Abstract
African Americans (AA) have the highest prevalence of hypertension, putting them at greater risk of cardiovascular disease and death. Previous studies have reported that, relative to Caucasian Americans (CA), healthy AA men have augmented pressor responses to sympatho-excitatory stressors. While important, these studies do not inform about the resting state and the influence of spontaneous changes in resting muscle sympathetic nerve activity (MSNA). Likewise, little is known regarding the transduction of MSNA into a vascular response at rest on a beat-to-beat basis. Accordingly, we tested the hypothesis that relative to CA, AA would exhibit greater vasoconstriction and pressor responses following spontaneous bursts of MSNA. Mean arterial pressure (MAP), common femoral artery blood flow, and MSNA were continuously recorded during 20 minutes of supine rest in 35 young healthy men (17 AA and 18 CA). Signal-averaging was used to characterize changes in leg (LVC) and total vascular conductance (TVC) and MAP following spontaneous MSNA bursts. AA demonstrated significantly greater decreases in LVC (AA: -15.0±1.0, CA: -11.5±1.2%; P=0.042) and TVC (AA: -8.6±0.9, CA: -5.1±0.4%; P=0.001) following MSNA bursts, which resulted in greater MAP increases (AA: +5.2±0.6, CA: +3.9±0.3 mmHg; P=0.04). These exaggerated responses in AA compared to CA were present whether MSNA bursts occurred in isolation (singles) or in combination (multiples) and were graded with increases in burst height. Collectively, these findings suggest that healthy young AA men exhibit augmented sympathetic vascular transduction at rest, and provide novel insight into potential mechanism(s) by which this population may develop hypertension later in life.
Keywords: race and ethnicity, vasculature, muscle sympathetic nerve activity, vascular conductance, blood pressure
Introduction
The prevalence and severity of hypertension in African Americans (AA) far exceeds any other racial group,1,2 and the development of hypertension typically occurs much earlier in life for these individuals.3 An increased incidence of high blood pressure amplifies the risk for cardiovascular disease, stroke, and death,4,5 and as such AA are at nearly double the risk of fatal stroke and heart disease-related deaths, compared to Caucasian Americans (CA).1 Despite these well-known racial disparities in cardiovascular health, the underlying mechanism(s) remain incompletely understood.
The sympathetic nervous system contributes importantly to cardiovascular regulation at rest by continuously altering vascular resistance and blood pressure on a heartbeat by heartbeat basis. Consequently, the increased prevalence of hypertension in AA may rely, in part, on the link between sympathetic nerve activity (SNA) and vascular responsiveness. In this regard, previous studies have demonstrated an augmented vasoconstrictor response to the infusion of α-adrenergic agonists in AA compared to CA.6 Furthermore, exaggerated increases in vascular resistance and blood pressure to sympatho-excitatory stressors have been reported in AA.7–10 However, findings have been equivocal.11,12 Additionally, while such experimental procedures reveal that large elevations in sympathetic outflow may increase vascular resistance more in AA than CA, these studies do not provide insight into the ability of the sympathetic nervous system to dynamically control vascular tone and blood pressure on a beat-to-beat basis under normal resting conditions, nor do they represent resting sympathetic vascular transduction.
With this background in mind, in the present study we made direct measures of muscle SNA (MSNA) combined with beat-to-beat arterial blood pressure and femoral artery blood flow to comprehensively investigate potential racial differences in resting sympathetic vascular transduction. There is a growing interest in assessing sympathetic vascular transduction at rest13–16 and herein, we employed a signal-averaging method previously developed17 and validated in our laboratory13–15 to characterize beat-to-beat changes in leg vascular conductance and blood pressure following spontaneous MSNA bursts. We tested the hypothesis that, relative to CA, AA would exhibit greater vasoconstriction and pressor responses following spontaneous bursts of MSNA.
Methods
Study population
We studied 35 young men (17 AA and 18 CA). Studies were performed at the University of Missouri (n=18 total: 9 AA, 9 CA) and the University of Texas at Arlington (n=17 total: 8 AA, 9 CA). Racial identification was determined by self-report and subjects were classified by identifying their parents as either both AA or both CA. All subjects were free from known neural, cardiovascular, respiratory, or metabolic disease and were nonsmokers. Participants were studied at least 3 hours post-prandial, and free from caffeine or alcohol for 12 hours and exercise for 24 hours. Each subject received verbal instructions and familiarization with all experimental measurements and procedures prior to data collection. Study procedures conformed to the Declaration of Helsinki and were approved by Institutional Review Boards at the University of Missouri and the University of Texas at Arlington. All subjects provided written informed consent prior to participation.
Experimental measures
Standard cardiovascular measures
A lead II ECG (Quinton Q710, Bothell, WA) was used to measure heart rate. Beat-to-beat blood pressure (BP) was measured continuously via finger photoplethysmography (Finapres Medical Systems, Amsterdam, Netherlands). In addition, BP was obtained through brachial artery sphygmomanometry (Welch Allyn, Skaneateles Falls, NY) to calibrate absolute BP readings from the Finapres.
Muscle sympathetic nerve activity (MSNA)
Multiunit MSNA recordings were obtained at the peroneal nerve, as previously performed in our laboratory.13,14,18,19 Briefly, a unipolar tungsten microelectrode was inserted percutaneously, and positioned into a fascicle of nerve fibers innervating the skeletal muscle vasculature. Signals were amplified, band-pass filtered (700-2,000 Hz), rectified, and integrated at a time constant of 0.1sec (Iowa Bioengineering, Iowa City, IA). The presence of MSNA was confirmed by a pulse synchronous signal that responded to an end-expiratory breath hold and stimulation of muscle, but not skin, afferents.
Leg blood flow
Common femoral artery diameter and velocity were obtained via duplex Doppler ultrasound (GE Logiq P5, Milwaukee, WI) as previously described by our laboratory.13,14 Briefly, a 9 or 11-MHz linear array transducer was selected for optimal image quality and positioned at the common femoral artery via a stereotactic clamp, 2-3 cm proximal to the bifurcation of the superficial and deep branches. Continuous measures of common femoral artery diameter and blood velocity were obtained in duplex mode at a pulsed frequency of 5 MHz. The sample volume encompassed the entire vessel lumen without extending beyond the walls, and the insonation angle was set at 60°. Due to an inability to obtain high quality Doppler ultrasound measures in 4 of the subjects, blood flow data include a total of 18 CA and 13 AA. Ultrasound images of the femoral artery and velocity waveform were acquired at 30 Hz using a custom LabVIEW program interfaced to the video output of the Doppler ultrasound machine. ECG, BP, and MSNA signals were sampled at 1,000 Hz and embedded as data streams into the AVI file allowing for simultaneous data acquisition of all cardiovascular variables. This custom LabVIEW program has been described in detail previously.13,14,20,21
Experimental protocol
Following instrumentation for all measurements, subjects rested for ∼15 minutes prior to any data collection. Neural and cardiovascular measures were then recorded continuously throughout a 20 minute baseline period in a dimly lit, temperature controlled room (21-22°C) while subjects rested quietly in the supine position. At the conclusion of each experiment, subjects were asked to provide a rating of their need to use the restroom on a scale of 0 to 10.
Data analysis
Common femoral artery diameter and blood velocity were analyzed via custom edge-detection software and synchronized beat-to-beat with ECG, BP, and MSNA signals in LabVIEW. Femoral blood flow was determined via continuous recordings of common femoral artery diameter and blood velocity, calculated as: π × (diameter/2)2 × mean blood velocity × 60. Leg vascular conductance (LVC) was determined by dividing femoral blood flow by mean arterial pressure (MAP), which was calculated as the integral of the arterial BP waveform. In addition, the arterial BP waveform was analyzed using the Modelflow method22 to estimate stroke volume, and calculate cardiac output from the product of stroke volume and heart rate. Total vascular conductance (TVC) was calculated by dividing cardiac output by MAP.
The sympathetic neurogram was analyzed on a beat-to-beat basis to determine the presence/absence of MSNA bursts. Bursts were identified via the following criteria: (1) greater than 3:1 signal-to-noise ratio, (2) burst morphology consistent with MSNA bursts, and (3) a pulse synchronous signal. As an index of resting sympathetic outflow, MSNA was quantified as burst frequency (bursts/min) and burst incidence (bursts/100 cardiac cycles).
A spike-triggered averaging methodology previously developed in our laboratory13–15 was used to assess beat-to-beat sympathetic vascular transduction. LVC and TVC were determined at the time of each MSNA burst, referred to as cardiac cycle 0, and followed for 10 subsequent cardiac cycles. This time frame was chosen based on the original work of Wallin and Nerhed17 demonstrating the peak increase in BP occurs at ∼5.5 seconds following a burst of MSNA. In addition, work from our group using phentolamine to block alpha adrenergic receptors has demonstrated the sympathetic origin of the responses, and that this window appropriately captures the time course and nadir changes in vascular conductance following spontaneous MSNA bursts.23 The absolute and percent changes in LVC and TVC over these 10 cardiac cycles were determined and averaged for each subject to provide a group mean (AA versus CA). This allowed for the characterization of the time course of the response in each group. Nadir responses were also determined as the largest decrease in LVC and TVC for each individual, to assess the magnitude of the response. Identical signal-averaging procedures following MSNA bursts were also performed for MAP to estimate sympathetic transduction, with respect to changes in BP. Lastly, we followed cardiac cycles which did not include an MSNA burst (i.e. non-bursts) and also signal-averaged LVC, TVC, and MAP for the subsequent 10 cardiac cycles.
To investigate the effect of burst patterning, MSNA bursts were further characterized as single bursts occurring in isolation (surrounded by cardiac cycles without MSNA bursts) or multiple bursts (directly adjacent to ≥ 1 other MSNA burst). As above, absolute and percent changes in LVC, TVC, and MAP were determined for each burst belonging to either a single burst or multiple burst, and then were signal-averaged for each subject to provide group means, as previously described.14
Because MSNA bursts heights vary throughout the recording period, we also examined the influence of burst amplitude on ensuing LVC, TVC, and MAP responses. For this analysis, burst amplitudes were normalized as a percentage of maximum burst height within the 20 minute recording, which was determined from the average of the 3 largest bursts. Next, sympathetic bursts were categorized into quartiles by their normalized burst heights, wherein Quartile 1 represented the smallest 25% of burst amplitudes and Quartile 4 represented the largest 25% of burst amplitudes. Signal-averaging procedures were then applied to all MSNA bursts in each of the height quartiles providing an absolute and percent change in LVC, TVC, and MAP for each subject, and these individual values were averaged to provide group means.
Statistics
Data were analyzed via a mixed-model ANOVA for group and time effects (SPSS, version 24), where group was a fixed variable and time was a repeated measure. When significant interactions were found, between-group post hoc comparisons were made using the Bonferroni correction. Between-group comparisons of subject characteristics or resting cardiovascular parameters were made using unpaired t-tests. All data are expressed as means±standard error (SEM) and significance was set a priori at P < 0.05.
Results
Baseline characteristics
Subjects were of similar height (AA: 177±2, CA: 179±2 cm; P = 0.35), weight (AA: 79±2, CA: 80±3 kg; P = 0.75), and body mass index (AA: 25±1, CA: 25±1 kg/m2; P = 0.85), however AA individuals were slightly younger (AA: 20±0, CA: 23±1 years; P = 0.01). As shown in Table 1, resting BP, HR, and MSNA were not different between groups (P > 0.05). Likewise, common femoral artery diameter, velocity, blood flow, and LVC did not differ between AA and CA groups (P > 0.05). At the end of each experiment, subjects' need to use the restroom, on a scale of 0 to 10, was not different between groups (AA: 0.8±0.5, CA: 1.6±0.5; P = 0.30).
Table 1.
Resting Cardiovascular and Neural Measurements.
| Measurement | AA | CA | P value |
|---|---|---|---|
| SBP (mmHg) | 125 ± 2 | 124 ± 3 | 0.75 |
| DBP (mmHg) | 69 ± 2 | 68 ± 2 | 0.69 |
| MAP (mmHg) | 88 ± 2 | 86 ± 2 | 0.66 |
| HR (beats·min-1) | 59 ± 2 | 60 ± 2 | 0.74 |
| Burst frequency (bursts·min-1) | 10 ± 1 | 12 ± 1 | 0.52 |
| Burst incidence (bursts·100 cardiac cycles-1) | 17 ± 0 | 19 ± 0 | 0.47 |
| Total activity (AU·min-1) | 529 ± 69 | 615 ± 45 | 0.30 |
| Mean femoral artery diameter (cm) | 0.91 ± 0.02 | 0.94 ± 0.03 | 0.44 |
| Mean femoral artery velocity (cm·sec-1) | 12.68 ± 1.60 | 9.54 ± 1.00 | 0.10 |
| Mean femoral artery blood flow (ml·min-1) | 496.6 ± 73.5 | 394.7 ± 45.3 | 0.22 |
| Mean femoral artery LVC (ml·min-1·mmHg-1) | 5.3 ± 0.9 | 4.5 ± 0.5 | 0.34 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate; AU, arbitrary units; LVC, leg vascular conductance
Effect of spontaneous MSNA bursts on LVC
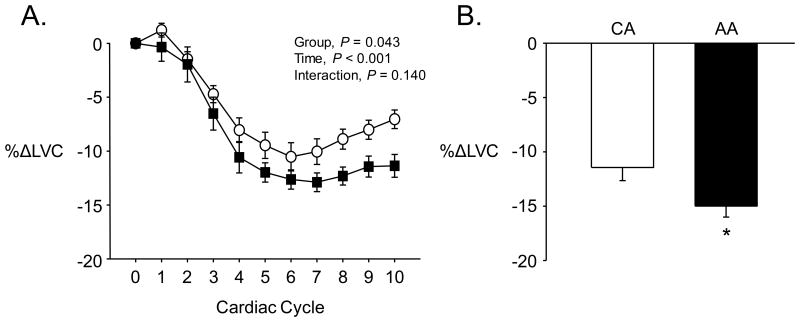
Figure 1A shows the beat-to-beat decreases in LVC following spontaneous bursts of MSNA in AA and CA groups. There was a clear and consistent decline in LVC over the 10 cardiac cycles following MSNA bursts in both groups (P < 0.001), with the reduction being significantly greater in the AA group compared to the CA group (Figure 1A). This greater vasoconstriction resulted in a larger nadir in LVC in the AA group when expressed as an absolute change (AA: -0.81±0.13, CA: -0.47±0.04 ml·min-1·mmHg-1; P = 0.009) or percent change (Figure 1B). In contrast to MSNA bursts, cardiac cycles which did not contain a burst of MSNA resulted in a slight increase in LVC (P < 0.001) but this was not different between groups (AA: +3.1±0.5, CA: +2.8±0.5%; P = 0.63).
Figure 1.
A) Percent decreases in leg vascular conductance (LVC) over the 10 cardiac cycles immediately following spontaneous MSNA bursts. B) Peak decreases in LVC following spontaneous MSNA bursts. AA = African American, denoted by filled symbols & bars; CA = Caucasian American, denoted by open symbols & bars; *P < 0.05, AA versus CA.
Effect of spontaneous MSNA bursts on TVC
The time course of beat-to-beat changes in TVC in response to MSNA bursts is shown in Figure 2A. Similar to LVC, TVC was significantly reduced following MSNA bursts (P < 0.001) and this response was greater in the AA versus CA group (Figure 2A). Consequently, AA exhibited a significantly larger nadir in TVC after MSNA bursts when expressed as an absolute change (AA: -6.4±0.9, CA: -4.3±0.4 ml·min-1·mmHg-1; P = 0.03) or percent change (Figure 2B). Conversely, cardiac cycles which lacked an MSNA burst resulted in an increase in TVC (P < 0.001) that was similar between groups (AA: +1.2±0.3, CA: +1.4±0.2%; P = 0.11).
Figure 2.
A) Percent decreases in total vascular conductance (TVC) over the 10 cardiac cycles immediately following spontaneous MSNA bursts. B) Peak decreases in TVC following spontaneous MSNA bursts. AA = African American, denoted by filled symbols & bars; CA = Caucasian American, denoted by open symbols & bars; *P < 0.05, AA versus CA.
Effect of spontaneous MSNA bursts on MAP
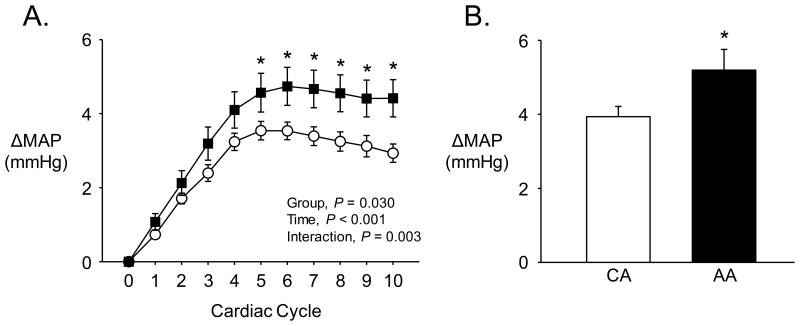
As shown in Figure 3A, spontaneous bursts of MSNA resulted in an increase in MAP (P < 0.001). This rise in MAP was heightened in the AA relative to the CA group (Figure 3A). Indeed, the peak increase in MAP was significantly greater in AA (Figure 3B). When cardiac cycles not associated with an MSNA burst were considered, MAP was significantly decreased (P < 0.001) and this response was similar between AA and CA (AA: -1.0±0.2, CA: -1.0±0.1 mmHg; P = 0.91).
Figure 3.
A) Absolute increases in mean arterial pressure (MAP) over the 10 cardiac cycles immediately following spontaneous MSNA bursts. B) Peak increase in MAP following spontaneous MSNA bursts. AA = African American, denoted by filled symbols & bars; CA = Caucasian American, denoted by open symbols & bars; *P < 0.05, AA versus CA.
Effect of MSNA burst pattern
Figure 4 depicts LVC, TVC, and MAP responses following MSNA bursts sorted by burst pattern into single or multiples. The AA group consistently demonstrated heightened responses compared to the CA group. For both singles and multiples, the peak decrease in LVC (Figure 4A) and TVC (Figure 4B), as well as the peak increase in MAP (Figure 4C) were all greater in the AA group.
Figure 4.
Peak changes in A) LVC, B) TVC, and C) MAP following spontaneous MSNA bursts which occur in isolation (Singles) or adjacent to other MSNA bursts (Multiples). AA = African American, denoted by filled bars; CA = Caucasian American, denoted by open bars.
Importantly for these analyses, over the 20 minute baseline period, the number of single MSNA bursts was not different between groups (AA: 97±9, CA: 111±8; P = 0.22). Additionally, the number of multiple burst occurrences was also not different between AA and CA. This was the case for occurrences of 2 bursts (AA: 36±6, CA: 36±4; P = 0.99), 3 bursts (AA: 6±2, CA: 8±2; P = 0.55), or 4+ bursts (AA: 2±0, CA: 2±0; P = 0.69). These data also highlight that for both groups, the occurrence of single MSNA bursts exceeded the number of multiple bursts.
Effect of MSNA burst amplitude
The influence of MSNA burst amplitude on LVC, TVC and MAP are presented in Figure 5. As burst amplitude increased, there was a graded decrease in LVC and TVC that was significantly greater in the AA group. Indeed, AA exhibited a greater nadir in LVC and TVC following MSNA bursts across all quartiles (Figure 5A & B). As an example, in Quartile 4 there was a -22.8±1.4% decrease in LVC in the AA group compared to -17.2±1.8% in the CA group (P = 0.030). Similarly, we observed a -12.2±1.4% decrease in TVC in the AA group in Quartile 4, whereas the CA group exhibited a -7.6±0.6% reduction (P = 0.003). Consistent with the greater vasoconstriction observed in the AA group across height quartiles, there was a progressive increase in MAP from Quartile 1 to Quartile 4 that was larger in the AA group (Figure 5C). Important for these group comparisons that include burst amplitude, the average normalized burst height was not different between AA and CA groups for all height quartiles (Group, P = 0.48; Quartile, P < 0.001; Interaction, P = 0.07).
Figure 5.
Peak changes in A) LVC, B) TVC, and C) MAP following spontaneous MSNA bursts from the smallest height quartile (Q1) to the largest (Q4). AA = African American, denoted by filled bars; CA = Caucasian American, denoted by open bars; *P < 0.05, AA versus CA.
Discussion
The current study provides novel insight into racial differences in resting sympathetic vascular transduction. We have demonstrated for the first time that, relative to CA men, AA men exhibit augmented decreases in LVC following spontaneous bursts of sympathetic outflow. This greater vasoconstriction in the leg contributed to greater reductions in TVC and rises in MAP following spontaneous MSNA bursts in the AA group. Collectively, our data indicate that otherwise healthy young AA men demonstrate exaggerated vasoconstriction and pressor responses to sympathetic outflow under normal resting conditions. These findings highlight a potential mechanism by which AA men may develop hypertension later in life.
In this regard, our findings are noteworthy in that prior to the development of hypertension and cardiovascular disease, young AA men exhibit augmented resting sympathetic vascular transduction. Importantly, MSNA burst frequency and incidence, traditional measures used to assess group differences in resting MSNA, were similar between groups. However, despite these similarities in resting MSNA, AA still exhibited greater vascular and pressor responses than CA. This is important because it highlights a distinction in sympathetic vascular function at rest that is not evident by quantification of resting MSNA alone. Interestingly, the AA group exhibited greater vasoconstriction and increased BP whether MSNA bursts occurred in isolation (i.e. singles) or in succession with other bursts (i.e. multiples). This is relevant because with increased frequency, as is the case with multiple bursts, there is likely greater norepinephrine (NE) release.24,25 Indeed, we found a greater vasoconstrictor and pressor response with multiple bursts compared to single bursts, that was consistently greater in the AA versus the CA group. Likewise, when burst amplitude, another index of NE release,26 was taken into consideration the vascular and BP responses to spontaneous MSNA bursts were also augmented in the AA group. This was evident within all height quartiles. Thus overall, whether the AA group had single or multiple bursts, or bursts of varying amplitude, we found greater reductions in LVC and TVC and larger increases in MAP. Taken together, our findings reveal that the observed racial differences in resting sympathetic vascular transduction were not specific to a particular MSNA burst pattern or size.
While our study design does not specifically allow us to determine the exact underlying mechanism(s) for the observed racial differences, our data lend some insight that warrants discussion. Considering that burst frequency, patterns, and amplitudes were all similar between groups, it is likely that NE release was not different for AA and CA24–26, however, prejunctional modulation of postganglionic NE release cannot be dismissed. Likewise, it is possible that racial differences in NE kinetics may exist in terms of NE reuptake and clearance. Interestingly, some27 but not all6 studies report greater NE spillover in AA at rest. Also, we cannot exclude the possibility that there are racial differences in the release of other neurotransmitters at the neurovascular junction (e.g. neuropeptide Y), which may lead to exaggerated sympathetic vascular transduction in the AA group. However, examining alterations in neurotransmitter release is technically challenging and to our knowledge has not been explored in this population. Further, given previous work demonstrating augmented α1-mediated vasoconstriction and blunted β-adrenergic vasodilation in AA,6 an altered adrenergic sensitivity may contribute to an exaggerated vascular reactivity in AA compared to CA. Lastly, several studies have previously demonstrated reduced vasodilator capacity in AA, as assessed by flow-mediated dilation,28,29 minimal vascular resistance,30,31 and intra-arterial infusion of endothelium-dependent and -independent vasodilators.32,33 This is relevant because it has been suggested that a diminished intrinsic vasodilator capacity may lessen the ability to dampen the effects of MSNA on BP.34,35 Nevertheless, given the time scale of the beat-to-beat changes in LVC following MSNA bursts, it will be challenging to pinpoint contributions of any or all of these factors to the heightened vasoconstriction and BP responses in AA compared to CA.
Augmented vasoconstriction, particularly under resting conditions, has significant cardiovascular consequences as it contributes to larger fluctuations in BP throughout normal daily life, which can lead to end-organ damage (e.g. cerebral, renal, etc.). In the present study, despite similar resting MAP, AA demonstrated a significantly greater MAP variability over the 20 minute resting period (AA: 6.1±0.3, CA: 4.9±0.3 standard deviations; P = 0.01). In addition, average minimum and maximum MAP ranged from 79±2 to 117±2 mmHg in the AA group and 74±2 to 105±2 mmHg in CA group. These data are noteworthy because they demonstrate that under basal conditions AA are facing greater BP at both the lower and the higher end of their BP range. Additionally striking, it appears that these group differences are particularly evident at the high end, wherein AA exhibit, on average, a 12 mmHg greater MAP. The impact of these larger BP surges on cardiovascular health cannot be overstated.
Perspectives
Our finding that vascular reactivity to spontaneous MSNA bursts is amplified in young healthy AA men under resting conditions provides key insight into potential sources for elevated cardiovascular risk in this racial group. Indeed, previous studies have demonstrated that AA develop hypertension earlier in life and at greater rates than individuals of Asian, Hispanic, or Caucasian descent.1–3 Importantly, the results presented herein provide early evidence that resting sympathetic vascular transduction is augmented, in advance of overt increases in vascular resistance, BP, and MSNA at rest. This exaggerated vascular reactivity and subsequent surges in BP may highlight a putative source of future cardiovascular risk in AA. Indeed, previous longitudinal work has provided evidence that greater BP variability is linked to increased incidence of hypertension later in life,36 and our findings provide an additional link between augmented neuro-vascular coupling and greater BP swings in young healthy AA. While it is unknown exactly how these BP surges may translate into cardiovascular disease as AA age, it is well established that MSNA increases with age.37 In this regard, if young healthy AA men already exhibit greater vasoconstriction and BP in response to resting MSNA, it is not unreasonable to presume that with age and increasing resting MSNA, exaggerated increases in vasoconstriction and BP over weeks/months/years would not only impart significant vascular damage but likely contribute to the development of hypertension. Indeed, even a mild increase in resting MSNA may result in AA individuals progressing to a hypertensive or pre-hypertensive state. Nevertheless, it should be noted that the development of hypertension is multi-factorial and complex, and not all cases involve elevated sympathetic outflow. However, of interest, in AA men there is no relationship between MSNA and body mass index indicating that even lean AA men have high sympathetic activity.38,39 Although the group we studied is younger than the subjects in these previous studies, these data and our current findings suggest a potential greater involvement of the sympathetic nervous system in the regulation of BP in AA men.
In conclusion, we demonstrate for the first time that AA have exaggerated sympathetic vascular transduction following spontaneous bursts of sympathetic activity, compared to CA at rest. Further, this augmented resting sympathetic vascular reactivity resulted in greater elevations in BP. In total, our findings suggest that AA exhibit an inappropriate sympathetically-mediated vasoconstriction under normal resting conditions compared to CA, highlighting a potential mechanism for the development of hypertension in AA.
What is New?
We demonstrate for the first time that, relative to CA men, AA men exhibited augmented vasoconstrictor and pressor responses following spontaneous bursts of MSNA.
What is Relevant?
This is relevant because AA are at greater risk for the development of hypertension and cardiovascular disease. These data provide novel insight into potential mechanism(s) by which AA men may develop hypertension later in life.
Summary.
Collectively, our findings demonstrate that healthy young AA men exhibit augmented sympathetic vascular transduction at rest. This may highlight a potential early source of impairment in sympathetic control of the vasculature in AA which contributes to the greater cardiovascular risk in this population.
Acknowledgments
The authors wish to thank the subjects for their time and participation in the study.
Sources of Funding: Support for this project was from NIH 1R15HL130906-01 (awarded to DMK) and NIH HL 127071 (awarded to PJF).
Footnotes
Conflicts of Interest/Disclosures: None.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. AHA Statistical Update Heart Disease and Stroke Statistics — 2015 Update. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension. 1995;25(3):305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 3.Voors AW, Webber LS, Berenson GS. Time course studies of blood pressure in children--the Bogalusa Heart Study. American journal of epidemiology. 1979;109(3):320–334. doi: 10.1093/oxfordjournals.aje.a112685. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB. Blood pressure as a cardiovascular risk factor: prevention and treatment. JAMA : the journal of the American Medical Association. 275(20):1571–6. [PubMed] [Google Scholar]
- 5.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 6.Stein CM, Lang CC, Singh I, He HB, Wood AJJ. Increased Vascular Adrenergic Vasoconstriction and Decreased Vasodilation in Blacks Additive Mechanisms Leading to Enhanced Vascular Reactivity. Hypertension. 2000;36:945–951. doi: 10.1161/01.hyp.36.6.945. [DOI] [PubMed] [Google Scholar]
- 7.Light KC, Obrist PA, Sherwood A, James SA, Strogatz DS. Effects of Race and Marginally Elevated Blood Pressure on Responses to Stress. Hypertension. 1987;10:555–563. doi: 10.1161/01.hyp.10.6.555. [DOI] [PubMed] [Google Scholar]
- 8.Adamopoulos D, Ngatchou W, Lemogoum D, Janssen C, Beloka S, Lheureux O, Kayembe P, Argacha J, Degaute J. Intensified Large Artery and Microvascular Response to Cold Adrenergic Stimulation in African Blacks. American Journal of Hypertension. 2009;22(9):958–963. doi: 10.1038/ajh.2009.106. [DOI] [PubMed] [Google Scholar]
- 9.Ray CA, Monahan KD. Sympathetic vascular transduction is augmented in young normotensive blacks. Journal of applied physiology. 2002;92(2):651–656. doi: 10.1152/japplphysiol.00788.2001. [DOI] [PubMed] [Google Scholar]
- 10.Calhoun Da, Mutinga ML, Collins aS, Wyss JM, Oparil S. Normotensive blacks have heightened sympathetic response to cold pressor test. Hypertension. 1993;22(6):801–5. doi: 10.1161/01.hyp.22.6.801. [DOI] [PubMed] [Google Scholar]
- 11.Johnson EH, Nazzaro P, Gilbert DC, Weder A, Jamerson K. Similarities in cardiovascular reactivity to behavioral stressors in African-American and white males. Ethnicity & disease. 1992;2(3):232–245. [PubMed] [Google Scholar]
- 12.McAdoo WG, Weinberger MH, Miller JZ, Fineberg NS, Grim CE. Race and gender influence hemodynamic responses to psychological and physical stimuli. Journal of hypertension. 1990;8(10):961–967. doi: 10.1097/00004872-199010000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Fairfax ST, Padilla J, Vianna LC, Davis MJ, Fadel PJ. Spontaneous bursts of muscle sympathetic nerve activity decrease leg vascular conductance in resting humans. American journal of physiology Heart and circulatory physiology. 2013;304(5):H759–66. doi: 10.1152/ajpheart.00842.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fairfax ST, Padilla J, Vianna LC, Holwerda SH, Davis MJ, Fadel PJ. Influence of spontaneously occurring bursts of muscle sympathetic nerve activity on conduit artery diameter. American journal of physiology Heart and circulatory physiology. 2013;305(6):H867–74. doi: 10.1152/ajpheart.00372.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vianna LC, Hart EC, Fairfax ST, Charkoudian N, Joyner MJ, Fadel PJ. Influence of age and sex on the pressor response following a spontaneous burst of muscle sympathetic nerve activity. American journal of physiology Heart and circulatory physiology. 2012;302(11):H2419–27. doi: 10.1152/ajpheart.01105.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Briant LJB, Burchell AE, Ratcliffe LEK, Charkoudian N, Nightingale AK, Paton JFR, Joyner MJ, Hart EC. Quantifying sympathetic neuro-haemodynamic transduction at rest in humans: insights into sex, ageing and blood pressure control. The Journal of Physiology. 2016;594(17):4753–4768. doi: 10.1113/JP272167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gunnar Wallin B, Nerhed C. Relationship between spontaneous variations of muscle sympathetic activity and succeeding changes of blood pressure in man. Journal of the Autonomic Nervous System. 1982;6(3):293–302. doi: 10.1016/0165-1838(82)90002-9. [DOI] [PubMed] [Google Scholar]
- 18.Holwerda SW, Vianna LC, Restaino RM, Chaudhary, Young CN, Fadel PJ. Arterial baroreflex control of sympathetic nerve activity and heart rate in patients with type 2 diabetes. American journal of physiology Heart and circulatory physiology2. 2017;311:H1170–H1179. doi: 10.1152/ajpheart.00384.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holwerda SW, Restaino RM, Manrique C, Lastra G, Fisher JP, Fadel PJ. Augmented pressor and sympathetic responses to skeletal muscle metaboreflex activation in type 2 diabetes patients. American journal of physiology Heart and circulatory physiology. 2016;310(2):H300–9. doi: 10.1152/ajpheart.00636.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padilla J, Simmons GH, Vianna LC, Davis MJ, Laughlin MH, Fadel PJ. Brachial artery vasodilatation during prolonged lower limb exercise: role of shear rate. Experimental physiology. 2011;96(10):1019–1027. doi: 10.1113/expphysiol.2011.059584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simmons GH, Padilla J, Young CN, Wong BJ, Lang JA, Davis MJ, Laughlin MH, Fadel PJ. Increased brachial artery retrograde shear rate at exercise onset is abolished during prolonged cycling : role of thermoregulatory vasodilation. J Appl Physiol. 2011;110:389–397. doi: 10.1152/japplphysiol.00936.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dyson KS, Shoemaker JK, Arbeille P, Hughson RL. Modelflow estimates of cardiac output compared with Doppler ultrasound during acute changes in vascular resistance in women. Experimental physiology. 2010;95(4):561–568. doi: 10.1113/expphysiol.2009.050815. [DOI] [PubMed] [Google Scholar]
- 23.Fairfax ST, Holwerda SW, Credeur DP, Zuidema MY, Medley JH, Dyke PC, Wray DW, Davis MJ, Fadel PJ. The role of α-adrenergic receptors in mediating beat-by-beat sympathetic vascular transduction in the forearm of resting man. The Journal of physiology. 2013;591.14:3637–3649. doi: 10.1113/jphysiol.2013.250894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esler MD, Hasking GJ, Willett IR, Leonard PW, Jennings GL. Noradrenaline release and sympathetic nervous system activity. Journal of hypertension. 1985;3(2):117–129. doi: 10.1097/00004872-198504000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Eisenhofer G, Cox HS, Esler MD. Parallel increases in noradrenaline reuptake and release into plasma during activation of the sympathetic nervous system in rabbits. Naunyn-Schmiedeberg's archives of pharmacology. 1990;342(3):328–335. doi: 10.1007/BF00169445. [DOI] [PubMed] [Google Scholar]
- 26.Ninomiya I, Malpas SC, Matsukawa K, Shindo T, Akiyama T. The amplitude of synchronized cardiac sympathetic nerve activity reflects the number of activated pre- and postganglionic fibers in anesthetized cats. Journal of the autonomic nervous system. 1993;45(2):139–147. doi: 10.1016/0165-1838(93)90125-e. [DOI] [PubMed] [Google Scholar]
- 27.Lang CC, Stein CM, Brown RM, Deegan R, Nelson R, He HB, Wood M, Wood a J. Attenuation of isoproterenol-mediated vasodilatation in blacks. The New England journal of medicine. 1995;333(3):155–160. doi: 10.1056/NEJM199507203330304. [DOI] [PubMed] [Google Scholar]
- 28.Campia U, Choucair WK, Bryant MB, Waclawiw MA, Cardillo C, Panza JA. Reduced endothelium-dependent and -independent dilation of conductance arteries in African Americans. Journal of the American College of Cardiology. 2002;40(4):754–760. doi: 10.1016/s0735-1097(02)02015-6. [DOI] [PubMed] [Google Scholar]
- 29.Alvarez Ja, Gower Ba, Calhoun Da, Judd SE, Dong Y, Dudenbostel T, Scholl J, Ashraf AP. Serum 25-hydroxyvitamin D and Ethnic Differences in Arterial Stiffness and Endothelial Function. Journal of clinical medicine research. 2012;4(3):197–205. doi: 10.4021/jocmr965w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bassett DR, Duey WJ, Walker AJ, Rowley ET, Bond V. Racial Differences in Maximal Vasodilatory of Forearm Resistance Vessels in Normotensive Young Adults. Am J Hypertens. 1992;5:781–786. doi: 10.1093/ajh/5.11.781. [DOI] [PubMed] [Google Scholar]
- 31.Hinderliter AL, Sager AR, Sherwood A, Light KC, Girdler SS, Willis PW. Ethnic Differences in Forearm Vasodilator Capacity. American Journal of Cardiology. 1996;78(2):208–211. doi: 10.1016/s0002-9149(96)90397-5. [DOI] [PubMed] [Google Scholar]
- 32.Stein CM, Lang CC, Nelson R, Brown M, Wood AJ. Vasodilation in black Americans: attenuated nitric oxide-mediated responses. Clinical pharmacology and therapeutics. 1997;62(4):436–443. doi: 10.1016/S0009-9236(97)90122-3. [DOI] [PubMed] [Google Scholar]
- 33.Ozkor MA, Rahman AM, Murrow JR, Kavtaradze N, Lin J, Manatunga A, Hayek S, Quyyumi AA. Differences in vascular nitric oxide and endothelium-derived hyperpolarizing factor bioavailability in blacks and whites. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014;34(6):1320–1327. doi: 10.1161/ATVBAHA.113.303136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hart EC, Wallin BG, Barnes JN, Joyner MJ, Charkoudian N. Sympathetic nerve activity and peripheral vasodilator capacity in young and older men. Am J Physiol Heart Circ Physiol. 2014;306:H904–H909. doi: 10.1152/ajpheart.00181.2013. [DOI] [PubMed] [Google Scholar]
- 35.Gamboa A, Figueroa R, Paranjape SY, Farley G, Diedrich A, Biaggioni I. Autonomic Blockade Reverses Endothelial Dysfunction in Obesity-Associated Hypertension. Hypertension. 2016;68(4):1004–1010. doi: 10.1161/HYPERTENSIONAHA.116.07681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen W, Srinivasan SR, Ruan L, Mei H, Berenson GS. Adult hypertension is associated with blood pressure variability in childhood in blacks and whites: the bogalusa heart study. Am J Hypertens. 2011;24(1):77–82. doi: 10.1038/ajh.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joyner MJ, Barnes JN, Hart EC, Wallin BG, Charkoudian N. Neural Control of the Circulation: How Sex and Age Differences Interact in Humans. Comprehensive Physiology. 2015;5:193–215. doi: 10.1002/cphy.c140005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abate NI, Mansour YH, Tuncel M, Arbique D, Chavoshan B, Kizilbash A, Howell-stampley T, Vongpatanasin W, Victor RG. Overweight and Sympathetic Overactivity in Black Americans. Hypertension. 2001;38:379–383. doi: 10.1161/01.hyp.38.3.379. [DOI] [PubMed] [Google Scholar]
- 39.Abbas A, Szczepaniak LS, Tuncel M, McGavock JM, Huet B, Fadel PJ, Wang Z, Arbique D, Victor R, Vongpatanasin W. Adiposity-independent sympathetic activity in black men. Journal of applied physiology. 2010;108(6):1613–1618. doi: 10.1152/japplphysiol.00058.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]