Abstract
Articular cartilage has a very limited ability to self-heal after injury or degeneration due to its low cellularity, poor proliferative activity, and avascular nature. Current clinical options are able to alleviate patient suffering, but cannot sufficiently regenerate the lost tissue. Biomimetic scaffolds that recapitulate the important features of the extracellular matrix (ECM) of cartilage are hypothesized to be advantageous in supporting cell growth, chondrogenic differentiation, and integration of regenerated cartilage with native cartilage, ultimately restoring the injured tissue to its normal function. It’s a challenge to support and maintain articular cartilage regenerated by bone marrow-derived mesenchymal stem cells (BMSCs), which are prone to hypertrophy and endochondral ossification after implanted in vivo. In the present work, a nanofibrous poly(L-lactic acid) (NF PLLA) scaffold developed by our group was utilized because of the desired highly porous structure, high interconnectivity, collagen-like NF architecture to support rabbit BMSCs for articular cartilage regeneration. We further hypothesized that Matrilin-3 (MATN3), a non-collagenous, cartilage-specific ECM protein, would enhance the microenvironment of the NF PLLA scaffold for cartilage regeneration and maintaining its property. To test this hypothesis, we seeded BMSCs on the NF PLLA scaffold with or without MATN3. We found that MATN3 suppresses hypertrophy in this 3D culture system in vitro. Subcutaneous implantation of the chondrogenic cell/scaffold constructs in a nude mouse model showed that pretreatment with MATN3 was able to maintain chondrogenesis and prevent hypertrophy and endochondral ossification in vivo. These results demonstrate that the porous NF PLLA scaffold treated with MATN3 represents an advantageous 3D microenvironment for cartilage regeneration and phenotype maintenance, and is a promising strategy for articular cartilage repair.
Keywords: Articular cartilage tissue engineering, Bone marrow-derived mesenchymal stem cells, Matrilin-3, Chondrogenesis, Hypertrophy, Endochondral ossification
Graphical abstract
Articular cartilage defects, caused by trauma, inflammation, or joint instability, may ultimately lead to debilitating pain and disability. Bone marrow-derived mesenchymal stem cells (BMSCs) are an attractive cell source for articular cartilage tissue engineering. However, chondrogenic induction of BMSCs is often accompanied by undesired hypertrophy, which can lead to calcification and ultimately damage the cartilage. Therefore, a therapy to prevent hypertrophy and endochondral ossification is of paramount importance to adequately regenerate articular cartilage. We hypothesized that MATN3 (a non-collagenous ECM protein expressed exclusively in cartilage) may improve regeneration of articular cartilage with BMSCs by maintaining chondrogenesis and preventing hypertrophic transition in an ECM mimicking nanofibrous scaffold. Our results showed that the administration of MATN3 to the cell/nanofibrous scaffold constructs favorably maintained chondrogenesis and prevented hypertrophy/endochondral ossification in the chondrogenic constructs in vitro and in vivo. The combination of nanofibrous PLLA scaffolds and MATN3 treatment provides a very promising strategy to generate chondrogenic grafts with phenotypic stability for articular cartilage repair.

1. Introduction
Articular cartilage defects, caused by trauma, inflammation, or joint instability, may ultimately lead to debilitating pain and disability given the body’s inability to sufficiently heal these injuries due to the lack of vascularity as well as low cell density and metabolism in cartilage tissue [1]. Current therapies such as abrasion arthroplasty, subchondral bone drilling or microfracture, transplantation of autograft, are reasonably effective at alleviating pain, but have their respective limitations including tissue hypertrophy and/or fibrosis [2]. Biomimetic scaffolds for tissue engineering provide a promising strategy to engineer cell/scaffold constructs that upon implantation, can integrate into native tissues and restore the original biological and mechanical functions of healthy tissue [3]. The goal of the current study is to develop a biomimetic scaffold microenvironment that supports chondrogenesis while preventing hypertrophy for in vivo cartilage repair.
An important consideration for tissue engineering using cell therapy is the cell source; cells must be chosen to meet the particular needs of the tissue being regenerated. Bone marrow-derived mesenchymal stem cells (BMSCs) are an attractive cell source for articular cartilage tissue engineering; they are easy to harvest, can expand quickly in vitro, and commit to chondrogenesis if stimulated with appropriate growth factors such as bone morphogenetic proteins (BMPs) and transforming growth factor-β (TGF-β). However, chondroinduction of BMSCs is often accompanied by undesired hypertrophy, which can lead to calcification and ultimately damage the cartilage [4] [5] [6]. Therefore, a therapy to prevent hypertrophy and endochondral ossification is of paramount importance to adequately regenerate articular cartilage tissue. Cartilage-specific ECM proteins have been proposed to play key roles in modulating cellular phenotypes during chondrogenesis of mesenchymal stem cells [7]. We have previously found that matrilin-3 (MATN3), a non-collagenous ECM protein expressed exclusively in cartilage, plays an important role in modulating chondrocyte differentiation [8], inhibiting premature chondrocyte hypertrophy [9], and regulating cartilage homeostasis [10]. MATN3 is endogenously expressed in cartilage tissue, and is therefore expected to be a safe therapeutic agent. We hypothesized that MATN3 may improve regeneration of articular cartilage with BMSCs by maintaining chondrogenesis and preventing hypertrophic transition.
In tissue engineering, scaffolds serve as a temporary replacement of the ECM to support cell seeding, regeneration, and the later integration of the new tissue with the adjacent host tissue. A successful biomimetic scaffold is capable of mimicking the natural ECM to facilitate cell recruiting and seeding, adhesion, proliferation, differentiation and tissue regeneration [11]. Our laboratory has developed a novel phase-separation technique to fabricate a 3D NF PLLA matrix with a macroporous structure [12]. These porous scaffolds also have nano-topographical features to imitate the ECM of native tissues and have been proven to support interactions between seeded cells and scaffolds, new ECM formation, and the intercellular connections in bone [13], nucleus pulposus [14], and cardiovascular tissue [15] [16]. Based on these findings, we hypothesized that the porous NF PLLA scaffolds would also facilitate the growth and differentiation of chondrogenic cells in 3D, and thereby promote articular cartilage regeneration.
We fabricated the porous NF PLLA scaffolds to investigate their ability to facilitate 3D BMSC growth and chondrogenic differentiation for articular cartilage formation. During the formation of cell/scaffold constructs in vitro, we added MATN3 to the culture medium to test its ability to maintain chondrogenesis and prevent hypertrophy. The cell/scaffold constructs were then tested in a subcutaneous implantation nude mouse model to determine if pretreatment with MATN3 could favorably support chondrogenic homeostasis of the engineered grafts in vivo.
2. Materials and Methods
2.1. Fabrication of Porous NF PLLA scaffolds
The porous NF PLLA scaffolds were fabricated according to the method previously reported by the Ma group [12]. Briefly, the PLLA (Boehringer Ingelheim, Ingelheim, Germany) with an inherent viscosity of approximately 1.6 dL/g was dissolved in tetrahydrofuran (10% w/v) at 60°C and cast into an assembled sugar template (composed of bound D-fructose spheres, 150–250 μm in diameter) under mild vacuum. The PLLA/D-fructose composites were phase-separated at −80°C overnight and then immersed into cyclohexane to exchange tetrahydrofuran for 2 days. The resulting composites were freeze-dried and the D-fructose spheres were leached out in distilled water to form the network with interconnected spherical pores. After freeze-drying again, the highly porous scaffolds were obtained and cut into circular discs with a diameter of 4 mm and thickness of 1.5 mm. The macro-image of the scaffold was taken under a Leica M165 FC stereo microscope (Leica Microsystems Inc., Buffalo Grove, IL).
2.2. Isolation and Culture of Rabbit Bone Marrow Mesenchymal Stem Cells (BMSCs)
Three New Zealand White Rabbits (Charles River Laboratories, Wilmington, MA) aged 8–10 weeks were anesthetized via intramuscularly injection of ketamine hydrochloride (50 mg/kg) and xylazine (10 mg/kg). After shaving and disinfecting the region, 6 mL of bone marrow was aspirated from the lateral condyle of the femur using an 18-gauge needle on a 10 mL syringe containing heparin (3000 U/mL). The bone marrow was immediately mixed with LG-DMEM (Gibco, Life Technologies Corporation, Grand Island, NY) containing 10% fetal bovine serum (FBS, Gibco) and centrifuged at 1500 rpm at 4°C for 5 min. The upper layer of fat tissue was discarded and the cells were re-suspended by LG-DMEM. The mononuclear cells (BMSCs) were separated from the cell suspension using Percoll Separating Medium (GE Healthcare Life Sciences) by centrifugation at 1800 rpm at 4°C for 20 min. The collected BMSCs were pooled and cultured with growth medium (LG-DMEM containing 10% FBS, 100 mg/mL streptomycin, and 100 U/mL penicillin) that was replaced twice a week. When cells reached 70–80% confluence, they were sub-passaged; cells prior to passage 3 were used in the following studies. The animal procedure was approved by the University of Michigan Committee on Use and Care of Laboratory Animals. Such obtained BMSCs are widely used as multipotent stem/progenitor cells for bone, cartilage and adipose tissue engineering [17], including in our lab [18].
2.3. Scanning Electron Microscopy (SEM) Observation
The blank PLLA scaffolds were first observed under a stereo microscope as described above, and then sputter-coated with gold before being imaged with a scanning electron microscope (SEM) at 15 kV with a JEOL-7800FLV SEM (JEOL, USA). After 1 and 3 days of culture, the cell/scaffold constructs were fixed with 2.5% phosphate-buffered glutaraldehyde (Sigma-Aldrich, St. Louis, MO) overnight at 4°C, and post-fixed with 1% osmium tetroxide (Sigma-Aldrich) for 1 h. These samples were then rinsed 3 times with PBS, dehydrated through a graded series of ethanol and dried using hexamethyldisilazane (HMDS, Sigma-Aldrich) as described previously [16]. After that, the samples were sputter-coated with gold and imaged at 15 kV with a JEOL-7800FLV SEM to observe cell adhesion, spreading, and ECM deposition in the scaffolds.
2.4. Cell Proliferation in the Scaffolds
For 3D culture, the porous NF PLLA scaffolds were pre-wet in 70% ethanol for 30 min, after which the internal air bubbles were expelled by mild vacuum [19]. After 3 washes with PBS and 2 with culture medium, each scaffold was seeded with 1×105 BMSCs. At days 1, 3, and 7 after seeding, the cell numbers in the scaffolds were compared by DNA assay as previously reported [20]. Briefly, the harvested cell/scaffold constructs were crushed using a plastic tissue pestle (Fisher Scientific, Pittsburg, PA) followed by gentle sonication on ice. Then lysed cells were acquired by proteinase K (0.5 mg/mL, Sigma) treatment at 56°C overnight. The resulting mixture was subjected to centrifugation and aliquots (40 μL) of the supernatants mixed with 160 μL Hoechst 33258 dye solution (0.1 g/mL, Sigma-Aldrich) were transferred to black flat-bottomed 96-well plates (Corning Costar, USA). The DNA content was compared spectrofluorometrically using a Varioskan Flash multimode reader (Thermo Scientific, Wyman Street Waltham, MA) at a wavelength of 460 nm (excitation wavelength of 360 nm). Non-seeded scaffolds were similarly treated as blank controls, the signals of which were then subtracted from the corresponding samples.
2.5. Chondrogenic Induction and Histological Evaluation
20 μL of BMSC suspension (3×107/mL) was seeded into 1 scaffold, and the cell/scaffold constructs were cultured with chondrogenic medium (HG-DMEM containing 1% FBS, 40 μg/mL proline, 0.1 μM dexamethasone, 0.1 mM ascorbic acid, 1× ITS Premix (BD Biosciences), and 10 ng/mL recombinant human TGF-β1 (PeproTech Inc., NJ, USA)). The MATN3 treatment was performed by adding 100 ng/mL MATN3 (R&D Systems, Minneapolis, MN, USA) to the chondrogenic medium to evaluate its effect during chondrogenesis. This dosage of matrilin-3 has been previously shown to be able to maintain chondrogenesis [10]. After 3 weeks of culture, the construct samples were fixed with ice-cold 4% paraformaldehyde for further Safranin O staining to evaluate chondrogenesis.
2.6. Hypertrophic Induction of the Cell/Scaffold Constructs
After 3 weeks of chondrogenic induction, the chondrogenic medium was changed into hypertrophic medium (HG-DMEM containing 1% FBS, 1 nM dexamethasone, 0.1 mM ascorbic acid, 1× ITS Premix (BD Biosciences), 10 mM β-glycerophosphate (Sigma-Aldrich), and 1 nM L-thyroxine (Sigma-Aldrich)) for 2 more weeks of culture. The MATN3 treatment was performed by adding 100 ng/mL MATN3 (R&D Systems, Minneapolis, MN, USA) to the hypertrophic medium to evaluate its effect during hypertrophy.
2.7. Biochemical Analysis for Glycosaminoglycans (GAGs) and DNA Content
Representative cell/scaffold constructs (n=3) in each group were crushed as described above. Then the mixture was digested by papain solution (125 μg/mL papain, 100 mM phosphate, 10 mM ethylenediaminetetraacetic acid (EDTA), 10 mM cysteine, pH 6.3) at 60°C overnight. The GAG content was determined via dimethylmethylene blue (DMMB, Sigma-Aldrich) assay to read the absorbance at 525 nm with bovine chondroitin sulfate (Sigma) as a standard. The GAG content was normalized by total DNA that was measured via Hoechst 33258 assay with calf thymus DNA (Sigma-Aldrich) as a standard.
2.8. Gene Expression Analysis by Real-time Polymerase Chain Reaction (Real-time PCR)
Total RNA was extracted from the cell/scaffold constructs by Trizol reagent (Life Technologies Corporation) using disposable plastic pestles (Fisher Scientific, Pittsburg, PA) to thoroughly crush the constructs. RNA concentration was determined from the optical absorbance of the extract at 260 nm. Complementary DNA (cDNA) was synthesized using the iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA) according to the manufacturer’s protocol. Real-time PCR was carried out using Taqman Universal PCR Master Mix (Applied Biosystems) with predesigned primers and probes (Applied Biosystems) for Col 2A1 (Assay ID:Oc03396134_m1), Aggrecan (Assay ID: AIY9X5B), and Col 10A1 (Assay ID: Oc04097225_s1). The reactions were performed using ABI 7500 Real-time PCR System (Applied Biosystems). The gene expression of interest was normalized by housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Assay ID: Oc03823402_g1) expression.
2.9. Alkaline phosphatase (ALP) Activity
The cell/scaffold constructs (n=3) were cultured with chondrogenic medium for 3 weeks and then were cultured with hypertrophic medium for 1 more week with or without MATN3 treatment. Samples were harvested on day 1, 3, 5 and 7 in hypertrophic culture medium. The ALP activity was determined using Alkaline Phosphatase assay kit (Anaspec) to read the absorbance at 405 nm with colorimetric alkaline phosphatase substrate (Anaspec) as a standard. The Alp content was normalized by total protein which was measured using Micro BCA protein assay kit (Thermo Scientific) with bovine serum albumin (Sigma) as a standard.
2.10. Subcutaneous Implantation
Three weeks after culture with or without MATN3 treatment in vitro, the subcutaneous implantation was performed in male athymic nude mice of 6–8 weeks old (Charles River Laboratories, Wilmington, MA) according to the protocol that was approved by the Use and Care of Laboratory Animals Committee at the University of Michigan. The surgery was performed under general anesthesia via inhalation of isoflurane. One midsagittal incision was made on the dorsa and a subcutaneous pocket was created on each side of the incision via blunt dissection. Cell/scaffold constructs pre-cultured in vitro were implanted subcutaneously into each pocket. Two samples were implanted in each mouse. After implantation, the incisions were closed with surgical staples. Eight weeks post-surgery, the mice were euthanized via CO2 asphyxiation followed by cervical dislocation, and the implants were harvested for the following histological and immunohistochemical studies.
2.11. Histological Assays
The harvested cell/scaffold constructs were fixed in 4% paraformaldehyde at 4°C overnight, followed by dehydration through a graded series of ethanol, clearing by xylene, and embedding in paraffin blocks. The cross-sections acquired at 5-μm thickness were dewaxed by xylene and a graded series of ethanol, and then stained with Safranin O to detect sulfated glycosaminoglycans (sGAG), Von Kossa staining to appraise ossification, and immunohistochemical staining to detect the expression of chondrogenic and hypertrophic makers. For immunohistochemistry, the dewaxed sections were treated with pepsin solution (FisherScientific) at room temperature for 20 min to retrieve antigens. The polyclonal goat anti-Col 2A1 (1:400), goat anti-HIF-1α (1:300), goat anti-Col 10A1 (1:400), goat anti-MMP-13 (1:200), and goat anti-Col 1A1 (1:300) were purchased from Santa Cruz Biotechnology. The polyclonal goat anti-Runx2 (1:500) was purchased from Cell Signaling Technology. All the above primary antibodies were visualized by the Anti-Goat HRP-AEC Cell & Tissue Staining Kit (R&D Systems). The histological images were taken under an Olympus BX53 microscope (Olympus Corporation, Tokyo, Japan). Von Kossa staining (3 images/group) was quantified using ImageJ (NIH, Bethesda, MD).
2.12. Statistics
Data were presented as mean ± standard deviation for 3 individuals. The experiments were performed in duplicate to ensure reproducibility. Statistical analysis for cell growth assay was performed by one-way ANOVA, and independent-samples Student’s t-tests assuming equal variance were performed for other assays using IBM SPSS Statistics Version 22 software. A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Essential Features of the NF PLLA Scaffolds to Support Cell Attachment and Growth
The biomimetic scaffolds used in this work were developed in the Ma laboratory to promote tissue regeneration by providing pores of the appropriate size and structure to mimic the native tissue ECM, which is integral to tissue regeneration [16]. These scaffolds have been shown to foster and direct cell attachment, migration, proliferation, and differentiation, as well as tissue formation and organization for targeted commitment towards the desired lineage [16]. The NF PLLA scaffolds used in this work showed a uniform porous structure with a high level of interconnectivity among the pores (Fig. 1A-C). The walls of the pores consisted of nanofibers (Fig. 1D) that mimic the natural collagen fibers in the ECM [14]. These features supported uniform cell retention in the pores and cell attachment onto the pore walls in the scaffolds 24h after seeding (Fig. 1E). After three days, cells adhered to the scaffold and produced ECM both along the NF walls and across the pores in the scaffolds (Fig. 1F), which is indicative of favorable environment for regeneration.
Fig. 1.
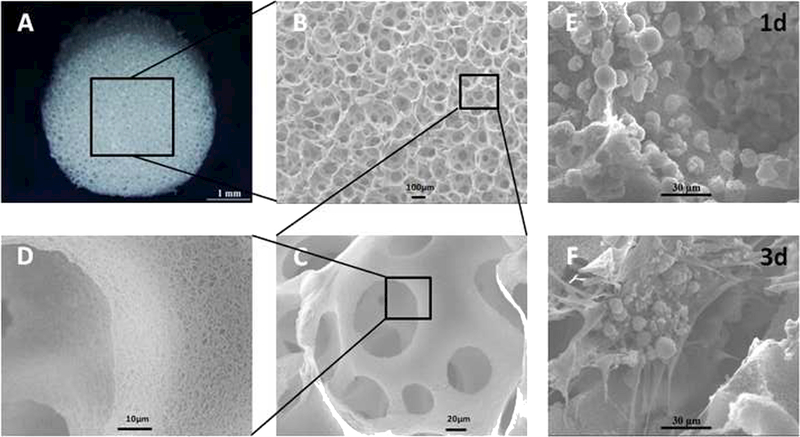
Gross and scanning electron microscopy (SEM) view of porous NF PLLA scaffolds as well as cell growth in the scaffolds. Gross (A) and SEM (B-D) observation showed that the PLLA scaffolds were fabricated with a uniform porous structure and high interconnectivity. The walls of the pores are nanofibrous. The BMSCs were retained in the pores, adhered to the walls, and started to secret ECM in the scaffolds at day 3 (E & F).
3.2. Cell Growth in the Scaffolds
Next, we determined if the porous scaffolds could support cell growth in 3D. The proliferation of BMSCs in the scaffolds was quantified using DNA content (Fig. 2). Significant proliferation was observed during the 7 days of culture.
Fig. 2.
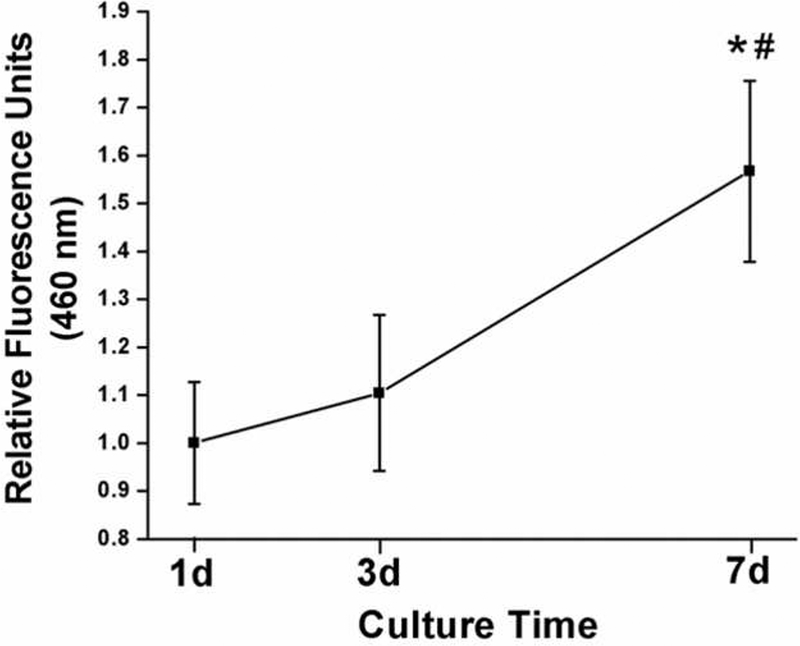
The proliferation of BMSCs in the NF PLLA scaffolds. Rabbit BMSCs were seeded into the porous PLLA scaffolds. After in culture for 1, 3, and 7 days, the cell/scaffold constructs were harvested and used to quantify cell proliferation (DNA content) with the Hoechst 33258 method. Here, * or # designate data that is significantly different from that of 1d or 3d, respectively.
3.3. MATN3 Facilitated BMSC Chondrogenic Differentiation and Hypertrophic Transition Suppression in Scaffolds in vitro
Next, we investigated whether the scaffolds could support the chondrogenic differentiation of the seeded BMSCs in 3D in vitro. The chondrogenic medium containing TGF-β1 successfully directed BMSCs into chondrogenesis as confirmed by positive Safranin O staining showing high expression of GAG after 3 weeks of culture (Fig. 3, upper panel). The control group showed no chondrogenesis as the cells appeared to be undergoing apoptosis due to the low level of FBS (1%) that was administered (Fig. 3, Control). Next, we investigated whether MATN3 treatment might benefit the chondrogenic differentiation of cells in 3D culture. We found that in the absence of TGF-β1 induction, MATN3 did not significantly initiate chondrogenesis (Fig. 3, MATN3). In the groups treated with TGF-β1, addition of MATN3 did not enhance chondrogenesis, as determined by Safranin O staining after 3 weeks of culture (Fig. 3, Chondro+MATN3). We subsequently treated the chondrogenic constructs with hypertrophic medium for 2 weeks to induce hypertrophy. The hypertrophic transition was confirmed by greatly decreased Safranin O staining compared with the chondrogenic constructs before hypertrophic induction (Fig. 3, Hyper). We found that MATN3 treatment was able to temper the reduction of chondrogenesis (Fig. 3, Hyper+MATN3), although Safranin O staining level did not reach those observed in the chondrogenic induction group at 3 weeks. This indicates that MATN3 treatment can maintain significant levels of chondrogenesis after hypertrophic induction but is not able to improve chondrogenesis alone. These observations were confirmed by quantification of GAG content (Fig. 3, lower panel) which showed an increase with time similar to the trend seen with Safranin O staining. As indicated in Fig. 3, the scaffolds supported GAG expression that reached a maximum after 3 weeks of chondrogenic induction. After 2 subsequent weeks of hypertrophic induction, the GAG expression decreased by 36.4%. Importantly, MATN3 treated samples were able to maintain significantly higher expression of GAG than the non-MATN3-treated group.
Fig. 3.
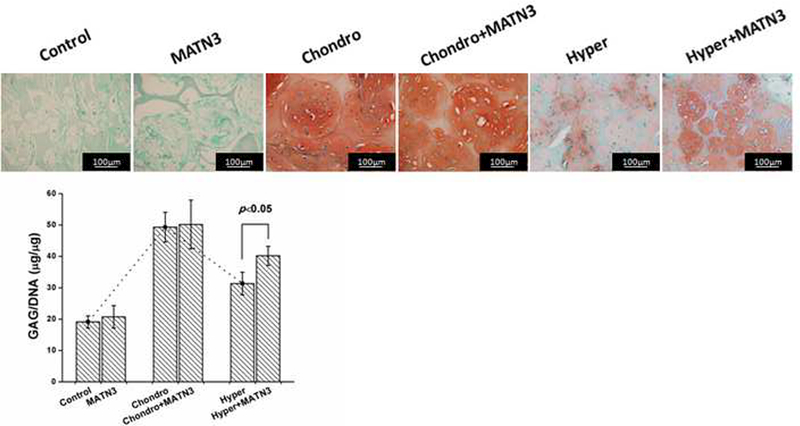
Safranin O staining and biochemical analysis to evaluate the maintenance of chondrogenesis and prevention of hypertrophy by MATN3 in the 3D culture system. The cell/scaffold constructs were cultured with growth medium or chondrogenic medium both with or without MATN3 treatment for 3 weeks. For the study of the effect of MATN3 on hypertrophy, a total culture time of 5 weeks was used: after the 3 weeks of chondrogenic induction, the chondrogenic medium was changed into hypertrophic medium (see Materials and Methods) for 2 more weeks of culture with or without MATN3 treatment (100 ng/mL MATN3). Safranin O staining was performed to detect glycosaminoglycans (GAG) expression (upper panel). Scale bar=100 μm. Biochemical analysis was performed to evaluate the GAG content that was normalized by total DNA (lower panel). Matrilin-3 did not affect the GAG production during the chondrogenic phase (the first 3 weeks in chondrogenic medium), but had a protective effect in reducing the negative effect in the hypertrophic phase (the 2 additional weeks of culture in hypertrophic medium).
The transitional profile of cell differentiation in the constructs at gene level was further investigated by real-time PCR (Fig. 4). After 3 weeks of chondrogenic induction, the MATN3 treatment did not increase Col 2A1 mRNA expression, but did show increased expression of another chondrogenic marker gene, Aggrecan. After 2 weeks of hypertrophic induction, MATN3 treatment also significantly suppressed hypertrophic marker gene Col 10A1 mRNA expression, though the decrease of Col 2A1 and Aggrecan mRNA expression observed in cultures without MTN3 was only partially correctec by MTN3.
Fig. 4.
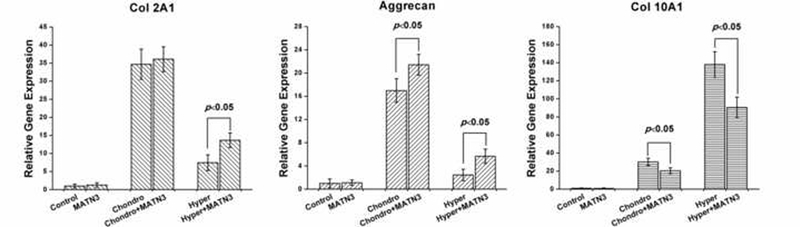
Real-time polymerase chain reaction (real-time PCR) to evaluate the maintenance of chondrogenesis and prevention of hypertrophy by MATN3 in the 3D culture system. Real-time PCR was performed on the samples harvested at different time points (as described in Fig. 3) to evaluate the gene expression of chondrogenic markers (Col 2A1and Aggrecan) and hypertrophic marker (Col 10A1). The gene expression of interest was normalized by housekeeping gene GAPDH expression.
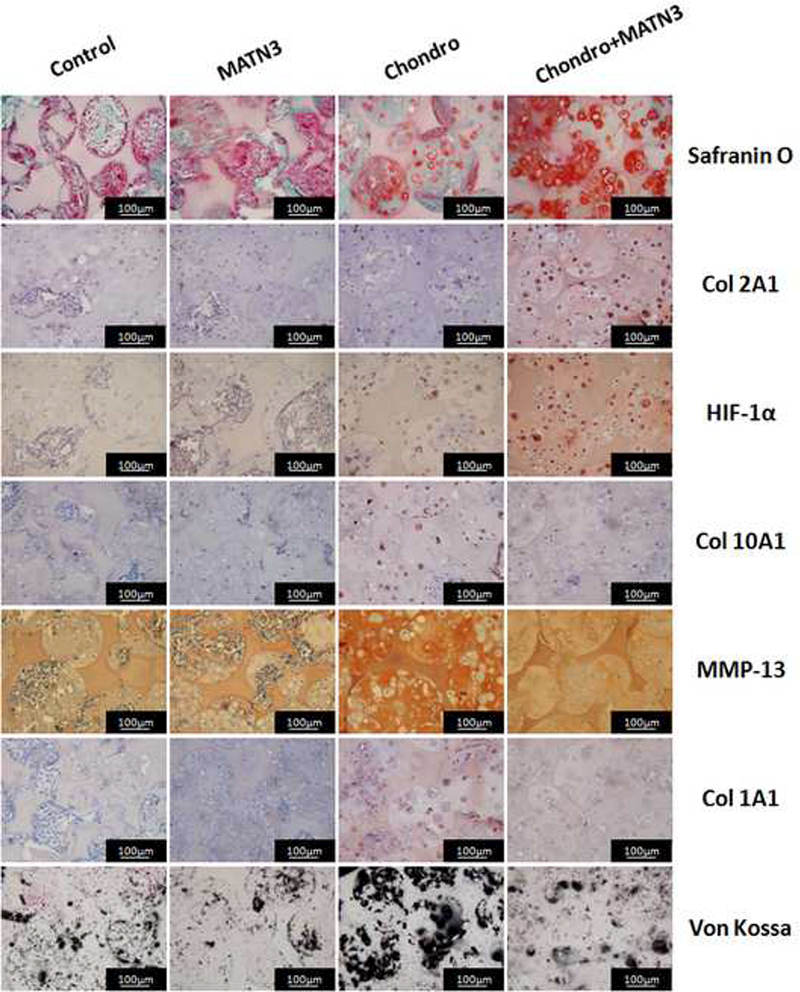
Immunohistochemical staining was performed to detect marker protein expression during chondrogenic and hypertrophic transitions in 3D culture (Fig. 5). Col 2A1 staining showed a trend similar to that seen in Safranin O staining. MATN3 treated samples showed no clear difference from non-treated groups after 3 weeks of chondrogenic induction, but MATN3 treated samples maintained higher levels of Col 2A1 expression after hypertrophic induction when compared to untreated cultures (Fig. 5, Col 2A1). The expression of hypoxia-inducible factor 1a (HIF-1α) in MATN3 treated samples undergoing chondrogenic induction showed a similar trend, expressing higher levels of HIF-1α after 3 weeks of culture compared to non-treated samples (Fig. 5, HIF-1a). Col 10A1, Runx2 and von Kossa staining confirmed the hypertrophic transition after 5 weeks of culture. MATN3 treatment resulted in significantly weaker Col 10A1, Runx2, and von Kossa staining after hypertrophic induction (Fig. 5, Col 10A1, Runx2, von Kossa). There was no detectable expression of protein MMP-13, another hypertrophic marker, at any time point during the process in this 3D culture system in vitro (Fig. 5, MMP-13). We also assessed the levels of osteogenesis and fibrosis in the constructs by staining for Col 1A1. After 2 more weeks of hypertrophic induction, some of the cells in the scaffolds showed Col 1A1 expression that was not clearly seen in MATN3-treated group, again indicating that MATN3 protected chondrogenesis (Fig. 5, Col 1A1). ALP activity of BMSCs during the one-week culture in hypertrophic medium with or without MATN3 was examined. The ALP activity was significantly reduced with MATN3 treatment at days 1, 3, and 5 compared to the group without MATN3 (Fig. 6). These results consistently indicated that MATN3 had protective effect against hypertrophy.
Fig. 5.
Staining to detect the expression of chondrogenic, hypertrophic, and osteogenic or fibrosis marker proteins in the cell/scaffold constructs in vitro. Staining was done on samples harvested (as described in Fig. 3) to evaluate the maintenance of chondrogenesis and prevention of hypertrophy by MATN3 in the 3D culture system. Scale bar=100 μm.
Fig. 6.

ALP activity of rabbit BMSCs/scaffold constructs cultured in hypertrophic medium with or without MATN3. The cell/scaffold constructs were cultured with chondrogenic medium for 3 weeks and then were cultured with hypertrophic medium for 1 more weeks with or without MATN3 treatment. ALP activity test was done on samples harvested on day1,3,5, and 7 in hypertrophic medium to evaluate the effect of MATN3 on the prevention of hypertrophy.
3.4. MATN3 Pretreatment Maintains Chondrogenesis and Prevents Hypertrophy and Endochondral Ossification in vivo
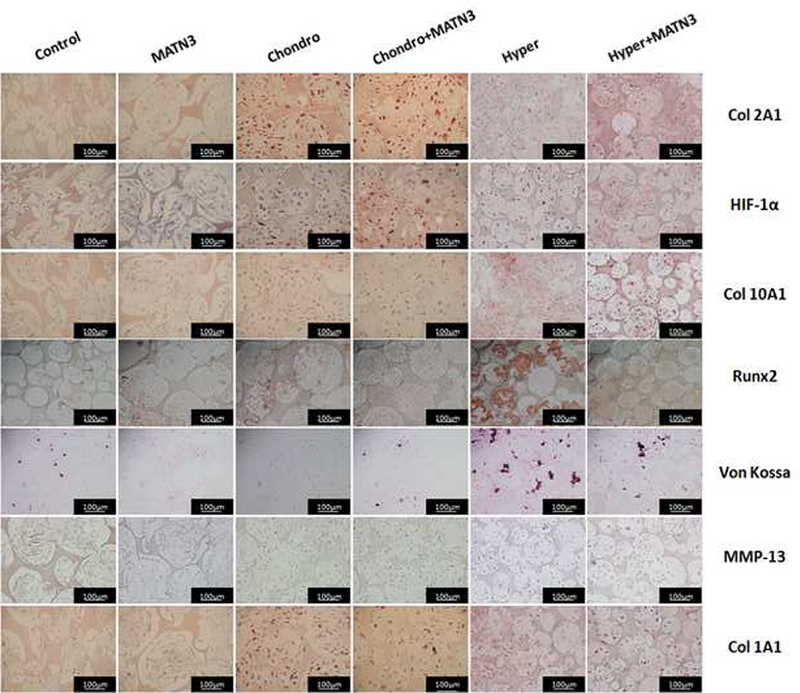
Next, we performed subcutaneous implantation of the pre-cultured construct into a nude mouse model to investigate the scaffold’s ability to enhance chondrogenesis of seeded cells and whether the MATN3 pretreatment showed protective effects in vivo. Safranin O staining showed that the pores in the implants pre-cultured with growth medium were colonized by host cells but not cartilage-like tissue (Fig. 7A, Control). Pretreatment with MATN3 resulted in some levels of GAG expression in the implants (Fig. 7A, MATN3). The constructs pre-cultured with chondrogenic medium alone showed low GAG expression (Fig. 7A, Chondro). In contrast, pretreatment with both chondrogenic medium and MATN3 in vitro successfully maintained GAG expression in the constructs in vivo (Fig. 7A, Chondro+MATN3). Similarly, immunohistochemistry staining showed that MATN3 treatment reduced the loss of both Col 2A1 and HIF-1α expression after implantation (Fig. 7A, Col 2A1). Additionally, the pretreatment with chondrogenic medium alone resulted in hypertrophic marker protein Col 10A1 expression in the constructs after subcutaneous implantation, but this was prevented by pretreatment with MATN3 (Fig. 7A, Col 10A1). Contrary to the in vitro study, which showed a lack of hypertrophic protein MMP-13, MMP-13 expression was detected in the chondrogenic induction alone group after implantation. This expression was significantly suppressed by pretreatment with MATN3 in vivo (Fig. 7A, MMP-13). Overall, hypertrophic transition was confirmed by both Col 10A1 and MMP-13 detection and exhibited a similar trend to the osteogenic or fibrotic transitions, which were confirmed by Col 1A1 detection. Von Kossa staining showed that there was weak ossification found in the groups that did not undergo chondrogenic induction. In contrast, substantial ossification was found in the chondrogenic induction alone group, but was decreased with MATN3 pretreatment (Fig. 7A, von Kossa; quantified in Fig. 7B).
Fig. 7.
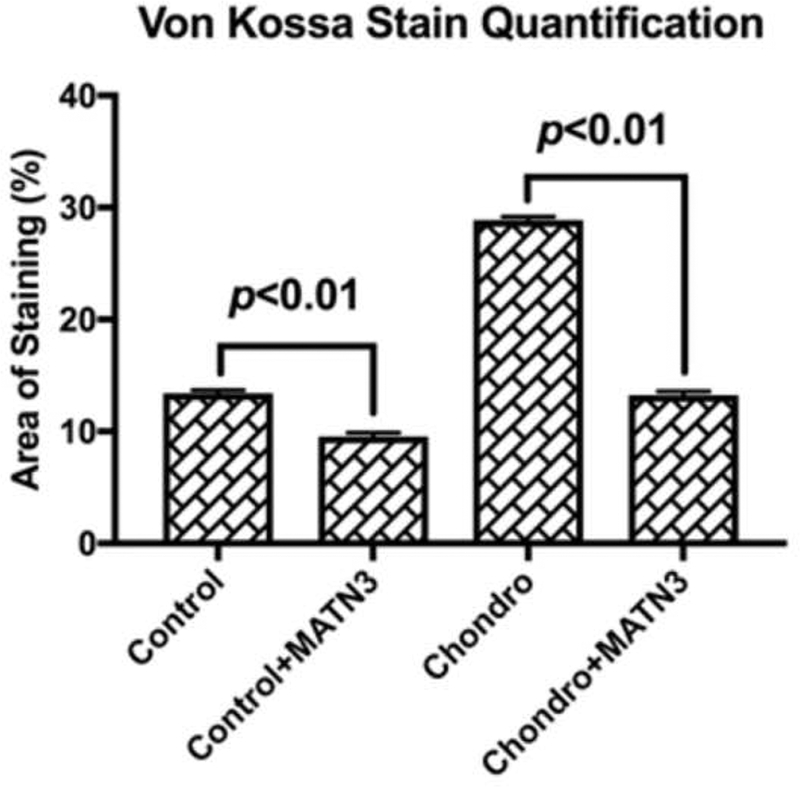
Pretreatment by MATN3 maintains chondrogenesis and prevents hypertrophy and endochondral ossification in subcutaneous implants. The cell/scaffold constructs were cultured with growth medium or chondrogenic medium with or without MATN3 treatment for 3 weeks in vitro. The constructs were then subcutaneously implanted in athymic nude mice. Eight weeks post-surgery, the mice were euthanized and the implants were harvested for Safranin O, Von Kossa, and immunohistochemical staining to evaluate the maintenance of chondrogenesis and prevention of hypertrophy and endochondral ossification by MATN3 treatment in the 3D constructs in vivo, Scale bar=100 μm (A). Quantification of mineralization based on von Kossa staining (B).
4. Discussion
The goal of the present study was to develop an efficient strategy to generate chondrogenic constructs with phenotypic stability for articular cartilage repair in vivo. Our data show that the porous NF PLLA scaffolds, by mimicking the physiological microenvironment, support the growth and chondrogenic differentiation of BMSCs in 3D. MATN3 has clearly shown beneficial effects on chondrogenic differentiation of BMSCs and preventive effects on cells’ hypertrophic transition in 3D both in vitro and in vivo. Furthermore, pretreatment with MATN3 helped maintain chondrogenesis while preventing hypertrophy and endochondral ossification in the chondrogenic cell/scaffold constructs in vivo.
In this study, the nanofibrous nature of the scaffolds mimics the structure of collagen fibers, a feature that was expected to aid in cartilage tissue regeneration. Our scaffolds were made with a highly interconnected, porous network structure that aids in the diffusion of oxygen and nutrients as well as waste removal. The open structure of the pores also benefits cell proliferation, communication, and reorganization as well as invasion of the host tissue [21]. The 3D culture showed uniform cell distribution after seeding and robust cell growth with sufficient integration to the pore walls in the scaffolds, indicating improved intercellular coupling and communication in the scaffolds. The chondrogenic differentiation of the cells was confirmed by substantial ECM production that covered the NF surface of the pore walls. These findings further support previous evidence that the NF networks were able to help regulate the structure and function of cells and tissues in the 3D scaffold via physical and biological signaling pathways [22] [23]. Moreover, the subcutaneous implantation showed that some of the host cells invaded the cell/scaffold constructs along the porous networks, indicating that the scaffolds’ porous architecture may facilitate in vivo integration with the adherent host tissue [16].
Cell source remains an important consideration in articular cartilage regeneration. While transplantation of chondrocytes has demonstrated clinical success in the treatment of injured knee joints [24] [25] [26] [27], donor-site morbidity and dedifferentiation of chondrocytes during expansion in vitro limited their use in clinical practice [4, 28, 29]. As previously mentioned, BMSCs have been proposed as a candidate cell source for cartilage tissue engineering due to their many advantages. It has been shown that at the onset of endochondral bone formation, mesenchymal cells of chondrogenic progeny undergo condensation followed by differentiation into chondrocytes. These chondrocytes then undergo a process of proliferation, maturation, and hypertrophy, followed by calcification of the ECM, cell death, and replacement by bone tissue [8]. Thus, a large hurdle to use BMSCs as the cell source for articular cartilage repair is to ensure the stability of the chondrogenic phenotype of the cell/scaffold constructs. As MATN3, a noncollagenous ECM protein, has been reported to play a regulatory role in cartilage homeostasis [10], we administered the chondrogenesis-protective MATN3 in the 3D constructs. MATN3 was not sufficient to promote chondrogenic differentiation program as it was found that only some chondrogenic gene markers, including Aggrecan, were increased in expression. It was found however, that treatment with MATN3 inhibited hypertrophic transition in 3D culture. Pretreatment with MATN3 also retained chondrogenic phenotype and prevented hypertrophic transition in a subcutaneous implantation model. The pretreatment with MATN3 also suppressed endochondral ossification in vivo as confirmed by Von Kossa staining. In cartilage tissue engineering, we do not propose to use a biphasic pre-differentiation protocol. Instead, we suggest pre-treating with MATN3 or incorporating it into 3D scaffolding to prevent MSCs from hypertrophy or endochondral ossification during cartilage repair.
MATN3 contains a von Willebrand factor A (vWFA) domain, 4 consecutive epidermal growth factor (EGF)-like repeats, and a single C-terminal coiled-coil domain [30]. As an ECM protein, MATN3 was thought to play a major structural role in forming a filamentous matrix network in cartilage by interacting with collagen fibrils, multiple proteoglycans, and other glycoproteins. It has been demonstrated that MATNs interact with small proteoglycans that bind TGF-β [31], which may in turn affect TGF-β signaling. Our group also performed extensive studies to explore the underlying mechanisms that address the chondroprotective properties of MATN3. As a novel BMP-2-binding protein and antagonist in the cartilage ECM, MATN3 may have the inherent ability to inhibit premature chondrocyte hypertrophy by suppressing BMP-2/Smad1 activity [9]. MATN3 was further found to play a novel regulatory role in cartilage homeostasis due to its capacity to induce anti-inflammatory cytokine IL-1 receptor antagonist (IL-1RA) expression, to upregulate anabolic gene expression of Col 2A1 and Aggrecan, to downregulate catabolic gene expression of ADAMTS-4, 5 and MMP-13, the matrix-degrading proteinases [10]. In the present study, we found MATN3 promoted expression of HIF-1α both in vitro and in vivo. HIF-1α has been reported to promote homeostatic pathways in the articular chondrocytes in the synovial joint [32]. HIF-1α supports normal cartilage ECM synthesis and chondrocyte differentiation [33] and promotes autophagy—all critical functions in articular cartilage homeostasis [34]. Thus, our study revealed that the MATN3 treatment possessed chondroprotective properties in this 3D engineering system through HIF-1α signaling pathway. However, further studies are needed to understand the underlying mechanisms.
We chose Col 10A1, Runx2, von Kossa, and MMP-13 to assess hypertrophy as reported in the literature [35, 36]. The results showed that hypertrophic medium increased Col 10, Runx2 and von Kossa, which is consistent with literature [35]. ALP as another hypertrophic indicator has been examined. Importantly, we found that MATN3 could reduce the expression of these hypertrophic markers and play a chondroprotective role. No clear alteration in MMP-13 expression was found in the hypertrophic culture with or without MATN3. However, increased expression of MMP-13, which has been reported to play a major role in the process of endochondral ossification [37], was detected in the BMSC/scaffold constructs in the subcutaneous implantation model. Therefore, pretreatment with MATN3 significantly inhibited this alteration in vivo. Furthermore, we found an increased expression of the osteogenic and fibrotic transition marker protein (Col I) during the late stage of 3D culture in vitro. As has been reported, chondrogenic induction of BMSCs is often accompanied by both hypertrophy and osteogenesis, which could lead to apoptosis and calcification [4] [5], ultimately leading to the failure of articular cartilage regeneration. Our study confirmed that MATN3 treatment favorably inhibited Col I expression in 3D culture as indicated by immunohistochemical staining.
MATN3, a water-soluble protein was administered to cells through the culture media used in 3D cell-culture on the NF PLLA scaffolds. Although the mechanism through which the chondroprotective properties of MATN3 work is largely unknown, we hypothesize that a continuous, controlled release of MATN3 may be beneficial to articular cartilage tissue engineering in vivo. Our lab has previously developed controlled release systems for both growth factors and microRNA from the NF PLLA scaffolds for soft tissue [38] [39], dentin [40], and bone [41] [42] tissue engineering. Future work will focus on developing a new system utilizing the NF PLLA scaffolds to control the release of MATN3 for articular cartilage tissue regeneration. While this study showed promising results, including the advantages of the porous NF PLLA scaffolds and the chondrogenesis-protective properties of MATN3, further studies of 3D culture in vitro and especially animal studies using an articular cartilage defect model are needed to confirm the efficacy of this therapy. Such studies will focus on the long-term function and phenotypic stability of the constructs, as well as their resistance to vascular invasion and calcification in vivo, and an investigation into the appropriate degradation rate of the scaffold to facilitate integration with the adjacent host tissue. With these studies, we hope to prove the efficacy, safety, and clinical relevance of the proposed therapy for which this paper lays the foundation.
5. Conclusions
We fabricated porous NF PLLA scaffolds to mimic the natural ECM in cartilage tissue to benefit the seeding, growth, and chondrogenic differentiation of BMSCs. The administration of MATN3 to the cell/scaffold constructs favorably maintained chondrogenesis and prevented hypertrophy/endochondral ossification in the chondrogenic constructs in vitro and in vivo. The combination of NF PLLA scaffolds and MATN3 pre-treatment provides a promising strategy to generate chondrogenic grafts with phenotypic stability for articular cartilage repair.
Articular cartilage defects, caused by trauma, inflammation, or joint instability, may ultimately lead to debilitating pain and disability. Bone marrow-derived mesenchymal stem cells (BMSCs) are an attractive cell source for articular cartilage tissue engineering. However, chondrogenic induction of BMSCs is often accompanied by undesired hypertrophy, which can lead to calcification and ultimately damage the cartilage. Therefore, a therapy to prevent hypertrophy and endochondral ossification is of paramount importance to adequately regenerate articular cartilage. We hypothesized that MATN3 (a non-collagenous ECM protein expressed exclusively in cartilage) may improve regeneration of articular cartilage with BMSCs by maintaining chondrogenesis and preventing hypertrophic transition in an ECM mimicking nanofibrous scaffold. Our results showed that the administration of MATN3 to the cell/nanofibrous scaffold constructs favorably maintained chondrogenesis and prevented hypertrophy/endochondral ossification in the chondrogenic constructs in vitro and in vivo. The combination of nanofibrous PLLA scaffolds and MATN3 treatment provides a very promising strategy to generate chondrogenic grafts with phenotypic stability for articular cartilage repair.
Acknowledgements
The authors gratefully acknowledge funding from NIH (DE022327, HL136231, HL114038: PXM; AR069383: YPC; and GM104937: QC).
Footnotes
Disclosure
There are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Vinatier C, Mrugala D, Jorgensen C, Guicheux J, Noel D. Cartilage engineering: a crucial combination of cells, biomaterials and biofactors. Trends Biotechnol. 2009;27:307–14. [DOI] [PubMed] [Google Scholar]
- [2].Santo VE, Gomes ME, Mano JF, Reis RL. Controlled release strategies for bone, cartilage, and osteochondral engineering--Part I: recapitulation of native tissue healing and variables for the design of delivery systems. Tissue Eng Part B Rev. 2013;19:308–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. [DOI] [PubMed] [Google Scholar]
- [4].Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, Aigner T, Richter W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–66. [DOI] [PubMed] [Google Scholar]
- [5].Mueller MB, Tuan RS. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 2008;58:1377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338:917–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pei M, Luo J, Chen Q. Enhancing and maintaining chondrogenesis of synovial fibroblasts by cartilage extracellular matrix protein matrilins. Osteoarthritis Cartilage. 2008;16:1110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].van der Weyden L, Wei L, Luo J, Yang X, Birk DE, Adams DJ, Bradley A, Chen Q. Functional knockout of the matrilin-3 gene causes premature chondrocyte maturation to hypertrophy and increases bone mineral density and osteoarthritis. Am J Pathol. 2006;169:515–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yang X, Trehan SK, Guan Y, Sun C, Moore DC, Jayasuriya CT, Chen Q. Matrilin-3 inhibits chondrocyte hypertrophy as a bone morphogenetic protein-2 antagonist. J Biol Chem. 2014;289:34768–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jayasuriya CT, Goldring MB, Terek R, Chen Q. Matrilin-3 induction of IL-1 receptor antagonist is required for up-regulating collagen II and aggrecan and down-regulating ADAMTS-5 gene expression. Arthritis Res Ther. 2012;14:R197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008;60:184–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wei G, Ma PX. Macroporous and nanofibrous polymer scaffolds and polymer/bone-like apatite composite scaffolds generated by sugar spheres. J Biomed Mater Res A. 2006;78:306–15. [DOI] [PubMed] [Google Scholar]
- [13].Smith LA, Liu X, Hu J, Ma PX. The enhancement of human embryonic stem cell osteogenic differentiation with nano-fibrous scaffolding. Biomaterials. 2010;31:5526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Feng G, Zhang Z, Jin X, Hu J, Gupte MJ, Holzwarth JM, Ma PX. Regenerating nucleus pulposus of the intervertebral disc using biodegradable nanofibrous polymer scaffolds. Tissue Eng Part A. 2012;18:2231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang Y, Hu J, Jiao J, Liu Z, Zhou Z, Zhao C, Chang LJ, Chen YE, Ma PX, Yang B. Engineering vascular tissue with functional smooth muscle cells derived from human iPS cells and nanofibrous scaffolds. Biomaterials. 2014;35:8960–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liu Q, Tian S, Zhao C, Chen X, Lei I, Wang Z, Ma PX. Porous nanofibrous poly(L-lactic acid) scaffolds supporting cardiovascular progenitor cells for cardiac tissue engineering. Acta Biomater. 2015;26:105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bakhtina A, Tohfafarosh M, Lichtler A, Arinzeh TL. Characterization and differentiation potential of rabbit mesenchymal stem cells for translational regenerative medicine. In Vitro Cell Dev Biol Anim. 2014;50:251–60. [DOI] [PubMed] [Google Scholar]
- [18].Zhang Z, Gupte MJ, Jin X, Ma PX. Injectable Peptide Decorated Functional Nanofibrous Hollow Microspheres to Direct Stem Cell Differentiation and Tissue Regeneration. Advanced Functional Materials. 2015;25:350–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hu J, Feng K, Liu X, Ma PX. Chondrogenic and osteogenic differentiations of human bone marrow-derived mesenchymal stem cells on a nanofibrous scaffold with designed pore network. Biomaterials. 2009;30:5061–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liu Q, Cen L, Yin S, Chen L, Liu G, Chang J, Cui L. A comparative study of proliferation and osteogenic differentiation of adipose-derived stem cells on akermanite and beta-TCP ceramics. Biomaterials. 2008;29:4792–9. [DOI] [PubMed] [Google Scholar]
- [21].Loh QL, Choong C. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng Part B Rev. 2013;19:485–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liu X, Jin X, Ma PX. Nanofibrous hollow microspheres self-assembled from star-shaped polymers as injectable cell carriers for knee repair. Nat Mater. 2011;10:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dvir T, Timko BP, Kohane DS, Langer R. Nanotechnological strategies for engineering complex tissues. Nat Nanotechnol. 2011;6:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bentley G, Biant LC, Carrington RW, Akmal M, Goldberg A, Williams AM, Skinner JA, Pringle J. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85:223–30. [DOI] [PubMed] [Google Scholar]
- [25].Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–95. [DOI] [PubMed] [Google Scholar]
- [26].Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grontvedt T, Solheim E, Strand T, Roberts S, Isaksen V, Johansen O. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86-A:455–64. [DOI] [PubMed] [Google Scholar]
- [27].Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85-A Suppl 2:17–24. [DOI] [PubMed] [Google Scholar]
- [28].Benz K, Breit S, Lukoschek M, Mau H, Richter W. Molecular analysis of expansion, differentiation, and growth factor treatment of human chondrocytes identifies differentiation markers and growth-related genes. Biochem Biophys Res Commun. 2002;293:284–92. [DOI] [PubMed] [Google Scholar]
- [29].Schnabel M, Marlovits S, Eckhoff G, Fichtel I, Gotzen L, Vecsei V, Schlegel J. Dedifferentiation-associated changes in morphology and gene expression in primary human articular chondrocytes in cell culture. Osteoarthritis Cartilage. 2002;10:62–70. [DOI] [PubMed] [Google Scholar]
- [30].Wagener R, Kobbe B, Paulsson M. Primary structure of matrilin-3, a new member of a family of extracellular matrix proteins related to cartilage matrix protein (matrilin-1) and von Willebrand factor. FEBS Lett. 1997;413:129–34. [DOI] [PubMed] [Google Scholar]
- [31].Wiberg C, Klatt AR, Wagener R, Paulsson M, Bateman JF, Heinegard D, Morgelin M. Complexes of matrilin-1 and biglycan or decorin connect collagen VI microfibrils to both collagen II and aggrecan. J Biol Chem. 2003;278:37698–704. [DOI] [PubMed] [Google Scholar]
- [32].Husa M, Liu-Bryan R, Terkeltaub R. Shifting HIFs in osteoarthritis. Nat Med. 2010;16:641–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Duval E, Leclercq S, Elissalde JM, Demoor M, Galera P, Boumediene K. Hypoxia-inducible factor 1alpha inhibits the fibroblast-like markers type I and type III collagen during hypoxia-induced chondrocyte redifferentiation: hypoxia not only induces type II collagen and aggrecan, but it also inhibits type I and type III collagen in the hypoxia-inducible factor 1alpha-dependent redifferentiation of chondrocytes. Arthritis Rheum. 2009;60:3038–48. [DOI] [PubMed] [Google Scholar]
- [34].Lafont JE, Talma S, Murphy CL. Hypoxia-inducible factor 2alpha is essential for hypoxic induction of the human articular chondrocyte phenotype. Arthritis Rheum. 2007;56:3297– 306. [DOI] [PubMed] [Google Scholar]
- [35.Tang Q, Zheng G, Feng Z, Tong M, Xu J, Hu Z, Shang P, Chen Y, Wang C, Lou Y, Chen D, Zhang D, Nisar M, Zhang X, Xu H, Liu H. Wogonoside inhibits IL-1beta induced catabolism and hypertrophy in mouse chondrocyte and ameliorates murine osteoarthritis. Oncotarget. 2017;8:61440–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ramezanifard R, Kabiri M, Hanaee Ahvaz H. Effects of platelet rich plasma and chondrocyte co-culture on MSC chondrogenesis, hypertrophy and pathological responses. EXCLI J. 2017;16:1031–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hosaka Y, Saito T, Sugita S, Hikata T, Kobayashi H, Fukai A, Taniguchi Y, Hirata M, Akiyama H, Chung UI, Kawaguchi H Notch signaling in chondrocytes modulates endochondral ossification and osteoarthritis development. Proc Natl Acad Sci U S A. 2013;110:1875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wei G, Jin Q, Giannobile WV, Ma PX. Nano-fibrous scaffold for controlled delivery of recombinant human PDGF-BB. J Control Release. 2006;112:103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jin Q, Wei G, Lin Z, Sugai JV, Lynch SE, Ma PX, Giannobile WV. Nanofibrous scaffolds incorporating PDGF-BB microspheres induce chemokine expression and tissue neogenesis in vivo. PLoS One. 2008;3:e1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wang W, Dang M, Zhang Z, Hu J, Eyster TW, Ni L, Ma PX. Dentin regeneration by stem cells of apical papilla on injectable nanofibrous microspheres and stimulated by controlled BMP-2 release. Acta Biomater. 2016;36:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wei G, Jin Q, Giannobile WV, Ma PX. The enhancement of osteogenesis by nano-fibrous scaffolds incorporating rhBMP-7 nanospheres. Biomaterials. 2007;28:2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhang X, Li Y, Chen YE, Chen J, Ma PX. Cell-free 3D scaffold with two-stage delivery of miRNA-26a to regenerate critical-sized bone defects. Nat Commun. 2016;7:10376. [DOI] [PMC free article] [PubMed] [Google Scholar]


