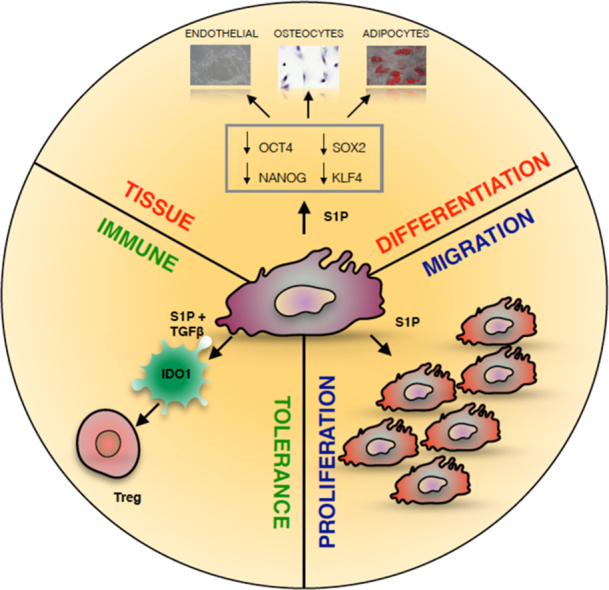
Abstract
Stem cells have high potential for cell therapy in regenerative medicine. We previously isolated stem cell types from human amniotic fluid, derived from prenatal amniocentesis. One type, characterized by a fast doubling time, was designated as fast human amniotic stem cells (fHASCs). These cells exhibited high differentiation potential and immunoregulatory properties. Sphingosine-1-phosphate (S1P) is a bioactive sphingolipid metabolite that influences stem-cell pluripotency, differentiation, mobility, and regulates immune functions. In this study, we investigated the influence of S1P on fHASC migration, proliferation, differentiation and immune regulatory functions. We found that fHASC stimulation with S1P potentiated their migratory and proliferative activity in vitro. Notably, short fHASC exposure to S1P enhanced their differentiation towards multiple lineages, including adipocytes, osteocytes and endothelial cells, an effect that was associated with downregulation of the main transcription factors involved in the maintenance of a stem-cell undifferentiated state. A specific crosstalk between S1P and tumor growth factor β1 (TGF-β1) has recently been demonstrated. We found that fHASC exposure to S1P in combination with TGF-β1 promoted the expression of the immune regulatory pathway of indoleamine 2,3-dioxygenase 1 (IDO1). In addition, human peripheral blood mononuclear cells, co-cultured with fHASCs treated with S1P and TGF-β1, expanded regulatory T-cells, via a mechanism requiring IDO1. Overall, this study demonstrates that S1P potentiates several properties in fHASCs, an effect that may be critical for exploiting the therapeutic potential of fHASCs and might explain the specific effects of S1P on stem cells during pregnancy.
Keywords: Sphingosine-1-phosphate, Amniotic Fluid Stem Cells, TGF-β1, Indoleamine 2, 3 dioxygenase
Graphical abstract
1. Introduction
Amniotic fluid cells derive from both fetal and extra-embryonic tissues. The majority of these cells are terminally differentiated and have limited proliferative capabilities (Cananzi et al., 2009). There appear to be both hematopoietic and non-hematopoietic stem-cell precursors in amniotic fluid (De Coppi et al., 2007, Prusa et al., 2003). We have isolated and characterized stem-cell types from second-trimester human amniotic-fluid samples, that were collectively indicated as human amniotic-fluid stem cells (HASCs) (Romani et al., 2015). Of those populations, one was characterized by a fast doubling time, and cells were thus designated as fHASCs (Romani et al., 2015). fHASCs express the main stromal markers, but also additional transcription factors (e.g., octamer-binding transcription factor 4, OCT4; Kruppel-like factor 4, KLF4; sex determining region Y-box 2, SOX2 and Nanog homeobox, NANOG) indicative of an undifferentiated state and pluripotency, as typically found in all embryonic stem cells (ESCs) (Jaenisch and Young, 2008). We found that fHASCs maintain their original phenotype under prolonged in vitro passaging, and that they were able to originate embryoid bodies. Moreover, fHASCs exhibited immunoregulatory properties when treated with interferon (IFN)-γ, and those depended on the induction of the immune regulatory pathway of indoleamine 2,3-dioxygenase 1 (IDO1) (Romani et al., 2015).
Sphingosine 1-phosphate (S1P), a potent bioactive sphingolipid metabolite, regulates diverse cellular processes (Inniss and Moore, 2006, Kim et al., 2003, Maceyka et al., 2012, Pebay et al., 2005, Spiegel and Milstien, 2002). S1P functions are mediated by five specific G protein-coupled S1P receptors (S1PR1–5), which initiate distinct downstream signalling pathways (Maceyka et al., 2012). The production of S1P is effected via two sphingosine-kinase isoforms (SK1 and SK2), and it occurs mostly at the membrane level – where the substrate resides – once translocation of the enzymes is triggered by cell-activating events (Taha et al., 2006).
Although S1P can be considered as a pleiotropic lipid mediator, involved in several biological functions – including regulation of cell proliferation, migration, cytoskeletal rearrangement, cell differentiation, and adhesion (Inniss and Moore, 2006, Pebay et al., 2005, Spiegel and Milstien, 2002) – recent evidence suggests that S1P is also an important mediator of both innate and adaptive immune responses (Rivera et al., 2008). In particular, local S1P elevation has been linked to the promotion of lymphocyte, dendritic-cell and macrophage differentiation (Rivera et al., 2008). Additional studies have demonstrated that S1P can activate the tumor growth factor β1 (TGF-β1) pathway (Lebman and Spiegel, 2008). TGFβ1 is produced by many cell types, and it induces a wide range of biological effects, which depend on the specific cell type as well as their state of differentiation (Massague and Gomis, 2006).
In pregnancy, S1P and the glycerophospholipid signalling molecule lysophosphatidic acid, are critical in the functional maturation of uterine endometrium in the decidual reaction, to support embryo implantation (Nagamatsu et al., 2014). The S1P pathway has also been implicated in placental immune function. Specifically, one study found that S1P inhibited the differentiation of human cytotrophoblasts into syncytiotrophoblasts, by a mechanism associated with intracellular cyclic adenosine monophosphate (cAMP) reduction (Johnstone et al., 2005). In addition, it has been reported that, before labor, human amniotic fluid contains significant amounts of S1P (Kim et al., 2003).
Although S1P is a potent bioactive lipid molecule, implicated in the regulation of pregnancy-related events, any direct effects of S1P on amniotic stem cells have not been investigated yet. In the present study, we investigated the influence of S1P on fHASC proliferation, survival, migration, differentiation and immune function. Specifically the effect of S1P in inducing immunregulatory pathways like that of IDO1 has never been investigated.
We found that S1P promoted fHASC proliferation, differentiation and immune activity. Understanding the role of S1P in fHASC functions may ultimately contribute to the identification of novel actions of S1P or synthetic analogues thereof (Xin et al., 2006) on stem cells, that could be instrumental in implementing fHASC therapeutic exploitation.
2. Materials and methods
2.1. fHASC isolation and culture
fHASCs were obtained from amniotic fluids of 15–17-weeks pregnant women (aged 35–40 years), who underwent amniocentesis during routine prenatal diagnosis. fHASC lines had a normal karyotype. The study was approved by the University of Perugia Bioethic Committee, and each participant provided informed consent for the secondary use of amniotic-fluid samples. Stem cells were isolated as described (Romani et al., 2015). Briefly, residual cells from prenatal diagnosis tests were cultured in mesenchymal stem-cell growth medium (MSCGM, LONZA Gaithersburg, MD, USA); the isolation consisted of selecting cultures containing cells with peculiar morphology and colony shape (Romani et al., 2015). These colonies were selected and cultured for several passages in vitro.
2.2. Flow cytometry
Phenotypical characterization of fHASCs was performed as follows. For fHASC surface antigen analysis, cells were harvested by trypsinization, washed with phosphate-buffered saline supplemented with FBS (3%) and stained with antibodies reacting to the following antigens: HLA A,B,C (Beckton Dickinson); class II DR, CD10, CD11b, CD14, CD29, CD34, CD38, CD44, CD49d, CD73, CD105 (Immunotools); CD90, CD117 (c-kit), SSEA3, SSEA4, (Biolegend). To detect the intracellular markers SSEA3, SSEA4 and CD117, cells were permeabilized with 0.2% (vol/vol) Triton-X (Sigma-Aldrich) for 30 min at room temperature before staining. Cells were acquired on an LSR Fortessa (BD Biosciences) flow cytometer, and analysed with FloJo software.
2.3. Cell proliferation and viability assay
fHASCs were cultured in selected stem-cell media, supplemented with delipidated serum, with or without different concentrations of externally added S1P. Cell proliferation assays were conducted using Click-iT™ EdU flow cytometry kits (Invitrogen), according to manufacturer's instructions. Briefly, fHASCs were collected by trypsinization and incubated with 10 μM EdU solution for 12 h. Cells were then fixed and permeabilized, and incubated with the Click-iT™ reaction mixture for 30 min at room temperature and analysed by flow cytometer. Cell viability was evaluated via reduction of 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyl-tetrazolium bromide (MTT), as described (Kanagasabai et al., 2010). Cell survival was calculated relative to that of control fHASCs, cultured in the absence of S1P, that was taken as the 100% value.
2.4. Reverse transcription and Quantitative Real-Time polymerase (RT-q-PCR) analysis
Total RNA was extracted from fHASCs by Trizol (Invitrogen) and used as a template for reverse transcription to cDNA. The cDNA was obtained by the RevertAid First Strand cDNA Synthesis Kit (Fermentas). All mRNA measurements were performed by RT-q-PCR analyses using Brilliant SYBR Green QPCR Master Mix 2 × (Stratagene) with the Mx3000P qPCR System (Stratagene), except for S1PRs, which were analysed using ABI Prism 7700 (Applied Biosystems), as previously described (Donati and Bruni, 2006). The reaction was performed following the manufacturer's recommendations. Each sample was normalized to β-actin (Invitrogen) or to ribosomal 18 S RNA. Final results, represented as relative gene expressions, were calculated using MxPro software (Stratagene). Each experiment was repeated at least three times. The 2-ΔΔCT method was applied, as a comparative method of quantitation, and data were normalized to β-actin RNA expression. Primer sequences are reported in Table 1.
2.5. Cellular fractionation
fHASCs treated with control media or S1P at various concentrations were washed twice with cold PBS, scraped, and collected by centrifugation (1000 × g). Cells were dispersed in a buffer solution containing 10 mM HEPES, pH 7.4, 1 mM EGTA, 1 mM EDTA, 250 mM sucrose, 5 mM NaN3, and protease inhibitors (1 mM 4-(2-aminoethyl) benzenesulfonyl fluoride (AEBSF), 0.3 μM aprotinin, 10 μg/ml leupeptin and 10 μg/ml pepstatin) and disrupted in a Dounce homogenizer (100 strokes). Lysates were centrifuged (10 min, 800 × g) and the resulting supernatants were centrifuged again at 200,000 × g for 1 h to separate cytosolic and total membrane fractions.
2.6. S1PRs and IDO1 Short interfering RNA (siRNA)
These procedures have been described previously (Matino et al., 2015). Briefly, the siRNA sequences specific for human IDO1 (sense, 5′-CCACGAUCAUGUGAACCCAtt-3′; antisense, 5′-UGGGUUCACAUGAUCGUGGat-3′) were selected, synthesized, and annealed by the manufacturer (Ambion). Additional sets of siRNA sequences specific for human IDO1 (sense, 5′-UCACCAAAUCCACGAUCAUUU-3′; antisense, 5′-PUAUGCGAAGAACACUGAAAUU-3′; and, sense, 5′-UUUCAGUGUUCUU- CGCAUAUU-3′; antisense, 5′-PUAUGCGAAGAACACUGAAAUU-3′) were from Dharmacon. siCONTROL Nontargeting Pool (D-001810–10-05, Dharmacon) was used as a control. siRNA sequences specific for:
S1PR1, 5′GAGUUAGUUCCUGUGAACA(dT)3′– 5′UGUUCACAGGAACUAACUC(dT)3′– 5′GACCAUUAUGUCUUCUGGA(dT)3′; UCCAGAAGACAUAAUGGUC(dT)3′;
S1PR2, 5′CAUCGUGCUAGGCGUCUUU(dT)3′– 5′AAAGACGCCUAGCACGAUG(dT)3′;
S1PR3, 5′CAGAAUUUGUGUUGCAGGU(dT)3′– 5′ACCUGCAACACAAAUUCUG(dT)3′– 5′CUUGUAUAUUCGUACAUCU(dT)3′– 5′AGAUGUACGAAUAUACAAG(dT)3′;
S1RP4, 5′GUCCUCAACUCGGCGGUCA(dT)3′– 5′UGACCGCCGGAGUUGAGGAC(dT)3′– 5′GCCUUUGACCGCUGCUCCA(dT)3′– 5′UGGAGCAGCGGUCAAAGGC(dT)3′;
and S1PR5, 5′GUCUUGCCGCUCUACGCCA(dT)3′ – 5′UGGCGUAGAGCGGCAAGAC3′(dT)3′
as well as MISSION® siRNA Universal Negative Control were from Sigma-Aldrich. For transfection, siRNAs (5 μg) in 30 μl of transfection buffer (20 mM HEPES, 150 mM NaCl, pH 7.4) were pipetted into a sterile Eppendorf tube. In a separate polystyrene tube, 6.7 μg of 1,2 dioleoyl-3-trimethylammonium-propane (DOTAP) was mixed with 30 μl of transfection buffer, and then both solutions were mixed gently by pipetting several times. After incubation at room temperature for 20 min, the mixture was added to 1 ml of complete medium, containing 106 cells and incubated for 24 h at 37 °C, either alone or in the presence of S1P. Cells were recovered, washed, and immediately used for in vitro experiments. siRNA treatment resulted in the complete disappearance of the relevant transcripts for S1PRs at 24–48 h (Supplemental Fig. 1) or IDO1 (Supplemental Fig. 4) Control treatments consisted of cells treated with negative control siRNA (Ambion or Sigma) or MISSION® siRNA Universal Negative Control. Efficiency of siRNA transfection was evaluated in terms of percentage of cells stained with Cyanine 3 (Cy3)-labeled siRNA negative control (Ambion). Transfection rates always exceeded 90%.
2.7. Differentiation of fHASCs in vitro
Cells were induced to differentiate in vitro under the conditions described below and RNA was then purified for RT-q-PCR analysis, to confirm lineage-specific gene expression.
2.7.1. Osteogenic cell differentiation
fHASCs, harvested at passage five, were cultured in Differentation Media BulletKits-Osteogenic (Lonza), according to manufacturer's instructions. Changes in the expression of genes, markers of osteogenic differentiation, namely, secreted Phospho-Protein 1 (SPP1), Bone Gamma-carboxyglutamate (Gla) Protein (BGLAP), and Runt-related transcription factor 2 (RUNX2) were assessed by RT-q-PCR using specific primers (Table 1). The differentiation potential for osteogenesis was assessed by mineralization of calcium accumulation on von Kossa staining (Abcam). Percent cell staining was determined by measuring von Kossa-positive stained areas relative to a total selected area, in five different images, using ImageJ software (NIH, Bethesda, MD, USA).
2.7.2. Adipogenic cell differentiation
HASCs, harvested as indicated above, were cultured in Differentiation Media Bullet Kits-Adipogenic (Lonza). Changes in the expression of specific genes, markers of adipogenic differentiation, such as Peroxisome Proliferator-Activated Receptor Gamma (PPARG), Lipo-Protein Lipase (LPL), and Fatty Acid Binding Protein 4 (FABP4) were determined by RT-q-PCR using specific primers (Table 1). The potential for adipogenic differentiation was assessed by Sudan III staining (Sigma-Aldrich), according to the manufacturer's instructions. Percentage of positive cell staining was recorded by measuring Sudan III-positive stained areas relative to a total selected area in five different images, using ImageJ software.
2.7.3. Endothelial cell differentiation
Cells were seeded at a density of 3000 cells/cm2 on plastic plates pre-coated with gelatin and maintained in culture for one month in endothelial cell medium-2 (EG-MTM-2, Clonetics; Cambrex Bioproducts) supplemented with 10% FBS, and Pen/Strep. Recombinant human BFGF (StemCell Technologies) was added every other day at the concentration of 2 ng/ml. Changes in the expression of specific genes, markers of endothelial differentiation such as Kinase Insert Domain Receptor (KDR) and Platelet and Endothelial Cell Adhesion Molecule 1 (PECAM1) were analysed by RT-q-PCR using specific primers (Table 1). For KDR and PECAM1 protein analysis, cells were harvested by trypsinization, washed with phosphate-buffered saline supplemented with FBS (3%) and stained with anti CD31 (PECAM1, Beckman Coulter) and CD309 (KDR, Beckman Coulter) antibody. Cells were acquired on an LSR Fortessa (BD Biosciences) flow cytometer, and analysed with FloJo software.
2.8. Cell migration
Cells were grown to reach 60–70% confluence, and then starved overnight in DMEM containing 0.1% BSA. The ChemoTx System was used for chemotaxis assay (NeuroProbe, Gaithersburg, MD). Briefly, cells were washed twice with PBS, and then incubated for 30 min at 37 °C in the presence of 1 μM calcein-AM. Stained cells were washed three times with PBS, and detached with trypsin. Approximately 8000 cells were placed directly onto chemotaxis filters. The bottom chambers were filled with DMEM containing different concentrations of S1P (0.01, 0.1, or 1 µM). Cells were incubated for 6 h at 37 °C. The non-migratory cells on the top of the filter were removed by gently wiping the filter with a cotton swab. Fluorescence of migrated cells into the bottom chamber was measured in a multi-well fluorescent plate reader (Fluoroskan Ascent FL, ThermoElectron Corporation, Excitation, 485 nm; Emission, 538 nm). Fluorescence was converted to numbers of cells based on a standard curve, generated by seeding known numbers of fHASCs at the bottom of the chamber.
2.9. Western blot analysis, kynurenine assay and TGF-β determination
Western blot analysis was performed according to standard procedures. A specific anti-SK1 polyclonal antibody (directed against the 16 carboxy-terminal amino acids of SK1) (kindly provided by Dr. Y. Banno, Gifu University School of Medicine, Japan) was used to detect endogenous SK1 in total cellular extracts. Rabbit polyclonal antibody generated against SK2 was a kind gift of Dr. S. Nakamura (Department of Molecular and Cellular Biology, Kobe University Graduate School of Medicine, Kobe, Japan). Equally loaded protein was checked by expression of the nonmuscle-specific β isoform of actin (β-actin monoclonal antibodies, Cytoskeleton Inc.).
Human IDO1 expression was detected by sequential immunoblotting with anti-IDO1 (Millipore) and anti–β-tubulin (Sigma-Aldrich) for normalization.
IDO1 functional activity was measured in vitro in terms of the ability to metabolize tryptophan to L-kynurenine, whose concentrations were measured by high-performance liquid chromatography (Romani et al., 2015; Pallotta et al., 2011). Briefly, fHASCs treated (24 h) or not with TGF-β1 (10 ng/ml), with S1P (1 µM) or a combination thereof were re-suspended in media containing 100 µM tryptophan (Sigma-Aldrich) and then incubated for 4 h at 37 °C. After incubation, the supernatant was collected and stored at − 80 °C for quantitation of kynurenine by HPLC. IDO1 activity was expressed as kynurenine concentration (µmol/l) in each sample (Pallotta et al., 2011). Human TGF-β1 was measured in culture supernatants by ELISA, using specific kits (R&D Systems and Abnova Corporation). The detection limit of the assay was 15 pg/ml.
2.10. fHASC-PBMC co-cultures and Treg analysis
Peripheral blood mononuclear cell (PBMC)-fHASC co-cultures were performed according to Romani et al. (2015) (Romani et al., 2015). Briefly, PBMCs (3 × 105/well) were activated with 5 µg/ml anti-CD3 mAb (clone OKT3) and co-cultured for 5 days with fHASCs, that had been pre-treated or not with 10 ng/ml TGF-β1, 1 µg/ml S1P or their combination for 24 h. Treg cells were evaluated by FACS. Briefly, cells were treated with rat anti-CD16/32 (2.4G2) for 30 min at 4 °C, for blocking Fc receptors, before assaying on LSRFortessa (BD BioSciences) flow cytometer, and then analysed by flowJo data analysis software. The following fluorochrome-conjugated mAbs were used, CD4 (RPA-T4), CD25 (BC 96) and FOXP3 (150D) (Biolegend).
2.11. Statistical analysis
All in vitro determinations are means ± S.D. from at least three independent experiments, unless otherwise indicated. For comparisons between two groups, unpaired, two tailed Student's t-test was used. For comparison of more than two groups, one-way ANOVA followed by Bonferroni for multiple comparison test was performed. All n values were computed by power analysis to yield a power of at least 80% with an α-level of 0.05. GraphPad Prism version 6.0 (San Diego, CA) was used for all analyses.
3. Results
3.1. S1P promotes fHASC migration
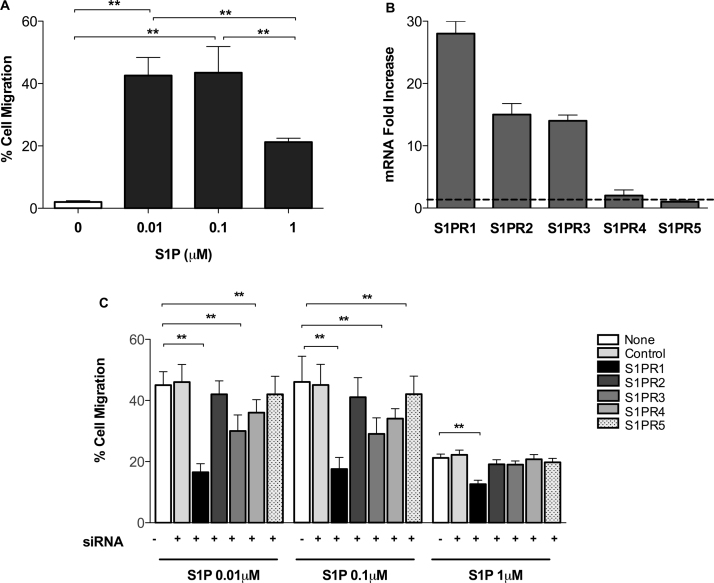
S1P is known to influence the migration of several cell types, including immune cells, bone-marrow stem cells, hematopoietic cells, and satellite cells (Calise et al., 2012, Golan et al., 2013, Kong et al., 2014, Li et al., 2009, Maceyka et al., 2012). We investigated the ability of fHASCs to migrate toward three different S1P concentrations (Fig. 1A). In particular, we found that fHASCs preferentially migrate toward the low concentrations (0.01 and 0.1 µM) of S1P (Fig. 1A), while higher S1P levels (1 µM) stimulated moderate fHASC migration (Fig. 1A).
Fig. 1.
S1P induces fHASC migration mostly via S1PR1, and moderately through SP1R3 and S1PR4. A) fHASC migration in response to three different S1P concentrations. Data are mean ± S.D. from 3 independent experiments. **P < 0.01, (ANOVA and Bonferroni multiple comparison test). B) S1PRs mRNA expression levels in fHASCs. Data (mean ± S.D.) are presented as normalized transcripts expression in the samples, relative to transcript expression of S1PR5 (in which fold change = 1; dotted line); one experiment representative of two. C) Effect of S1PR1–5 silencing on S1P-induced fHASC migration. Data are means ± S.D. from three independent experiments. ** P < 0.01, ANOVA followed by Bonferroni multiple comparison test.
S1P-associated chemotactic activity is mediated by activation of specific S1PRs (Calise et al., 2012). S1PR1, S1PR2 and S1PR3 are mostly involved in S1P chemotactic activity in specific cell types, such as T cells and B cells (Proia and Hla, 2015). High-level expression of S1PRs has been detected in a variety of cell types (Brinkmann, 2007). Accordingly, we found that fHASCs expressed all five S1PRs mRNAs (Fig. 1B). In particular, S1PR1 was the most expressed receptor in fHASCs, whereas an intermediate expression was found for S1PR2 and S1PR3, and only low levels were detected for S1PR4 and S1PR5 (Fig. 1B).
To test the functional roles of individual S1PRs on fHASC migration, we used the siRNA technique (Fig. 1C and Supplemental Fig. 1). Transfection of fHASCs with a S1PR1-specific siRNA, but not with a control siRNA, significantly reduced fHASC migration in response to S1P gradients (Fig. 1C). Moreover, transduction of fHASCs with S1PR3- or S1PR4-specific siRNAs moderately reduced fHASC migration, but only toward low S1P (0.01 and 0.1 µM) concentrations (Fig. 1C). In contrast, inhibition of S1PR2 and S1PR5 had no impact on fHASC migration. (Fig. 1C). Overall, these results suggest that low S1P gradients may favor fHASC recirculation mainly through S1PR1 activation.
3.2. S1P induces fHASC proliferation and favors stem-cell lineage differentiation
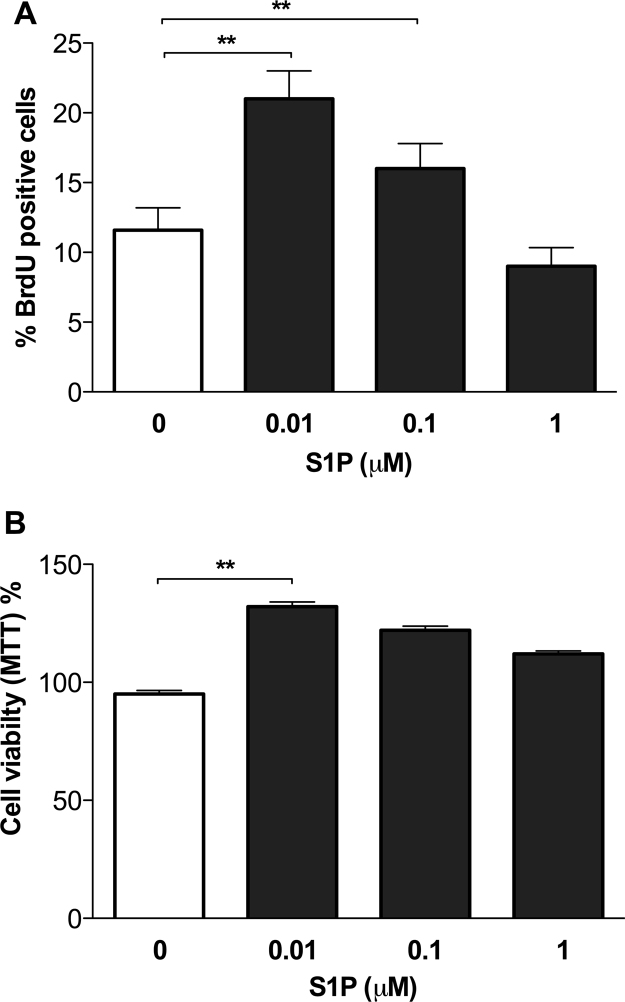
S1P has potent actions on embryonic-cell differentiation and proliferation (Price et al., 2009). We assessed the ability of S1P to promote fHASC proliferation. To this purpose, fHASCs were cultured for 24 h in selected stem-cell media, supplemented with delipidated serum, either as such – to eliminate endogenous S1P – or containing three individual, externally added S1P concentrations (0.01, 0.1, or 1 μM). Similarly to what showed for migration, we found a negative correlation between fHASC proliferation or viability and S1P concentration in media (Fig. 2). In particular, the lowest S1P concentration (0.01 μM) yielded the highest fHASC proliferation and viability (Fig. 2 A-B). Next, we evaluated S1P effect on the expression of several fHASC surface markers. To this purpose, fHASCs were cultured for 24 h in media supplemented with delipidated serum alone or containing different S1P concentrations. We found that S1P did not alter the expression of major stem cell markers such as HLA-ABC (Human Leucocyte Antigens ABC), CD10 (Common Acute Lymphoblastic Leukemia Antigen), CD29 (integrin β1-chain), CD44 (hyaluronan receptor), CD73 (ecto-50-nucleotidase), CD90 (Thy-1 membrane glycoprotein), and CD105 (endoglin) (Supplemental Fig. 2). Moreover, S1P did not induce any change in the expression of HLA DR (Antigen D-Related) or other haematopoietic markers (i.e., CD11b, CD14, CD34, CD38) (Supplemental Fig. 2). These results suggest that S1P affects fHASC migration and viability without altering major stem-cell surface markers.
Fig. 2.
S1P promotes fHASC proliferation and viability. A) Evaluation of fHASC proliferation in response to different S1P concentrations. fHASCs were cultured in selected stem cell media, supplemented with delipidated serum, with or without different concentrations of S1P for 24 h, then harvested and incubated with 10 μM EdU solution for 12 h. Percentage of BrDu positive cells was evaluated by flow cytometry. Data are means ± S.D. of three independent experiments. **P < 0.01, ANOVA and Bonferroni multiple comparison test. B) Evaluation of cell growth using the MTT Assay. fHASCs were cultured as in a, the percent survival values for each sample were calculated relative to the vehicle-treated controls (taken as the 100% value). Data are means ± S.D. from three independent experiments, each performed in triplicate. * * P < 0.01, ANOVA followed by Bonferroni multiple comparison test.
3.3. S1P facilitates fHASC differentiation towards distinct major cell lineages
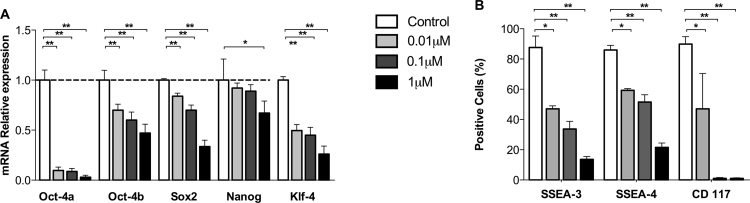
We previously reported that fHASCs have an ability to differentiate towards the adipogenic and osteogenic lineages, when cultured under specific conditions (Romani et al., 2015). Because S1P has been shown to condition stem-cell pluripotency (Smith et al., 2013), we evaluated the impact of S1P on fHASC lineage differentiation. Notably, we found that 24 h pre-treatment of fHASCs with S1P significantly reduced mRNA expression of the major transcription factors associated with stem-cell pluripotency (Fig. 3A-B). Considering the role of S1P in stem-cell differentiation and tissue plasticity (Arya et al., 2014, Price et al., 2015), we tested the effect of S1P on fHASC commitment and differentiation towards three different cell lineages, namely, adipogenic, osteogenic and endothelial.
Fig. 3.
S1P decreases the expression of specific pluripotent transcription factors in fHASCs. fHASCs were cultured in selected stem-cell media, supplemented with delipidated serum in the presence or in the absence of three different S1P concentrations for 24 h. A) Analysis of expression of pluripotent markers by real-time PCR in fHASCs. Data (mean ± S.D of three experiments) are presented as normalized transcript expression in the samples relative to normalized transcript expression in control cultures (i.e., in basal medium), in which fold change = 1 (dotted line). *P < 0.1, **P < 0.01, ANOVA followed by Bonferroni's correction. B) Analysis of percent expression of selected pluripotent markers by FACS in fHASCs. Data are means ± S.D. of three independent experiments. * P < 0.1, **P < 0.01, ANOVA and Bonferroni multiple comparison test.
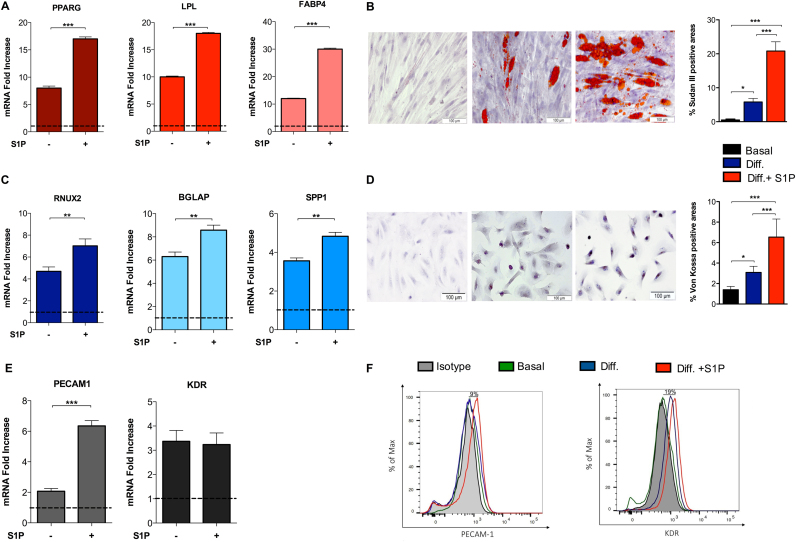
For this purpose, we analysed the expression of markers specific for adipogenic (e.g., PPRAG, LPL, FABP4), osteogenic (e.g., RUNX2, SPP1, BGLAP), and endothelial (e.g., PECAM1, KDR) lineages, after culturing fHASCs in selected cell-differentiation media or in basal stem-cell media (Fig. 4A-F). Under these conditions, we found that 24-h fHASC pre-treatment with S1P (1 µM) – the concentration promoting the highest downregulation of the major pluripotency transcription factors – but not with vehicle resulted in a significant increase in adipocyte (Fig. 4A) and osteocyte (Fig. 4C) specific mRNA transcripts. We confirmed these results by specific immunostaining. Specifically, fHASC pre-treatment with S1P led to increased Sudan III cytoplasmic staining (adipogenic differentiation) (Fig. 4B), and to more intense von Kossa staining (osteogenic differentiation) (Fig. 4D), relative to control cultures. Notably, we found that fHASC pretreatment with S1P, prior to their culture in differentiation media, was an important requirement for improving fHASC lineage differentiation. This effect was not observed when S1P was added to the stem-cell differentiation media (Supp. Fig. 3A-C). Similarly, we demonstrated that fHASC pretreatment with S1P significantly increased the expression of the endothelial marker PECAM1, both at the mRNA and protein levels (Fig. 4E-F). Altogether, these results suggested that S1P downregulates the main pluripotency markers and facilitates fHASC differentiation toward specific lineages.
Fig. 4.
S1P increases fHASC differentiation towards specific cell lineages. fHASCs were pre-treatment with 1 μM S1P for 24 h in basal stem-cell culture medium then cultured in specific differentiation media for adipogenic, osteogenic or endothelial cell lineage differentiation. A) mRNA levels of specific adipogenic markers. Data (means ± S.D) are presented as normalized transcript expressions in the samples relative to normalized transcript expression in control medium in one experiment representative of two. ***P < 0.001 by ANOVA followed by Bonferroni multiple comparison test. B) representative pictures of fHASC differentiation into adipocytes monitored with Sudan III staining for lipid droplets and nuclear staining with Mayer's hematoxylin, scale bar = 100 μm. Sudan III positive stained areas (means ± S.D.) were determined. C) mRNA levels of specific osteogenic markers. Data (means ± S.D.) are showed as normalized transcript expressions in the samples relative to normalized transcript expression in samples cultured in control medium. One experiment representative of two. **P < 0.01 by ANOVA followed by Bonferroni multiple comparison test. D) Representative pictures of fHASC differentiation in osteocytes monitored with Von Kossa staining of calcium deposition in the extracellular matrix in terminal osteoblast differentiation and nuclear staining with Mayer's hematoxylin, scale bar = 100 μm. Von Kossa positive stained areas (means ± S.D) were determined. E) mRNA levels of specific endothelial markers. Data (means ± S.D) presented as normalized transcript expressions in the samples relative to normalized those in samples cultured in control medium. One experiment representative of two. ***P < 0.001 by Student's t-test. F) Expression of endothelial cell differentiation markers by FACS, in one representative experiment of three.
3.4. S1P increases TGF-β1 release while TGF-β1 promotes S1P kinase re-localization in fHASCs
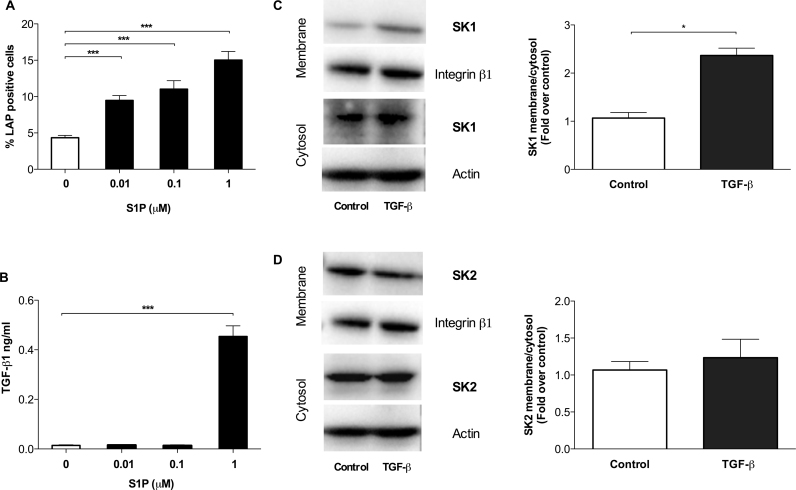
It has previously been reported that S1P treatment of fibroblasts activates the TGF-β1 signalling pathway (Watterson et al., 2007). To ascertain whether S1P would stimulate TGF-β1 release from fHASCs, cells were cultured for 24 h in stem-cell media, supplemented with delipidated serum, either as such or containing three different concentrations of S1P (e.g., 0.01, 0.1, or 1 μM). Then, the expression of latency associated protein (LAP) – the TGF-β1's precursor on cell surface – and secreted TGF-β1 in supernatants were measured. We found that S1P significantly increased LAP expression in fHASCs, in a dose-dependent manner, relative to control cells (Fig. 5A). In contrast, a significant increase in active TGF-β1 could be detected only in supernatants from fHASCs treated with the highest S1P concentration (1 μM) (Fig. 5B), which suggested that only specific S1P levels will promote the expression of both precursor and secreted forms of TGF-β1 in fHASCs.
Fig. 5.
Positive regulation of TGF-β1 by S1P and SK1 subcellular localization by TGF-β1 in fHASCs. Quantification of latency-associated peptide (LAP) on cell surface of fHASCs by FACS (A) and of secreted TGF-β1 by ELISA (B) in fHASCs cultured for 24 h in stem cell medium, supplemented with delipidated serum, either as such or containing different concentrations of S1P. Data are means ± S.D. of three independent experiments. **P < 0.01, ***P < 0.001; ANOVA and Dunnett test (treatments vs. medium control). Effect of TGF-β1 on SK1 and SK2 subcellular localization. Serum fHASCs were incubated with 10 ng/ml TGF-β1 for 10 min. Western analysis of SK1 (C) and SK2 (d) was performed in membrane and cytosolic fractions. Representative Western blots and relative histograms depicting densitometric analysis of immunoblots of SK1 (C) or SK2 (D) bands in three independent experiments. Data reported as fold increase over untreated control of the membrane/cytosol ratio for SK1 and for SK2. * P < 0.05, Student's t-test.
Cell-dependent production of S1P is effected by two sphingosine-kinase isoforms (i.e., SK1 and SK2), and it occurs mostly at the membrane level, once translocation of the enzymes is triggered by specific cell-activating events (11). It has previously been reported that myoblast stimulation with TGF-β1 results in specific upregulation of SK1 enzymatic activity (Cencetti et al., 2010). Therefore, we investigated whether TGF-β1 conditioning could activate the same pathway in fHASCs as well. We found that TGF-β1 treatment caused increased SK1 accumulation on fHASC plasma membrane (Fig. 5C), suggesting a potential for TGF-β1–enriched medium to increase S1P production by fHASCs. Notably, under the same conditions, SK2 expression – the other kinase involved in sphingosine phosphorylation (Hait et al., 2006) – was not affected (Fig. 5D). Overall, these results demonstrated the occurrence of feedback regulation and of a positive loop between S1P and TGF-β1 in fHASCs, which could be instrumental in specific fHASC-dependent functions.
3.5. Cooperativity between S1P and TGF-β1 activates IDO1-dependent immune regulatory effects in fHASCs
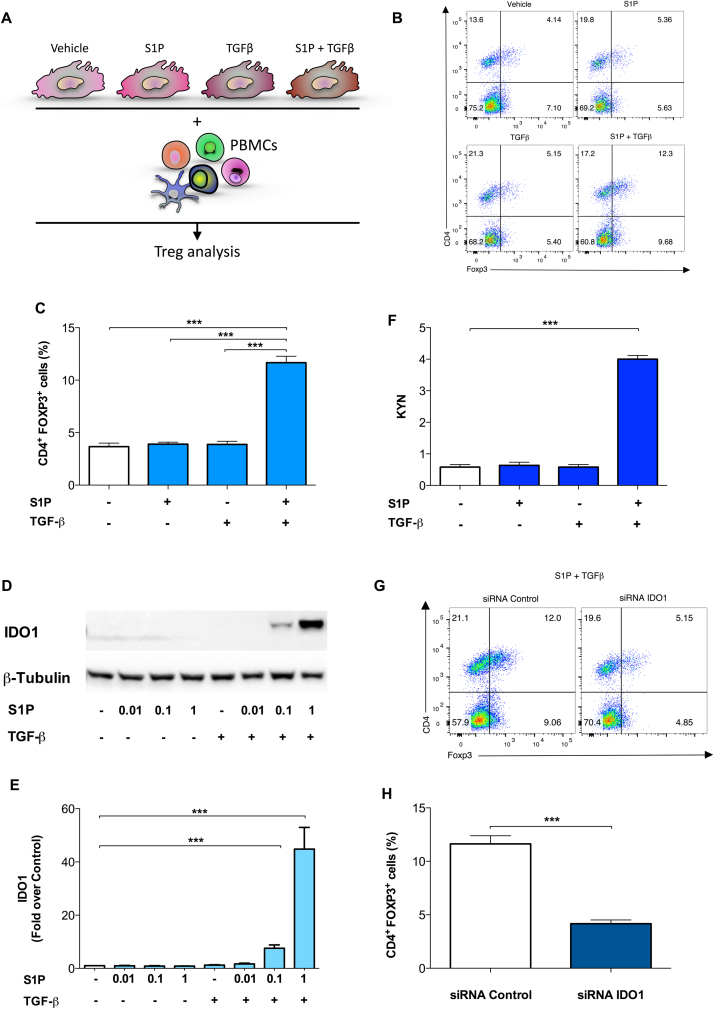
S1P is now emerging as an important mediator of both innate and adaptive immunity (Spiegel and Milstien, 2011). Moreover, both S1P and TGF-β1 have been shown to activate immune-regulatory functions in several immune cells (Aoki et al., 2016, Fujio et al., 2016). Based on the existence of a specific crosstalk between S1P and TGF-β1 in fHASCs (Fig. 5), we assessed its potential to promote immune regulatory functions in fHASCs. Accordingly, fHASCs, pre-conditioned with S1P or TGF-β1, each alone or in combination, were assessed for their ability to induce human Treg cells in PBMC–fHASC co-cultures, using vehicle-treated fHASCs as a control (Fig. 6A). Unlike IFN-γ (Romani et al., 2015), stimulation of fHASCs with S1P or TGF-β1 alone did not result in a significant increase in CD4+ FOXP3+ (Treg) cells in vitro (Fig. 6B-C). However, PBMCs co-cultured with fHASCs pre-conditioned with a combination of S1P and TGF-β1 significantly increased Treg levels, implying the occurrence of a synergistic S1P/ TGF-β1-dependent immune regulatory action (Fig. 6B-C). We have previously reported that IFN-γ–stimulated fHASCs increase Treg cells through the induction of the immune regulatory enzyme IDO1 (Romani et al., 2015). Therefore, we analysed IDO1 expression after treatment of fHASCs with S1P, TGF-β1 or their combination. Similarly, to what found for Treg induction (Fig. 6b-c), induction of IDO1 protein (Fig. 6D-E) and activity (Fig. 6F) could be detected only when fHASCs were treated with a combination of selected concentrations of S1P and TGF-β1. In contrast, treatment with either molecule alone resulted in no detectable effects (Fig. 6D-F). Next, to test for any IDO1 requirement for Treg induction, we transfected fHASCs with either IDO1-specific or control siRNA targeting, before combined treatment of fHASCs with S1P and TGF-β1 (S1P/TGF-β1). Notably, as previously reported for IFN-γ (Romani et al., 2015), we found that transfection of fHASCs with the IDO1-specific siRNA impaired Treg generation via S1P/TGF-β1 pre-conditioned fHASCs (Fig. 6G-H). These results suggested that, as in a different setting (Romani et al., 2015), IDO1 represents a crucial checkpoint pathway required for Treg generation by fHASCs. Moreover, these data may be the first to describe a synergic action between S1P and TGF-β, resulting in functional expression of the immunoregulatory IDO1 pathway.
Fig. 6.
S1P promotes IDO1-dependent immune regulatory properties in fHASCs. fHASCs were preconditioned with S1P or TGF-β1, or their combination, using vehicle-stimulated fHASCs as a control. A) Scheme of treatment and analysis. B) Representative results of CD4+FOXP3+ cell frequency (top right quadrants) among total CD4+ T cells, cultured for 5 days with fHASCs treated as indicated. Representative results from one experiment of three. C) CD4+ FOXP3+ cell frequency in cultures established as in b; n = 5. Means ± S.D. (three independent experiments). ***P < 0.001; Dunnett test (treatments vs. vehicle control). D) IDO1 protein expression in fHASCs treated with different concentrations of S1P or TGF-β1, or their combination, as determined by immunoblotting. Representative results from one of three independent experiments. E) Densitometric analysis of IDO1 immunoblots in three independent experiments. Data are represented as means ± S.D. (n = 5); **P < 0.01; ANOVA followed by Bonferroni multiple comparison test. β-tubulin was used as protein loading control. F) IDO1 enzymatic activity by kynurenine quantification in fHASCs treated with different concentrations of S1P or TGF-β1, or their combination, as determined by HPLC. ***P < 0.001; Dunnett test (treatments vs. vehicle control). G) Representative results of CD4+ FOXP3+ cell frequency (top right quadrants) among PBMC cells, cultured for 5 days with fHASCs (preconditioned with S1P and TGF-β1) that had been transfected with an IDO1 siRNA (IDO1 siRNA) or a control siRNA (c siRNA). H) CD4+ FOXP3+ cell frequency in cultures established as in g; n = 5. Means ± S.D. (three independent experiments). Data are means ± S.D. of three independent experiments performed in triplicate. ***P < 0.001; Student's t-test (IDO1 siRNA vs. vehicle c siRNA).
4. Discussion
S1P is a pleiotropic lipid mediator whose effects may be particularly interesting in improving specific functions of stem cells. Specifically, S1P can increase proliferation, survival, and differentiation of several stem cell types (Calise et al., 2012, Donati et al., 2007, Lu et al., 2015), including human embryonic stem cells (Inniss and Moore, 2006, Pebay et al., 2005).
fHASCs are a recently-identified stem-cell subtype present in human amniotic fluid, characterized by a fast doubling time and the ability to differentiate into several cell lineages (Romani et al., 2015). Yet, the effects of S1P on fHASCs are still unknown. Here we provide evidence for several biological roles of S1P on fHASCs, suggesting that S1P may represent an interesting means of potentiating selected functions of this stem-cell type. Specifically, we found that S1P improves proliferation, survival and migration of fHASCs. Somewhat different from previous studies, we demonstrated that maximum increase in fHASC proliferative and migratory capacities did selectively occur with low concentrations of S1P (e.g., 10 nM); conversely, a hundred times higher level of S1P resulted poorly or not effective. These results could be due to selective recruitment of specific S1PRs, more abundantly expressed by fHASCs that therefore require lower S1P concentrations. Accordingly, although we found that fHASCs expressed transcripts of all five S1PRs, high S1PR mRNA levels were found only for S1PR1.
In lymphocytes, S1PR1 activation is required for S1P migratory stimulation (Matsuyuki et al., 2006). Agonism of S1PR1 by the selective small molecule SEW2871 boosts cell mobilization, while functional antagonism of S1PR1 with FTY720 sequesters hematopoietic cells in bone marrow (Golan et al., 2012). In fHASCs, we found that, S1PR1-specific siRNA abrogated the effect of S1P on migration, whereas silencing of the other S1PRs (2−5) had no effect. S1PR1, S1PR2, and S1PR3 are coupled to different and opposing signalling cascades. For example, S1PR1 is coupled to inhibitory G protein (Gi) and has been shown to activate extracellular signal regulated kinase (ERK), PI3K, and Rac, whereas S1PR2 is coupled to Gi/o, Gq, and G12/13 and signals in part through JNK and Rho (Watterson et al., 2005). Moreover, activation of S1PR2 is thought to directly oppose S1PR1-induced proliferation and migration through a Rho-dependent pathway and decreased Rac. Therefore, although not addressed in this study, it is possible that a specific S1PR1-mediated signalling cascade is required for S1P-mediated fHASC migration. Another potential explanation for the low S1P concentration required for inducing fHASC migration might be traced to the selective S1PR1 downregulation occurring with high S1P concentrations. Accordingly, lymphocyte egress from LNs is blocked upon inhibition or loss of S1P lyase, which increases total lymph node S1P by almost a hundred fold and down-modulates S1PR1 on T cells (Schwab et al., 2005).
Migration and differentiation of stem cells are crucial for tissue regeneration following injury. Previous studies have shown that S1P may have a decisive role in those events, being upregulated in damaged tissues in response to organ and tissue injuries (Bendall and Basnett, 2013, Golan et al., 2012, Ogle et al., 2016, Ratajczak et al., 2014). S1P has been implicated in events regulating pluripotency and differentiation in both adult and embryonic stem cells and in various species (Kobayashi et al., 2010, Pitson and Pebay, 2009). S1P, in combination with platelet-derived growth factor (PDGF), was shown to maintain hESCs undifferentiated, in Gi-, ERK-, and SphK-dependent mechanisms (Pebay et al., 2005). Conversely, it has been shown that S1P promotes the differentiation of human umbilical cord mesenchymal stem cells into cardiomyocytes under the designated culturing conditions (Zhao et al., 2011). Other studies have reported that SP1 can be an effective factor inducing endothelial cell proliferation (English et al., 2001). Similar to previous data in human ESCs (Avery et al., 2008), here we found that S1P would foster fHASC differentiation into different lineages, an effect likely associated with downregulation of the main transcription factors indicative of stem-cell pluripotency. Although we did not find changes in specific stem cell markers shortly after S1P pretreatment, we reported a downregulation of the main surface stem cell markers when the cells differentiated toward specific cell types. Therefore, our data suggest that, depending on the specific stem-cell type or other selected cell-extrinsic differentiation factors, S1P may be able to maintain both stem cell pluripotency and favor stem cell differentiation. Specifically fHASCs represent also a novel behavior in response to S1P only pretreatment of the cells with S1P facilitated cell differentiation but this effect was lost when S1P was added concomitant with the differentiation medium, this represent a novel action of S1P.
S1P is now emerging as an important mediator of multiple aspects of both innate and adaptive immunity (Spiegel and Milstien, 2011). It has been shown that some of the biological effects of S1P overlap with those of specific cytokines and growth factors, and this overlapping can be traced to a bidirectional loop, namely, an ability of S1P to trans-activate growth factor and cytokine signalling cascades and the capacity of growth factors and cytokines to trans-activate S1P signalling pathways (Lebman and Spiegel, 2008). More recent studies have demonstrated that S1P can trans-activate the TGF-β1 pathway (Sauer et al., 2004). Moreover, cross-talk between S1P and TGF-β1 signalling pathways has also been observed in renal mesangial cells (Cencetti et al., 2013, Donati et al., 2009, Xin et al., 2006, Xin et al., 2004). Similarly, here we found that treatment with S1P resulted in a significant increase of TGF-β1 in fHASCs. Although our study did not investigate the specific molecular pathway leading to such an increase, it is possible that selected S1PRs signalling events may intercept specific transcription factors leading to TGF-β1 production.
Both S1P and TGF-β1 are crucial mediators of regulatory functions in several immune cells (Aoki et al., 2016, Fujio et al., 2016). Here we detected the occurrence of a crosstalk between S1P and TGF-β1 in fHASCs, leading to specific induction of the immune regulatory enzyme IDO1, which was in turn instrumental for in-vitro generation of human Treg cells. It has been previously reported that TGF-β1 is critical for IDO1 induction in dendritic cells (Pallotta et al., 2011). Conversely, here we demonstrated that fHASCs exposed to TGF-β1 were instead unable to increase IDO1 expression. In contrast, when TGF-β1 was used in combination with S1P, high levels of IDO1 could be detected in fHASCs as well. It is possible that, differently from what found in DCs (Pallotta et al., 2011), fHASCs require the combined effects of TGF-β1 and S1P for efficient IDO1 expression. Such combined effects may be required for activation or reinforcement of selected downstream signalling events, known to affect IDO1 expression in other cells, including the non-canonical nuclear factor-κB (NF-κB) or the Signal Transducers and Activators of Transcription (STAT) pathways (Pallotta et al., 2011, Rosowski et al., 2014). Future studies are required to investigate whether such pathways are activated by the combined treatment of fHASCs with S1P and TGF-β1. In fact this may represent a novel means to activate IDO1 in specific cell types.
In conclusion, we demonstrate that S1P activates multiple effects in fHASCs leading to increased migratory activity, promotion of cell differentiation toward multiple lineages and immune-regulatory functions. The implications of such findings are of interest. On the one side, S1P is present in amniotic fluid and our data may suggest that it may potentiate specific stem cell functions during pregnancy. On the other side, this study reveals novel potential and druggable effects of S1P functioning. Currently, S1P analogues, including 2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol (FTY720) fingolimod and FTY720 phosphate, offer therapeutic potentials in autoimmune diseases, such as multiple sclerosis (Brinkmann et al., 2010), where FTY720 is mainly considered to inhibit trafficking of immune cells to the central nervous system. Our results suggest that the effects of S1P and potentially of its analogues may extend beyond the effects on specific immune cells and may involve potentiation of specific stem-cell functions that could be instrumental in tissue regeneration and other pathological conditions.
Acknowledgments
This study was supported by a Telethon Research Grant GGP17094 to F.F, a National Multiple Sclerosis Society Grant: PP-1603-08205 to F.F and the Fondazione Cassa di Risparmio Grant 2017.0189.021 to V.N.T.
Acknowledgments
Author information
Department of Experimental Medicine, University of Perugia, Polo Unico Sant'Andrea delle Fratte, Piazzale Gambuli, Bldg C, 4th Fl, 06132 Perugia, Italy
Footnotes
We wish to confirm that there are no known conflicts of interest associated with this publication. We confirm that the manuscript has been read and approved by all named authors and that, there are no other persons who satisfied the criteria for authorship but are not listed.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ejphar.2018.06.005.
Appendix A. Supplementary material
Supplementary material
References
- Aoki M., Aoki H., Ramanathan R., Hait N.C., Takabe K. Sphingosine-1-phosphate signaling in immune cells and inflammation: roles and therapeutic potential. Mediat. Inflamm. 2016;2016:8606878. doi: 10.1155/2016/8606878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya D., Chang S., DiMuzio P., Carpenter J., Tulenko T.N. Sphingosine-1-phosphate promotes the differentiation of adipose-derived stem cells into endothelial nitric oxide synthase (eNOS) expressing endothelial-like cells. J. Biomed. Sci. 2014;21:55. doi: 10.1186/1423-0127-21-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery K., Avery S., Shepherd J., Heath P.R., Moore H. Sphingosine-1-phosphate mediates transcriptional regulation of key targets associated with survival, proliferation, and pluripotency in human embryonic stem cells. Stem Cells Dev. 2008;17:1195–1205. doi: 10.1089/scd.2008.0063. [DOI] [PubMed] [Google Scholar]
- Bendall L.J., Basnett J. Role of sphingosine 1-phosphate in trafficking and mobilization of hematopoietic stem cells. Curr. Opin. Hematol. 2013;20:281–288. doi: 10.1097/MOH.0b013e3283606090. [DOI] [PubMed] [Google Scholar]
- Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol. Ther. 2007;115:84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Brinkmann V., Billich A., Baumruker T., Heining P., Schmouder R., Francis G., Aradhye S., Burtin P. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- Calise S., Blescia S., Cencetti F., Bernacchioni C., Donati C., Bruni P. Sphingosine 1-phosphate stimulates proliferation and migration of satellite cells: role of S1P receptors. Biochim. Biophys. Acta. 2012;1823:439–450. doi: 10.1016/j.bbamcr.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Cananzi M., Atala A., De Coppi P. Stem cells derived from amniotic fluid: new potentials in regenerative medicine. Reprod. Biomed. Online. 2009;18(Suppl 1):17–27. doi: 10.1016/s1472-6483(10)60111-3. [DOI] [PubMed] [Google Scholar]
- Cencetti F., Bernacchioni C., Nincheri P., Donati C., Bruni P. Transforming growth factor-beta1 induces transdifferentiation of myoblasts into myofibroblasts via up-regulation of sphingosine kinase-1/S1P3 axis. Mol. Biol. Cell. 2010;21:1111–1124. doi: 10.1091/mbc.E09-09-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cencetti F., Bernacchioni C., Tonelli F., Roberts E., Donati C., Bruni P. TGFbeta1 evokes myoblast apoptotic response via a novel signaling pathway involving S1P4 transactivation upstream of Rho-kinase-2 activation. FASEB J. 2013;27:4532–4546. doi: 10.1096/fj.13-228528. [DOI] [PubMed] [Google Scholar]
- De Coppi P., Bartsch G., Jr., Siddiqui M.M., Xu T., Santos C.C., Perin L., Mostoslavsky G., Serre A.C., Snyder E.Y., Yoo J.J., Furth M.E., Soker S., Atala A. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- Donati C., Bruni P. Sphingosine 1-phosphate regulates cytoskeleton dynamics: implications in its biological response. Biochim. Biophys. Acta. 2006;1758:2037–2048. doi: 10.1016/j.bbamem.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Donati C., Cencetti F., De Palma C., Rapizzi E., Brunelli S., Cossu G., Clementi E., Bruni P. TGFbeta protects mesoangioblasts from apoptosis via sphingosine kinase-1 regulation. Cell. Signal. 2009;21:228–236. doi: 10.1016/j.cellsig.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Donati C., Cencetti F., Nincheri P., Bernacchioni C., Brunelli S., Clementi E., Cossu G., Bruni P. Sphingosine 1-phosphate mediates proliferation and survival of mesoangioblasts. Stem Cells. 2007;25:1713–1719. doi: 10.1634/stemcells.2006-0725. [DOI] [PubMed] [Google Scholar]
- English D., Garcia J.G., Brindley D.N. Platelet-released phospholipids link haemostasis and angiogenesis. Cardiovasc. Res. 2001;49:588–599. doi: 10.1016/s0008-6363(00)00230-3. [DOI] [PubMed] [Google Scholar]
- Fujio K., Komai T., Inoue M., Morita K., Okamura T., Yamamoto K. Revisiting the regulatory roles of the TGF-beta family of cytokines. Autoimmun. Rev. 2016;15:917–922. doi: 10.1016/j.autrev.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Golan K., Kollet O., Lapidot T. Dynamic cross talk between S1P and CXCL12 regulates hematopoietic stem cells migration, development and bone remodeling. Pharmaceuticals. 2013;6:1145–1169. doi: 10.3390/ph6091145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan K., Vagima Y., Ludin A., Itkin T., Cohen-Gur S., Kalinkovich A., Kollet O., Kim C., Schajnovitz A., Ovadya Y., Lapid K., Shivtiel S., Morris A.J., Ratajczak M.Z., Lapidot T. S1P promotes murine progenitor cell egress and mobilization via S1P1-mediated ROS signaling and SDF-1 release. Blood. 2012;119:2478–2488. doi: 10.1182/blood-2011-06-358614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait N.C., Oskeritzian C.A., Paugh S.W., Milstien S., Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim. Biophys. Acta. 2006;1758:2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Inniss K., Moore H. Mediation of apoptosis and proliferation of human embryonic stem cells by sphingosine-1-phosphate. Stem Cells Dev. 2006;15:789–796. doi: 10.1089/scd.2006.15.789. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567–582. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone E.D., Chan G., Sibley C.P., Davidge S.T., Lowen B., Guilbert L.J. Sphingosine-1-phosphate inhibition of placental trophoblast differentiation through a G(i)-coupled receptor response. J. Lipid Res. 2005;46:1833–1839. doi: 10.1194/jlr.M500095-JLR200. [DOI] [PubMed] [Google Scholar]
- Kanagasabai R., Karthikeyan K., Vedam K., Qien W., Zhu Q., Ilangovan G. Hsp27 protects adenocarcinoma cells from UV-induced apoptosis by Akt and p21-dependent pathways of survival. Mol. Cancer Res. 2010;8:1399–1412. doi: 10.1158/1541-7786.MCR-10-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.I., Jo E.J., Lee H.Y., Cha M.S., Min J.K., Choi C.H., Lee Y.M., Choi Y.A., Baek S.H., Ryu S.H., Lee K.S., Kwak J.Y., Bae Y.S. Sphingosine 1-phosphate in amniotic fluid modulates cyclooxygenase-2 expression in human amnion-derived WISH cells. J. Biol. Chem. 2003;278:31731–31736. doi: 10.1074/jbc.M300625200. [DOI] [PubMed] [Google Scholar]
- Kobayashi N.R., Hawes S.M., Crook J.M., Pebay A. G-protein coupled receptors in stem cell self-renewal and differentiation. Stem Cell Rev. 2010;6:351–366. doi: 10.1007/s12015-010-9167-9. [DOI] [PubMed] [Google Scholar]
- Kong Y., Wang H., Lin T., Wang S. Sphingosine-1-phosphate/S1P receptors signaling modulates cell migration in human bone marrow-derived mesenchymal stem cells. Mediat. Inflamm. 2014;2014:565369. doi: 10.1155/2014/565369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebman D.A., Spiegel S. Cross-talk at the crossroads of sphingosine-1-phosphate, growth factors, and cytokine signaling. J. Lipid Res. 2008;49:1388–1394. doi: 10.1194/jlr.R800008-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Kong Y., Wang H., Wang S., Yu H., Liu X., Yang L., Jiang X., Li L., Li L. Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. J. Hepatol. 2009;50:1174–1183. doi: 10.1016/j.jhep.2009.01.028. [DOI] [PubMed] [Google Scholar]
- Lu W., Xiu X., Zhao Y., Gui M. Improved proliferation and differentiation of bone marrow mesenchymal stem cells into vascular endothelial cells with sphingosine 1-phosphate. Transplant. Proc. 2015;47:2035–2040. doi: 10.1016/j.transproceed.2015.05.032. [DOI] [PubMed] [Google Scholar]
- Maceyka M., Harikumar K.B., Milstien S., Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012;22:50–60. doi: 10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J., Gomis R.R. The logic of TGFbeta signaling. FEBS Lett. 2006;580:2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Matino D., Gargaro M., Santagostino E., Di Minno M.N., Castaman G., Morfini M., Rocino A., Mancuso M.E., Di Minno G., Coppola A., Talesa V.N., Volpi C., Vacca C., Orabona C., Iannitti R., Mazzucconi M.G., Santoro C., Tosti A., Chiappalupi S., Sorci G., Tagariello G., Belvini D., Radossi P., Landolfi R., Fuchs D., Boon L., Pirro M., Marchesini E., Grohmann U., Puccetti P., Iorio A., Fallarino F. IDO1 suppresses inhibitor development in hemophilia A treated with factor VIII. J. Clin. Investig. 2015;125:3766–3781. doi: 10.1172/JCI81859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyuki H., Maeda Y., Yano K., Sugahara K., Chiba K., Kohno T., Igarashi Y. Involvement of sphingosine 1-phosphate (S1P) receptor type 1 and type 4 in migratory response of mouse T cells toward S1P. Cell. Mol. Immunol. 2006;3:429–437. [PubMed] [Google Scholar]
- Nagamatsu T., Iwasawa-Kawai Y., Ichikawa M., Kawana K., Yamashita T., Osuga Y., Fujii T., Schust D.J. Emerging roles for lysophospholipid mediators in pregnancy. Am. J. Reprod. Immunol. 2014;72:182–191. doi: 10.1111/aji.12239. [DOI] [PubMed] [Google Scholar]
- Ogle M.E., Olingy C.E., Awojoodu A.O., Das A., Ortiz R.A., Cheung H.Y., Botchwey E.A. Sphingosine-1-phosphate receptor-3 supports hematopoietic stem and progenitor cell residence within the bone marrow niche. Stem Cells. 2016 doi: 10.1002/stem.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallotta M.T., Orabona C., Volpi C., Vacca C., Belladonna M.L., Bianchi R., Servillo G., Brunacci C., Calvitti M., Bicciato S., Mazza E.M., Boon L., Grassi F., Fioretti M.C., Fallarino F., Puccetti P., Grohmann U. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat. Immunol. 2011;12:870–878. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- Pebay A., Wong R.C., Pitson S.M., Wolvetang E.J., Peh G.S., Filipczyk A., Koh K.L., Tellis I., Nguyen L.T., Pera M.F. Essential roles of sphingosine-1-phosphate and platelet-derived growth factor in the maintenance of human embryonic stem cells. Stem Cells. 2005;23:1541–1548. doi: 10.1634/stemcells.2004-0338. [DOI] [PubMed] [Google Scholar]
- Pitson S.M., Pebay A. Regulation of stem cell pluripotency and neural differentiation by lysophospholipids. Neuro-Signals. 2009;17:242–254. doi: 10.1159/000231891. [DOI] [PubMed] [Google Scholar]
- Price M.M., Kapitonov D., Allegood J., Milstien S., Oskeritzian C.A., Spiegel S. Sphingosine-1-phosphate induces development of functionally mature chymase-expressing human mast cells from hematopoietic progenitors. FASEB J. 2009;23:3506–3515. doi: 10.1096/fj.08-128900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price S.T., Beckham T.H., Cheng J.C., Lu P., Liu X., Norris J.S. Sphingosine 1-phosphate receptor 2 regulates the migration, proliferation, and differentiation of mesenchymal stem cells. Int. J. Stem Cell Res. Ther. 2015:2. doi: 10.23937/2469-570x/1410014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proia R.L., Hla T. Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy. J. Clin. Investig. 2015;125:1379–1387. doi: 10.1172/JCI76369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusa A.R., Marton E., Rosner M., Bernaschek G., Hengstschlager M. Oct-4-expressing cells in human amniotic fluid: a new source for stem cell research? Hum. Reprod. 2003;18:1489–1493. doi: 10.1093/humrep/deg279. [DOI] [PubMed] [Google Scholar]
- Ratajczak M.Z., Suszynska M., Borkowska S., Ratajczak J., Schneider G. The role of sphingosine-1 phosphate and ceramide-1 phosphate in trafficking of normal stem cells and cancer cells. Expert Opin. Ther. Targets. 2014;18:95–107. doi: 10.1517/14728222.2014.851671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J., Proia R.L., Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat. Rev. Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani R., Pirisinu I., Calvitti M., Pallotta M.T., Gargaro M., Bistoni G., Vacca C., Di Michele A., Orabona C., Rosati J., Pirro M., Giovagnoli S., Matino D., Prontera P., Rosi G., Grohmann U., Talesa V.N., Donti E., Puccetti P., Fallarino F. Stem cells from human amniotic fluid exert immunoregulatory function via secreted indoleamine 2,3-dioxygenase1. J. Cell. Mol. Med. 2015;19:1593–1605. doi: 10.1111/jcmm.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosowski E.E., Nguyen Q.P., Camejo A., Spooner E., Saeij J.P. Toxoplasma gondii Inhibits gamma interferon (IFN-gamma)- and IFN-beta-induced host cell STAT1 transcriptional activity by increasing the association of STAT1 with DNA. Infect. Immun. 2014;82:706–719. doi: 10.1128/IAI.01291-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B., Vogler R., von Wenckstern H., Fujii M., Anzano M.B., Glick A.B., Schafer-Korting M., Roberts A.B., Kleuser B. Involvement of Smad signaling in sphingosine 1-phosphate-mediated biological responses of keratinocytes. J. Biol. Chem. 2004;279:38471–38479. doi: 10.1074/jbc.M313557200. [DOI] [PubMed] [Google Scholar]
- Schwab S.R., Pereira J.P., Matloubian M., Xu Y., Huang Y., Cyster J.G. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- Smith G.S., Kumar A., Saba J.D. Sphingosine phosphate lyase regulates murine embryonic stem cell proliferation and pluripotency through an S1P2/STAT3 signaling pathway. Biomolecules. 2013;3:351–368. doi: 10.3390/biom3030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S., Milstien S. Sphingosine 1-phosphate, a key cell signaling molecule. J. Biol. Chem. 2002;277:25851–25854. doi: 10.1074/jbc.R200007200. [DOI] [PubMed] [Google Scholar]
- Spiegel S., Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 2011;11:403–415. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha T.A., Hannun Y.A., Obeid L.M. Sphingosine kinase: biochemical and cellular regulation and role in disease. J. Biochem. Mol. Biol. 2006;39:113–131. doi: 10.5483/bmbrep.2006.39.2.113. [DOI] [PubMed] [Google Scholar]
- Watterson K.R., Lanning D.A., Diegelmann R.F., Spiegel S. Regulation of fibroblast functions by lysophospholipid mediators: potential roles in wound healing. Wound Repair Regen. 2007;15:607–616. doi: 10.1111/j.1524-475X.2007.00292.x. [DOI] [PubMed] [Google Scholar]
- Watterson K.R., Ratz P.H., Spiegel S. The role of sphingosine-1-phosphate in smooth muscle contraction. Cell. Signal. 2005;17:289–298. doi: 10.1016/j.cellsig.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Xin C., Ren S., Eberhardt W., Pfeilschifter J., Huwiler A. The immunomodulator FTY720 and its phosphorylated derivative activate the Smad signalling cascade and upregulate connective tissue growth factor and collagen type IV expression in renal mesangial cells. Br. J. Pharmacol. 2006;147:164–174. doi: 10.1038/sj.bjp.0706452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin C., Ren S., Kleuser B., Shabahang S., Eberhardt W., Radeke H., Schafer-Korting M., Pfeilschifter J., Huwiler A. Sphingosine 1-phosphate cross-activates the Smad signaling cascade and mimics transforming growth factor-beta-induced cell responses. J. Biol. Chem. 2004;279:35255–35262. doi: 10.1074/jbc.M312091200. [DOI] [PubMed] [Google Scholar]
- Zhao Z., Chen Z., Zhao X., Pan F., Cai M., Wang T., Zhang H., Lu J.R., Lei M. Sphingosine-1-phosphate promotes the differentiation of human umbilical cord mesenchymal stem cells into cardiomyocytes under the designated culturing conditions. J. Biomed. Sci. 2011;18:37. doi: 10.1186/1423-0127-18-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material