Abstract
Background
Heart rate variability (HRV) has emerged as a predictor of later cardiac risk. This study tested whether pregnancy complications that may have long-term offspring cardiac sequelae are associated with differences in HRV at birth, and whether these HRV differences identify abnormal cardiovascular development in the postnatal period.
Methods
98 sleeping neonates had 5-minute electrocardiogram recordings at birth. Standard time and frequency domain parameters were calculated and related to cardiovascular measures at birth and three months of age.
Results
Increasing prematurity, but not maternal hypertension or growth restriction, was associated with decreased HRV at birth as demonstrated by a lower root mean square of the difference between adjacent NN intervals (rMSSD), low (LF) and high frequency power (HF) with decreasing gestational age (p<0.001, p=0.009 and p=0.007 respectively). We also demonstrated a relative imbalance between sympathetic and parasympathetic tone compared to term infants. However, differences in autonomic function did not predict cardiovascular measures at either time-point.
Conclusions
Altered cardiac autonomic function at birth relates to prematurity rather than other pregnancy complications and does not predict cardiovascular developmental patterns during the first three months post birth. Long-term studies will be needed to understand relevance to cardiovascular risk.
Introduction
Heart rate variability (HRV) analysis provides a non-invasive measure of cardiac autonomic function based on variation in the QRS to QRS (RR or normal to normal NN interval) interval sequence of the electrocardiogram (ECG). The derived metrics of HRV allow evaluation of sympathetic and parasympathetic balance within the autonomic nervous system (ANS) and the ability of the sinoatrial node to adapt to extrinsic signals. In a multitude of well-designed studies decreased HRV has emerged as a strong predictor of cardiac risk in adults and death in patients at increased cardiovascular risk. (1–3) Interestingly, attenuation in HRV is also evident in infants born preterm (4) with dysfunction being greater in those with higher clinical illness scales (5) or pathological problems such as respiratory distress syndrome, (6) birth asphyxia, (6) intraventricular haemorrhage (6) and small-for gestational age (7). Pregnancy complications, in particular, preterm birth and maternal hypertension, have been found to associate with an increased risk of cardiovascular disease in later life (8) and the offspring display a distinct cardiovascular phenotype characterised by microvascular rarefaction and cardiac hypertrophy (9–11).
These cardiac and vascular patterns become evident during the first three months of life when differences in autonomic function have been identified in preterm infants(10, 12, 13). Therefore, we investigated, for the first time, using short ECG recordings in a large cohort of newborn infants, whether differences in neonatal HRV relates just to prematurity or are found in other pregnancy complications linked with later cardiovascular disease. Furthermore, we studied whether altered HRV may be a marker of abnormal in utero or postnatal cardiac and vascular development in these infants.
Methods
Study overview
Between 2011 and 2015, 600 mothers being cared for by Oxford University Hospitals NHS Foundation Trust were identified by their clinical care team and invited to take part in one or more of a portfolio of studies coordinated by the Oxford Cardiovascular Clinical Research Facility. These studies were designed to investigate the impact of pregnancy complications on cardiovascular development during fetal and neonatal life and used a stratified recruitment approach to ensure balanced representation of preterm and term birth as well as hypertensive and normotensive pregnancies.
To study heart rate variability, cardiac and microvascular development, we used available electrocardiogram (ECG), echocardiographic and in vivo microvascular datasets from birth and three months of age (Figure 1) from participants in the EPOCH programme (Effect of Pregnancy on Offspring Cardiovascular Health study - approved by South Central Berkshire Research Ethics Committee ref. 11/SC/0006, UKCRN/clinical trials ref. NCT01888770).
Figure 1.
Overview of study design investigating whether (1) pregnancy complications had an effect on heart rate variability at birth and (2) if heart rate variability at birth predicted cardiovascular development at birth or at 3 months of age as measured by macrovascular, microvascular and cardiac assessments in the offspring. HRV indicates heart rate variability; SDNN standard deviation of the NN intervals; rMSSD root mean square of the difference between adjacent NN intervals; HF high frequency; LF low frequency and LV left ventricular.
All mothers gave written informed consent for involvement of their children in accordance with the Declaration of Helsinki, including permission to access maternal and offspring clinical records and link data between studies. Mothers below the age of 16 years were excluded from the study as were those with chronic cardiovascular conditions prenatally, including hypertension. Infants were excluded if they had evidence of congenital cardiovascular disease (with the exception of Persistent Ductus Arteriosus and Atrial Septal Defect), chromosomal abnormalities or genetic disorders. Prolonged resuscitation at birth, intraventricular haemorrhage and ventilatory support during the time of assessment did not constitute specific exclusion criteria, although no infants in our cohort fell into these categories.
Clinical data collection and characterisation of pregnancy complications
Characterisation of pregnancy complications and perinatal data related to the clinical care of the infant was extracted from medical records and questionnaires in a standardised way across studies by the same data collection team (CA, ED, YK). Data collection details are available from https://clinicaltrials.gov (NCT01888770). Postmenstrual age at time of measurements was calculated relative to gestational age defined at first trimester ultrasound. Hypertensive pregnancy (HTN) diagnosis (pregnancy induced hypertension, preeclampsia (PET)) was defined according to ISSHP guidelines(14). Z-scores for birthweight were calculated using the International Standard size at birth reference charts from the INTERGROWTH-21st Project(15, 16) using their online application (https://intergrowth21.tghn.org/global-perinatal-package/intergrowth-21st-comparison-application/). Small for gestational age (SGA) was defined as a birthweight below the 10th centile.
Cardiovascular measures
Cardiovascular measurements were performed within four weeks of birth and again at three months of age in a temperature-controlled room, with the infant at rest, either in their mother’s arms, or in a crib. At both the birth and three month assessments, weight was measured using digital scales (Charder Model MS4200) to the nearest 0.01kg with the infant fully naked. Head circumference was measured with a tape measure to nearest mm. At birth and three months, three blood pressure measurements were recorded on the right calf with an automated digital monitor (Dinamap technology® V100) using appropriate sized cuffs and were averaged for analysis. Brachial-femoral pulse wave velocity (PWV) in order to study arterial stiffness was measured by fitting brachial and femoral cuffs a known distance apart using an oscillometry device (Vicorder, Skidmore Medical, Taunton, UK). Methods for echocardiography and in vivo microvascular imaging have been published previously and can be found in Supplemental Methods (online).(10, 17)
Heart rate variability
Data acquisition
At the birth assessment all babies had a short 5-10 minute ECG taken between feeds, lying down without use of a pacifier. The Shimmer® device was used to acquire the data which was connected via Bluetooth to an Android mobile smartphone. The ECG collected consisted of two-lead signals with a 256Hz sampling frequency and 12bit quantization levels. Data was collected through an android app,(18) processed and stored on the phone before being transferred to a server. Details of whether the baby was asleep or awake were noted and the recording was stopped if there was excessive restlessness or crying.
Data processing
The ECG signals were then processed in order to extract the RR interval time-series, but also a Signal Quality Index (SQI). These features were extracted using previously published techniques.(19) The SQI was used in order to select the “best” five minute segment, and the RR interval time-series from this segment was kept for heart rate variability (HRV) analysis.
Heart Rate Variability analysis
The five minute RR interval segment was used to extract HRV features. These features were extracted using the HRV toolkit which has been validated and is freely available online.(20) Processing included detection and extraction of the normal to normal (NN) interval time-series and automated outlier removal for rejection of artefactual RR points. Calculated HRV features were based on basic time-domain HRV statistics used in the literature, specifically, the standard deviation of the NN intervals (SDNN) and root mean square of the difference between adjacent NN intervals (rMSSD). The frequency-domain features were extracted using the Lomb periodogram, eliminating the need for evenly sampled data in contrast to the traditional Fast Fourier Transformation. The benefit of this is that sections of the recordings in which there are gaps or extreme noise in the data can be omitted. Parameters included the total spectral power of all NN intervals between 0.05 and 0.2 Hz (low frequency power, LF), the total spectral power of all NN intervals between 0.2 and 1 Hz (high frequency power, HF) and the ratio of low to high frequency power (LF/HF ratio) using cut offs previously suggested in the literature for the neonatal population.(21) In order to be able to standardise these measures, only recordings during which the babies were asleep throughout were included in analysis.
Statistical Analysis
Statistical analysis was performed using SPSS Version 22 (IBM, Armonk, NY) and GraphPad Prism 6.0 (La Jolla, CA). Comparison between groups for continuous variables was carried out using a two-tailed, independent samples t-test for normally distributed variables and Mann Whitney U test for non-normally distributed data. Bivariate regression models were performed using a forced entry method and unstandardized B coefficients and 95% confidence intervals are reported. The sample size n=33 preterm and n=65 term offspring provided us with 80% power at a significance level of α=0.05 to detect a difference of at least 0.85 standard deviations between groups. P-values less than 0.05 were considered statistically significant.
Results
Study Population Characteristics
The technology for assessment of HRV became available during the course of recruitment to the EPOCH study and therefore, out of the 266 infants in the full neonatal cohort, 140 had an ECG taken at birth. Of these, 3 were unanalysable due to poor signal quality and 39 were excluded as the infant was awake or restless during acquisition. Maternal and offspring demographic and anthropometric characteristics in the cohort are presented in Table 1 with subgroup characteristics available in Supplemental Table S1 (online).
Table 1.
Cohort Characteristics
| n = 98 | |
|---|---|
| Maternal Demographics & Anthropometrics | |
| Maternal age at delivery, years | 33.0±4.6 |
| BMI at booking, kg/m2 | 25.4±5.1 |
| Smokers, n (%) | 2 (2) |
| Maternal hypertension during pregnancy, n (%) | 46 (47) |
| Offspring Birth Characteristics | |
| Gestational age at delivery, weeks | 37.9±2.9 |
| Males, n (%) | 48 (49) |
| Birth ordera | 1±1 |
| Caesarean section, n (%) | 36 (37) |
| Antenatal steroids, n (%) | 28 (29) |
| Offspring Physiological Measures at Birth | |
| Head circumference, cms | 33.5±2.2 |
| Birthweight, grams | 2964±754 |
| Birthweight Z-score | 0.10±1.0 |
| sBP, mmHg | 81±15 |
| dBP, mmHg | 44±10 |
| Pulse Wave Velocity, (m/sec) | 5.5±1.5 |
| Offspring Physiological Measures at 3 months | |
| Weight, grams | 5763±950 |
| Head circumference, cms | 40.5±1.6 |
| sBP, mmHg | 97±12 |
| dBP, mmHg | 53±13 |
| Pulse Wave Velocity, (m/sec) | 6.5±1.7 |
Mean±Standard Deviation unless stated otherwise
Median±Interquartile range
BMI indicates body mass index; sBP systolic blood pressure and dBP diastolic blood pressure
Pregnancy complications and heart rate variability
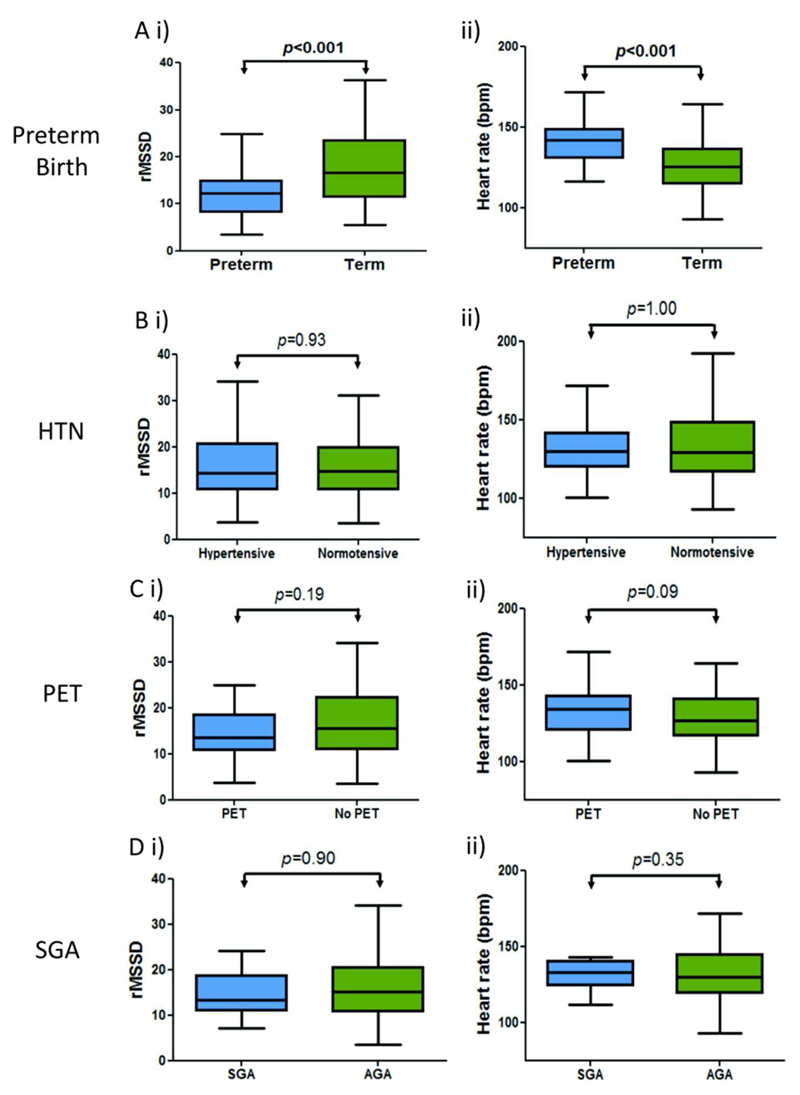
Those born preterm had a higher heart rate and lower heart rate variability than those born term (Figure 2A). There was a positive association between SDNN, rMSSD, LF and HF and gestational age at birth; although the association with SDNN failed to reach significance after adjusting for postnatal age at assessment and offspring sex (Table 2). There was a negative correlation between LF/HF ratio and gestational age at birth even after adjustment. There was no significant difference in heart rate or heart rate variability parameters between those born to mothers with or without maternal hypertension (Figure 2B and Table 2). We separately analysed those whose mothers had a more severe hypertensive disorder of pregnancy, classified as preeclampsia, and found no differences in neonatal heart rate variability in this group (Figure 2C). Furthermore, there were no significant associations between birthweight z-score and any HRV parameter (Table 2), or any difference between those classified as small or appropriate for gestational age (Figure 2D). Due to the small numbers of preterm babies in our cohort we were not powered to detect differences between subgroups, for example preterm small for gestational age versus preterm appropriate for gestational age. We therefore performed additional regression analyses to clarify the lack of relationship with pregnancy complications by studying the relationship between rMSSD at birth, correcting for postnatal age at assessment, offspring sex as well as gestatinal age at birth (Supplemental Table S2 (online)). We found that even after adjusting for physiological changes in autonomic balance due to gestational age, there were still no correlations between other pregnancy complications with HRV.
Figure 2.
Boxplots demonstrating (A) a significantly decreased rMSSD and increased heart rate in offspring born preterm but no significant difference in those exposed to maternal hypertension (B), preeclampsia (C) or those born small for gestational age (D). rMSSD indicates root mean square of the difference between adjacent NN intervals; HTN hypertensive pregnancy; PET preeclamptic pregnancy; SGA small for gestational age and AGA appropriate for gestational age.
Table 2.
Multivariable Regression Coefficients for Heart Rate Variability Parameters at Birth and Pregnancy Complications
| Gestational age at birth (weeks) | Maternal Hypertension | Birthweight z-score | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | 95% CI | p-value | B | 95% CI | p-value | B | 95% CI | p-value | |
| SDNN | 0.76 | -0.13-1.66 | 0.09 | 2.30 | -2.92-7.53 | 0.38 | -1.92 | -4.32-0.47 | 0.11 |
| rMSSD | 0.99 | 0.43-1.54 | 0.001 | 0.24 | -3.17-3.65 | 0.89 | -0.41 | -1.98-1.17 | 0.61 |
| LF | 23.50 | 6.02-40.98 | 0.009 | 61.16 | -42.84-165.16 | 0.25 | -41.59 | -89.25-6.07 | 0.09 |
| HF | 19.25 | 5.51-33.00 | 0.007 | 9.28 | -73.32-91.87 | 0.82 | -10.85 | -48.96-27.27 | 0.57 |
| LF/HF ratio | -0.20 | -0.34- -0.06 | 0.005 | 0.34 | -0.49-1.17 | 0.42 | 0.15 | -0.23-0.53 | 0.44 |
B unstandardized coefficient presented with 95% CI after correcting for postnatal age at assessment and offspring sex SDNN indicates standard deviation of the NN intervals; rMSSD root mean square of the difference between adjacent NN intervals; LF low frequency and HF high frequency
We additionally studied whether particular perinatal clinical features, including time on ventilation or APGAR score, predicted autonomic dysfunction in those born preterm but were not able to identify specific markers of more deranged function except for a borderline association between caesarean delivery and a greater rMSSD (Table 3). When subgroup analysis was performed on only babies that were born by caesarean section, there was no correlation between rMSSD and whether the delivery took place pre or during labour after adjusting for offspring sex and postmenstrual age at assessment (B=2.40 95%CI(-3.74-8.52), p=0.43)).
Table 3.
Multivariable Regression Coefficients for Perinatal Clinical Features and rMSSD at Birth
| rMSSD | |||
|---|---|---|---|
| B | 95% CI | p-value | |
| Maternal smoking | -3.29 | -14.98-8.39 | 0.58 |
| Antenatal steroids | 0.11 | -0.02-0.24 | 0.58 |
| Caesarean section | 3.63 | -7.16- -0.10 | 0.04 |
| Apgar score at 5 mins | 0.20 | -2.06-2.46 | 0.86 |
| Days of oxygen | 0.16 | -1.04-1.36 | 0.80 |
| Postnatal infection | -1.28 | -7.69-5.13 | 0.69 |
B unstandardized coefficient presented with 95% CI after correcting for postmenstrual age at assessment (gestational age at birth plus age at assessment) and offspring sex
rMSSD root mean square of the difference between adjacent NN intervals
Relationship with cardiovascular structure and function at birth and three months of age
We studied whether heart rate variability was a predictor of other cardiovascular developmental differences at birth or related to those known to be found in preterm offspring, specifically postnatal cardiac hypertrophy and microvascular rarefaction. We based analysis on associations with rMSSD at birth, as the HRV parameter with the strongest association with gestational age. However, there were no association with these parameters (Table 4) even when the cohort was split into preterm and term groups (data not shown).
Table 4.
Multivariable Regression Coefficients for Cardiovascular Development in Early Postnatal Life and rMSSD at Birth
| Birth | 3 Months | |||||
|---|---|---|---|---|---|---|
| B | 95% CI | p-value | B | 95% CI | p-value | |
| Macrovascular | ||||||
| sBP (mmHg) | -0.11 | -0.47-0.25 | 0.55 | 0.07 | -0.23-0.38 | 0.63 |
| dBP (mmHg) | -0.03 | -0.27-0.20 | 0.78 | -0.03 | -0.34-0.28 | 0.85 |
| PWV (m/s) | -0.01 | -0.05-0.03 | 0.63 | 0.01 | -0.03-0.05 | 0.60 |
| Microvascular | ||||||
| TVD (mm/mm2) | -0.04 | -0.17-0.09 | 0.56 | 0.02 | -0.08-0.11 | 0.75 |
| Cardiac | ||||||
| Left ventricular mass index | 0.00 | -0.09-0.09 | 1.00 | -0.04 | -0.18-0.12 | 0.60 |
| Ejection fraction (%) | 0.06 | -0.23-0.34 | 0.69 | 0.20 | -0.05-0.46 | 0.11 |
| Lateral E/E’ ratio | 0.002 | -0.07-0.07 | 0.96 | -0.06 | -0.11-0.01 | 0.07 |
B unstandardized coefficient presented with 95% confidence intervals (CI) after correcting for postnatal age at assessment and offspring sex rMSSD indicates root mean square of the difference between adjacent NN intervals; sBP systolic blood pressure; dBP diastolic blood pressure; PWV pulse wave velocity and TVD total vessel density
Discussion
In this study we have demonstrated that heart rate variability parameters derived from short length ECG recordings in the first week of life are significantly associated with gestational age at birth. HRV is decreased in preterm infants compared to term counterparts with reduced parasympathetic activity(22) and a relative imbalance between sympathetic and parasympathetic tone compared to term infants.(23) In contrast, we found no association between HRV and exposure to maternal hypertension or fetal growth restriction within our cohort. We have also found no evidence that HRV at birth associates with patterns of cardiovascular development in the early postnatal period that we have previously reported in those born preterm.(10, 17)
Our findings are consistent with several previous studies that have also observed reduced HRV at birth in those born preterm.(4) These changes could have been established in response to specific in utero stressors linked with the preterm birth. Alterations in maternal heart rate variability are seen in pathological pregnancies; with preeclampsia having been associated with reduced maternal heart rate variability, which worsens as the pregnancy progresses.(3) Interestingly, there is evidence that this is of relevance to the child as maternal autonomic heart rate modulation relates to fetal heart rate patterns in hypertensive pregnancies.(3) However, our data suggests that any links between maternal and fetal heart rate do not persist after delivery in those born to hypertensive pregnancies, irrespective of the severity or classification of the hypertensive disorder.
Furthermore, even when there is evidence of fetal compromise, with a reduced birthweight z-score or classification as small for gestational age (SGA), if there are any in utero differences in autonomic function, they are not evident after birth although, interestingly, adults born SGA have been shown to have sympathetic nerve hyperactvity.(24) A reason for this might be that those in our cohort born SGA were constitutionally small rather than being growth restricted and were therefore unlikely to exhibit autonomic dysfunction. Data which would have differentiated between SGA and fetal growth restriction was unfortunately not available for our cohort. This was because babies were recruited postnatally and problems with growth were not suspected prior to birth in the majority of cases. Therefore, in utero measures of placental function had not been carried out by their clinical team. An alternative explanation for our, and others’, findings in those born preterm is that they merely reflect a relative functional immaturity in ANS activity. The fetal autonomic nervous system develops progressively throughout pregnancy(25), with more rapid development of the parasympathetic branch(26), and therefore differences in ANS function at birth would be expected, proportional to gestational age.
Nevertheless, this functional immaturity at birth could still have pathological significance. Previous studies have demonstrated deficits in HRV parameters in the preterm population may persist after birth up to term equivalent age (27), which suggests normal development may require the fetus to remain in utero until term.(7, 26) Another potential hypothesis for this delayed or arrested maturation is disordered anatomical and cellular development of the nervous system (28) or disruption of neuropeptide synthesis caused by inflammatory events (29) which are more common in the preterm population. HRV has been shown to be altered in conditions such as intraventricular haemorrhage(30) as well as being an indicator of the severity of clinical conditions such as respiratory distress syndrome (6), and clinical illness scales(5). Nutritional,(31) environmental (32) or iatrogenic stress (33) in the ex utero environment has also been potentially linked with abnormal ANS development. This may explain the borderline association between vaginal delivery and decreased HRV and vaginal delivery as a caesarean section may mitigate some of the delivery stress accompanying complicated vaginal deliveries. However, there were no significant associations between rMSSD and Apgar score at 5 minutes (Table 3) and no correlation between HRV and whether the caesarean section was performed pre or during labour.
In our cohort, perinatal conditions were not related to HRV and measures at birth were not predictive of cardiac and vascular structure and function at birth or the postnatal changes in these parameters we have reported in these preterm offspring. Therefore, altered ANS function is unlikely to explain these cardiovascular developmental differences in those born preterm. The lack of importance of ANS function may be because our premature subgroup had an average gestational age of 34.4 weeks and the frequency of severe clinical postnatal conditions was low. The ANS of late preterm infants matures more quickly after birth(34) and previous studies that showed continued reduction in HRV at term equivalent age have tended to be in the more extreme preterm infants born prior to 32 weeks(35). Therefore it remains possible a functional immaturity of the ANS has a greater impact on cardiovascular development for the more extreme preterm infant.
An alternative explanation might be that HRV, does not sufficiently assess sympathetic function in spite of it being a sensitive measure of overall autonomic imbalance which allows for evaluation of a proportion of cardiovagal function. Therefore, predominance of pathology in one of the ANS branches might be obscured by compensatory interactions with the other ANS branch not captured by HRV analysis. (36) Since our data demonstrate that overall cardiac autonomic dysfunction relates to increasing prematurity but appears not to associate with altered cardiovascular development it clearly highlights the necessity of follow up research to elucidate whether separate assessment of sympathetic and parasympathetic functional integrity (including non-cardiac measures) might provide additional insights into the mechanisms whereby birth complications such as preterm birth affect the development of the cardiovascular system.
Whether the differences in autonomic function we observe at birth in those born preterm could be of relevance to the increased risk of hypertension in adulthood, independent of changes in cardiac and vascular phenotype,(37) remains to be seen in future studies. HRV attenuation has long been implicated in adult cardiovascular disease states such as acute myocardial infarction(1) and congestive heart failure (2). However, one longer term study of preterm offspring HRV found early differences were attenuated by two years of age and equivalent to term born offspring by six to seven years of age(27), suggesting there would need to be a re-emergence of HRV differences in adult life.
Studies of ANS function in neonatal populations need to confront several challenges. We used state-of-the art, small, remote monitoring technology to capture data but our analysis needed to be based on short length recordings. However, the other measures we have derived from these short recordings have previously been shown to correlate well with parameters measured from longer recordings in adults.(38) Measurement of HRV is also only an indirect measurement of autonomic function but structural measures of the ANS, such as skin nerve biopsies, are not feasible in the neonatal population. In addition, specific measurements of sympathetic function such as by measuring muscle sympathetic nerve activity by microneurography (39) would be technically challenging in this age group which might constitute one of the major challenges in future studies of the specific role of the integrity of both ANS branches in cardiovascular development. We used data on sleeping infants so as to control external conditions and stimulation to make it easier to interpret differences in HRV parameters between groups of subjects. However, we did not differentiate between active and quiet sleep states using a simultaneous electroencephalogram but instead stopped recordings during periods of observed unrest. Nevertheless, a previous study has suggested in healthy term neonates that there is no difference in HRV measures between groups when divided into behavioural states during sleep(25) with a close agreement between low mean heart rate and quiet sleep and high mean heart rate and active sleep in infants.(40) Mean respiration rate during the recording was also not recorded although, again, a previous study has suggested this may not correlate with HRV indices.(25) In summary, heart rate variability at birth is significantly associated with gestational age at birth with increasing prematurity resulting in increased differences in autonomic function compared to term infants as suggested by reduced time and frequency domain heart rate variability parameters. No associations between HRV and maternal hypertension or fetal growth restriction were found. In addition, we found no evidence that autonomic function at birth had an impact on cardiovascular development in the early postnatal period, but whether it in part explains the long-term risk of hypertension in offspring exposed to pregnancy complications remains to be seen.
Supplementary Material
Acknowledgments
We are grateful to all the pregnant women and babies who participated in this study.
Statement of Financial Support
This work was supported by the British Heart Foundation (FS/11/65/28865) to Professor Leeson. Additional grants were received from the National Institute for Health Research Oxford Biomedical Research Centre and Oxford British Heart Foundation Centre for Research Excellence.
Footnotes
Disclosure Statement
The authors have no conflicts of interest to declare.
Category of Study
Clinical study
References
- 1.Lombardi F, Sandrone G, Pernpruner S, et al. Heart rate variability as an index of sympathovagal interaction after acute myocardial infarction. Am J Cardiol. 1987;60:1239–1245. doi: 10.1016/0002-9149(87)90601-1. [DOI] [PubMed] [Google Scholar]
- 2.Casolo G, Balli E, Fazi A, Gori C, Freni A, Gensini G. Twenty-four-hour spectral analysis of heart rate variability in congestive heart failure secondary to coronary artery disease. Am J Cardiol. 1991;67:1154–1158. doi: 10.1016/0002-9149(91)90887-q. [DOI] [PubMed] [Google Scholar]
- 3.Swansburg ML, Brown CA, Hains SM, Smith GN, Kisilevsky BS. Maternal cardiac autonomic function and fetal heart rate in preeclamptic compared to normotensive pregnancies. Can J Cardiovasc Nurs. 2005;15:42–52. [PubMed] [Google Scholar]
- 4.Patural H, Pichot V, Jaziri F, et al. Autonomic cardiac control of very preterm newborns: a prolonged dysfunction. Early Hum Dev. 2008;84:681–687. doi: 10.1016/j.earlhumdev.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein B, Fiser DH, Kelly MM, Mickelsen D, Ruttimann U, Pollack MM. Decomplexification in critical illness and injury: relationship between heart rate variability, severity of illness, and outcome. Crit Care Med. 1998;26:352–357. doi: 10.1097/00003246-199802000-00040. [DOI] [PubMed] [Google Scholar]
- 6.Prietsch V, Knoepke U, Obladen M. Continuous monitoring of heart rate variability in preterm infants. Early Hum Dev. 1994;37:117–131. doi: 10.1016/0378-3782(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 7.Spassov L, Curzi-Dascalova L, Clairambault J, et al. Heart rate and heart rate variability during sleep in small-for-gestational age newborns. Pediatr Res. 1994;35:500–505. [PubMed] [Google Scholar]
- 8.Lewandowski AJ, Davis EF, Yu G, et al. Elevated blood pressure in preterm-born offspring associates with a distinct antiangiogenic state and microvascular abnormalities in adult life. Hypertension. 2015;65:607–614. doi: 10.1161/HYPERTENSIONAHA.114.04662. [DOI] [PubMed] [Google Scholar]
- 9.Lewandowski AJ, Augustine D, Lamata P, et al. Preterm heart in adult life: cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation. 127:197–206. doi: 10.1161/CIRCULATIONAHA.112.126920. [DOI] [PubMed] [Google Scholar]
- 10.Yu GZ, Aye CY, Lewandowski AJ, et al. Association of Maternal Antiangiogenic Profile at Birth With Early Postnatal Loss of Microvascular Density in Offspring of Hypertensive Pregnancies. Hypertension. 2016;68:749–759. doi: 10.1161/HYPERTENSIONAHA.116.07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewandowski AJ, Bradlow WM, Augustine D, et al. Right ventricular systolic dysfunction in young adults born preterm. Circulation. 2013;128:713–720. doi: 10.1161/CIRCULATIONAHA.113.002583. [DOI] [PubMed] [Google Scholar]
- 12.Lazdam M, de la Horra A, Diesch J, Kenworthy Y, et al. Unique blood pressure characteristics in mother and offspring after early onset preeclampsia. Hypertension. 2012;60:1338–1345. doi: 10.1161/HYPERTENSIONAHA.112.198366. [DOI] [PubMed] [Google Scholar]
- 13.Davis EF, Lewandowski AJ, Aye C, et al. Clinical cardiovascular risk during young adulthood in offspring of hypertensive pregnancies: insights from a 20-year prospective follow-up birth cohort. BMJ Open. 2015;5:e008136. doi: 10.1136/bmjopen-2015-008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP) Hypertens Pregnancy. 2001;20:IX–XIV. doi: 10.1081/PRG-100104165. [DOI] [PubMed] [Google Scholar]
- 15.Villar J, Ismail LC, Victora CG, Growth IFN et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384:857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 16.Villar J, Giuliani F, Fenton TR, Ohuma EO, Ismail LC, Kennedy SH, Consortium I-s INTERGROWTH-21st very preterm size at birth reference charts. Lancet. 2016;387:844–845. doi: 10.1016/S0140-6736(16)00384-6. [DOI] [PubMed] [Google Scholar]
- 17.Aye CYL, Lewandowski AJ, Lamata P, et al. Disproportionate cardiac hypertrophy during early postnatal development in infants born preterm. Pediatr Res. 2017 doi: 10.1038/pr.2017.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oster J, Behar J, Colloca R, Li Q, Clifford GD. Open source Java-based ECG analysis software and Android app for atrial fibrillation screening. Computing in Cardiology. 2013:731–734. [Google Scholar]
- 19.Johnson AE, Behar J, Andreotti F, Clifford GD, Oster J. Multimodal heart beat detection using signal quality indices. Physiological measurement. 2015;36:1665. doi: 10.1088/0967-3334/36/8/1665. [DOI] [PubMed] [Google Scholar]
- 20.Goldberger AL, Amaral LA, Glass L, et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101:E215–220. doi: 10.1161/01.cir.101.23.e215. [DOI] [PubMed] [Google Scholar]
- 21.Giddens DP, Kitney RI. Neonatal heart rate variability and its relation to respiration. J Theor Biol. 1985;113:759–780. doi: 10.1016/s0022-5193(85)80192-2. [DOI] [PubMed] [Google Scholar]
- 22.Pomeranz B, Macaulay RJ, Caudill MA, et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol. 1985;248:H151–153. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- 23.Electrophysiology TFotESoCatNASoPa. Heart rate variability. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 24.Boguszewski MC, Johannsson G, Fortes LC, Sverrisdottir YB. Low birth size and final height predict high sympathetic nerve activity in adulthood. J Hypertens. 2004;22:1157–1163. doi: 10.1097/00004872-200406000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Longin E, Schaible T, Lenz T, Konig S. Short term heart rate variability in healthy neonates: normative data and physiological observations. Early Hum Dev. 2005;81:663–671. doi: 10.1016/j.earlhumdev.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Gagnon R, Campbell K, Hunse C, Patrick J. Patterns of human fetal heart rate accelerations from 26 weeks to term. Am J Obstet Gynecol. 1987;157:743–748. doi: 10.1016/s0002-9378(87)80042-x. [DOI] [PubMed] [Google Scholar]
- 27.De Rogalski Landrot I, Roche F, Pichot V, et al. Autonomic nervous system activity in premature and full-term infants from theoretical term to 7 years. Auton Neurosci. 2007;136:105–109. doi: 10.1016/j.autneu.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Horner SM, Murphy CF, Coen B, Dick DJ, Harrison FG, Vespalcova Z, Lab MJ. Contribution to heart rate variability by mechanoelectric feedback. Stretch of the sinoatrial node reduces heart rate variability. Circulation. 1996;94:1762–1767. doi: 10.1161/01.cir.94.7.1762. [DOI] [PubMed] [Google Scholar]
- 29.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 30.Watkins TW, Horns KM, Harvadi DG, Sohacy R, Woodward PJ, Milley JR. Heart rate variability: relationship to IVH in VLBW neonates (abstract) Pediatr Res. 1996;39:251A. [Google Scholar]
- 31.Lonsdale D. Sudden infant death syndrome requires genetic predisposition, some form of stress and marginal malnutrition. Med Hypotheses. 2001;57:382–386. doi: 10.1054/mehy.2001.1363. [DOI] [PubMed] [Google Scholar]
- 32.Goto K, Mirmiran M, Adams MM, et al. More awakenings and heart rate variability during supine sleep in preterm infants. Pediatrics. 1999;103:603–609. doi: 10.1542/peds.103.3.603. [DOI] [PubMed] [Google Scholar]
- 33.Thiriez G, Bouhaddi M, Mourot L, et al. Heart rate variability in preterm infants and maternal smoking during pregnancy. Clin Auton Res. 2009;19:149–156. doi: 10.1007/s10286-009-0003-8. [DOI] [PubMed] [Google Scholar]
- 34.Henslee JA, Schechtman VL, Lee MY, Harper RM. Developmental patterns of heart rate and variability in prematurely-born infants with apnea of prematurity. Early Hum Dev. 1997;47:35–50. doi: 10.1016/s0378-3782(96)01767-7. [DOI] [PubMed] [Google Scholar]
- 35.Scher MS, Steppe DA, Dokianakis SG, Sun M, Guthrie RD, Sclabassi RJ. Cardiorespiratory behavior during sleep in full-term and preterm neonates at comparable postconceptional term ages. Pediatr Res. 1994;36:738–744. doi: 10.1203/00006450-199412000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Houle MS, Billman GE. Low-frequency component of the heart rate variability spectrum: a poor marker of sympathetic activity. Am J Physiol. 1999;276:H215–223. doi: 10.1152/ajpheart.1999.276.1.H215. [DOI] [PubMed] [Google Scholar]
- 37.de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, Belfort MB. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59:226–234. doi: 10.1161/HYPERTENSIONAHA.111.181784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bigger JT, Fleiss JL, Rolnitzky LM, Steinman RC. The ability of several short-term measures of RR variability to predict mortality after myocardial infarction. Circulation. 1993;88:927–934. doi: 10.1161/01.cir.88.3.927. [DOI] [PubMed] [Google Scholar]
- 39.Grassi G, Spaziani D, Seravalle G, et al. Effects of amlodipine on sympathetic nerve traffic and baroreflex control of circulation in heart failure. Hypertension. 1999;33:671–675. doi: 10.1161/01.hyp.33.2.671. [DOI] [PubMed] [Google Scholar]
- 40.Nugent ST, F J. Spectral analysis of heart rate variability in children and young adults during sleep and awake states. Proc. 14th International Conf. IEEE/EMBS; 1992. pp. 2640–2641. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.