivated researchers to pay more attention to the development of advanced polymeric optical biomaterials.
3. Bacteria cell-based biomaterials
Among existing biomaterial candidates, one of the most promising is the components inherent to biological organisms. Without introducing any foreign materials, such biomaterials have the distinct advantages of being inherently biocompatible, biodegradable, and resorbable. The inevitable interaction between biological cells and light inspired researchers to develop biophotonic components using living cells, which let cells serve simultaneously as optical devices and testing samples for signal sensing and detection.
E. coli became the first living cell type that was used to fabricate optical waveguides due to its unique rod-shaped structure. Biological optical waveguides were formed directly with E. coli cells using an optical trapping strategy through an abrupt tapered optical fiber (ATF) to trap multiple E. coli cells to form highly ordered organization (Fig. 1a–c). Using this facile strategy, E. coli based biological optical waveguides with variable lengths (4.6–54.5 μm) were developed. The resulting biological optical waveguides had a propagation loss of 0.23 dB/μm for the 980 nm light, and presented good light propagation performances (Fig. 1d) [26]. In addition to single branched E. coli based biological optical waveguides, multi-branched (two-branched and three-branched) biological optical waveguides were also constructed from E. coli using a four-segment tapered optical fiber (4-STF). Through coupling a 644 nm laser into the 4-STF, direct observation of light propagation with the multi-branched waveguides was successfully conducted. Subsequently, the stability and robustness of the waveguides were studied in a microfluidic channel. During the experiments, branched E. coli cell waveguides could be flexibly moved without destroying their structures, and remained stable and reversible in a dynamic environment with perturbations. This study demonstrates that the resulting robust branched structures have potential as multidirectional waveguides and beam splitters for light delivery to different destinations in biological systems (Fig. 1e–f) [27]. This novel optical trapping strategy provides an interesting avenue to integrate optical devices with biological systems and enables direct sensing and detection of biological signals.
Fig. 1.
Living Biomaterials. (a) Experimental scheme of the fabrication of a bio-WG by trapping E. coli using a 980 nm laser; (b) SEM image of E. coli bacteria used to form the bio-WG; (c) SEM image of the ATF used to form the bio-WG, inset shows the enlarged view of the ATF tip; (d) Microscope image of the 644 nm red light transmitted via the bio-WG (white arrows indicate output points of the transmitted light). Reprinted with permission from Ref. [26]. Copyright 2013, American Chemical Society. (e) Experimental scheme of the formation of branched E. coli cell structures via 980 nm laser. Reprinted with permission from Ref. [27]. Copyright 2015, John Wiley and Sons.
Alternatively, a family of marine bacteria cells (cyanobacteria) based optical device was studied to explore nonlinear optical response in biological media. By deliberately altering the host environment of the cyanobacteria, tunable nonlinear interactions can lead to altered deep light penetration and enhanced light scattering through the bacterial suspension, while maintaining the viability of the bacteria cells [28].
Bacteria cell-based biomaterials have irreplaceable advantages and promising roles for biophotonic technology development due to their natural biocompatibility, which minimizes safety concerns for in vivo applications. However, they also contain significant drawbacks that are difficult to overcome. For instance, the small sizes of cells critically limited the sizes of biophotonic devices made by them. Also, the manufacturing conditions of these biomaterials are very strict, and only a limited number of approaches can be applied to fabricate biophotonic devices. Therefore, more efforts are required from researchers to either improve the abilities to control bacteria cell-based biomaterials or incorporate other materials including naturally derived biomaterials and synthetic biomaterials.
4. Naturally derived polymers
Naturally derived biomaterials have excellent biocompatibility, biodegradability, and remodeling capabilities, making them promising for the repair and replacement of damaged tissues and organs as well as for other implantations [29]. Many natural materials also present favorable optical properties, including high transparency and light guiding efficiency, making them ideal for biophotonic applications.
4.1. DNA
DNA is a biopolymer chain comprising four deoxynucleoside monomers (Cytosine (C), Guanine (G), Adenine (A), and Thymine (T)) that are connected by phosphodiester bonds. DNAs can either remain in the single-stranded polymer chain or complementarily hybridize into double-stranded helix structures. Due to their favorable biocompatibility and unique structural properties, DNAs are ideal building platforms for designing and assembling new biomaterials for cell-related applications, such as diagnosis, drug delivery, and protein production [30].
In light of the manipulability of DNA, waveguides based on DNA biomaterials have been prepared. With a PDMS micro-chamber system, liquid waveguides based on DNA and DNA complex (DNA complex with cetyltrimethylammonium (DNA-CTMA)) solutions were developed and were able to deliver light. Thin-film waveguides with DNA-CTMA complex were also fabricated using a PDMS molding channel and spin-coater, and the films had high light transmission and controllable thicknesses. Although DNA-based photonics have not been widely studied, due to the unique structures, biological functionalities, programmability, and manipulability, DNA biomaterials based thin-film waveguides, optical amplifiers and resonator devices could have important applications, especially in medical devices [31].
4.2. Silk
Silks are natural protein fibers produced by insect larvae of silkworms, moths, butterflies, and spiders. Their hydrophobic B-sheet structures grant them robust mechanical strength and toughness [32,33]. Molecular designability and moderate aqueous processing allow them to be engineered with chemical and biological functionalities; they can be combined with incorporated dyes, growth factors, peptides, or cells [34,35]. The high fabrication flexibility of silk has led to the creation of silk-based films, gels, and 3D porous scaffolds for in vitro and in vivo biomedical applications. In addition, silk films with the thickness between 20 and 100 μm were found to be ideal for optical devices and biophotonic applications, owing to their low surface roughness (<5 nm rms) and high transparency (>95%) across the visible range [34,36].
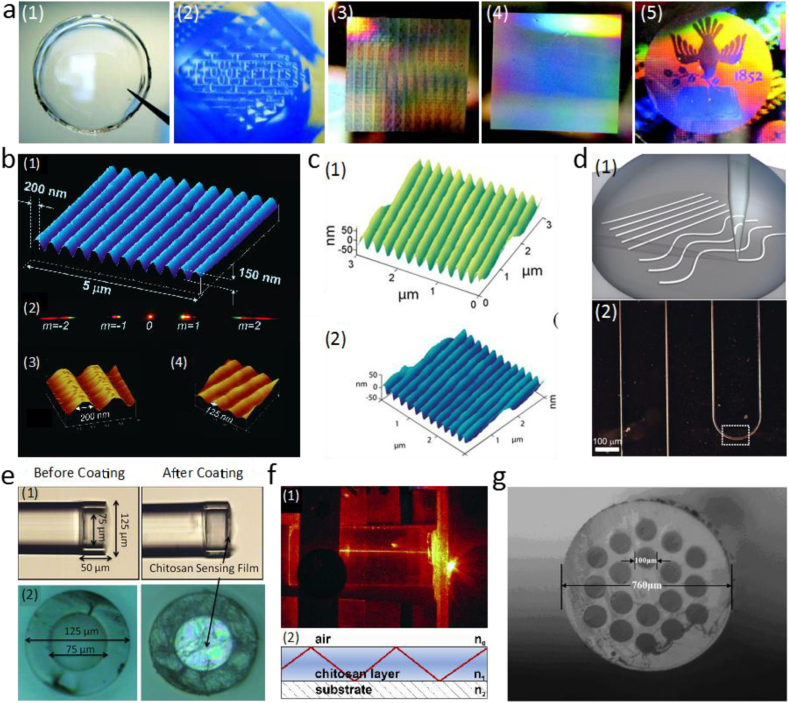
Over the past several decades, silk has been developed into diffraction gratings, lenses, and waveguides for light delivery and in vivo implanting applications (Fig. 2a) [34]. By inducing crystallization of films on appropriate substrates, nanopatterned silk optical elements, including lenses, microlens arrays, diffraction gratings, and pattern generators were developed. Surface morphology of these optics was controlled down to 125 nm with fidelity over large length scales (Fig. 2b). Films with thicknesses ranging between 30 and 50 μm were found to exhibit a consistent refractive index of 1.55. Transmission across the visible wavelength of silk films with thicknesses of 100 μm was measured to approach 100%. Incorporation of bioactive components including hemoglobin and peroxidase during processing led to biocompatible silk optical fibers with both high optical quality and biological functionalities [42]. A simple modified soft lithography method was also developed to fabricate silk fibroin based nano- and micro-patterned films. This technique allows the creation of optically transparent and mechanically robust films with a sub-40 nm transverse pattern resolution, which promotes the development of highly efficient materials with intricate 2-D and 3-D nano- and micro-patterns [43]. Further, a cost effective and high throughput nanoprinting process was developed to print nanopatterned (<50 nm) silk films in short periods of time (seconds to minutes) with low printing pressure (50 psi) (Fig. 2c). Nanoprinted lysed red blood cell doped silk solution was used to develop a self-sensing optofluidic device [44]. With advanced understanding and novel fabrication techniques, researchers recently presented an approach to develop a single implantable, biocompatible, and biodegradable optical device combining enhanced imaging of malignancies (through optical transduction), therapy (through drug stabilization and delivery), and quantitative feedback (through drug delivery imaging within the device) based on silk. This high quality and multifunctional device demonstrates a next-generation concept of personalized medicine and provides a platform for new medical device designs [22].
Fig. 2.
Naturally derived polymers. (a) 50 μm thick optical silk elements ((1) a clear film, (2) an image through a microlens array, (3) a 2D diffractive optical element, (4) a diffraction grating, and (5) a white light hologram). Reprinted by permission from Springer Customer Service Centre GmbH: Springer Nature, Nature Photonics [34], (A new route for silk, Fiorenzo G. Omenetto, David L. Kaplan). Copyright 2008. (b) (1, 3, 4) AFM image of a nanopatterned silk optical grating, and (2) diffracted orders from white light propagation through the grating. Reprinted with permission from Ref. [42]. Copyright 2008, American Chemical Society. (c) AFM images of (1) a grating imprinted on a silk film and (2) a grating master pattern. Reprinted with permission from Ref. [44]. Copyright 2010, John Wiley and Sons. (d) (1) Schematic of direct-write assembly of silk waveguides and (2) optical images of printed silk waveguides. Reprinted with permission from Ref. [36]. Copyright 2009, John Wiley and Sons. (e) Chitosan coted sensor before and after coating. Reprinted from Sensors and Actuators B: Chemical, Vol 169, L.H. Chen,T. Li,C.C. Chan,R. Menon,P. Balamurali,M. Shaillender,B. Neu,X.M. Ang,P. Zu,W.C. Wong,K.C. Leong, Chitosan based fiberoptic Fabry–Perot humidity sensor, 167–172, Copyright (2012), with permission from Elsevier [46]. (f) (1) Images and (2) schematic structure of chitosan acetate based POW. Reprinted from Chemical Engineering Journal, Vol 244, Alexander Mironenko, Evgeny Modin, Alexander Sergeev, Sergey Voznesenskiy, Svetlana Bratskaya, Fabrication and optical properties of chitosan/Ag nanoparticles thin film composites, 457–463, Copyright (2014), with permission from Elsevier [48]. (g) Cross section of a cellulose MPOF. Reprinted from Optics Communications, Vol 283, Dongdong Li, Lili Wang, Cellulose acetate polymer film modified microstructured polymer optical fiber towards a nitrite optical probe, 2841–2844, Copyright (2010), with permission from Elsevier [52].
Through direct-write assembly of a highly concentrated silk aqueous solution, pure and rhodamine 6G doped microscale silk optical waveguides with controlled structure and composition were fabricated (Fig. 2d). The optical losses (measured using a He:Ne laser at 633 nm) for straight and wavy pure silk waveguides surrounded by air were 0.25 dB/cm and 0.81 dB/cm, respectively. The optical loss in a 1-cm-long doped waveguide was estimated to be < 0.1 dB/cm. The direct ink writing approach provides a way to create sophisticated biophotonic devices with varied geometries and biological functionalities [36]. Although silk materials present low optical loss in air, the interaction of the light guided by these waveguides with variable and complex surrounding tissues at in vivo condition causes scattering and absorption optical losses, and thus light cannot be efficiently delivered to or collected from deep tissues, which hinders the capability to perform in vivo deep tissue diagnosis, treatment, and post-surgical monitoring with single-material waveguides. To address this issue, a biocompatible step-index optical fiber composed of a silk core (n = 1.54) and a silk hydrogel (n = 1.34) cladding was developed with a propagation loss of approximately 2 dB/cm. The core/cladding structure of the resulting optical fiber provided less sensitivity to the surrounding environment, and this system could be used to perform organ-scale light delivery [35].
4.3. Chitosan
Chitosan is a biocompatible and biodegradable natural hydrophilic polysaccharide. Due to the presence of abundant hydroxyl and amino side groups, chitosan provides reversible water adsorption and good binding to both cationic and anionic forms of noble and transition metal ions, altering optical properties. Combined with its superior film forming properties, chitosan thus presents a promising candidate for optical waveguides and sensors [45].
Chitosan has been used as a sensitive coating at the surface of a waveguide to form a low-finesse Fabry–Perot humidity sensor. The detectable relative humidity (RH) of this sensor ranges from 20%RH to 95%RH with satisfactory repeatability and stability (Fig. 2e). A fast response time of 380 m s is accomplished with a sensitivity of 0.13 nm/%RH, due to the miniature size of the chitosan film [46]. Additionally, chitosan was also applied to form the waveguide as a sensing element of a relative humidity sensor [47]. The resulting integrated-optical sensors based on waveguide films of both salt and neutral forms of chitosan have high sensitivity and short response time.
The refractive index of chitosan films can be tuned through doping polymer layers with Ag+ ions followed by ‘‘in situ’’ reduction and formation of Ag nanoparticles. Varying Ag+ ion concentration and doping time allows precise control over the doping level. The chitosan/Ag thin films with Ag content up to 3% presented no sign of opaqueness. The refractive index of the films at a wavelength of 633 nm was increased from 1.53 to 1.69 with increased Ag volume fractions up to 2.55%. Light propagation study didn't show significant scattering losses at Ag volume fractions up to 1.3%, which corresponds to the maximum refractive index of 1.58. With an increased refractive index, chitosan/Ag composite films can be used for planar optical waveguides (POW) and optical sensor substrates (Fig. 2f) [48]. Furthermore, Chitosan/Au and chitosan/Ag nanocomposites were fabricated via the absorption and reduction of [AuCl4]− and Ag+ ions on 150-nm-thick spin-coated chitosan films. Applying the Chitosan/Au and chitosan/Ag nanocomposites as sensitive layers onto planar optical waveguides allowed detection of H2S gas at concentration ranges of 0.1–100 ppm and 5–300 ppm. These H2S gas sensors presented high sensitivity, selectivity and reproducibility of the response signal [49].
The above studies did not include direct biomedical applications of chitosan-based optical devices. However, its biocompatible and biodegradable nature provides a great potential for chitosan to be used in biophotonic applications.
4.4. Cellulose
Cellulose, as a naturally occurring polysaccharide, has also been applied for fabricating optical fibers and sensors due to its high transmittance of visible light and favorable permeability for water and ions [50]. The hydroxyl groups of cellulose can be partially or fully reacted with various chemicals to form cellulose derivatives with useful properties, including cellulose esters of cellulose butyrate, cellulose acetate, and cellulose triacetate. A novel microstructured polymer optical fiber from two types of biodegradable cellulose that have different glass transition temperatures was fabricated with a porous double-cladding structure in which two cellulose butyrate tubes are separated with hydroxypropyl cellulose powder particles. The inner core is a cellulose tube with a hole that can be collapsed for laser delivery, or left open for potential drug delivery. The microstructured cellulose fiber allows the integration of optical, microfluidic, and drug release functionalities [51].
Researchers have also applied cellulose materials as a coating film for microstructured polymer optical fiber (MPOF) to develop optical probes (Fig. 2g). For instance, a novel nitrite (NO2−) detection probe was made by applying rhodamine 6G (Rh 6G)-doped cellulose acetate (CA) on the sidewall of array holes in a MPOF. The calibration graph of fluorescence intensity versus nitrite concentration was linear in the range of 2.0 × 10−4–5.0 × 10−3 g/ml. With high sensitivity, satisfactory nitrite sensing in real samples was demonstrated [52]. Another MOPF modified with eosin-cellulose acetate (CA) was developed by using a liquid-phase coating for gaseous ammonia fluorescence sensing. The response of the sensor is linear within a wide range of concentrations from 50 to 400 ppm with a fast response of 500 m s. This work presents new possibilities for optical gas sensing with MPOFs [50].
4.5. Agarose
Agarose is a polysaccharide derived from agar, and it has been widely used as a biocompatible material for DNA separation, cell encapsulation, and tissue regeneration. Since the tunable optical refractive index of agarose hydrogels depends on their concentrations (a change of ∼0.001 in refractive index with a concentration change of 0.5% w∕v), agarose serves as an attractive optical material. For example, hydrogel/cell hybrid optical waveguides were developed by encapsulating live cells within agarose hydrogels, and the resulting cell encapsulated hydrogel had an average light propagation loss of ∼12–13 dB∕cm. Optimization of optical properties is still needed in future studies, e.g. by adjusting gel concentrations. Although there were still limitations of the agarose hydrogel based optical waveguides, they can serve as a potential platform to incorporate various biological subjects, such as protein and DNA, in order to enable novel light delivery and biosensing applications [53].
5. Synthetic polymers
Natural biomaterials are created by nature without strict process control and high replicability, thus they typically possess many limitations, such as nonuniform material properties, limited sources, and poor design flexibility, despite their good biocompatibility. On the other hand, synthetic biomaterials offer much better material designability, more controllable and adjustable physical/chemical/biological properties, and practically unlimited sources, which enable their widespread use in biomedical applications. Also, the optical properties of synthetic biomaterials can be tuned and optimized to meet the requirements of various optical devices. Here, we discuss several popular synthetic non-degradable and degradable polymeric biomaterials that have been used in biophotonic applications.
5.1. Synthetic non-degradable polymers
5.1.1. Polyethylene glycol (PEG)
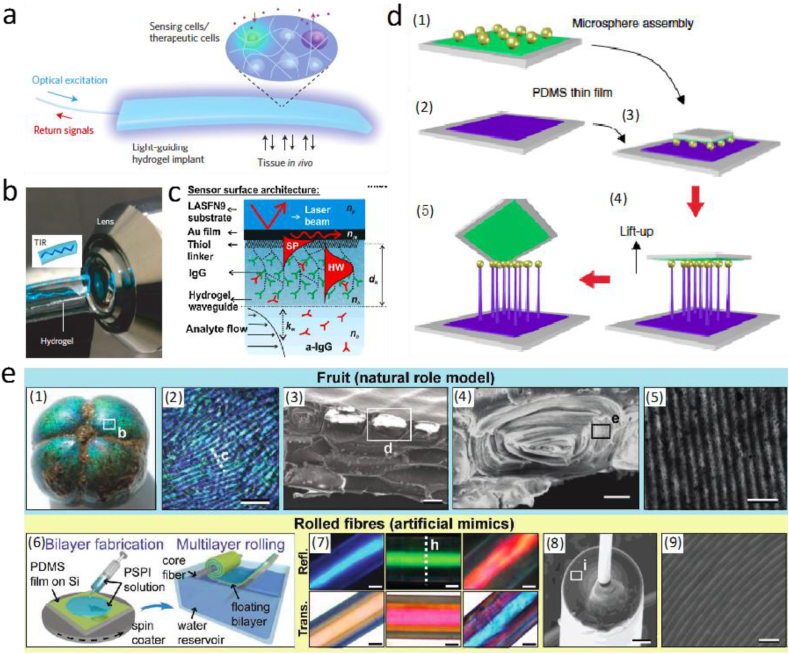
PEG hydrogels can easily contain more than 95 wt% water, approximating the mechanics and consistency of soft tissues. Their biocompatibility enables PEG hydrogels to be involved in various biomedical applications, including tissue engineering scaffolds and controlled release carriers [54]. Recently, researchers also studied the potential photonic functionalities of PEG hydrogels. By adjusting molecular weight, water content, and shape, the optimal design parameters can be determined to achieve desired optical properties and functionalities. For example, optical PEG hydrogel waveguides that offer high mechanical flexibility, high transparency, and excellent low-loss light-guiding properties were manufactured and applied for cell encapsulation (Fig. 3a–b). The cell-containing PEG hydrogels were implanted subcutaneously in mice, and shown to offer efficient light-guiding properties with an optical power loss <6 dB, which is slightly higher than that measured in air (1 dB/cm). After implantation for 3 and 8 days, the cell-integrated hydrogels presented favorable biocompatibility, high cell viability, and low optical transmittance decrease (<1 dB/cm). Later, the fiber-optic cell-containing hydrogel also worked together with an intrinsic cellular cytotoxicity sensor, heat-shock-protein 70 (hsp70), to detect cellular toxicity of Cadmium (Cd)-based quantum dots in vivo. The real-time in vivo readout was qualitatively consistent with the values measured ex vivo, which confirmed the accuracy of the hydrogels for nanotoxicity sensing. PEG hydrogels containing glucagon-like peptide-1 (GLP-1) secreting cells were also applied for optogenetic therapy of diabetic mice. GLP-1 is an incretin to promote glucose homeostasis through enhancing glucose-dependent insulin secretion. Under the illumination of blue light (455 nm), the implanted cell-containing hydrogels were able to release optogenetically synthesized GLP-1 to promote glucose homeostasis. After the implantation of these hydrogels, light-exposed animals presented significantly improved glucose homeostasis than the non-illuminated group, which proved the optogenetic therapy effect of the cell-containing PEG hydrogels. These studies demonstrate the potential of PEG hydrogels as a platform technology for health diagnosis and disease therapy [25].
Fig. 3.
Synthetic non-degradable polymers: (a) Schematic of a flexible light guiding PEG hydrogel with encapsulated cells acting as sensors and cytokine and hormone producers in vivo; (b) Demonstration of TIR within the PEG hydrogel. Reprinted by permission from Springer Customer Service Centre GmbH: Springer Nature, Nature Photonics [25], (Light-guiding hydrogels for cell-based sensing and optogenetic synthesis in vivo, Myunghwan Choi, Jin Woo Choi, Seonghoon Kim, Sedat Nizamoglu, Sei Kwang Hahn et al.). Copyright 2013. (c) Schematic of the surface architecture of a poly (N-isopropylacrylamide) (PNIPAAm) hydrogel based antibody sensor. Reprinted from Biosensors and Bioelectronics, Vol 25, Yi Wang, ChunJen Huang, Ulrich Jonas, Tianxin Wei, Jakub Dostalek, Wolfgang Knoll, Biosensor based on hydrogel optical waveguide spectroscopy, 1663–1668, Copyright (2010), with permission from Elsevier [55]. (d) Fabrication of microsphere-tipped PDMS MSMP via direct drawing. Reprinted by permission from Springer Customer Service Centre GmbH: Springer Nature, Nature Communications [58], (Microsphere-assisted fabrication of high aspect-ratio elastomeric micropillars and waveguides, Jungwook Paek, Jaeyoun Kim). Copyright 2014. (e) (1) Margaritaria nobilis fruit, (2) elongated blue surface cells from the fruit surface, (3) SEM of stacked cells in the outer endocarp layers, (4) SEM of concentric flattened cylindrical structure of single cells, (5)TEM image of the layered architecture within a single cell, (6) schematic of the fabrication of artificial photonic fibers, (7) optical images of PDMS based rolled-up multilayer fibers, (8) SEM of the cross section of multilayer fiber, and (9) SEM of the individual cladding layers. Reprinted with permission from Ref. [63]. Copyright 2013, John Wiley and Sons.
5.1.2. Polyacrylamide (PAM)
Polyacrylamide (PAM) is a polymer formed from acrylamide monomers. Due to its high hydrophilicity, crosslinked PAM can be used to form soft hydrogels when hydrated. PAM-based hydrogels are chemically designable and biologically inert, and have been widely applied in medical devices. In combination with their optical transparency and mechanical flexibility, PAM hydrogels were also used as biophotonic sensors.
For example, a PAM gel containing 3-phenylboronic acid was applied on the end of an optical fiber as a glucose-selective optical sensor. The working mechanism of this sensor was based on glucose-induced contraction of the PAM gel. The fabricated hydrogel sensors showed reversibility (in both equilibrium swelling and swelling kinetics), high temperature dependency (about 4-fold faster swelling with temperature increased from 25 °C to 37 °C), excellent reproducibility, and minimal interference from other analytes. Successful glucose measurements conducted in blood plasma indicate that the developed PAM hydrogel sensor is a great candidate for continuous monitoring of glucose in blood [37].
In addition to the PAM gel sensors via physical volume changes, a label-free biosensor based on the measurement of binding-induced refractive index variations by hydrogel optical waveguide spectroscopy (HOWS) was also developed for molecular analyte detection. This biosensor was prepared by using a surface plasmon resonance (SPR) optical setup in which a poly (N-isoproprylacrylamide) (PNIPAAm) hydrogel film modified with analyte binding molecules is attached on a metallic surface (Fig. 3c). The PAM hydrogel worked as both a binding matrix and an optical waveguide. The HOWS sensor achieved an order of magnitude improved sensing resolution and an increased binding capacity, as well as a 5-fold increase in the lower limit of IgG molecule detection compared to regular SPR biosensors [55].
PAM hydrogels were also used for sensing multi-signals. For instance, portable sensors for both pH sensing and inflammatory C-reactive protein (CRP) detecting were applied to achieve continuous monitoring of wound healing. For pH sensing, the PAM hydrogels swell or shrink in response to surrounding pH changes, and pH measuring range can be adjusted by changing chemical compositions of the PAM hydrogels. For the CRP detection, the hydrogels were designed with functional phosphorylcholine receptors that specifically recognize the targeting protein. The variation of pH and CRP concentrations would lead to tuning of optical properties, such as refractive index, and changes of PAM hydrogels, which work as sensing principles of the hydrogel sensors. The resulting sensors were applied for blood serum testing, demonstrating a reversible pH measuring range of 6–8 and CRP detecting concentrations between 1 and 100 μg/ml. The obtained portable sensing device is suitable for wearable biosensors, enabling remote, real time in situ sensing of wound healing or other ambulatory cares [56].
Based on the above studies, PAM hydrogels have versatile designing possibilities to provide variable chemical structures, mechanical properties, and multi-functionalities. These designs are able to enable different forms of optical sensing devices, presenting great potentials for biophotonic applications.
5.1.3. Polydimethylsiloxane (PDMS)
PDMS is a silicone elastomer that is very attractive for optical device development due to its high optical transparency, chemical inertness, mechanical flexibility, and facile fabrication process. The optical properties of PDMS slightly depend on its preparation process, such as curing temperatures and time. At 25 °C, PDMS polymers have a refractive index of 1.4118 at the wavelength of 598 nm and 1.3997 at the wavelength of 1554 nm. The optical absorption loss of PDMS is lower than 5 dB/cm in a broad wavelength range (400–1600 nm) [57].
Soft lithography is typically used to fabricate PDMS devices; however, this method faces challenges for making circularly-shaped waveguides with small diameters and long lengths. Therefore, researchers developed a modified fiber drawing method from partially cured PDMS to fabricate PDMS optical fibers in a wide range of diameters (few micrometers to few millimeters) and lengths (tens of centimeters). With this method, PDMS can be fabricated into a variety of geometrical structures, including multimode optical fibers, looped and twisted fiber resonators, and optical fiber couplers [57]. This technology will significantly promote the development of PDMS-based optical devices. A direct drawing-based technique was applied for the fabrication of PDMS-based micropillars (Fig. 3d) [58]. Although the material is extremely soft, this technique allowed the elastomeric micropillars to reach a high aspect-ratio of 100 and a height of 2000 μm. It also enabled in situ integration of microspheres at the tips of the micropillars. The resulting devices were then applied as airflow sensors to detect sound waves with variable flow-rates and directions, and the sensing resolution could achieve a mms−1 scale. In addition, PDMS was used to fabricate a hybrid diffractive optical element, named G-Fresnel, that can both focus light and disperse the constituent wavelengths, for developing compact optical spectrometers [59,60]. The G-Fresnel based spectrometer design can enable integration with smartphone technology for quantitative colorimetric assay (e.g. Bradford assay) and for detecting hemoglobin concentration through diffuse reflectance spectroscopy [61,62].
Inspired by the hierarchical photonic architecture in individual cells of the fruit of the tropical plant Margaritaria nobilis, multilayer fibers were produced by initially forming a bilayer of the two constituent materials, polydimethylsiloxane (PDMS) and polyisoprene-polystyrene triblock copolymer (PSPI), with a sufficiently high refractive index contrast (PDMS: 1.41 ± 0.02; PSPI: 1.54 ± 0.02) (Fig. 3e). The multilayer structured fibers were fabricated by combining the spin coating and multilayer rolling methods. The band-gap center frequency of the hollow fibers can be tuned by adjusting the individual film thicknesses prior to the rolling process, which can shift into the range of near-UV or near-infrared wavelengths. The multilayer fibers are promising for applications in mechanically tunable light guides or for optical strain sensing given the flexibility in the selection and design of constituent materials and their unique combination of mechanical and optical properties [63].
Currently, PDMS has only been applied to fabricate nano- or micro-scaled biophotonic devices due to current available fabrication technologies. In the future, new technologies will help extend PDMS based applications. Alternatively, PDMS can be combined with other materials to develop new biomedical and photonic devices.
5.2. Synthetic biodegradable polymers
Synthetic biodegradable polymers can be degraded and absorbed or cleared by the human body after they have fulfilled their desired functions without the need of further surgical removal. As such they are critical components of many biomedical strategies, including tissue engineering, drug delivery, gene therapy, and bioimaging [23]. In addition, their degradation profiles, mechanical properties, optical properties, and other chemical or biological functionalities are easier to control and design compared to natural materials. Therefore, multiple synthetic biodegradable materials have been developed for implantable biophotonic devices.
5.2.1. Polylactone biomaterials
Aliphatic polyesters of poly (lactic acid) (PLA), poly (glycolic acid) (PGA) and their copolymer poly (lactic-co-glycolic acid) (PLGA) are U.S. Food and Drug Administration (FDA) approved polymers for therapeutic devices usages. They were chosen for initial exploratory biomedical applications and they have been widely utilized as implantable and injectable materials for drug delivery and tissue regeneration [64]. PLA, PGA, and PLGA polymers can be completely degraded through hydrolysis and enzymolysis, and the degraded products can be resorbed or removed by the body with minimal systemic toxicity. The degradation rate, mechanical properties, and biofunctionalities of lactide and glycolide-based polymers or their copolymers can be adjusted by controlling the molecular weight, lactide/glycolide unit ratios, enantiomeric forms, or through modification with additional moieties or end groups. Furthermore, lactide and glycolide-based materials can be transparent and have a typical refractive index of about 1.47. As such, PLA, PGA, and PLGA polymers are very attractive biomaterials for developing medical optical devices.
During the past several years, optical waveguides made with PLA were studied. For example, researchers designed and fabricated a comb-shaped planar PLA based waveguide [65]. This PLA waveguide was applied to conduct photochemical tissue bonding (PTB) treatment for a full-thickness (>10 mm) skin incision, which achieved nearly 10-fold increase of the tissue area that can be accomplished with conventional PTB using surface illumination (Fig. 4a). This approach allowed watertight crosslinking between wound tissues to be formed in situ without removing the PLA waveguides. This technology is expected to impact widespread applications associated with photomedicine, including health monitoring, PDT and drug delivery. Besides the organ scale waveguides, a PLA-based optical microneedle array (OMNA) was also manufactured by press-molding PLA for percutaneous light delivery. The fabricated 11 by 11 array of needles (1.6 mm in length) were positioned at a spacing of 1 mm. In the transmittance study with an LED source (400–650 nm), the OMNA enabled a transmittance enhancement of 3-folds. In the light penetration measurement, the OMNA could deliver 8.5% of the input power at the wavelength of 491 nm through a bovine muscle slice (3.1 mm), while the light transmission was only 0.85% by direct light illumination without the device. When the tissue was changed to a porcine muscle slice with a thickness of 2.7 mm, OMNA had a 32% light transmission, which was more than 4 times higher than that without OMNA (7.6%). With proven light delivery efficiency, this novel device is able to increase the effective treatment depth of anti-microbial blue light therapy in human skin from 1.2-1.5 mm to 2.4–2.6 mm [66].
Fig. 4.
Synthetic biodegradable polymers. (a) (1) Biopolymer waveguide bundle displaying increasing photoactivation within dyed porcine skin (indicated by the propagation of photobleaching in the dye), and (2) schematic of the experimental procedure of waveguide-assisted photochemical tissue bonding using the biodegradable waveguide [65]. (b) (1) Bright field image and (2) fluorescence image of Vitamin B microspheres on a PLLA substrate. Reprinted with permission from Ref. [67]. Copyright 2013, John Wiley and Sons. (c) Citrate based step index optical fiber demonstrating (1) flexibility, (2) core cladding structure, (3, 4) light guidance, and (5) image projection. Reprinted from Biomaterials, Vol 143, Dingying Shan, Chenji Zhang, Surge Kalaba, Nikhil Mehta, Gloria B. Kim, Zhiwen Liu, Jian Yang, Flexible biodegradable citrate-based polymeric step-index optical fiber, 142–148, Copyright (2017), with permission from Elsevier [24].
Another interesting biomedical optical application with lactide and glycolide-based materials was biomaterial lasers. In one study, PLA films were used as a super-hydrophobic pattern to allow the formation of a vitamin B2 (riboflavin) microdroplet-based whispering gallery mode resonator (Fig. 4b). The resulting biomaterial laser had a low lasing threshold on the order of tens of nanojoules, and the enhanced light-matter interaction enabled it with great potential in biophotonic applications including biosensors with high sensitivity [67]. Moreover, individual PLA and PLGA polymers themselves were applied to fabricate whispering gallery mode lasers [68]. Biodegradable PLGA microparticle lasers were able to show lasing inside blood with an efficiency similar to that achievable with operation in water, meaning the red blood cells and other constituents of blood did not degrade the lasing effect. Researchers also delivered PLA microbeads into porcine skin tissues at a depth of ∼100 μm using a tattoo machine, and successful lasing was observed when the skin tissue was illuminated with a 532 nm laser. Bio-lasers in blood and tissues could significantly promote direct in vivo and in situ diagnosis and health monitoring.
Although lactide and glycolide-based polymers have been involved in a variety of biophotonic applications, only basic material designs and limited functionalities were studied so far. Biomaterials with more advanced properties, including flexible mechanical properties, drug delivery, and biosensing, are expected to be involved in the development of biophotonics.
5.2.2. Citrate-based biomaterials
Current synthetic biodegradable materials used for optical waveguides have limited processability and designability, leading to low-efficiency in vivo light delivery and limited functionalities. There is an urgent need of a versatile material platform that can simultaneously meet the diversified requirements of optical (tunable refractive indices, low optical loss), mechanical (tunable mechanical flexibility for tissue compliance), and biological (biocompatibility, biodegradability and bioactivity) functionalities. To address this problem, a citrate-based biomaterial platform has been explored. Citrate-based biomaterials are a group of polymers synthesized by reacting citric acid with different diols and/or amino acids through a facile one-pot polycondensation reaction [69,70]. Flexible chemical and design properties have enabled citrate-based biomaterials with tunable degradation rates (from a few days to over one year), adjustable mechanical strengths (tens of Pa to hundreds of MPa), alterable optical properties, and various functionalities (fluorescent, adhesive, antimicrobial, antioxidant, etc.). For more than a decade, the ease of fabrication has allowed versatile applications of citrate-based biomaterials for bioadhesive [71], tissue engineering [72,73], drug delivery [74], fluorescent imaging [75,76], and biosensing [77,78].
Recently, by leveraging the designability of citrate chemistry and processability of citrate-based biomaterials, two bioelastomers, poly (octamethylene citrate) (POC) and poly (octamethylene maleate citrate) (POMC), with minor chemical structure differences, were used to develop a step-index optical fiber. The small structure differences between the two materials leads to a higher refractive index of POMC than that of POC (Δn∼0.003), which is similar to that between the cladding and the core of conventional silica optical fibers, over a broad range of wavelength from 300 to 1000 nm. However, the two citrate-based materials still possess close mechanical properties, with the initial modulus of 4.35 ± 0.51 (POMC) and 3.79 ± 0.45 (POC) MPa, as well as matched biodegradation profiles after degradation for 4 weeks. A two-step fabrication method was developed to manufacture the step-index optical fiber with POMC as the core material and POC as the cladding material (Fig. 4c (1)–(4)). Briefly, in the first step, a stainless steel wire with a diameter of 500 mm was used as a mold to prepare POC cladding layer. In the second step, POMC was infiltrated into the fabricated POC cladding tube using an air pressure pump to prepare the fiber core. The resulting step-index optical fibers were biocompatible, biodegradable, and mechanically flexible (initial modulus of 3.39 ± 0.31 MPa; elongation of 61.49 ± 5.81%). In addition, the optical fibers exhibited a low transmission loss of 0.4 dB/cm. In the in vitro light transmission study under a porcine tissue, the optical fiber showed light delivery ability of both 473 and 633 nm lasers at different bending angles (0, 30 and 90°). The fiber also demonstrated in vivo deep tissue detection and fluorescence sensing capability with a rat animal model. Moreover, in vitro image transmission through the fiber indicated the feasibility of the citrate-based optical fiber for image delivery, enhancing its potential for future deep tissue implantation and in vivo image transmission (Fig. 4c(5)) [24].
The biodegradable citrate-based step-index optical fiber can serve as an attractive candidate for organ-scale light delivery, in vivo imaging, and biosensing. In addition, with the citrate-based material platform, the refractive indices, degradation profiles, and mechanical properties of core and cladding materials can be further tailored by modifying material chemical structures depending on specific optical requirements. Citrate-based polymers can be further modified with various chemical or biological functionalities through their pendant carboxyl and hydroxyl groups both during and post synthesis. Therefore, citrate based biodegradable step-index optical fibers may become enabling tools for diverse biomedical applications where light delivery, imaging, light therapy, and/or sensing are desired.
6. Combinations of materials
Depending on the desired optical, mechanical, or biofunctional requirements of specific biophotonic applications, a single biomaterial may not be able to satisfy all the demands. Thus, optical devices made from different materials have been developed.
For instance, core-clad step-index hydrogel optical fibers were prepared by applying PEG hydrogel as the core material and alginate hydrogel as the cladding material (Fig. 5a–b). The resulting optical fibers possess a propagation loss of 0.32 ± 0.02 dB/cm in air and 0.42 ± 0.01 dB/cm in tissue at 492 nm, demonstrating high light guiding efficiency both in vitro and in vivo (Fig. 5c). In addition, the hydrogel core allowed the incorporation of various functional materials, including organic dyes and nanoparticles, for fluorescence generation, photothermal heating, and light amplification [79].
Fig. 5.
Combinations of biomaterials. – (a) Schematic of the fabrication of PEG/Ca-Alginate core-cladded optical fibers; (b) Phase contrast images of core-cladded fibers with variable sizes; (c) Light guidance of a core-cladded fiber (1) in air and (2) between porcine tissue slices. Reprinted with permission from Ref. [79]. Copyright 2015, John Wiley and Sons. (d) Mechanical properties of alginate-polyacrylamide hydrogel optical fibers: (1) Photos of 3X stretching of a hydrogel fiber, (2) light transmission through a hydrogel fiber in the relaxed (left) and stretched (right) conditions, and (3) illustration of strain dependent optical loss; (e) Alginate-polyacrylamide fiber with 3 sensing regions using rose Bengal (RB), methylene blue (MB), and fluorescein (FL) dyes. Reprinted with permission from Ref. [80]. Copyright 2016, John Wiley and Sons. (f) A glucose sensitive hydrogel optical fiber implanted in porcine tissue; (g) Schematic of the design of the glucose sensitive hydrogel optical fibers. Reprinted with permission from Ref. [81]. Copyright 2017, John Wiley and Sons.
Highly stretchable and tough step-index optical fibers made of optimized alginate-polyacrylamide (PAM) hydrogels were also fabricated (Fig. 5d–e). The relatively low propagation loss of 0.45 dB/cm in air can provide efficient light guiding. Due to their elastic nature, the step-index optical fibers were able to reach an axial strain of 700% and then relaxed over multiple cycles. Combining the unique physical and optical properties of the fibers, novel strain sensing with a large dynamic strain range of 120% was demonstrated. The hydrogel fibers have potential for applications including wearable sensors and implantable therapy-enabling devices [80].
Hydrogel optical fibers, containing a poly (acrylamide-co-poly (ethylene glycol) diacrylate) p (AM-co-PEGDA) core and a Ca alginate cladding, with 3-(acrylamido)phenylboronic acid (3-APBA) molecules incorporated into the core for sensing glucose were also developed (Fig. 5f–g). Quantitative readouts were obtained from measuring the intensity changes of transmitted light through the hydrogel optical fibers, which correlated with glucose variations. The biocompatibleand flexible hydrogel optical fiber sensors demonstrated reproducible, real-time glucose sensing within the glucose concentration range in diabetes. These optical fibers show great potential for continuous in vivo glucose sensing for diabetes patients [81].
Due to the complex and sensitive biological system, as well as increased degenerative diseases, there is a growing need for advanced biophotonic techniques complemented with theranostic functionalities. Combining different materials with specific functionalities might be an efficient strategy for developing advanced biophotonic devices.
7. Conclusion
This review discusses the advances of natural and synthetic polymeric biomaterials for the design and development of biophotonic devices, and their applications for imaging, sensing and therapy. Bacteria cell-based living biomaterials naturally possess superior biocompatibility. However, their small size, limited modification and fabrication methods hinder their application fields as optical materials. Biomaterials composed of naturally occurring DNA, silk, chitosan, cellulose and agarose have demonstrated advantages including biocompatibility, degradability, and impressive mechanics, leading to the development of various in vivo biomedical devices. Despite these advantages, naturally derived materials still suffer from variability and limited designability. Synthetic materials offer similar biocompatibility and degradation to natural materials, while providing a high degree of modification and fabrication potential, leading to a large number of effective materials for optical sensing in situ. In particular, synthetic material systems have been leveraged to develop a wide range of multifunctional devices combining diagnosis, sensing, and therapeutic potential. Synthetic materials can also combine optical capabilities with drug release, wound closure, and other modalities. The development of such polymeric materials and material combinations has great potential in clinical diagnosis and treatment. Advances in optical materials with biologically relevant properties and combining multiple functionalities promise to translate traditional optical technology into the biomedical field, leading to improved patient outcomes and expanding the range of treatment options for clinicians.
Acknowledgement
This work was supported in part by National Institutes of Health awards (EB024829, CA182670, AR072731).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Baker M.J., Hughes C.S. Morgan & Claypool Publishers; 2016. Hollywood K a. Biophotonics: Vibrational Spectroscopic Diagnostics. [Google Scholar]
- 2.Prasad P.N. John Wiley & Sons; 2004. Introduction to Biophotonics. [Google Scholar]
- 3.Yang S.T., Cao L., Luo P.G. Carbon dots for optical imaging in vivo. J. Am. Chem. Soc. 2009;131(32):11308–11309. doi: 10.1021/ja904843x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stender A.S., Marchuk K., Liu C. Single cell optical imaging and spectroscopy. Chem. Rev. 2013;113(4):2469–2527. doi: 10.1021/cr300336e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu H., Isikman S.O., Mudanyali O. Optical imaging techniques for point-of-care diagnostics. Lab a Chip. 2013;13(1):51–67. doi: 10.1039/c2lc40864c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan X., White I.M., Shopova S.I. Sensitive optical biosensors for unlabeled targets: a review. Analytica Chimica Acta. 2008;620(1):8–26. doi: 10.1016/j.aca.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribaut C., Loyez M., Larrieu J.C. Cancer biomarker sensing using packaged plasmonic optical fiber gratings: towards in vivo diagnosis. Biosens. Bioelectron. 2017;92:449–456. doi: 10.1016/j.bios.2016.10.081. [DOI] [PubMed] [Google Scholar]
- 8.Balaji A., Zhang J. Electrochemical and optical biosensors for early-stage cancer diagnosis by using graphene and graphene oxide. Cancer nanotechnology. 2017;8(1):10. doi: 10.1186/s12645-017-0035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panesar S., Weng X., Neethirajan S. Toward point-of-care diagnostics of breast cancer: development of an optical biosensor using quantum dots. IEEE sensors letters. 2017;1(4):1–4. [Google Scholar]
- 10.Cennamo N., Varriale A., Pennacchio A. An innovative plastic optical fiber-based biosensor for new bio/applications. The case of celiac disease. Sensor. Actuator. B Chem. 2013;176:1008–1014. [Google Scholar]
- 11.Feng K., Qiu L.P., Yang Y. Label-free optical bifunctional oligonucleotide probe for homogeneous amplification detection of disease markers. Biosens. Bioelectron. 2011;29(1):66–75. doi: 10.1016/j.bios.2011.07.068. [DOI] [PubMed] [Google Scholar]
- 12.Doong R.A., Lee P.S., Anitha K. Simultaneous determination of biomarkers for Alzheimer's disease using sol–gel-derived optical array biosensor. Biosens. Bioelectron. 2010;25(11):2464–2469. doi: 10.1016/j.bios.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Debele T.A., Peng S., Tsai H.C. Drug Carrier for photodynamic cancer therapy. Int. J. Mol. Sci. 2015;16(9):22094–22136. doi: 10.3390/ijms160922094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcez A.S., Fregnani E.R., Rodriguez H.M. The use of optical fiber in endodontic photodynamic therapy. Is it really relevant? Laser Med. Sci. 2013;28(1):79–85. doi: 10.1007/s10103-012-1073-8. [DOI] [PubMed] [Google Scholar]
- 15.Ma X., Qu Q., Zhao Y. Targeted delivery of 5-aminolevulinic acid by multifunctional hollow mesoporous silica nanoparticles for photodynamic skin cancer therapy. ACS Appl. Mater. Interfaces. 2015;7(20):10671–10676. doi: 10.1021/acsami.5b03087. [DOI] [PubMed] [Google Scholar]
- 16.Wang C., Tao H., Cheng L., Liu Z. Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials. 2011;32(26):6145–6154. doi: 10.1016/j.biomaterials.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Wang J., Zhu G., You M. Assembly of aptamer switch probes and photosensitizer on gold nanorods for targeted photothermal and photodynamic cancer therapy. ACS Nano. 2012;6(6):5070–5077. doi: 10.1021/nn300694v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oakley E., Bellnier D.A., Hutson A. Surface markers for guiding cylindrical diffuser fiber insertion in interstitial photodynamic therapy of head and neck cancer. Laser Surg. Med. 2017;49(6):599–608. doi: 10.1002/lsm.22644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee K.S., Andraud C., Tamada K. Feature issue introduction: biophotonic materials and applications. Opt. Mater. Express. 2016;6(5):1747–1750. doi: 10.1364/BOE.7.002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.West J.L., Halas N.J. Engineered nanomaterials for biophotonics applications: improving sensing, imaging, and therapeutics. Annu. Rev. Biomed. Eng. 2003;5(1):285–292. doi: 10.1146/annurev.bioeng.5.011303.120723. [DOI] [PubMed] [Google Scholar]
- 21.Tong L., Gattass R.R., Ashcom J.B. Subwavelength-diameter silica wires for low-loss optical wave guiding. Nature. 2003;426(6968):816–819. doi: 10.1038/nature02193. [DOI] [PubMed] [Google Scholar]
- 22.Tao H., Kainerstorfer J.M., Siebert S.M. Implantable, multifunctional, bioresorbable optics. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109(48):19584–19589. doi: 10.1073/pnas.1209056109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian H., Tang Z., Zhuang X. Biodegradable synthetic polymers: preparation, functionalization and biomedical application. Prog. Polym. Sci. 2012;37(2):237–280. [Google Scholar]
- 24.Shan D., Zhang C., Kalaba S. Flexible biodegradable citrate-based polymeric step-index optical fiber. Biomaterials. 2017;143:142–148. doi: 10.1016/j.biomaterials.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi M., Choi J.W., Kim S. Light-guiding hydrogels for cell-based sensing and optogenetic synthesis in vivo. Nat. Photon. 2013;7(12):987–994. doi: 10.1038/nphoton.2013.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xin H., Li Y., Liu X. Escherichia coli-based biophotonic waveguides. Nano Letters. 2013;13(7):3408–3413. doi: 10.1021/nl401870d. [DOI] [PubMed] [Google Scholar]
- 27.Xin H., Li Y., Li B. Bacteria-based branched structures for bionanophotonics. Laser Photon. Rev. 2015;9(5):554–563. [Google Scholar]
- 28.Bezryadina A., Hansson T., Gautam R. Nonlinear self-action of light through biological suspensions. Phys. Rev. Lett. 2017;119(5):058101. doi: 10.1103/PhysRevLett.119.058101. [DOI] [PubMed] [Google Scholar]
- 29.Ha T.L.B., Quan T.M., Vu D.N. Regenerative Medicine and Tissue Engineering. Intech; 2013. Naturally Derived Biomaterials: Preparation and Application. [Google Scholar]
- 30.Peng S., Derrien T.L., Cui J., Xu C., Luo D. From cells to DNA materials. Mater. Today. 2012;15(5):190–194. [Google Scholar]
- 31.Jung W., Paulson B., Choi K. 2013. Fabrication and Characteristics of Thin-film Waveguides Based on DNA Biomaterials. Optical Processes in Organic Materials and Nanostructures II. 88270V-88270V-6. International Society for Optics and Photonics. [Google Scholar]
- 32.Vepari C., Kaplan D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007;32(8):991–1007. doi: 10.1016/j.progpolymsci.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tao H., Kaplan D.L., Omenetto F.G. Silk materials–a road to sustainable high technology. Adv. Mater. 2012;24(21):2824–2837. doi: 10.1002/adma.201104477. [DOI] [PubMed] [Google Scholar]
- 34.Omenetto F.G., Kaplan D.L. A new route for silk. Nat. Photon. 2008;2(11):641–643. [Google Scholar]
- 35.Applegate M.B., Perotto G., Kaplan D.L. Biocompatible silk step-index optical waveguides. Biomed. Optic Express. 2015;6(11):4221–4227. doi: 10.1364/BOE.6.004221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parker S.T., Domachuk P., Amsden J. Biocompatible silk printed optical waveguides. Adv. Mater. 2009;21(23):2411–2415. [Google Scholar]
- 37.Tierney S., Falch B.M.H., Hjelme D.R. Determination of glucose levels using a functionalized hydrogel-optical fiber biosensor: toward continuous monitoring of blood glucose in vivo. Anal. Chem. 2009;81(9):3630–3636. doi: 10.1021/ac900019k. [DOI] [PubMed] [Google Scholar]
- 38.Sudarsan V. Elsevier Inc; 2012. Optical Materials: Fundamentals and Applications. [Google Scholar]
- 39.Shabahang S., Kim S., Yun S.H. Light-guiding biomaterials for biomedical applications. Adv. Funct. Mater. 2018:1706635. doi: 10.1002/adfm.201706635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Froggatt M., Moore J. High-spatial-resolution distributed strain measurement in optical fiber with Rayleigh scatter. Appl. Optic. 1998;37(10):1735–1740. doi: 10.1364/ao.37.001735. [DOI] [PubMed] [Google Scholar]
- 41.Bassan P., Byrne H.J., Bonnier F., Lee J., Dumas P., Gardner P. Resonant Mie scattering in infrared spectroscopy of biological materials–understanding the ‘dispersion artefact’. Analyst. 2009;134(8):1586–1593. doi: 10.1039/b904808a. [DOI] [PubMed] [Google Scholar]
- 42.Lawrence B.D., Cronin-Golomb M., Georgakoudi I. Bioactive silk protein biomaterial systems for optical devices. Biomacromolecules. 2008;9(4):1214–1220. doi: 10.1021/bm701235f. [DOI] [PubMed] [Google Scholar]
- 43.Perry H., Gopinath A., Kaplan D.L. Nano- and micropatterning of optically transparent, mechanically robust, biocompatible silk fibroin films. Adv. Mater. 2008;20(16):3070–3072. [Google Scholar]
- 44.Amsden J.J., Domachuk P., Gopinath A. Rapid nanoimprinting of silk fibroin films for biophotonic applications. Adv. Mater. 2010;22(15):1746–1749. doi: 10.1002/adma.200903166. [DOI] [PubMed] [Google Scholar]
- 45.Sergeev A.A., Voznesenskiy S.S., Bratskaya S.Y. Investigation of humidity influence upon waveguide features of chitosan thin films. Physics Procedia. 2012;23:115–118. [Google Scholar]
- 46.Chen L.H., Li T., Chan C.C. Chitosan based fiber-optic Fabry–Perot humidity sensor. Sensor. Actuator. B Chem. 2012;169:167–172. [Google Scholar]
- 47.Voznesenskiy S.S., Sergeev A.A., Mironenko A.Y. Integrated-optical sensors based on chitosan waveguide films for relative humidity measurements. Sensor. Actuator. B Chem. 2013;188:482–487. [Google Scholar]
- 48.Mironenko A., Modin E., Sergeev A. Fabrication and optical properties of chitosan/Ag nanoparticles thin film composites. Chem. Eng. J. 2014;244:457–463. [Google Scholar]
- 49.Mironenko A.Y., Sergeev A.A., Nazirov A.E. H 2 S optical waveguide gas sensors based on chitosan/au and chitosan/ag nanocomposites. Sensor. Actuator. B Chem. 2016;225:348–353. [Google Scholar]
- 50.Peng L., Yang X., Yuan L. Gaseous ammonia fluorescence probe based on cellulose acetate modified microstructured optical fiber. Optic Commun. 2011;284(19):4810–4814. [Google Scholar]
- 51.Dupuis A., Guo N., Gao Y. Prospective for biodegradable microstructured optical fibers. Opt. Lett. 2007;32(2):109–111. doi: 10.1364/ol.32.000109. [DOI] [PubMed] [Google Scholar]
- 52.Li D., Wang L. Cellulose acetate polymer film modified microstructured polymer optical fiber towards a nitrite optical probe. Optic Commun. 2010;283(14):2841–2844. [Google Scholar]
- 53.Jain A., Yang A.H.J., Erickson D. Gel-based optical waveguides with live cell encapsulation and integrated microfluidics. Opt. Lett. 2012;37(9):1472–1474. doi: 10.1364/OL.37.001472. [DOI] [PubMed] [Google Scholar]
- 54.Lin C.C., Anseth K.S. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharmaceut. Res. 2009;26(3):631–643. doi: 10.1007/s11095-008-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y., Huang C.J., Jonas U. Biosensor based on hydrogel optical waveguide spectroscopy. Biosens. Bioelectron. 2010;25(7):1663–1668. doi: 10.1016/j.bios.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 56.Pasche S., Angeloni S., Ischer R. vol 57. Trans Tech Publications; 2008. Wearable Biosensors for Monitoring Wound Healing. Advances in Science and Technology; pp. 80–87. [Google Scholar]
- 57.Martincek I., Pudis D., Chalupova M. Technology for the preparation of PDMS optical fibers and some fiber structures. IEEE Photon. Technol. Lett. 2014;26(14):1446–1449. [Google Scholar]
- 58.Paek J., Kim J. Microsphere-assisted fabrication of high aspect-ratio elastomeric micropillars and waveguides. Nat. Commun. 2014;5:3324. doi: 10.1038/ncomms4324. [DOI] [PubMed] [Google Scholar]
- 59.Yang C., Shi K., Edwards P., Liu Z. Demonstration of a PDMS based hybrid grating and Fresnel lens (G-Fresnel) device. Optic Express. 2010;18(23):23529–23534. doi: 10.1364/OE.18.023529. [DOI] [PubMed] [Google Scholar]
- 60.Yang C., Edwards P., Shi K., Liu Z. Proposal and demonstration of a spectrometer using a diffractive optical element with dual dispersion and focusing functionality. Opt. Lett. 2011;36(11):2023–2025. doi: 10.1364/OL.36.002023. [DOI] [PubMed] [Google Scholar]
- 61.Zhang C., Cheng G., Edwards P., Zhou M.D., Zheng S., Liu Z. G-Fresnel smartphone spectrometer. Lab a Chip. 2016;16(2):246–250. doi: 10.1039/c5lc01226k. [DOI] [PubMed] [Google Scholar]
- 62.Edwards P., Zhang C., Zhang B., Hong X., Nagarajan V.K., Yu B., Liu Z. Smartphone based optical spectrometer for diffusive reflectance spectroscopic measurement of hemoglobin. Sci. Rep. 2017;7(1):12224. doi: 10.1038/s41598-017-12482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kolle M., Lethbridge A., Kreysing M. Bio-inspired band-gap tunable elastic optical multilayer fibers. Adv. Mater. 2013;25(15):2239–2245. doi: 10.1002/adma.201203529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anderson J.M., Shive M.S. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 2012;64:72–82. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 65.Nizamoglu S., Gather M.C., Humar M. Bioabsorbable polymer optical waveguides for deep-tissue photomedicine. Nat. Commun. 2016;7 doi: 10.1038/ncomms10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim M., An J., Kim K.S. Optical lens-microneedle array for percutaneous light delivery. Biomed. Optic Express. 2016;7(10):4220–4227. doi: 10.1364/BOE.7.004220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nizamoglu S., Gather M.C., Yun S.H. All-biomaterial laser using vitamin and biopolymers. Adv. Mater. 2013;25(41):5943–5947. doi: 10.1002/adma201300818. [DOI] [PubMed] [Google Scholar]
- 68.Humar M., Dobravec A., Zhao X. Biomaterial microlasers implantable in the cornea, skin, and blood. Optica. 2017;4(9):1080–1085. doi: 10.1364/OPTICA.4.001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tran R.T., Yang J., Ameer G.A. Citrate-based biomaterials and their applications in regenerative engineering. Annu. Rev. Mater. Res. 2015;45:277–310. doi: 10.1146/annurev-matsci-070214-020815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guo J., Xie Z., Tran R.T. Click chemistry plays a dual role in biodegradable polymer design. Adv. Mater. 2014;26(12):1906–1911. doi: 10.1002/adma.201305162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo J., Kim G.B., Shan D. Click chemistry improved wet adhesion strength of mussel-inspired citrate-based antimicrobial bioadhesives. Biomaterials. 2017;112:275–286. doi: 10.1016/j.biomaterials.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guo J., Wang W., Hu J. Synthesis and characterization of anti-bacterial and anti-fungal citrate-based mussel-inspired bioadhesives. Biomaterials. 2016;85:204–217. doi: 10.1016/j.biomaterials.2016.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Su L.C., Xu H., Tran R.T. In situ re-endothelialization via multifunctional nanoscaffolds. ACS Nano. 2014;8(10):10826–10836. doi: 10.1021/nn504636n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li J., Tian Y., Shan D. Neuropeptide YY 1 receptor-mediated biodegradable photoluminescent nanobubbles as ultrasound contrast agents for targeted breast cancer imaging. Biomaterials. 2017;116:106–117. doi: 10.1016/j.biomaterials.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 75.Yang J., Zhang Y., Gautam S. Development of aliphatic biodegradable photoluminescent polymers. Proc. Natl. Acad. Sci. Unit. States Am. 2009;106(25):10086–10091. doi: 10.1073/pnas.0900004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu J., Guo J., Xie Z. Fluorescence imaging enabled poly (lactide-co-glycolide) Acta Biomaterialia. 2016;29:307–319. doi: 10.1016/j.actbio.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim J.P., Xie Z., Creer M. Citrate-based fluorescent materials for low-cost chloride sensing in the diagnosis of cystic fibrosis. Chem. Sci. 2017;8(1):550–558. doi: 10.1039/c6sc02962k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang C., Kim J.P., Creer M. A smartphone-based chloridometer for point-of-care diagnostics of cystic fibrosis. Biosens. Bioelectron. 2017;97:164–168. doi: 10.1016/j.bios.2017.05.048. [DOI] [PubMed] [Google Scholar]
- 79.Choi M., Humar M., Kim S. Step-index optical fiber made of biocompatible hydrogels. Adv. Mater. 2015;27(27):4081–4086. doi: 10.1002/adma.201501603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo J., Liu X., Jiang N. Highly stretchable, strain sensing hydrogel optical fibers. Adv. Mater. 2016;28(46):10244–10249. doi: 10.1002/adma.201603160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yetisen A.K., Jiang N., Fallahi A. Glucose-sensitive hydrogel optical fibers functionalized with phenylboronic acid. Adv. Mater. 2017;29(15) doi: 10.1002/adma.201606380. [DOI] [PMC free article] [PubMed] [Google Scholar]