Abstract
Background
Recent studies indicate that excess total body adipose contributes to exercise intolerance in heart failure with preserved ejection fraction (HFpEF). However, the impact of the pattern of regional (abdominal, cardiac, intermuscular) adipose deposition on exercise intolerance in HFpEF is unknown.
Methods
We measured total body (dual-energy x-ray absorptiometry) and regional adipose (magnetic resonance imaging), peak oxygen uptake (peak VO2), 6-minute walk distance (6MWD), short physical performance battery (SPPB), and leg press power in 100 older obese HFpEF patients and 61 healthy controls (HC), and adjusted for age, gender, race, and body surface area.
Results
Peak VO2 (15.7±0.4 vs. 23.0±0.6 ml/kg/min; p<0.001), 6MWD (427±7 vs. 538±10 m; p<0.001), SPPB (10.3±0.2 vs. 10.9±0.2; P<0.05) and leg power (117±5 vs. 152±9 watts, p=0.004) were significantly lower in HFpEF versus HC. Total fat mass, total percent fat, abdominal subcutaneous fat, intra-abdominal fat, and thigh intermuscular fat were significantly higher, while epicardial fat was significantly lower in HFpEF versus HC. After adjusting for total body fat, intra-abdominal fat remained significantly higher while epicardial fat remained significantly lower in HFpEF. Abdominal subcutaneous fat, thigh subcutaneous fat, and thigh intermuscular fat/skeletal muscle ratio were inversely associated, while epicardial fat was directly associated, with peak VO2, 6MWD, SPPB, and leg power. With multiple stepwise regression, intra-abdominal fat was the strongest independent predictor of peak VO2 and 6MWD.
Conclusions
In metabolic/obese HFpEF, the pattern of regional adipose deposition may have important adverse consequences beyond total body adipose. Interventions targeting intra-abdominal and intermuscular fat could potentially improve exercise intolerance.
Clinical Trial Registration
www.clinicaltrials.gov Identifier: NCT00959660
Keywords: Heart failure with preserved ejection fraction, Aging, Obesity, Adipose, Physical Function, Exercise
Introduction
Heart failure (HF) with preserved ejection fraction (HFpEF) is the fastest growing form of HF and is associated with high morbidity and mortality.(1) Exercise intolerance, manifested as severe exertional dyspnea and fatigue, is a hallmark of chronic HFpEF and is associated with reduced quality-of-life (QoL).(2,3) The mechanisms of exercise intolerance are incompletely understood, however it appears that abnormalities in non-cardiac, systemic factors are important contributors in addition to cardiac function.(3–9)
Obesity is a major independent risk factor for development of HF,(10) and >80% of HFpEF patients are overweight/obese.(11,12) Increased adiposity promotes inflammation, hypertension, dyslipidemia and insulin resistance and impairs cardiac, vascular, pulmonary, and skeletal muscle function, all of which contribute to the pathophysiology of HFpEF.(7,12–15) Multiple lines of evidence suggest that excess body adipose tissue contributes to reduced peak exercise oxygen uptake (peak VO2) in HFpEF.(12,14–16) Adipose-induced inflammation has wide-ranging adverse effects including coronary and systemic microvascular endothelial dysfunction, capillary rarefaction and impaired skeletal muscle mitochondrial function and protein synthesis that result in reduced skeletal muscle oxygen delivery and extraction.(7,9,14,16) Emerging data suggest that, in addition to the amount of total body adipose tissue, the specific location of adipose tissue may play a role in adverse outcomes, including exercise intolerance.(4,14,17,18) However, the impact of adipose distribution on exercise performance has not been systematically examined in HFpEF.
We aimed to test the hypothesis that older obese HFpEF patients have significantly greater abdominal, cardiac, and intermuscular fat compared to healthy, age-matched controls (HC), out of proportion to total body fat, and that these abnormalities are associated with objective measures of physical function. Therefore, we performed a prospective study in HFpEF patients and HCs using dual-energy x-ray absorptiometry (DEXA) to assess total body adipose mass and magnetic resonance imaging (MRI) for regional adipose mass, and cardiopulmonary exercise testing, 6-minute walk distance (6MWD), and lower extremity muscle power to comprehensively assess physical function.
Methods
Study Participants
As previously described,(13) patients were interviewed and examined by a board certified cardiologist and met these inclusion criteria: ≥60 years of age; body mass index ≥ 30 kg/m2; signs and symptoms of HF defined by National Health and Nutrition Examination Survey score ≥ 3,(19) the criteria by Rich et al.,(20) or both; left ventricular (LV) ejection fraction ≥50%; no segmental wall motion abnormalities; and no significant ischemic or valvular heart disease, pulmonary disease, anemia, or other disorder that could explain the patients’ symptoms.(2,21,22) HCs were recruited from the community and excluded if they had any chronic medical illness, were on any chronic medication, had current complaints or an abnormal physical examination (including blood pressure ≥ 140/90 mmHg), had abnormal results on the screening tests (echocardiogram, electrocardiogram, cardiopulmonary exercise testing), or regularly undertook vigorous exercise.(2,22) The study was approved by the Wake Forest School of Medicine Institutional Review Board. All participants provided written informed consent.
Outcome Measures
Outcomes were assessed and images analyzed by individuals blinded to participant group.
LV Morphology and Function
As previously described, LV mass and volumes were assessed by cardiac MRI (1.5 T Siemens Avanto scanner) from a series of multi-slice, multi-phase gradient-echo sequences positioned perpendicular to the LV long axis, spanning apex to base.(13) The epi- and endocardial borders of each slice were traced manually at end-diastole and end-systole, and volumes were calculated by Simpson’s rule. LV stroke volume and EF were calculated from standard formulae.(13)
As previously described, LV filling patterns, mitral annulus velocity, and pulse-wave velocity were assessed by Doppler echocardiography (iE33, Philips Ultrasound).(13)
Body Composition
Total body fat and lean mass were measured by DEXA (Hologic Delphi QDR) using standardized protocols as previously described.(3,4,13)
Regional scans of the thigh, abdomen, and heart were performed by MRI as previously described.(4,13,23) Images were transferred to a dedicated workstation and analyzed using commercially available software (Sliceomatic, Tomovision, Montreal, Quebec) as previously described.(4,13,23) Cross-sectional thigh areas of skeletal muscle (SM), subcutaneous fat (SCF), intermuscular fat (IMF), and bone were measured with images taken at a constant location of the mid-thigh of the left leg. Total thigh area was calculated as the sum of SCF, IMF, SM and bone, and thigh compartment (TC) area was calculated as the sum of SM, IMF, and bone. Abdominal images were taken from the tenth thoracic to the second sacral vertebrae positioned every 3 cm to cover the entire abdomen. A single slice at the level of the second lumbar vertebra was used for determination of abdominal fat measurements including SCF, intra-peritoneal and retroperitoneal fat. Intra-abdominal fat was calculated as the sum of intra-peritoneal and retroperitoneal fat. For epicardial (within the pericardium) and paracardial (outside the pericardium) fat volumes, axial images were acquired from the diaphragm to the aortic arch using a prospectively ECG-gated, T1-weighted, breath-held, black-blood, single-shot, turbo-spin echo sequence. Pericardial fat was calculated by summing epicardial and paracardial fat.(23)
Physical Function
As previously described, cardiopulmonary exercise testing was performed on a treadmill using the modified Naughton (HFpEF) or the Modified Bruce (HC) protocol using standardized instructions and encouragement to achieve a peak symptom-limited exhaustive effort.(13,24) Continuous expired gas analysis was performed during exercise and averaged over 15-second intervals (Medgraphics Ultima, Medical Graphics Corp.).(2,13,24) Peak VO2 was calculated as the average of the last 30 seconds during peak exercise.(2,13,24) Ventilatory anaerobic threshold (VAT) and ventilation/carbon dioxide output (VE/VCO2) slope were assessed as previously described.(21,24)
Six-minute walk distance was assessed as described by Guyatt et al.(25) The short physical performance battery (SPPB) is a validated, reliable measure of physical function in older populations and is strongly predictive of disability, hospitalization, nursing home readmission, and death.(26,27) It consists of three subtasks: standing balance, 4-meter walking speed at usual pace, and time to rise from a chair five times.(26) Leg press power (Watts) was assessed using the Nottingham power rig.(13) Leg power was chosen due to its strong correlation with disability and other adverse outcomes with aging.(28) Muscle quality was calculated as leg power divided by thigh muscle area from the MRI scans.(13)
Statistical Analysis
All analyses were performed with SAS Enterprise Guide (SAS Institute), version 7.1. All outcomes were tested at the 5% 2-sided level of significance. Patient characteristics are presented as mean and standard deviation or frequency and percent. Comparisons of patient characteristics were made by independent samples t-tests for continuous variables and Fisher’s exact or Chi Square tests for categorical variables. Outcome measures are presented as unadjusted (mean ± standard deviation) and adjusted (least squares mean±standard error), with a p-value for each. Unadjusted values were compared using independent samples t-tests, while adjusted values were compared using analysis of covariance with age, gender, race, and body surface area as covariates; a supplemental analysis adjusted for total body fat. Associations between regional fat and physical function measures were made by Spearman correlations and adjusted for body surface area. Multiple linear regression models were constructed to predict physical function using stepwise selection with age, gender, and race forced into the model since these are potential confounders in predicting physical function. The following regional fat measures showing consistent significance in bivariate analyses were included as candidate variables in the stepwise selection process: abdominal SCF, intra-abdominal fat, thigh IMF, and epicardial fat. Effect sizes were reported as partial R-square.
Results
Participant Characteristics
Clinical characteristics of the HFpEF patients and HC are shown in Table 1. HFpEF patients were clinically stable New York Heart Association Class II/III and had typical characteristics of HFpEF, including female preponderance, increased body mass index, increased LV mass and concentric remodeling, increased left atrial size, and Doppler diastolic dysfunction with increased E/e′ ratio.
Table 1.
Patient characteristics
| Variable | HFpEF (n=100) | HC (n=61) | p-Value |
|---|---|---|---|
| Age (years) | 66.5 ± 5.2 | 69.3 ± 7.4 | 0.011 |
| Women | 81 (81) | 38 (62) | 0.010 |
| White | 55 (55) | 58 (95) | <0.001 |
| Weight (kg) | 105.5 ± 17.9 | 74.5 ± 16.4 | <0.001 |
| Body mass index (kg/m2) | 39.3 ± 6.1 | 25.9 ± 4.9 | <0.001 |
| Body surface area (m2) | 2.1 ± 0.2 | 1.8 ± 0.2 | <0.001 |
| NYHA class | |||
| II | 60 (60) | -- | -- |
| III | 40 (40) | -- | -- |
| Ejection Fraction (%) | 61.1 ± 6.0 | 59.0 ± 4.8 | 0.030 |
| LV mass (g) | 214 ± 60 | 129 ± 35 | <0.001 |
| LV mass index (g/m2) * | 102 ± 25 | 70 ± 14 | <0.001 |
| Relative wall thickness | 0.57 ± 0.12 | 0.38 ± 0.05 | <0.001 |
| Left atrial diameter (cm) | 4.0 ± 0.5 | 3.4 ± 0.6 | <0.001 |
| Diastolic Filling Pattern † | |||
| Normal | 2 (2) | 48 (80) | |
| Impaired relaxation | 87 (87) | 12 (20) | |
| Pseudonormal | 9 (9) | 0 (0) | <0.001 |
| Restrictive | 1 (1) | 0 (0) | |
| Indeterminate | 0 (0) | 0 (0) | |
| e′ (cm/s) | 6.2 ± 1.5 | 7.9 ± 1.6 | <0.001 |
| E/e′ ratio | 13.1 ± 3.7 | 9.3 ± 2.2 | <0.001 |
| History of atrial fibrillation | 3 (3%) | -- | -- |
| History of established CAD | 11 (11%) | -- | -- |
| History of hypertension | 95 (95) | -- | -- |
| History of diabetes mellitus | 35 (35) | -- | -- |
| Blood Pressure (mmHg) | |||
| Systolic | 135 ± 14 | 123 ± 11 | <0.001 |
| Diastolic | 78 ± 8 | 75 ± 7 | 0.018 |
| Medications | |||
| Diuretics | 76 (76) | -- | -- |
| ARBs | 35 (35) | -- | -- |
| ACE inhibitors | 37 (37) | -- | -- |
| Beta blockers | 40 (40) | -- | -- |
| Ca2+ channel blockers | 35 (35) | -- | -- |
| Nitrates | 9 (9) | -- | -- |
Values are mean ± SD, or n (%);
LV mass indexed to body surface area.
Diastolic filling pattern determined according to American Society of Echocardiography criteria; Abbreviations: NYHA, New York Heart Association; LV, left ventricle; e′, early mitral annulus velocity (septal); E, E-wave velocity; CAD, coronary artery disease; ARB, angiotensin receptor blocker; ACE, angiotensin-converting enzyme.
Exercise Performance Measures
Peak exercise VO2 (in ml/min, and indexed to either body weight, lean body mass or leg lean mass), carbon dioxide production, and heart rate were significantly lower in HFpEF versus HC, while ventilation/carbon dioxide slope was significantly higher (Table 2). Peak exercise respiratory exchange ratio (an objective, reliable measure of participant effort) was similar in HFpEF versus HC. There were no significant differences in peak exercise systolic or diastolic blood pressure (Table 2). The 6MWD, SPPB total score, and leg press power (absolute or indexed to thigh muscle area) were also significantly lower in HFpEF patients compared to HC (Table 3).
Table 2.
Exercise performance measures
| Variable | Unadjusted Mean ± SD |
Adjusted* LSMean ± SE |
||||
|---|---|---|---|---|---|---|
| HFpEF | HC | p-Value | HFpEF | HC | p-Value | |
| Peak VO2 | ||||||
| ml/min | 1509 ± 313 | 1852 ± 608 | <0.001 | 1464 ± 35 | 1927 ± 48 | <0.001 |
| ml/kg/min | 14.5 ± 2.6 | 25.0 ± 7.0 | <0.001 | 15.7 ± 0.4 | 23.0 ± 0.6 | <0.001 |
| ml/kg lean/min† | 27.7 ± 5.2 | 37.4 ± 7.6 | <0.001 | 28.3 ± 0.7 | 36.6 ± 0.9 | <0.001 |
| ml/kg leg lean/min† | 87.4 ± 17.9 | 117.8 ± 24.1 | <0.001 | 90.9 ± 2.3 | 112.3 ± 3.0 | <0.001 |
| Peak carbon dioxide production (ml/min) | 1701 ± 403 | 2123 ± 712 | <0.001 | 1650 ± 43 | 2207 ± 60 | <0.001 |
| Peak respiratory exchange ratio | 1.12 ± 0.08 | 1.15 ± 0.09 | 0.073 | 1.12 ± 0.01 | 1.15 ± 0.01 | 0.18 |
| Ventilatory anaerobic threshold (ml/min) | 1014 ± 244 | 1115 ± 373 | 0.063 | 956 ± 25 | 1210 ± 35 | <0.001 |
| Ventilation/carbon dioxide slope | 29.45 ± 3.99 | 28.56 ± 3.57 | 0.16 | 30.11 ± 0.41 | 27.46 ± 0.57 | 0.001 |
| Peak heart rate (beats/min) | 139 ± 18 | 153 ± 13 | <0.001 | 139 ± 2 | 153 ± 3 | <0.001 |
| Peak systolic blood pressure (mmHg) | 177 ± 18 | 179 ± 18 | 0.39 | 176 ± 2 | 182 ± 3 | 0.13 |
| Peak diastolic blood pressure (mmHg) | 78 ± 9 | 76 ± 8 | 0.13 | 77 ± 1 | 78 ± 1 | 0.62 |
| 6-minute walk distance (m) | 412 ± 73 | 563 ± 70 | <0.001 | 427 ± 7 | 538 ± 10 | <0.001 |
| SPPB total score | 10.0 ± 1.7 | 11.3 ± 0.8 | <0.001 | 10.3 ± 0.2 | 10.9 ± 0.2 | 0.044 |
| SPPB Balance score | 3.8 ± 0.6 | 4.0 ± 0.2 | 0.010 | 3.8 ± 0.1 | 4.0 ± 0.1 | 0.15 |
| SPPB Gait speed score | 3.8 ± 0.5 | 4.0 ± 0.0 | <0.001 | 3.8 ± 0.0 | 3.9 ± 0.1 | 0.30 |
| SPPB Chair rise score | 2.5 ± 1.2 | 3.3 ± 0.7 | <0.001 | 2.6 ± 0.1 | 3.0 ± 0.2 | 0.12 |
| Leg press power (W) | 112 ± 53 | 163 ± 67 | <0.001 | 117 ± 5 | 152 ± 9 | 0.004 |
| Leg muscle quality (W/cm2)‡ | 0.91 ± 0.33 | 1.45 ± 0.52 | <0.001 | 0.97 ± 0.05 | 1.28 ± 0.08 | 0.004 |
Raw data are presented as mean ± SD;
Adjusted for age, race, gender, and body surface area and presented as least square means ± standard error;
kg of lean body mass and kg of leg lean mass were measured by dual energy x-ray absorptiometry;
Leg muscle quality is leg power divided by thigh muscle area. SPPB = Short Physical Performance Battery.
Table 3.
Total and regional body composition measures
| Variable | Unadjusted Mean ± SD |
Adjusted* LSMean ± SE |
||||
|---|---|---|---|---|---|---|
| HFpEF | HC | p-Value | HFpEF | HC | p-Value | |
| DEXA | ||||||
| Total Mass (kg) | 102.4 ± 14.2 | 75.7 ± 16.3 | <0.001 | 94.4 ± 0.6 | 88.0 ± 0.8 | <0.001 |
| Total Fat Mass (kg) | 46.7 ± 10.2 | 24.1 ± 9.4 | <0.001 | 41.0 ± 0.7 | 32.9 ± 0.9 | <0.001 |
| Total Percent Fat | 45.5 ± 6.4 | 31.5 ± 8.5 | <0.001 | 42.8 ± 0.6 | 35.6 ± 0.8 | <0.001 |
| Total Lean Mass (kg) | 53.3 ± 9.1 | 49.3 ± 11.0 | 0.016 | 51.1 ± 0.4 | 52.8 ± 0.6 | 0.041 |
| Total Percent Lean | 52.2 ± 6.3 | 65.5 ± 8.2 | <0.001 | 54.7 ± 0.6 | 61.6 ± 0.8 | <0.001 |
| MRI | ||||||
| Abdominal SC fat (cm2) | 377.8 ± 145.2 | 147.7 ± 84.5 | <0.001 | 312.9 ± 12.8 | 236.9 ± 15.9 | 0.002 |
| Intra-peritoneal fat (cm2) | 116.4 ± 61.5 | 68.8 ± 60.4 | <0.001 | 110.0 ± 6.4 | 77.7 ± 8.0 | 0.007 |
| Retroperitoneal fat (cm2) | 94.4 ± 51.3 | 47.3 ± 35.0 | <0.001 | 95.8 ± 4.1 | 45.4 ± 5.1 | <0.001 |
| Intra-abdominal fat (cm2) | 210.8 ± 107.9 | 116.1 ± 90.0 | <0.001 | 205.7 ± 9.7 | 123.1 ± 12.1 | <0.001 |
| Abdominal SC fat/Intra-abdominal fat ratio | 2.34 ± 1.73 | 1.91 ± 1.31 | 0.10 | 1.94 ± 0.18 | 2.46 ± 0.22 | 0.11 |
| Total thigh area (cm2) | 316.8 ± 80.4 | 205.3 ± 47.3 | <0.001 | 277.0 ± 5.6 | 270.7 ± 7.7 | 0.56 |
| Thigh SC fat (cm2) | 165.2 ± 74.8 | 73.8 ± 41.9 | <0.001 | 132.9 ± 5.4 | 126.7 ± 7.5 | 0.55 |
| TC area (cm2) | 151.6 ± 28.7 | 131.5 ± 32.1 | <0.001 | 144.1 ± 2.1 | 143.9 ± 3.0 | 0.96 |
| Femur (cm2) | 4.3 ± 0.7 | 5.9 ± 1.0 | <0.001 | 4.2 ± 0.1 | 6.1 ± 0.1 | <0.001 |
| Thigh IMF (cm2) | 25.9 ± 9.2 | 14.4 ± 5.9 | <0.001 | 23.2 ± 0.9 | 18.9 ± 1.2 | 0.014 |
| Thigh IMF/TC (%) | 17.1 ± 5.2 | 11.3 ± 4.8 | <0.001 | 16.2 ± 0.6 | 12.9 ± 0.8 | 0.003 |
| Thigh SM (cm2) | 121.5 ± 25.7 | 111.2 ± 30.5 | 0.029 | 116.8 ± 2.0 | 118.9 ± 2.8 | 0.58 |
| Thigh SM/TC (%) | 80.0 ± 5.1 | 84.0 ± 5.0 | <0.001 | 80.9 ± 0.6 | 82.6 ± 0.8 | 0.11 |
| Thigh IMF/SM ratio | 0.22 ± 0.08 | 0.14 ± 0.07 | <0.001 | 0.21 ± 0.01 | 0.16 ± 0.01 | 0.009 |
| Paracardial fat (cm3) | 63.1 ± 39.6 | 53.9 ± 36.7 | 0.19 | 63.6 ± 4.1 | 53.1 ± 5.5 | 0.18 |
| Epicardial fat (cm3) | 36.0 ± 17.7 | 54.9 ± 20.6 | <0.001 | 36.3 ± 2.3 | 54.5 ± 3.0 | <0.001 |
| Pericardial fat (cm3) | 99.0 ± 53.0 | 108.8 ± 52.9 | 0.31 | 99.8 ± 5.6 | 107.6 ± 7.6 | 0.48 |
Raw data are presented as mean ± SD;
Adjusted for age, race, gender, and body surface area and presented as least square means ± standard error; Intra-abdominal fat = Intra-peritoneal + Retroperitoneal fat; SC fat = Subcutaneous Fat; TC Area = Thigh Compartment Area; SM = Skeletal Muscle; IMF = Intermuscular Fat; TC area = SM + IMF + Femur. Total thigh area = TC Area + Thigh SC fat. Pericardial fat = Paracardial fat + Epicardial fat.
Total and Regional Body Composition Measures
By DEXA, total body mass, total body fat mass, and percent fat were significantly higher, while total lean (muscle) mass and percent lean mass were significantly lower in HFpEF compared to HC (Table 3). By MRI, the following patterns of regional fat deposition were observed in HFpEF versus HC (Table 3): abdominal SCF and intra-abdominal fat were significantly higher; thigh IMF, thigh IMF/thigh compartment (TC,%), thigh IMF/SM ratio were significantly higher while thigh SCF was similar; epicardial fat was 33% lower (p<0.001) while pericardial and paracardial fat were similar. Even when adjusted for total body fat, intra-abdominal fat remained significantly higher while epicardial fat remained significantly lower in HFpEF versus HC; additionally, abdominal SCF/intra-abdominal fat ratio became significantly lower in HFpEF versus HC. Furthermore, there was a significant association between intra-abdominal fat and epicardial fat in the HFpEF patients (r=0.37, p=0.001) but not in the HC (r=0.16, p=0.26).
Relationships between body composition and exercise tolerance and physical function
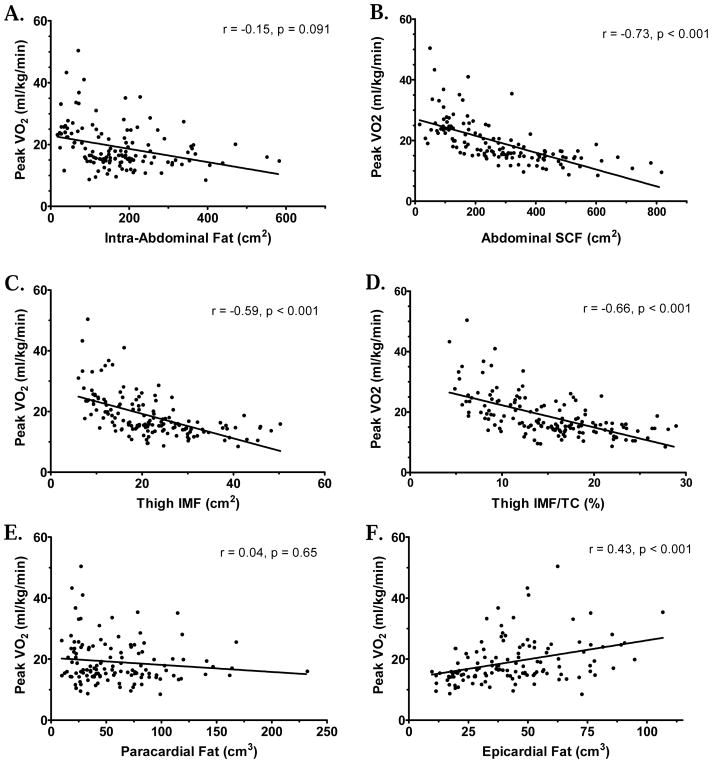
All measures of physical function were inversely related to abdominal and thigh SCF, thigh IMF, thigh IMF/TC (%), and thigh IMF/SM ratio, and positively related to thigh SM/TC (%) and epicardial fat (Tables 4a–4b, Figure 1). Peak VO2 showed trends (p<0.10) for inverse relationships with intra-peritoneal, retroperitoneal, and intra-abdominal fat. When adjusted for total body fat, peak VO2 (ml/min and ml/kg/min), ventilatory anaerobic threshold, and leg press power remained positively related to epicardial fat.
Table 4a.
Relationships of cardiac fat, abdominal fat and thigh composition with Peak VO2
| Variable | Peak VO2 (ml/min) | Peak VO2 (ml/kg/min) | Peak VO2 (ml/kg lean/min) | Peak VO2 (ml/kg leg lean/min) | ||||
|---|---|---|---|---|---|---|---|---|
| Corr. | P-Value | Corr. | P-Value | Corr. | P-Value | Corr. | P-Value | |
| Abdominal SCF (cm2) | −0.60 | <0.001 | −0.73 | <0.001 | −0.36 | <0.001 | −0.35 | <0.001 |
| Intra-peritoneal fat (cm2) | −0.12 | 0.19 | −0.13 | 0.17 | −0.16 | 0.073 | −0.08 | 0.38 |
| Retroperitoneal fat (cm2) | −0.15 | 0.10 | −0.21 | 0.024 | −0.30 | <0.001 | −0.19 | 0.036 |
| Intra-abdominal fat (cm2) | −0.13 | 0.15 | −0.15 | 0.091 | −0.24 | 0.010 | −0.13 | 0.15 |
| Thigh SCF (cm2) | −0.63 | <0.001 | −0.71 | <0.001 | −0.34 | <0.001 | −0.39 | <0.001 |
| Thigh IMF (cm2) | −0.46 | <0.001 | −0.59 | <0.001 | −0.39 | <0.001 | −0.39 | <0.001 |
| Thigh IMF/TC (%) | −0.57 | <0.001 | −0.66 | <0.001 | −0.40 | <0.001 | −0.40 | <0.001 |
| Thigh SM (cm2) | 0.51 | <0.001 | 0.46 | <0.001 | 0.19 | 0.034 | 0.20 | 0.026 |
| Thigh SM/TC (%) | 0.54 | <0.001 | 0.62 | <0.001 | 0.35 | <0.001 | 0.36 | <0.001 |
| Thigh IMF/SM ratio | −0.57 | <0.001 | −0.66 | <0.001 | −0.39 | <0.001 | −0.39 | <0.001 |
| Paracardial fat (cm3) | 0.00 | 0.96 | 0.04 | 0.65 | −0.06 | 0.50 | 0.03 | 0.72 |
| Epicardial fat (cm3) | 0.36 | <0.001 | 0.43 | <0.001 | 0.28 | 0.002 | 0.32 | <0.001 |
| Pericardial fat (cm3) | 0.17 | 0.060 | 0.23 | 0.013 | 0.10 | 0.28 | 0.18 | 0.050 |
Table 4b.
Relationships of cardiac fat, abdominal fat and thigh composition with other exercise performance measures
| Variable | Ventilatory Anaerobic Threshold (ml/min) | 6-Minute Walk Distance (m) | Total SPPB Score | Leg Press Power (W) | ||||
|---|---|---|---|---|---|---|---|---|
| Corr. | P-Value | Corr. | P-Value | Corr. | P-Value | Corr. | P-Value | |
| Abdominal SCF (cm2) | −0.39 | <0.001 | −0.56 | <0.001 | −0.33 | <0.001 | −0.67 | <0.001 |
| Intra-peritoneal fat (cm2) | −0.00 | 0.97 | −0.12 | 0.23 | −0.03 | 0.78 | 0.05 | 0.60 |
| Retroperitoneal fat (cm2) | −0.08 | 0.40 | −0.19 | 0.054 | −0.08 | 0.43 | 0.07 | 0.51 |
| Intra-abdominal fat (cm2) | −0.02 | 0.80 | −0.15 | 0.13 | −0.04 | 0.65 | 0.08 | 0.41 |
| Thigh SCF(cm2) | −0.42 | <0.001 | −0.58 | <0.001 | −0.38 | <0.001 | −0.63 | <0.001 |
| Thigh IMF (cm2) | −0.29 | 0.001 | −0.46 | <0.001 | −0.33 | <0.001 | −0.36 | <0.001 |
| Thigh IMF/TC (%) | −0.37 | <0.001 | −0.54 | <0.001 | −0.40 | <0.001 | −0.49 | <0.001 |
| Thigh SM (cm2) | 0.35 | <0.001 | 0.39 | <0.001 | 0.41 | <0.001 | 0.57 | <0.001 |
| Thigh SM/TC (%) | 0.35 | <0.001 | 0.50 | <0.001 | 0.40 | <0.001 | 0.48 | <0.001 |
| Thigh IMF/SM ratio | −0.37 | <0.001 | −0.54 | <0.001 | −0.40 | <0.001 | −0.50 | <0.001 |
| Paracardial fat (cm3) | −0.00 | 0.97 | −0.02 | 0.84 | 0.01 | 0.94 | 0.15 | 0.12 |
| Epicardial fat (cm3) | 0.27 | 0.002 | 0.36 | <0.001 | 0.21 | 0.034 | 0.43 | <0.001 |
| Pericardial fat (cm3) | 0.13 | 0.16 | 0.16 | 0.12 | 0.09 | 0.39 | 0.30 | 0.002 |
Data adjusted for Body Surface Area and presented as Spearman correlation coefficients. P value indicates significance for each correlation for the overall sample (HFpEF and HC). SCF = Subcutaneous fat; Intra-abdominal fat = Intra-peritoneal + Retroperitoneal fat; SM = Skeletal Muscle; IMF = Intermuscular Fat; TC = Thigh Compartment; SPPB = Short Physical Performance Battery. Pericardial fat = Paracardial fat + Epicardial fat.
Figure 1. Peak VO2 (ml/kg/min) versus Regional Fat Measures.
Regression plots for baseline measures of Peak VO2 (ml/kg/min) versus (A) Intra-Abdominal Fat, (B) Abdominal SCF, (C) Thigh IMF, (D) Thigh IMF as Percent of Thigh Compartment (TC), (E) Paracardial Fat, (F) Epicardial Fat. Similar results were found for non-indexed Peak VO2 (ml/min) as well. Regional fat measures obtained using MRI. Thigh compartment calculated as Intermuscular Fat+Skeletal Muscle+Bone. Spearman r and p values additionally included for reference.
Multiple linear regression with age, gender, race forced in showed that intra-abdominal fat was the strongest independent predictor of peak VO2 and 6MWD with partial R-square of 0.36 for peak VO2 and 0.2 for 6MWD, respectively (Table 5), while abdominal subcutaneous and epicardial fat remained significant but modest predictors (inverse for epicardial).
Table 5.
Predictors of Physical Function
| Peak VO2 (ml/kg/min) | 6-minute Walk Distance (m) | Leg Press Power (W) | ||||
|---|---|---|---|---|---|---|
| Baseline Characteristic | Partial R-Square | p-value | Partial R-Square | p-value | Partial R-Square | p-value |
| Age | 0.140 | <0.001 | 0.156 | <0.001 | 0.053 | 0.021 |
| Gender | 0.355 | <0.001 | 0.093 | <0.001 | 0.293 | <0.001 |
| Race | 0.086 | 0.001 | 0.005 | 0.43 | 0.015 | 0.23 |
| Intra-abdominal Fat | 0.358 | 0.003 | 0.200 | <0.001 | -- | -- |
| Abdominal SCF | 0.077 | <0.001 | 0.182 | <0.001 | 0.075 | 0.006 |
| Epicardial Fat | 0.034 | 0.046 | 0.033 | 0.048 | -- | -- |
Age, Gender, Race forced into model; Thigh IMF did not reach significance for Peak VO2 or 6-minute Walk Distance.
Thigh IMF, Intra-abdominal Fat, nor Epicardial Fat reached significance for Leg Press Power.
Discussion
Multiple recent studies have shown that excess total body adipose is a key contributor to the severely reduced peak VO2 in the metabolic/obese HFpEF phenotype.(3,4,8,9,15) Prior studies in other disorders suggest that the location of excess adipose has effects independent of total body adipose, and that regional adipose may be an important determinant of impaired cardiac function and exercise intolerance.(12,14,15,23) However, the impact of regional adipose in HFpEF is unkown. In this study, we examined the distribution of adipose tissue in HFpEF patients compared to HCs and the relationships of regional adipose depots with multiple objective measures of exercise tolerance and physical function. The major new findings of this study include that older, obese HFpEF patients have relatively larger depots of abdominal and thigh adipose, and smaller amounts of epicardial fat compared to HC. There was also a significant association between intra-abdominal fat and epicardial fat in the HFpEF patients but not in the HCs. Additional novel findings are that abdominal SCF, thigh SCF, and thigh IMF/SM ratio were inversely associated with all objective measures of physical function, including peak VO2, 6MWD, ventilatory anaerobic threshold, SPPB, and leg power, while epicardial fat was directly associated with these physical function measures. In multiple linear regression, intra-abdominal fat yielded the highest partial R-square among all the regional fat measures and was the strongest independent predictor of peak VO2 and 6MWD. These data suggest that in HFpEF, the pattern of regional adipose distribution may have important adverse consequences beyond those of total body adipose.
Previous studies in other disorders showed that Increased abdominal fat is associated with concentric remodeling, LV diastolic dysfunction and adverse subclinical changes in LV mechanics.(29–33) Obokata et al. recently reported that compared to non-obese HFpEF and controls, obese HFpEF patients had more concentric LV remodeling, greater right ventricular dysfunction, increased right and left heart filling pressures, impaired pulmonary vasodilation and reduced peak VO2 during maximal supine cycle exercise.(15) We confirm and significantly extend these findings by showing, in a relatively large cohort of older, obese HFpEF patients compared to HC, that obese HFpEF have increased left atrial size, LV mass, relative wall thickness, and impaired Doppler measures of diastolic function, as well as impairments in multiple objective measures of physical function, including peak VO2, VAT, SPPB, and leg muscle power, and by examining the relationships of physical function with multiple measures of total and regional adipose using both DEXA and MRI.
Increased adiposity promotes inflammation, hypertension, dyslipidemia and insulin resistance, all of which contribute to the pathophysiology of HFpEF.(13,16) Moreover, adipose-induced inflammation has wide-ranging adverse effects including coronary and systemic microvascular endothelial dysfunction, capillary rarefaction and impaired skeletal muscle mitochondrial function and protein synthesis that results in reduced skeletal muscle oxygen delivery and utilization.(7,9,14) A consequence of adipose-mediated inflammatory endothelial dysfunction and capillary rarefaction is that it reduces O2 delivery to the active muscles resulting in fatigue even during low-level exercise.(7,14,22) Indeed, a novel finding of our study was that excess abdominal adipose was inversely associated with peak VO2, VAT, 6MWD, and leg muscle power. Taken together, these data suggest that central adiposity may be an important contributor to the impaired exercise tolerance and physical function in older, obese HFpEF patients.
In addition to multiple novel findings, our results confirm, in a much larger group of subjects, our prior reports that increased thigh IMF area and IMF/SM ratio are significantly related to the severely reduced peak VO2 in older obese HFpEF patients,(3,4) suggesting that abnormal skeletal muscle composition and its adverse consequences contribute to exercise intolerance in HFpEF.(34,35) We extend these findings by demonstrating that increased thigh SCF, IMF, IMF/TC ratio, and thigh IMF/SM ratio were associated with other objective measures of impaired physical function, including lower VAT, SPPB, 6MWD, and leg power. The mechanisms whereby increased thigh IMF is associated with increased anaerobic metabolism during submaximal exercise may be related to the deleterious effect excess adiposity has on skeletal muscle mitochondrial function. Specifically, Bharadwaj et al. reported that thigh fat volume, thigh SCF and thigh IMF were negatively associated with skeletal muscle citrate synthase activity in sedentary older individuals.(36) Moreover, we previously reported that older HFpEF patients with obesity have decreased skeletal muscle oxidative fibers, enzymes, capillarity and mitochondrial function that result in decreased diffusive O2 transport and impaired peak and reserve oxygen utilization by the active muscles,(4,7–9,22,37) confirming findings reported in an animal model of HFpEF.(37)
In contrast to our pre-specified hypothesis, we found that epicardial fat, even when adjusted for body surface area or for total body fat, was substantially (33%) lower in HFpEF versus HC. However, these results are consistent with multiple prior studies in patients with HF with reduced EF (HFrEF) which showed significantly reduced epicardial fat.(38,39) They differ, though, from Obokata et al. who reported finding greater epicardial fat thickness in non-obese and obese HFpEF participants compared to controls.(15) The discrepancy between studies may be due to the techniques used to quantify epicardial fat. Obokata used echocardiography to measure one-dimensional thickness of epicardial fat at a single point adjacent to the right ventricular free wall. We used MRI to acquire volumetric measures of epicardial and paracardial fat around the entire heart.(40) Adipose tissue provides an extremely high signal-to-noise ratio for MRI whereas it tends to scatter ultrasound waves, degrading signal-to-noise for echocardiography. Furthermore, echocardiography overestimates epicardial fat and underestimates paracardial fat compared to MRI(41), likely due to difficulty in distinguishing the pericardium. Finally, volumetric measurements by MRI are not only more accurate and reproducible than by echocardiography, they preclude systematic errors from measuring epicardial fat thickness at a single point that are due to individual differences in epicardial fat distribution, such as occurs in HF patients from redistribution of epicardial fat due to LV remodeling.(40,42)
While our study does not address the potential mechanism(s) for reduced epicardial fat observed in obese HFpEF, prior studies in HFrEF have highlighted that epicardial fat plays a major role in regulating fatty acids to cardiac muscle as a local energy source, and that the heart can switch fuel sources in response to stress.(43) For example, in the setting of obesity and diabetes, myocardial fatty acid β-oxidation increases at the expense of glucose utilization.(44) Given the high prevalence of impaired glucose tolerance in metabolic/obese HFpEF, it seems possible that increased reliance on fatty acid β-oxidation may, at least partly, explain the observed reduction in epicardial fat in HFpEF (and in prior studies of HFrEF) compared to HC’s.
In contrast to abdominal and thigh fat which were inversely related to physical function, epicardial adipose was directly associated with all physical function measures, and remained a significant, independent, positive predictor of physical function even after adjusting for total body fat. This suggests that, unlike abdominal and thigh fat, epicardial adipose may not adversely impact physical function in HFpEF. This would be consistent with data regarding epicardial fat and myocardial energy utilization discussed above.
The finding of a significant association between intra-abdominal fat and epicardial fat in the HFpEF patients but not in the HCs further supports the validity of our key finding that obese HFpEF may have a unique pattern of regional fat distribution.
Clinical implications
Reducing total body adipose via bariatric surgery can prevent incident HF and improve exercise intolerance in established HFrEF.(12,13) Our data suggest that in obese/metabolic HFpEF, in addition to reducing total fat mass, additional benefit may be realized by targeting specific fat depots, such as intra-abdominal and intermuscular adipose. Indeed, dietary weight loss significantly improved symptoms, exercise capacity, and quality-of-life in older obese HFpEF patients, and these benefits were related to improved body and regional composition, including reduced fat mass and improved thigh muscle mass/intermuscular fat ratio.
Strengths and Limitations
Our study has several strengths, including prospective design, relatively large numbers of HFpEF and HCs, comprehensive measures of body composition by both DEXA and MRI, and multiple objective measures of physical function. Our study also has potential limitations. Although DEXA can potentially overestimate fat and lean mass in presence of edema, our HFpEF patients were well-compensated without overt fluid overload. Moreover, our MRI assessments confirm and extend our DEXA results by demonstrating regional differences in fat distribution between HFpEF and HC. Average BMI in the HC was lower than HFpEF, and even though we adjusted for body size, our data do not definitively prove that the pattern of regional fat distribution we identified is specific to obese HFpEF, which would require a control group matched for BMI and comorbidities.
Conclusions
In patients with the metabolic/obese HFpEF phenotype, in addition to total body adipose, the pattern of regional adipose deposition may have important adverse consequences for HFpEF and its primary manifestation, exercise intolerance. Interventions targeting specific adipose depots, such as intra-abdominal and thigh intermuscular fat, have the potential to improve the clinically important outcome of exercise intolerance.
Acknowledgments
Funding: Supported in part by the following grants from the National Institutes of Health: R01AG18917, R01AG045551, R01HL107257, P30-AG21331, and UL1TR001420 (Dr. Kitzman); R15NR016826 (Dr. Haykowsky); K01AG033652 (Dr. Brinkley); and R01HL093713 (Dr. Nicklas). Also supported by the Kermit G. Phillips II Chair in Cardiovascular Medicine of Wake Forest School of Medicine (Dr. Kitzman) and the Moritz Endowed Chair in Geriatrics in the College of Nursing and Health Innovation (Dr. Haykowsky).
Abbreviations List
- 6MWD
six minute walk distance
- DEXA
dual energy x-ray absorptiometry
- EF
ejection fraction
- HC
age-matched healthy control
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- IMF
intermuscular fat
- LV
left ventricle
- MRI
magnetic resonance imaging
- NYHA
New York Heart Association
- SCF
subcutaneous fat
- SM
skeletal muscle
- SPPB
short physical performance battery
- TC
thigh compartment
- VAT
ventilatory anaerobic threshold
- VO2
oxygen consumption
Footnotes
Disclosures: Dr. Kitzman has been a consultant for Relypsa, Abbvie, GlaxoSmithKline, St. Luke’s Medical Center, DCRI, and Corvia Medical; received grants from Novartis and St. Luke’s Medical Center; and owns stock in Gilead Sciences.
Competency in Medical Knowledge
Metabolic/obese HFpEF patients have excess intra-abdominal and intemuscular adipose that is independently related to exercise capacity, beyond total body adipose.
Translational Outlook
Strategies to differentially impact regional adipose could improve exercsie intolerance in metabolic/obese HFpEF.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288(17):2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 3.Haykowsky MJ, Brubaker PH, Morgan TM, Kritchevsky S, Eggebeen J, Kitzman DW. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: role of lean body mass. J Gerontol A Biol Sci Med Sci. 2013;68(8):968–975. doi: 10.1093/gerona/glt011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haykowsky M, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. 2014;113:1211–1216. doi: 10.1016/j.amjcard.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhella PS, Prasad A, Heinicke K, et al. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13(12):1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhakal BP, Malhotra R, Murphy RM, et al. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail. 2015;8:286–294. doi: 10.1161/CIRCHEARTFAILURE.114.001825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haykowsky MJ, Tomczak CR, Scott JM, Patterson DI, Kitzman DW. Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J Appl Physiol. 2015;119:739–744. doi: 10.1152/japplphysiol.00049.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molina AJ, Bharadwaj MS, Van HC, et al. Skeletal Muscle Mitochondrial Content, Oxidative Capacity, and Mfn2 Expression Are Reduced in Older Patients With Heart Failure and Preserved Ejection Fraction and Are Related to Exercise Intolerance. JACC Heart Fail. 2016;4(8):636–645. doi: 10.1016/j.jchf.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitzman DW, Nicklas B, Kraus WE, et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306(9):H1364–H1370. doi: 10.1152/ajpheart.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347(5):305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 11.Haass M, Kitzman DW, Anand IS, et al. Body Mass Index and Adverse Cardiovascular Outcomes in Heart Failure Patients with Preserved Ejection Fraction: Results from the I-PRESERVE Trial. Circ Heart Fail. 2011;4(3):324–331. doi: 10.1161/CIRCHEARTFAILURE.110.959890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitzman DW, Shah SJ. The HFpEF Obesity Phenotype: The Elephant in the Room. J Am Coll Cardiol. 2016;68:200–203. doi: 10.1016/j.jacc.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 13.Kitzman DW, Brubaker P, Morgan T, et al. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA. 2016;315(1):36–46. doi: 10.1001/jama.2015.17346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah SJ, Kitzman DW, Borlaug BA, et al. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation. 2016;134(1):73–90. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obokata M, Reddy YN, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19. doi: 10.1161/CIRCULATIONAHA.116.026807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitzman DW, Lam C. Obese heart failure with preserved ejection phenotype: from Pariah to Central Player. Circulation. 2017;136:20–23. doi: 10.1161/CIRCULATIONAHA.117.028365. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Li S, Feuers RJ, Buffington CK, Cowan GS. Influence of body fat distribution on oxygen uptake and pulmonary performance in morbidly obese females during exercise. Respirology. 2001;6:9–13. doi: 10.1046/j.1440-1843.2001.00290.x. [DOI] [PubMed] [Google Scholar]
- 18.Bacchi E, Negri C, Tarperi C, et al. Relationships between cardiorespiratory fitness, metabolic control, and fat distribution in type 2 diabetes subjects. Acta Diabetol. 2014;51:369–375. doi: 10.1007/s00592-013-0519-1. [DOI] [PubMed] [Google Scholar]
- 19.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20:301–306. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 20.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney R. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190–1195. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 21.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail. 2010;3(6):659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58(3):265–274. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brinkley TE, Leng X, Chughtai HL, et al. Periaortic fat and cardiovascular risk: a comparison of high-risk older adults and age-matched healthy controls. Int J Obes (Lond) 2014;38(11):1397–1402. doi: 10.1038/ijo.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott JM, Haykowsky MJ, Eggebeen J, Morgan TM, Brubaker PH, Kitzman DW. Reliability of peak exercise testing in patients with heart failure with preserved ejection fraction. Am J Cardiol. 2012;110(12):1809–1813. doi: 10.1016/j.amjcard.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guyatt GH, Sullivan M, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–923. [PMC free article] [PubMed] [Google Scholar]
- 26.Guralnik JM, Simonsick EM, Ferrucci L, et al. A Short Physical Performance Battery Assessing Lower Extremity Function: Association With Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 27.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foldvari M, Clark M, Laviolette LC, et al. Association of muscle power with functional status in community-dwelling elderly women. J Gerontol A Biol Sci Med Sci. 2000;55:M192–M199. doi: 10.1093/gerona/55.4.m192. [DOI] [PubMed] [Google Scholar]
- 29.Neeland IJ, Gupta S, Ayers CR, et al. Relation of regional fat distribution to left ventricular structure and function. Circ Cardiovasc Imaging. 2013;6:800–807. doi: 10.1161/CIRCIMAGING.113.000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park J, Kim NH, Kim SH, et al. Visceral adiposity and skeletal muscle mass are independently and synergistically associated with left ventricular structure and function: the Korean Genome and Epidemiology Study. Int J Cardiol. 2014;176:951–955. doi: 10.1016/j.ijcard.2014.08.108. [DOI] [PubMed] [Google Scholar]
- 31.Bello NA, Cheng S, Claggett B, et al. Association of weight and body composition on cardiac structure and function in the ARIC Study (Atherosclerosis Risk in Communities) Circ Heart Fail. 2016;9(8):e002978. doi: 10.1161/CIRCHEARTFAILURE.115.002978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F. Relation between epicardial adipose tissue and left ventricular mass. Am J Cardiol. 2004;94(8):1084–1087. doi: 10.1016/j.amjcard.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 33.Selvaraj S, Martinez EE, Aguilar FG, et al. Association of Central Adiposity With Adverse Cardiac Mechanics: Findings From the Hypertension Genetic Epidemiology Network Study. Circ Cardiovasc Imaging. 2016;9(6) doi: 10.1161/CIRCIMAGING.115.004396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss K, Schar M, Panjrath GS, et al. Fatigability, Exercise Intolerance, and Abnormal Skeletal Muscle Energetics in Heart Failure. Circ Heart Fail. 2017;10(7) doi: 10.1161/CIRCHEARTFAILURE.117.004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitzman DW, Haykowsky MJ, Tomczak CR. Making the Case for Skeletal Muscle Myopathy and Its Contribution to Exercise Intolerance in Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2017;10(7) doi: 10.1161/CIRCHEARTFAILURE.117.004281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bharadwaj MS, Tyrrell DJ, Leng I, et al. Relationships between mitochondrial content and bioenergetics with obesity, body composition and fat distribution in healthy older adults. BMC Obes. 2015;2:40. doi: 10.1186/s40608-015-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bowen TS, Rolim NP, Fischer T, et al. Heart failure with preserved ejection fraction induces molecular, mitochondrial, histological, and functional alterations in rat respiratory and limb skeletal muscle. Eur J Heart Fail. 2015;17:263–272. doi: 10.1002/ejhf.239. [DOI] [PubMed] [Google Scholar]
- 38.Doesch C, Haghi D, Fluchter S, et al. Epicardial adipose tissue in patients with heart failure. J Cardiovasc Magn Reson. 2010;12(40) doi: 10.1186/1532-429X-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khawaja T, Greer C, Chokshi A, et al. Epicardial fat volume in patients with left ventricular systolic dysfunction. Am J Cardiol. 2011;108(3):397–401. doi: 10.1016/j.amjcard.2011.03.058. [DOI] [PubMed] [Google Scholar]
- 40.Nelson AJ, Worthley MI, Psaltis PJ, et al. Validation of cardiovascular magnetic resonance assessment of pericardial adipose tissue volume. J Cardiovasc Magn Reson. 2009;11:15. doi: 10.1186/1532-429X-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sicari R, Sironi AM, Petz R, et al. Pericardial rather than epicardial fat is a cardiometabolic risk marker: an MRI vs echo study. J Am Soc Echocardiogr. 2011;24:1156–1162. doi: 10.1016/j.echo.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 42.Fluchter S, Haghi D, Dinter D, et al. Volumetric assessment of epicardial adipose tissue with cardiovascular magnetic resonance imaging. Obesity (Silver Spring) 2007;15:870–878. doi: 10.1038/oby.2007.591. [DOI] [PubMed] [Google Scholar]
- 43.Antonopoulos AS, Antoniades C. The role of epicardial adipose tissue in cardiac biology: classic concepts and emerging roles. J Physiol. 2017;595:3907–3917. doi: 10.1113/JP273049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fukushima A, Lopaschuk GD. Acetylation control of cardiac fatty acid beta-oxidation and energy metabolism in obesity, diabetes, and heart failure. Biochim Biophys Acta. 2016;1862:2211–2220. doi: 10.1016/j.bbadis.2016.07.020. [DOI] [PubMed] [Google Scholar]