Abstract
Background
Enamel hypoplasia (EH) increases risk for dental caries and also is associated with vitamin D deficiencies. This pilot study evaluates the feasibility to determine the association of human maternal circulating vitamin D concentrations during pregnancy and EH in infant’s teeth that develop in utero.
Methods
A pilot population of 37 children whose mothers participated in a RCT of vitamin D supplementation during pregnancy was evaluated. Major outcome was EH and major exposure was maternal monthly serum circulating 25(OH)D concentrations during pregnancy. EH was assessed using the Enamel Defect Index and digital images made by a ProScope High Resolution™ handheld digital USB microscope at 50x magnification.
Results
During initial 8 weeks of study, 29/37 children had evaluable data with mean age of 3.6 ± 0.9 years; 48% male; and 45% White, 31% Hispanic, and 24% Black. EH was identified in 13 (45%) of the children. Maternal mean 25(OH)D concentrations were generally lower for those children with EH.
Conclusions
Preliminary results suggest follow-up of children of mothers in a vitamin D supplementation RCT during pregnancy provides an important approach to study the etiology of EH in the primary teeth. Further study is needed to discern thresholds and timing of maternal serum 25(OH)D concentrations during pregnancy associated with absence of EH in teeth that develop in utero. Potential dental public health implications for prevention of early childhood caries via sound tooth structure as related to maternal vitamin D sufficiency during pregnancy need to be determined.
Keywords: Vitamin D, Vitamin D Deficiency, Enamel Hypoplasia, Tooth, Deciduous, Pregnancy
1. Introduction
Enamel hypoplasia (EH) is an abnormal and localized area of less tooth enamel that may or may not be visible to the unassisted eye (1). This developmental defect is characterized as an enamel surface reduced in thickness and with pits, grooves or areas of the defect having a smooth outline (2). Current research of EH is important because of the increasing evidence that EH is a significant risk factor for early childhood dental caries in the primary teeth of children (ECC) (3-5).
A role for vitamin D in hypoplastic tooth development is evidenced by EH found in association with vitamin D deficiencies that occurred during the time of tooth development. In general, vitamin D deficiencies can result from either lack of an exogenous factor (e.g. sunlight or nutrition), and/or lack of an endogenous factor (e.g. hereditary defects in vitamin D metabolism). There are a few published experiments of nutritional vitamin D deficiencies during tooth development causing EH in animals. These animal experiments were first conducted in puppies by May Mellanby in the early 1900’s (6) and later in the 1970’s using rats (7). Observations of EH in humans with inherited defects of vitamin D metabolism, i.e. hypophosphatemic vitamin D-resistant rickets, were published beginning in 1989 (8-10). Hypophosphatemic vitamin D-resistant rickets is an X-linked dominant trait that is reported to affect about 1:20,000 - 25,000 of those with the genetically determined forms of hypophosphatemic osteomalacia (11-13). Children with this inherited trait may exhibit EH in both their primary and permanent dentition (8, 14-18). There are at least two even more rare types of hereditary defects in humans involving vitamin D enzyme and receptor abnormalities, and they too may exhibit dental defects (19-21).
To study the role of vitamin D in the development of EH in the human primary dentition, one begins with the pregnancy environment. At approximately the 13th week from conception, the human primary maxillary central incisor teeth begin calcification (22, 23). These tooth crowns are mostly formed by birth and therefore these teeth can serve as a record of the pregnancy environment. The primary central incisor teeth are normally erupted into the oral cavity by 12-18 months of age. For those reasons we considered EH of the human primary maxillary central incisor teeth as a suitable outcome for this pilot study of the role of maternal vitamin D during pregnancy in EH. For measure of the maternal prenatal vitamin D there is evidence of a positive and significant correlation between a pregnant woman’s 25-hydroxyvitamin D (calcidiol, calcifediol, or 25-hydroxyvitamin D abbreviated 25(OH)D) concentration and the neonatal concentration at birth (24, 25). Those findings suggest that during pregnancy, the maternal serum circulating 25(OH)D concentrations correspond to those of the fetus, and that maternal concentrations could be used as surrogate marker for those of the developing fetus (25). Though the associated factors and mechanisms are not clearly described for the role of vitamin D in tooth development, evidence from mouse models suggest 1,25-dihydroxy vitamin D3 (an active metabolite of 25(OH)D) functions to upregulate the vitamin D receptor that in turn could induce structural gene products, including calcium-binding proteins and several extracellular matrix proteins (e.g., enamelins, amelogenins, dentin sialoglycoproteins and dentine phosphoproteins), resulting in dentin and enamel formation (26). A more current review suggests and discusses more specific mechanisms to explain vitamin D deficiencies and tooth defects (27). However, what is not known or not available in the published literature is a demonstration of the relationship or association of human maternal total circulating vitamin D concentrations during pregnancy and EH in the child’s teeth that develop in utero. This pilot study begins to address that gap in the knowledge.
2. Subjects and methods
2.1. Selection of subjects
The study population is the 170 child-mother pairs of the mothers who participated in a single-center, double-blind, randomized clinical trial of vitamin D supplementation during pregnancy (28). In brief description of the mothers’ study, healthy women at least 16 years of age at consent and at fewer than 16 weeks’ gestation with a singleton pregnancy were eligible to participate. Those with preexisting calcium or parathyroid conditions or who required chronic diuretic or cardiac medication therapy, including calcium channel blockers, or who suffered chronic hypertension, or with active thyroid disease other than those on thyroid supplements with normal endocrine function were excluded. The cohort excluded mothers with known calcium metabolism dysfunction, liver or kidney problems, or gastrointestinal malabsorption problems (28).
The pilot study sample presented in this report includes 37 child-mother pairs from the initial 8 weeks of imaging the children’s teeth. Of the 37 children, 8 were excluded for the following reasons: camera was not ready (n=2), child was uncooperative (n=2), primary maxillary central incisors were crowned or extracted due to dental caries (n=2), or digital images were not read (n=2), leaving a final sample size of 29. The mean age of the children at their dental visit was 3.6 ± 0.9 years and 14 (48%) were male. By race/ethnicity, 13 of the children were White (45%), 9 (31%) Hispanic and 7 (24%) Black. The 29 children contributed 56 of a possible maximum of 58 primary maxillary teeth for study, with 1 sound tooth missing due to exfoliation and 1 sound tooth missing due to trauma. For the 56 teeth, 148 of the168 possible images of incisal, middle and cervical locations were available for scoring for EH. Reasons for not scoring a location was that the image was not completely captured and/or too blurred to score.
2.2. Enamel hypoplasia outcome measure and vitamin D exposure measure
The major outcome is the presence of EH in the primary maxillary central incisors of the child. Digital images of the buccal surfaces of the children’s maxillary central incisors were made in vivo using a ProScope High Resolution™ handheld digital USB microscope with a fixed focal length and 50x magnification (see Figure 1). These digital images were later evaluated by two examiners who scored the teeth for presence or absence of EH using the Enamel Defect Index (EDI) (2). EH was scored as presence or absence at three locations (incisal third, middle third, and cervical third) on each primary maxillary central incisor. Each child had two central incisors for a maximum of 6 scored tooth locations for each child. Children with primary maxillary central incisors that were missing or filled due to dental caries were excluded from the analyses. Examiners were blinded to the maternal vitamin D status.
Figure 1.
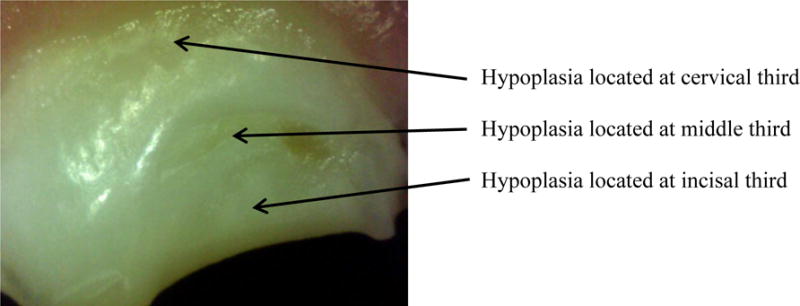
Enamel Hypoplasia of Primary Maxillary Central Incisor, ProScope HR™ 50x
The major exposure was the woman’s total circulating serum 25(OH)D measured by a rapid, direct radioimmunoassay developed in the Hollis laboratory and manufactured by Diasorin Corporation (Stillwater, MN, USA) (29). Clinical guidelines define vitamin D deficiency as below 20 ng/ml (50nmol/liter), vitamin D insufficiency as 21-19 ng/ml (52.5-72.5 nmol/liter) and serum circulating 25(OH)D concentrations for sufficiency are recommended to be above 30 ng/ml (75 nmol/liter) (30). A circulating 25(OH)D level of approximately 30 ng/ml is required to normalize calcium excretion into the urine and equilibrate intestinal calcium absorption (28, 31). Vitamin D supplementation (400, 2000 or 4000 IU D3/day until delivery) began at week 16 for all women. Each woman had 8 monthly 25(OH)D concentration values measured from 12 - 40 weeks of gestation. Missing data for the maternal monthly 25(OH)D concentrations were imputed using the average of the previous and subsequent monthly values, or the previous value was carried forward when the last monthly value was missing. This method of imputation is biologically plausible because the half-life of 25(OH)D is about 2-3 weeks. For this pilot study, there were 9 imputed values for the 9 missing of 232 monthly values, or 3.9% in the dataset. The mean difference in the mean concentrations of 25(OH)D when using the imputed values and without the imputed values was -.06, and was not significantly different from zero (Wilcoxon Signed Rank t-test, 0.5781).
2.3 Statistical analysis
We conducted a case control analysis of the data. Characteristics of the children with and without EH and their mothers were compared for potential confounders for fetal development of EH, e.g. pregnancy health status and delivery information as were available from the existing dataset. Characteristics were compared using Student’s t-test, Wilcoxon rank sum, and Fisher’s exact test. SAS version 9 was used for the analyses (32).
To begin to investigate the timing or critical months for the development of the EH, the monthly mean maternal 25(OH)D concentrations for those children with and without EH were tabulated. At specific weeks’ gestation, mean concentrations are presented at the child level and then again at the level of the location level (incisal, middle and/or cervical thirds) on the buccal surface of the primary maxillary tooth.
2.4 Ethics approval
The study was approved by the Institutional Review Board of the Medical University of South Carolina (approval numbers HR#10727 and #19641).
3. Results
EH was found in 13 (45%) of the children. Hypoplasia was determined by the presence of hypoplasia at any of the 6 possible locations on either of his/her two primary maxillary central incisors. Seventeen children had the maximum 6 locations scored, 4 children had 5 locations scored, 3 had 4 locations scored, 4 had 3, and 1 child had 2 locations scored. Location of the EH resulted in 5 children with hypoplasia at the incisal third, 9 with EH at the middle third, and 7 children with EH at the cervical third. For 4 children, the EH was located in the same third on both primary maxillary central incisors, and two of those 4 children had hypoplasia at two contingent locations on their primary maxillary central incisors. Two examiners scored the digital images with intra-examiner reliability of 90% and 91%, and inter-examiner percent agreement was 89%. Discrepant scores were discussed until agreement was met for the assigned value.
Table 1 presents characteristics of the 13 children with and 16 children without EH. The group with EH is slightly younger by age and by count of erupted teeth. Gender, mode of delivery and level of neonatal care was similar amongst the two groups. The mean serum circulating 25(OH)D concentration during the pregnancy for the women of children with EH was slightly lower than for the women of children without EH. Statistical test results are presented as will be performed for the full dataset of 170 children of the child-mother pairs (33).
Table 1.
Children with and without enamel hypoplasia by explanatory variables: Median or mean (standard deviation), or % of total.
| With enamel hypoplasia n=13 | Without enamel hypoplasia n=16 | Comparison (p-value) | |
|---|---|---|---|
|
| |||
| Maternal and child race/ethnicity | 0.3‡ | ||
| White | 7/13 = 54% | 6/16 = 38% | |
| Hispanic | 2/13 = 15% | 7/16 = 44% | |
| Black | 4/13 = 31% | 3/16 = 19% | |
|
| |||
| Maternal age, years | 28.5 (5.5) | 28.7 (6.5) | 0.95* |
|
| |||
| Maternal 25(OH)D (ng/ml) 12 – 40 weeks of gestation | |||
| 32.1 (13.6) | 33.6 (12.8) | 0.76* | |
|
| |||
| Maternal pregnancy complication | 13 none | 12 none, 2 minor, 1 moderate, 1 severe | Insufficient |
|
| |||
| Mode of delivery | 11 vaginal, 2 C-section | 13 vaginal, 3 C-section | Insufficient |
|
| |||
| Level of neonatal care | 13 normal newborn | 16 normal newborn | Insufficient |
|
| |||
| RCT treatment group 1 | 6 | 5 | Insufficient |
| 2 | 3 | 5 | |
| 3 | 4 | 6 | |
|
| |||
| Child sex | 7/13 = 54% F | 8/16 = 50% F | 0.8‡ |
|
| |||
| Child age (years) | 3.4±0.8 | 3.75±0.9 | 0.31** |
|
| |||
| Child tooth count (average) | 19.0 (3.2) | 19.6 (1.1) | 0.87* |
Fisher’s exact test,
Student’s t-test,
Wilcoxon rank sum test presented where normality assumption was not met, median presented.
Table 2 displays the mothers’ mean serum circulating 25(OH)D concentrations by weeks of gestation for the children with and without EH, and again for those children by the specific location of the hypoplasia (incisal, middle, and cervical) on their teeth. The overall increase in the mean 25(OH)D concentrations by weeks of gestation reflects the RCT of maternal vitamin D supplementation during pregnancy. Vitamin D supplementation began after baseline measures at 12 weeks of gestation. The mean 25(OH)D concentration at 12 weeks for the women is 22.5 SD 10.2 ng/ml with a range from 5.8 ng/ml to 45.5 ng/ml. At 12 weeks of gestation the 25(OH)D concentrations were lower for the mothers of children with EH and this is also true by location of EH on the tooth. With the children grouped by hypoplasia at a specific location on their teeth (incisal, middle, and cervical), 21/24 of the mothers’ mean 25(OH)D concentrations for those with children with hypoplasia were lower compared to those without hypoplasia. For the middle buccal tooth location, the mothers’ mean 25(OH)D concentrations were lower at all weeks of gestation for those children with EH compared to those without EH. In general and throughout the table, the mean serum circulating 25(OH)D concentrations for women of children with EH were lower compared to women of children without EH. Although not significant, children with EH were 1.29 times more likely to have mothers with lower 25(OH)D concentrations during pregnancy compared to children with no EH (unadjusted odds ratio = 1.29 with a corresponding 95% CI (0.1432, 11.5432)).
Table 2.
Maternal mean serum circulating 25(OH)D concentrations (ng/ml) at designated weeks of gestation and by presence and by location of enamel hypoplasia on the buccal surface the child’s primary maxillary central incisors.
| Child | Incisal Location | Middle Location | Cervical Location | |||||
|---|---|---|---|---|---|---|---|---|
| Weeks of Gestation | Hypo n=13 |
No Hypo n=16 |
Hypo n=5 |
No Hypo n=24 |
Hypo n=9 |
No Hypo n=20 |
Hypo n=7 |
No Hypo n=22 |
| 12 | 19.7 | 24.8 | 19.2* | 23.2 | 18.7 | 24.2 | 19.2 | 23.6 |
| 16 | 30.3 | 28.2 | 29.1 | 29.2 | 28.2 | 29.6 | 29.7 | 29.0 |
| 20 | 31.7 | 24.6 | 29.1 | 32.2 | 28.0 | 33.3 | 33.3 | 31.1 |
| 24 | 33.5 | 35.7 | 37.0 | 34.3 | 32.3 | 35.8 | 30.0 | 36.3 |
| 28 | 34.9 | 36.4 | 30.9 | 36.7 | 30.1 | 38.2 | 35.6 | 35.7 |
| 32 | 38.9 | 38.2 | 34.3 | 39.4 | 35.5 | 39.8 | 39.5 | 38.2 |
| 36 | 35.3 | 37.1 | 34.8 | 36.6 | 35.5 | 36.7 | 31.9 | 37.7 |
| 40 | 32.2 | 36.9 | 32.5 | 35.3 | 29.0 | 37.5 | 28.5 | 36.8 |
Bolded values plotted in Figure 2.
Figure 2 graphs the mean maternal 25(OH)D values for the incisal, buccal, and cervical locations of EH selected by estimated gestational weeks of calcification. In other words, the bolded values from Table 2 (weeks 12 and 16 for incisal third values, weeks 20-32 for middle third, and weeks 36-40 for cervical values) were selected to estimate the location on the tooth that would reflect the timing of tooth crown calcification and the circulating 25(OH)D concentration. In general, the maternal mean 25(OH)D concentrations are lower for those children with EH compared to those without EH.
Figure 2.
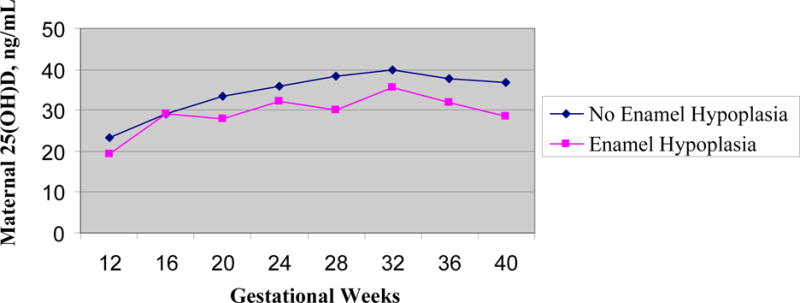
Children with and without enamel hypoplasia by maternal mean circulating serum 25(OH)D (ng/ml) and by gestational weeks of pregnancy. Hypoplasia of incisal location of teeth plotted for gestational weeks 12-16, hypoplasia of middle location plotted for weeks 20-32, and cervical hypoplasia plotted for weeks 36-40 (plots from bolded values of Table 2).
4. Discussion
This pilot study describes the first systematic approach to provide evidence about the role of prenatal maternal vitamin D in the development of hypoplastic primary maxillary teeth. Findings from this pilot study provide feasibility support for our continued study of vitamin D during pregnancy and the etiology of EH for those teeth that develop in utero.
Our novel approach was possible in part because of the available data from the RCT of vitamin D supplementation during pregnancy. Though results of maternal vitamin D exposure and child dental outcome were not the primary aim of that study (33), we did find that the presence of EH in the primary maxillary central incisors of the 2-5 year olds of this pilot study tended to correspond with lower maternal serum circulating 25(OH)D concentrations during pregnancy. Findings in this pilot study demonstrated that EH was evident across treatment groups and whether this is because the hypoplasia is determined by 12 weeks’ gestation or reflects the supplementation by treatment group will be explored in the larger dataset. Evidence from this pilot study of 29 children supports further investigation of the dental information that we have for the child-mother pairs of the RCT.
For an example of the results of a prospective approach to the longitudinal data, characteristics of the pregnant women e.g. mean serum 25(OH)D during pregnancy (16-40 weeks) with mean 25(OH)D concentration <15 ng/ml during pregnancy (n= 4) versus those with a mean ≥ 32 ng/ml (n=16) and considered sufficient were calculated. Using these deficient or sufficient cut-off levels, EH was evident in 50% (2/4) of the children of the mothers with <15 ng/ml during pregnancy compared to 44% (7/16) of those with a mean ≥ 32 ng/ml and there was no statistically significant association (Fisher’s exact test p-value = 1). The relative risk of developing EH for children of mothers with <15 ng/ml during pregnancy compared to children of mothers with 25(OH)D ≥32 ng/ml was 1.14 (.37, 3.53). The pilot sample size precludes accurate estimates yet serves as an example for analyses of the full dataset.
Our findings do not contradict the 1980 report of the secondary outcome of dental enamel defects in children whose mothers had participated in a study of vitamin D during pregnancy. Those authors found a highly significant difference in the number of children with dental enamel defects (15/31, 48%), born to unsupplemented (control) mothers of a vitamin D supplementation during pregnancy trial compared to only 2/30 (7%) of mothers who had received 400 IU vitamin D2 supplementation beginning at the 12th week of pregnancy (34). Limitations of that study include that the serum circulating 25(OH)D was measured only at the 24th and 34th weeks and at delivery, and also that the defect of dental enamel is not further described (34).
A strength of this pilot study is the relatively accessible pregnant woman for measure of the enclosed environment of the fetus. For example we did not consider dental fluorosis in the primary maxillary central incisors when scoring for EH. There is evidence for passive placental transfer when maternal fluoride intake was low; however the placenta serves as at least a partial diffusion barrier to fluoride and therefore fluorosis was not considered when classifying the primary maxillary central incisors for EH in this pilot study (35-38).
Variability in the maternal mean serum circulating 25(OH)D concentrations was apparent in children with and without EH, and also when related to specific locations on the teeth for children with hypoplasia. Additional aspects of timing during pregnancy for developmental hypoplasia were brought to focus by the variability of the maternal serum circulating 25(OH)D concentrations. As shown in Table 2 at 12 weeks of gestation, the mothers of the children with hypoplasia had lower mean concentrations compared to children without hypoplasia. This suggests that more elegant temporal and spatial statistical methods are needed to better portray the hypothesized relationship of defect timing for location of defect during tooth development in the larger study. Likewise we plan to more fully explore potentially confounding variables including race, maternal medication used during pregnancy, infant oral strep mutans, infant dental behaviors and feeding habits as potentially influential for interpretation of findings.
The relationship of the location of EH on the tooth and timing of tooth development reflecting the pregnancy environment is approached with the graph of Figure 2. Forensic evidence from 19 fetal and infant cases in Turkey provides estimates for the initial time of calcification for the primary maxillary central incisor as 13.7 weeks for females and 15.8 weeks for males (22). To approximate the relationship of maternal serum circulating 25(OH)D and developmental calcification of the maxillary central incisor we chose weeks 12 and 16 to reflect the incisal third, weeks 20-32 for the middle third, and weeks 36-40 to reflect the cervical third. Recognizing the limitations of this pilot sample size none the less, this approach to estimate the impact of the timing is innovative as related to development of EH and subsequent risk for early childhood caries. The pattern of hypoplasia (and decay) on the maxillary central incisors is distinct and present or absent in the horizontal locations of the tooth (39). These patterns of EH reflect the pregnancy environment at specific weeks of gestation. The maternal-fetal relationship for non-defective dental enamel needs to be further investigated in future research.
A major limitation of this pilot study is the small sample size and lack of statistical inference from results (33). Using an analysis of two independent proportions test and equal numbers of participants in each group of hypoplasia and no hypoplasia, with an assumed proportion of 0.6 hypoplasia in the low 25(OH)D concentration group we would need 58 subjects to detect an effect size (risk ratio) of 1.5 and 20 participants to detect an effect size of 1.67 (Likelihood Ratio Test with alpha = .05, power = 80%). Further study of the full dataset from which this pilot originated is justified to provide more robust evidence of the association of maternal vitamin D during pregnancy and EH in the primary maxillary incisors of the child.
The purpose of this pilot study is to inform feasibility and identify modifications for future research. Vitamin D insufficiency in women of childbearing years is well-documented (40-44). The intent of reporting the preliminary findings of this pilot study is to demonstrate an approach to investigate maternal vitamin D status during pregnancy that likely impacts biological mechanisms e.g. development of sound tooth structure, which in turn influences risk for ECC.
5. Conclusions
This pilot study of the relationship of maternal monthly total circulating 25(OH)D concentration during pregnancy and EH in the primary maxillary central incisors of the child provides an approach for future research of EH etiology. Though limited by the small sample size, findings of the initial 29 children of the dataset suggest evidence to support additional analyses of the 170 child-mother pairs and statistical testing of the relationship of maternal 25(OH)D concentrations and child EH. The potential dental public health implications for prevention of the developmental defect EH via maternal vitamin D sufficiency during pregnancy need to be determined.
Acknowledgments
We are grateful to the study coordinators Judith Shary, MS, Pamela G. Smith, BSN, and Martha Murphy of the Medical University of South Carolina, Department of Pediatrics; and for support from Grant Award Numbers UL1RR029882, RR01070, T35DE007337, P20RR017696, and 5R01HD043921 from the National Institutes of Health; The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health; Support also in part by the American Association for Dental Research Student Research Fellowship; Thrasher Research Fund; and MUSC James B. Edwards College of Dental Medicine.
Footnotes
Conflict of interest
Dr. Hollis had support from DiaSorin, S.p.A. for serving as an academic consultant.
All authors disclose that there is no actual conflict of interest including any financial, personal or other relationships with other people or organizations for the submitted work that could inappropriately influence, or be perceived to influence, their work.
References
- 1.Mellanby M. The Effect of Diet on the Resistance of Teeth to Caries. Proc R Soc Med. 1923;16:74–82. doi: 10.1177/003591572301601131. Odontol Sect. Epub 1923/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brook AH, Elcock C, Hallonsten A-L, Poulson S, Andreasen J, Koch G, Yeung CA, Dosanjh T. The development of a new index to measure enamel defects. In: Brook A, editor. Dental Morphology. Sheffield: Sheffield Academic Press; 2001. pp. 59–66. [Google Scholar]
- 3.Hong L, Levy SM, Warren JJ, Broffitt B. Association between enamel hypoplasia and dental caries in primary second molars: a cohort study. Caries Res. 2009;43(5):345–53. doi: 10.1159/000231571. Epub 2009/08/04. doi: 000231571 [pii] 10.1159/000231571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliveira AFB, Chaves AMB, Rosenblatt A. The influence of enamel defects on the development of early childhood caries in a population with low socioeconomic status: a longitudinal study. Caries Res. 2006;40(4):296–302. doi: 10.1159/000093188. [DOI] [PubMed] [Google Scholar]
- 5.Hujoel PP. Vitamin D and dental caries in controlled clinical trials: systematic review and meta-analysis. Nutr Rev. 2012;71(2):88–97. doi: 10.1111/j.1753-4887.2012.00544.x. Epub 2013/01/30. [DOI] [PubMed] [Google Scholar]
- 6.Mellanby M. Dental structure in dogs. London: His Majesty’s Stationary Office; 1929. Diet and the teeth: An experimental study. Part I. (Medical Research Council, Series 140). [Google Scholar]
- 7.Yoshiki S, Yanagisawa T, Suda T, Sasaki S. The role of vitamin D in the mineralization of dentin in rats made rachitic by a diet low in calcium and deficient in vitamin D. Calcif Tissue Res. 1974;15(4):295–302. doi: 10.1007/BF02059064. Epub 1974/01/01. [DOI] [PubMed] [Google Scholar]
- 8.Seow WK, Romaniuk K, Sclavos S. Micromorphologic features of dentin in vitamin D-resistant rickets: correlation with clinical grading of severity. Pediatr Dent. 1989;11(3):203–8. Epub 1989/09/01. [PubMed] [Google Scholar]
- 9.Seeto E, Seow WK. Scanning electron microscopic analysis of dentin in vitamin D-resistant rickets–assessment of mineralization and correlation with clinical findings. Pediatr Dent. 1991;13(1):43–8. [PubMed] [Google Scholar]
- 10.Dimitri P, Bishop N. Rickets. Paediatr Child Health. 2007;17(7):279–87. [Google Scholar]
- 11.Davis M, Stanbury SW. The rheumatic manifestation of metabolic bone disease. Clin Rheum Dis. 1981;7:595–646. [Google Scholar]
- 12.Hanna JD, Niimi K, Chan JC. X-linked hypophosphatemia. Genetic and clinical correlates. Am J Dis Child. 1991;145(8):865–70. doi: 10.1001/archpedi.1991.02160080041018. Epub 1991/08/01. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen H, Tenenhouse HS. Mendelian hypophosphatemias. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 7th. New York: McGraw-Hill; 1995. pp. 3717–45. [Google Scholar]
- 14.Archard HO. The dental defects of vitamin D-resistant rickets. Birth Defects: Orig Artic Ser. 1971;7(7):196–9. [PubMed] [Google Scholar]
- 15.Tracy WE, Steen JC, Steiner JE, Buist NR. Analysis of dentine pathogenesis in vitamin D–resistant rickets. Oral Surg Or Med Or Path. 1971;32(1):38–44. doi: 10.1016/0030-4220(71)90248-9. Epub 1971/07/01. [DOI] [PubMed] [Google Scholar]
- 16.Fraser D, Nikiforuk G. The etiology of enamel hypoplasia in children–a unifying concept. J Int Assoc Dent Child. 1982;13(1):1–11. Epub 1982/06/01. [PubMed] [Google Scholar]
- 17.Abe K, Ooshima T, Lily TS, Yasufuku Y, Sobue S. Structural deformities of deciduous teeth in patients with hypophosphatemic vitamin D-resistant rickets. Oral Surg Or Med Or Path. 1988;65(2):191–8. doi: 10.1016/0030-4220(88)90165-x. [DOI] [PubMed] [Google Scholar]
- 18.Goodman JR, Gelbier MJ, Bennett JH, Winter GB. Dental problems associated with hypophosphataemic vitamin D resistant rickets. Int J of Paediatr Dent. 1998;8(1):19–28. doi: 10.1046/j.1365-263x.1998.00059.x. [DOI] [PubMed] [Google Scholar]
- 19.Arnaud C, Maijer R, Reade T, Scriver CR, Whelan DT. Vitamin D dependency: an inherited postnatal syndrome with secondary hyperparathyroidism. Pediatrics. 1970;46(6):871–80. Epub 1970/12/01. [PubMed] [Google Scholar]
- 20.Zambrano M, Nikitakis NG, Sanchez-Quevedo MC, Sauk JJ, Sedano H, Rivera H. Oral and dental manifestations of vitamin D-dependent rickets type I: report of a pediatric case. Oral Surg Oral Med O. 2003;95(6):705–9. doi: 10.1067/moe.2003.116. [DOI] [PubMed] [Google Scholar]
- 21.Beck-Nielsen SS, Brock-Jacobsen B, Gram J, Brixen K, Jensen TK. Incidence and prevalence of nutritional and hereditary rickets in southern Denmark. Eur J Endocrinol. 2009;160(3):491–7. doi: 10.1530/EJE-08-0818. Epub 2008/12/20. doi: EJE-08-0818 [pii]10.1530/EJE-08-0818. [DOI] [PubMed] [Google Scholar]
- 22.Sema AP, Nergis C, Rukiye D, Murat Y. Age determination from central incisors of fetuses and infants. Forensic Sci Int. 2009;184:15–20. doi: 10.1016/j.forsciint.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Berkowitz BKB, Holland GR, Moxham BJ. Color atlas and textbook of oral anatomy, histology and embryology. 2nd. St. Louis: Mosby-Year Book; 1992. [Google Scholar]
- 24.Hillman LS, Haddad JG. Human perinatal vitamin D metabolism. I. 25-Hydroxyvitamin D in maternal and cord blood. J Pediatr. 1974;84(5):742–9. doi: 10.1016/s0022-3476(74)80024-7. Epub 1974/05/01. [DOI] [PubMed] [Google Scholar]
- 25.Hollis BW, Pittard WB., 3rd Evaluation of the total fetomaternal vitamin D relationships at term: evidence for racial differences. J Clin Endocrinol Metab. 1984;59(4):652–7. doi: 10.1210/jcem-59-4-652. [DOI] [PubMed] [Google Scholar]
- 26.Slavkin HC, Hu CC, Sakakura Y, Diekwisch T, Chai Y, Mayo M, Bringas P, Jr, Simmer J, Mak G, Sasano Y, et al. Gene expression, signal transduction and tissue-specific biomineralization during mammalian tooth development. Crit Rev Eukaryot Gene Expr. 1992;2(4):315–29. Epub 1992/01/11. [PubMed] [Google Scholar]
- 27.Foster BL, Nociti FH, Somerman MJ. The Rachitic Tooth. Endocr Rev. 2013:er20131009. doi: 10.1210/er.2013-1009. Epub 2013/08/14. doi: er.2013-1009 [pii]10.1210/er.2013-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;26(10):2341–57. doi: 10.1002/jbmr.463. Epub 2011/06/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem. 1993;39(3):529–33. Epub 1993/03/01. [PubMed] [Google Scholar]
- 30.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30. doi: 10.1210/jc.2011-0385. Epub 2011/06/08. [DOI] [PubMed] [Google Scholar]
- 31.Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22(2):142–6. doi: 10.1080/07315724.2003.10719287. [DOI] [PubMed] [Google Scholar]
- 32.SAS Institute Inc, editor. SAS/STAT. Version 9 of the SAS System for Windows. Cary, NC: SAS Institute Inc; 2002-2004. Copyright © [2002-2010] [Google Scholar]
- 33.Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res. 2011;45(5):626–9. doi: 10.1016/j.jpsychires.2010.10.008. Epub 2010/11/03. doi: S0022-3956(10)00292-X [pii]10.1016/j.jpsychires.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cockburn F, Belton NR, Purvis RJ, Giles MM, Brown JK, Turner TL, Wilkinson EM, Forfar JO, Barrie WJ, McKay GS, Pocock SJ. Maternal vitamin D intake and mineral metabolism in mothers and their newborn infants. Br Med J. 1980;281(6232):11–4. doi: 10.1136/bmj.281.6232.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richter H, Kierdorf U, Richards A, Kierdorf H. Dentin abnormalities in cheek teeth of wild red deer and roe deer from a fluoride-polluted area in Central Europe. Ann Anat. 2010;192(2):86–95. doi: 10.1016/j.aanat.2009.12.003. Epub 2010/02/09. doi: S0940-9602(10)00018-X [pii]10.1016/j.aanat.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Montherrat-Carret L, Perrat-Mabilon B, Barbey E, Bouloc R, Boivin G, Michelet A, Magloire H. Chemical and X-ray analysis of fluoride, phosphorus, and calcium in human foetal blood and hard tissues. Arch Oral Biol. 1996;41(12):1169–78. doi: 10.1016/s0003-9969(96)00033-7. Epub 1996/12/01. doi: S0003-9969(96)00033-7 [pii] [DOI] [PubMed] [Google Scholar]
- 37.Toyama Y, Nakagaki H, Kato S, Huang S, Mizutani Y, Kojima S, Toyama A, Ohno N, Tsuchiya T, Kirkham J, Robinson C. Fluoride concentrations at and near the neonatal line in human deciduous tooth enamel obtained from a naturally fluoridated and a non-fluoridated area. Arch Oral Biol. 2001;46(2):147–53. doi: 10.1016/s0003-9969(00)00104-7. Epub 2001/02/13. doi: S0003-9969(00)00104-7[pii] [DOI] [PubMed] [Google Scholar]
- 38.Ron M, Singer L, Menczel J, Kidroni G. Fluoride concentration in amniotic fluid and fetal cord and maternal plasma. Eur J Obstet Gynecol Reprod Biol. 1986;21(4):213–8. doi: 10.1016/0028-2243(86)90018-3. Epub 1986/04/01. [DOI] [PubMed] [Google Scholar]
- 39.Caufield PW, Li Y, Bromage TG. Hypoplasia-associated severe early childhood caries–a proposed definition. J Dent Res. 2012;91(6):544–50. doi: 10.1177/0022034512444929. Epub 2012/04/25. doi: 0022034512444929 [pii] 10.1177/0022034512444929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schroth RJ, Lavelle C, Tate R, Bruce S, Billings RJ, Moffatt ME. Prenatal Vitamin D and Dental Caries in Infants. Pediatrics. 2014 doi: 10.1542/peds.2013-2215. Epub 2014/04/23. [DOI] [PubMed] [Google Scholar]
- 41.Nesby-O’Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988-1994.[see comment] Am J Clin Nutr. 2002;76(1):187–92. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 42.Ginde AA, Sullivan AF, Mansbach JM, Camargo CA., Jr Vitamin D insufficiency in pregnant and nonpregnant women of childbearing age in the United States. Am J Obstet Gynecol. 2010;202(5):436 e1–8. doi: 10.1016/j.ajog.2009.11.036. Epub 2010/01/12. doi: S0002-9378(09)02210-8 [pii]10.1016/j.ajog.2009.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schroth RJ, Lavelle CLB, Moffatt MEK. Review of vitamin D deficiency during pregnancy: who is affected? Int J Circumpolar Health. 2005;64(2):112–20. doi: 10.3402/ijch.v64i2.17964. [DOI] [PubMed] [Google Scholar]
- 44.Parlak M, Kalay S, Kalay Z, Kirecci A, Guney O, Koklu E. Severe vitamin D deficiency among pregnant women and their newborns in Turkey. J Matern Fetal Neonat Med. 2014:1–12. doi: 10.3109/14767058.2014.924103. Epub 2014/05/16. [DOI] [PubMed] [Google Scholar]


