Summary
Congenital aniridia manifests as total or partial absence of the iris caused most commonly by mutations in PAX6, FOXC1, PITX2, and CYP1B1. Recently two new genes, FOXD3 and TRIM44, have also been implicated in isolated studies. We discuss the genotype-phenotype correlations for the main implicated genes. Classic aniridia is a panocular condition, which includes aniridia, cataract, corneal pannus, foveal, and optic nerve hypoplasia associated with mutations in the PAX6 gene. Classical aniridia is due to PAX6 mutations, while other genes contribute to aniridia-like phenotypes. We review the challenges involved in the management of aniridia, and discuss various surgical interventions. The clinical importance of defining the genotype in cases of congenital aniridia has become acutely apparent with the advent of possible therapies for classical aniridia, which are discussed.
Keywords: aniridia, congenital, aniridia-associated keratopathy, goniotomy, cataract
Aniridia is an ocular disorder in which there is total or partial hypoplasia of the iris tissue. It was first described by Barrata in 1818 [1]. In this review, we discuss the etiology, presentation, complications, management and possible future developments of this disorder.
Etiology
Aniridia may be congenital or acquired. Acquired aniridia is almost always secondary to trauma and usually unilateral [2]. Congenital aniridia is described as partial or total absence of iris, but gonioscopy almost always reveals an iris stump [3]. For congenital partial or total aniridia, mutations in the following genes have been reported; PAX6, FOXC1, PITX2, CYP1B1, FOXD3 and TRIM44 (Table 1).
Table 1.
Etiology of partial and total aniridia
| Phenotype: Partial aniridia |
|---|
|
Etiology: PAX6 [73] [69] FOXC1 [88] PITX2 [89] CYP1B1 [32] |
| Phenotype: Total aniridia (iris stump on gonioscopy) |
|
Etiology: PAX6 [81] FOXC1 [33] CYP1B1 [90] FOXD3 [28] TRIM44 [31] |
numbered references for FOXC1 and CYP1B1 match to case orders in the text
PAX6
This gene encodes a paired domain DNA-binding transcription factor that has been shown to be critical in eye formation and important for neural, pancreas and olfactory system development [4]. In humans, the gene is located on chromosome 11p13, the transcript encompasses 14 exons over 27.4 kb of genomic DNA, and the coding region starts from exon 4. It has two major isoforms, which are canonical Pax6 and Pax6(5a) [5], that have been shown to activate different targets [6, 7], and a paired-less form that may regulate amacrine populations in the retina [8, 9]. Apart from expression in the developing and mature eye (Figure 1), Pax6 is also expressed in the developing forebrain, gut, pineal gland, β-cells of the pancreas and olfactory epithelium [4].
Figure 1. Expression pattern of Pax6, FoxC1 and Pitx2 in the early and late eye.
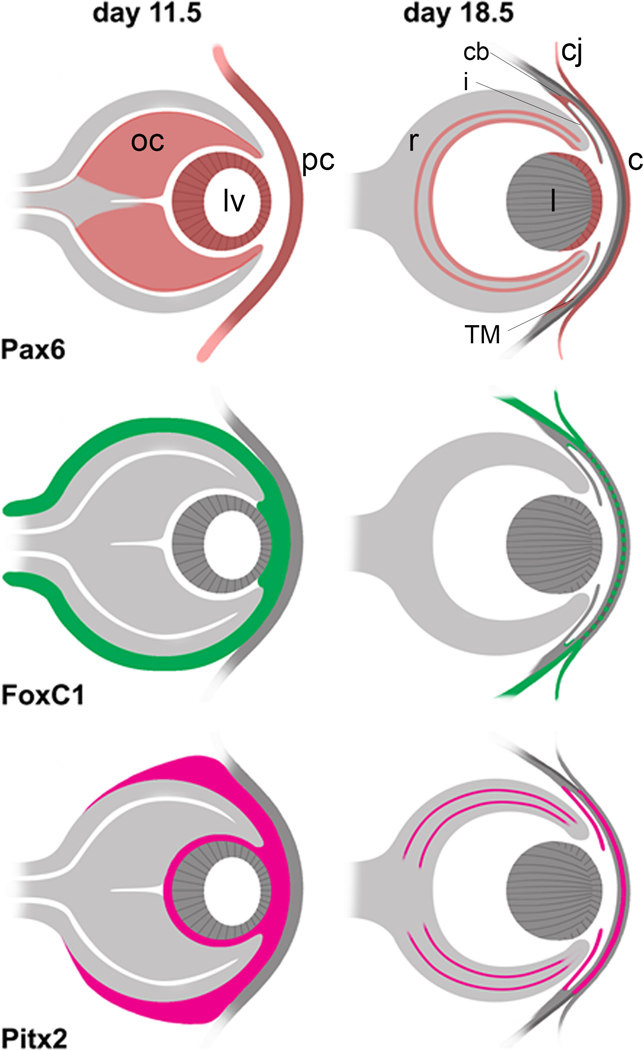
The expression patterns for Pax6, FoxC1, and Pitx2 are presented based on studies in mice and humans [82–87]. Early expression is shown at embryonic day 11.5 (E11.5), which represents day 33 in human development, and late expression is shown at E18.5, which is close to prenatal in humans. For Pax6, early expression is seen in the lens vesicle (lv), optic cup (oc), and presumptive cornea (pc) [86]. Expression later in the prenatal is restricted to the ganglion cell layer and inner and outer portions of the inner nuclear layer of the retina (r), where the latter points to expression specifically in the amacrine and horizontal cells [85]. At this stage, Pax6 is also expressed in the epithelia of the cornea (c), conjunctiva (cj), lens (l), ciliary body (cb), iris (i) and trabecular meshwork (TM). For both FoxC1 and Pitx2, expression in the early eye is seen in the neural crest cells of the periocular mesenchyme that surrounds the oc, in the intracellular space between the lv and pc, and the intracellular space between the inner oc and lv [84]. In the prenatal eye, FoxC1 expression is reduced to the conjunctival epithelium, sclera and trabecular meshwork [82] and Pitx2 expression is reduced to the iris [83], and according to a study of expression in human eyes is expressed in the corneal epithelium, the ciliary body non-pigmented layer and the nuclear layers of the retina [87]. Pitx2 is present in the corneal stroma but FoxC1 is much reduced in this layer [82, 83].
Since PAX6 is expressed in the cornea, lens, iris and retina, it is not surprising that heterozygous mutations result in a panocular condition affecting the cornea, lens, iris, retina and optic nerve (see later). Haploinsufficiency of the protein arising from deleterious mutations results in aniridia (Table 1). The PAX6 mutation database currently reports 376 novel mutations [10], with heterozygous mutations primarily resulting in aniridia in about 90% of cases [11], where intragenic mutations account for two-third of cases and chromosomal rearrangements for one-third of cases. Ninety-four percent of all intragenic point mutations leads to either a premature termination codon (PTC), C-terminal extensions (CTE), or amino acid substitutions [12]. The importance of this will become critically clear when we discuss future developments. In a fraction of classical aniridia patients, the mutation is located outside the PAX6 structural gene and, in particular, in its transcriptional control regions [13, 14].
Two-thirds of all aniridic cases due to PAX6 mutation are familial displaying autosomal dominant inheritance and the remaining are sporadic [11]. Sporadic mutations arise from de novo gene mutations or deletions. Sporadic deletions of PAX6 contiguous with WT1 (about 700 kb apart) often lead to Wilms Tumor, aniridia, genitourinary anomalies, and mental retardation (WAGR) syndrome [15], which Wilms’ tumor, aniridia, genitourinary anomalies, and intellectual disability (replacing the former term mental retardation). Wilms’ tumor develops through somatic loss of both WT1 alleles in the kidney, or more rarely the gonad; therefore, the extent of the WAGR phenotype is dependent on the length of the deletion.
FOXC1
This gene product is a member of the forkhead domain/winged helix (FHD) class of transcription factors that play essential roles in embryonic development, such as cellular differentiation and proliferation [16]. In humans, the gene is located on chromosome 6p25, contains 1 exon and spans 3.9 kb of genomic DNA. In the developing eye, FoxC1 is expressed in the periocular mesenchyme, with later expression in the corneal stroma, sclera, conjunctival epithelium, and trabecular meshwork (Figure 1) [17]. Extra-ocular expression includes the heart, kidney, peripheral blood leukocytes, and prostate.
Mutations in this gene typically produce ocular phenotypes, but may manifest extra-ocular phenotypes such as malar hypoplasia, hypodontia, umbilical hernia and intracranial anomalies, such as cerebellar vermis hypoplasia or Dandy-Walker syndrome. Similar to PAX6, haploinsufficiency in FOXC1 adversely affects development of the ocular anterior segment leading to the distinct Axenfeld-Rieger syndrome (ARS; see later). The type of FOXC1 mutations that gives rise to ARS includes missense mutations in the FHD, frameshift and nonsense mutations, and whole gene deletions/duplications. More than 50 mutations in FOXC1 have been found to cause ARS [18]. Mutations in the FOXC1 gene have also been identified in another eye disorder called iridogoniodysgenesis type 1, where the anterior segment of the eye is primarily involved, and is associated with underdevelopment of the iris and an elevated risk of glaucoma.
A number of mutations or deletions of FOXC1 that results in aniridia, usually with severe glaucoma, have been reported (Table 1). The first is a case of total aniridia and subtle iris hypoplasia in a month-old female and her mother, respectively, due to a heterozygous p.Met161Lys FOXC1 missense mutation. The second a case of aniridia from a heterozygous p.Trp152Gly missense mutation in a newborn male, and the third a case of total aniridia from homozygosity for a missense mutation, p.Pro297Ser, in a female patient. The fourth reports acquired peripheral circumferential iris degeneration from a heterozygous deletion, p.Tyr81_Pro95del, in a month old female. The fifth describes a newborn female and 6-day old male with a deletion of 1.5Mb at 6p25.3p25.2 and duplication of 46XY, dup(6)(25.1p25.3), respectively, that both encompass FOXC1 leading to total aniridia-like symptoms in both patients.
PITX2
This gene belongs to the bicoid class of homeodomain proteins known as RIEG/PITX and its function includes development of the eye anterior segment [19], tooth development, left-right determination, early morphogenesis and arrhythmogenesis during cardiac development [20], and myogenesis [21]. The gene in humans is located at chromosome 4q25, and the coding region contains three exons spanning 5.68 kb of genomic DNA. It is expressed mainly in the neural crest tissue; therefore, it is present in the anterior segment of the developing eye (Figure 1), the developing extraocular muscles, branchial arches, developing teeth, and the maxillary, and mandibular epithelium. Its expression also extends to the umbilicus, limb buds, heart, gut, and anterior pituitary gland.
PITX2 was the first ARS gene to be identified [22]. Mutations in PITX2 and FOXC1 account for approximately 40% of ARS occurrences, where PITX2 mutants usually present with ocular, dental, and umbilical anomalies and FOXC1 mutants associate with isolated ocular or ocular, heart, and/or hearing defects. PITX2 mutations tend to group in the homeodomain and C-terminal region, and result in complete or partial loss of function. Gain-of-function mutations, deletions of coding exons/upstream-regulatory regions, and chromosomal translocations have been reported, but are rare.
A report describes iris hypoplasia in a father with classical symptoms of ARS from a heterozygous FOXC1 frameshift mutation p.Ala204ArgfsX111, and his wife with isolated bilateral posterior embryotoxon and iris heterochromia from a heterozygous missense mutation in the critical OAR domain (homologous to drosophila otp, aristaless, and murine rax) known to interact with FOXC1 [23, 24]. Their female daughter had a severe anterior segment disorder of extruding lenses, thin cornea and severe iris hypoplasia. One other report describes a case of severe iris hypoplasia in an individual without foveal hypoplasia due to a mutation in PITX2 described as a IVS 2 (−1), G→C mutation (Table 1).
CYP1B1
This gene encodes the enzyme cytochrome P450, family 1, subfamily B, polypeptide 1 - a member of the cytochrome P450 superfamily that participates in oxidative biochemical reactions important to many processes in the body. It resides on chromosome 2p22 in humans and consists of three exons spanning 8.5 kb of genomic DNA, with the coding region starting from the second exon [25]. It is expressed in numerous fetal and adult tissues, including the parenchymal and stromal tissues of the brain, breast, cervix, kidney, lymph nodes, prostate, uterus, ovary, heart, placenta, lung and skeletal muscle. It is also detected in ocular tissues. Human CYP1B1 is more highly expressed in the fetus than the adult. It is expressed in the fetal ciliary epithelium at 26 days post‐conception before anterior chamber development [26]. Ocular expression is also observed in the fetal corneal epithelium, keratocytes and iris stromal cells but not in adult eyes. It is observed in the non‐pigmented ciliary epithelium, iris pigmented epithelium and iris dilator muscle both in fetuses and adults. Its expression could not be detected in fetal or adult trabecular meshwork.
Mutations in CYP1B1 occur in 87% of familial and 27% of sporadic cases of primary congenital glaucoma (PCG) worldwide [27]. It is not well understood how defects in the CYP1B1 enzyme cause signs and symptoms of glaucoma. Cases of bilateral congenital glaucoma with aniridia due to mutations in CYP1B1 have been previously reported (Table 1). The first was a 4-year old boy presented with bilateral buphthalmos, abnormal discoloration, central corneal scarring and absent iris. The optic nerve was cupped and pale in the right eye and retinal detachment was observed in the left eye; both due to uncontrolled glaucoma. An autozygosity scan using autoSNPa and sequencing revealed homozygosity for a previously reported CYP1B1 mutation, p.Arg145ProfsX4. The second study involved 67 families with 46 having primary PCG. All had bilateral buphthalmos with corneal scarring, and eight additionally had mild ectropion uveae with partial aniridia. Genetic testing revealed that two of these eight had the same homozygous mutations in CYP1B1, specifically p.Asn252Lys. The third case involved three sisters with PCG, where two of them also had bilateral aniridia. Genetic screening showed a novel homozygous mutation in CYP1B1, p.Ser485Phe, for these two sisters.
FOXD3
This gene expresses another member of the family of transcription factors characterized by a forkhead/winged helix domain (FHD), but has an important role in the induction, development and migration of neural crest cells [28], formation of melanocytes, maintenance of stem cells, their ability to self-renew without differentiating, and as a marker for embryonic stem cells [29]. It is located on chromosome 1p31.3 in humans, and is encoded by 1 exon spanning 2 kb of genomic DNA. The gene is primarily expressed in neural crest cells.
In humans, mutations in this gene are usually associated with vitiligo, an autoimmune skin condition characterized by progressive patchy depigmentation [30]. In a different study where 310 probands were screened for mutations in FOXD3, in addition to Pax6 and others described in this section, four mutations in conserved regions of FOXD3 segregated with aniridia or Peters’ anomaly (Table 1) [28]. These mutations are p.Thr16Met (heterozygous), p.Pro120Leu, p.Asn173His, and p.Arg273_Gly276dup, where the p.Asn173His mutation disrupts the highly conserved helix 2 of the DNA-binding domain.
TRIM44
This gene translates a tripartite motif-containing protein family member thought to function as a ubiquitin E3 ligase, where ubiquitination can result in either degradation or change the activity of target proteins. In humans it is located on chromosome 11p13, downstream of the loci for PAX6, and is encoded by 5 exons spanning 155 kb genomic DNA. Little is known about its normal expression pattern.
A recent study revealed four heterozygous missense mutations in all seven affected individuals of a four generation Chinese pedigree with aniridia [31]. Two of the mutations were in PAX6, but were found not to affect its regulation. Two of the mutations were in PAX6, but were found not to affect its regulation. The two mutations in TRIM44 were shown to suppress endogenous PAX6 expression when transfected into human lens epithelial cells due to overexpression of TRIM44 protein product, which the authors speculated mediates degradation of PAX6 through recruitment of other TRIM E3 ligases, resulting in an aniridia phenotype.
Clinical manifestations and management
Aniridia can be categorized into two groups: classic aniridia, which is associated with mutations in PAX6 [32], and aniridia-like, which is associated with mutations in genes other than PAX6 [33].
Classic aniridia
Ocular findings for this condition include partial or near total absence of iris, cataract, aniridia-associated keratopathy (ARK), glaucoma, foveal hypoplasia, optic disk hypoplasia and nystagmus making it truly panocular (Table 2).
Table 2.
Features of classic aniridia
| Condition | Previous studies [35] | Schanilec et al., 2014 [35] | Chang et al., 2014 [63] | Singh et al., 2014 [91] |
|---|---|---|---|---|
| Nystagmus | 81.8–95% | 76% | 68% | N/A |
| Cataract | 50–80% | 56% | 53% | 40.3% |
| Glaucoma | 6–75% | 64% | 20 OH**** | 36.3% |
| ARK | 20–64% | 48% | 69% | 59.9% |
| Dry eye | 94% | 1% | N/A | N/A |
| FH | 10.7–54.5% | 20% | 91% | 87.7% |
| ONH | 10.7–75% | 20% | N/A | N/A |
ARK: aniridia-associated keratopathy; FH: foveal hypoplasia; ONH: optic nerve hypoplasia; OH: ocular hypertension
ARK
The cornea is clear at birth but invariably has a degree of peripheral pannus (Figure 2) that stains with fluorescein. Over the next two decades these patients develop keratopathy [34, 35], which is not surprising as the PAX6 gene is responsible for embryonic and postnatal development of the cornea (Figure 1). A prior study showed defective cell adhesions, altered cell-to-cell junctions, and wider intercellular spaces due to defective desmosomes in the corneal epithelium [36].
Figure 2. Aniridia showing absence of iris and peripheral pannus.
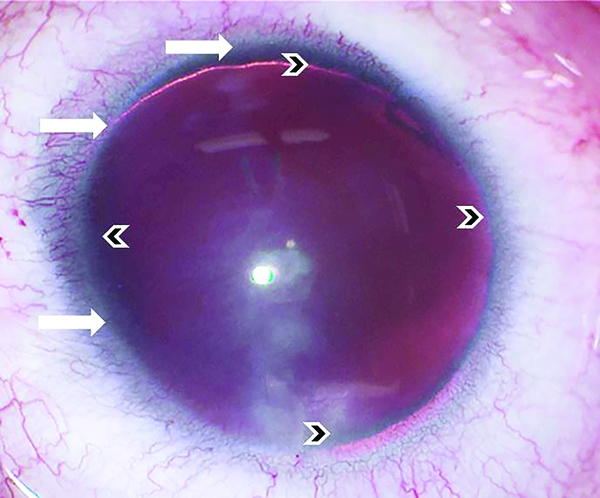
Eye of 20-year-old female showing corneal and limbal changes. Indicated are presence of pannus and absence of a well demarcated limbus (white arrows), in addition to absence of the iris (small black arrows).
An in vivo study of the central cornea with a confocal microscopy revealed that the morphology of the epithelium varies according to the severity of the disease. In most advanced cases, goblet cells were present together with total loss of corneal epithelium, while milder cases showed an intact epithelium [37]. At the early stage of keratopathy, studies have shown an increased central corneal thickness, focal opacities at the level of the basal epithelium, and decreased sensitivity of the cornea [38]. Absence of the limbal stem cell niche and conjunctivalisation of the limbal region leads to limbal stem cell deficiency (LSCD) that clearly has a role in ARK. Meibomian gland dysfunction in form of stenosed or atrophic glands was also seen, which leads to defective lipid layer formation resulting in evaporative dry eye that exacerbates the demise of the ocular surface [39].
Clinically, patients present in early adulthood with symptoms of blurry vision, redness, dry eye, and over the years with gradual opacification of the cornea from the periphery to the center [40]. These changes lead to progressive loss of vision. Keratopathy has been graded on basis of symptoms and signs like photophobia, erosions, and vascular pannus in three stages (Table 3) [41]. Recently, evaluation of the limbal palisades using confocal microscopy has allowed correlation between ARK and palisade integrity [42].
Table 3.
Staging of aniridia-related keratopathy
| Stage | Erosion/ulcer | Pannus | Signs & symptoms | Others |
|---|---|---|---|---|
|
1 |
2 recurring erosions/ulcer in 6 months | <1mm from limbal arch | Slight epiphora and photophobia | Small disorder in absorption of fluorescein |
|
2 |
> 3 recurring erosions/ulcer in 6 months | ≥ peripheral half of the cornea +/− sub epithelial fibrosis | Constant red eye, epiphora, photophobia | Permanent instability of tear film |
|
3 |
Permanent signs of corneal erosions | Central cornea involved | Constant red eye, epiphora, photophobia and loss of vision | Permanent instability of tear film |
Management of ARK depends on the severity of the presenting symptoms and signs. After having established that evaporative dry eye can exacerbate the unhealthy ocular surface, lubricants and anti-inflammatory drops may delay the progression of ARK. In mild to moderate cases of ARK, autologous serum facilitated in maintaining tear film stability and decreasing the frequency of corneal erosion [43]. However, as the disease progresses most patients require surgical interventions. Penetrating keratoplasty (PK) has not been very successful, because of high rate of opacification of the graft [44]. Since LSTD is the main cause of keratopathy, kerato-limbal allograft (KLAL) has shown success in maintaining a stable ocular surface for at least a year [44]. Secondary PK or keratoprosthesis on these eyes that have undergone KLAL, with continuation of the ocular and systemic immunosuppression, demonstrated better outcomes, but long-term stability and maintenance of the corneal clarity was inconsistent [44]. Another study reported a 5-year follow-up in cases of aniridia. The interesting finding in this cohort was that they had subtle iris defects, minimal foveal hypoplasia, and absence of nystagmus. This aniridia variant showed good success with KLAL alone; however, no palisade evaluation was undertaken [45]. Since the ocular surface in advanced cases of ARK is very poor, there is a corresponding increase in rate of rejection of any transplant procedures. A study done on ocular surface stem cell transplantation (OSST) looked at the causes of rejection; 158 patients had undergone OSST with mean follow-up of five years and mean age of 41.7yrs [46]. Aniridia was present in 46% of the patients and the commonest procedure performed was KLAL (80%). All the patients were administered topical and systemic immunosuppressive therapy. Rejection occurred in 31.1% of the patients and the mean time to rejection was 19.3 months. They observed that the etiology did not play a major role in rejection; the important factors for rejection were young age and noncompliance with systemic immunosuppression. These patients needed regular follow-up examinations, as future rejections could not be ruled out.
In recent years, keratoprosthesis has been performed as a primary procedure in cases where there is low probability of transplant success. Outcomes were studied in 26 eyes with aniridia that underwent type one Boston keratoprosthesis, of which 19 eyes had this as the primary procedure for corneal pathology [47]. The mean age was 56 yrs (SD; 11yrs), and the mean follow-up time was 28.7 months. The common complication that occurred in these eyes was the emergence of a retro-prosthetic membrane, where nine of them required neodymium-doped–yttrium aluminum garnet (Nd:YAG) laser membranotomy. They observed visual acuity (>20/200) was maintained in the primary procedure group better than the secondary group. They also had 77% (20/26 eyes) retention rate, while 6 eyes needed replacement of the keratoprosthesis. Glaucoma was diagnosed in 21 eyes before keratoprosthesis surgery and 5 eyes had undergone glaucoma surgery. Post-keratoprosthesis, 23 eyes were diagnosed with glaucoma. Three of them underwent implant surgery. Four eyes developed phthisis due to severe hypotony, choroidal detachment, retinal detachment, and unspecified infection. Similar complications have been reported previously [48]. Eyes that underwent multiple intraocular surgeries developed a progressive fibrosis in the form of a membrane called anterior fibrosis syndrome [49]. The fibrous membrane grew from the rudimentary iris stump and extended anteriorly, thereby pushing the intraocular lens implants forward. This, in turn, caused endothelial touch and posteriorly extended to cover the ciliary body leading to hypotony. Histopathologically, this membrane showed a mixture of hypo cellular fibrous tissue, consisting of immature collagen fibers with mature collagen fibers with absence of glial tissue, corneal epithelium and endothelium and lens tissue. All the eyes in this study had undergone cataract extraction with iris diaphragm implants, except for one case that had an acrylic sulcus implant. All of the eyes had additional corneal or glaucoma procedures, with an average of total three intraocular surgeries. Close and long-term monitoring of patients with aniridia who have undergone ocular surgeries is necessary to detect rejections or complications.
Glaucoma
Glaucoma in aniridia occurs in early adulthood, but may also occur in infants and toddlers [50]. The incidence ranges from 6% to 75% [1]. The angle in subjects with aniridia was studied by performing serial gonioscopies over a span of 18 years, where it was observed that all cases always had an iris stump [3]. The trabecular meshwork and ciliary processes were visible posterior to the stump. It was noted that patients who developed glaucoma had irregular strands arising from the iris stroma and attaching it to the angle wall. These attachments became thicker and moved forward, causing obscuration of the trabecular meshwork, scleral spur and the ciliary body. Consequently, the iris stump tilted and the angle gradually closed. A study of the angle using an ultrasound biomicroscope showed that along with iris hypoplasia, the ciliary body was also hypoplastic [51]. There was anterior inclination of the ciliary body and an anteriorly placed lens. Initial management is usually with medical therapy, as most of them require surgery at a later stage.
Prophylactic goniosurgery in aniridic patients has been reported [52], with an 89% success rate without topical drops [53]. The mean follow-up in the last report was 9.5 yrs. Trabeculotomy as an initial procedure has been advocated for patients with high intraocular pressure (IOP) [54], with a 83% success rate in patients who underwent the surgery as the initial procedure. The average age in this study was 4.5yrs and the follow-up was 11.6yrs. A recent study with Ahmed valve implant as the primary procedure showed good IOP control when assessed at a mean period of 37.4 months post surgery [55]. A different study documented high IOP and greater number of surgical interventions as the cause of poor visual outcome in aniridic glaucoma eyes, with the probability of blindness estimated to be 69.8% at the end of five years [56]. To address the difficulty in treating aniridic glaucoma, perhaps a future study showing prophylactic goniosurgey performed at an early age and then comparing various other management options to control increases in IOP may aid in a better understanding of the long-term management of glaucoma.
Cataract
Cataracts are seen in 50 to 85 % of aniridic patients [57]. They are detected at infancy, but become visually significant in the first decade or early adulthood. The morphology of the lens opacities seen are anterior polar, posterior polar and subcapsular cataracts (Figure 3) [58, 59].
Figure 3. Anterior polar cataract and subcapsular lens opacities.
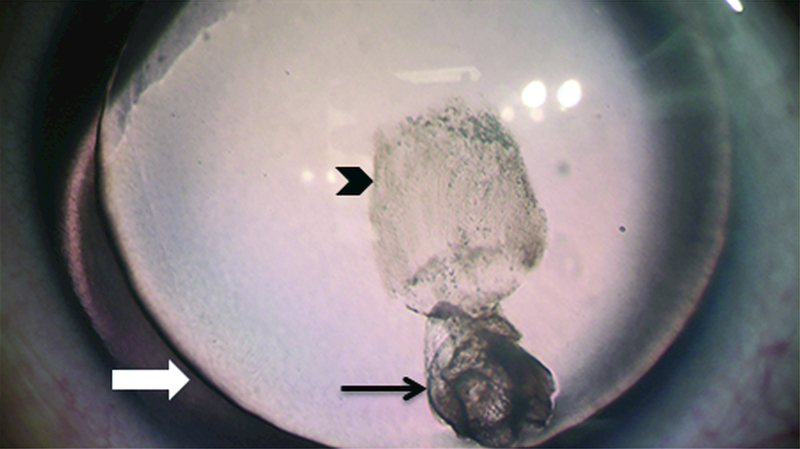
3-year-old male showing lens changes. Subcapsular opacity (small black arrow), anterior polar lens opacity (long black arrow) and absence of the iris (white arrow) are shown.
Cataract surgery in patients with aniridia comes with unique challenges. Preoperative intraocular lens power calculation can be difficult due to the unstable ocular surface, nystagmus and polar cataracts. Incision size and length need careful consideration to avoid causing or exacerbating LSCD in these patients [60]. Study of the lens capsule showed there was 50% reduction in thickness of the capsule in young patients when compared to normal controls [61]. Due to a hypoplastic iris resulting in absence of a sulcus in aniridia, in-the bag implantation is the only option in these cases. If the anterior capsule is unstable, posterior capsule optic capture must be performed. Iris reconstruction in aniridia can be a challenging task. Outcomes of black iris diaphragm lens in congenital aniridia cases have been studied, where it was found that glaucoma was the most common complication in these eyes who required further surgeries for control of IOP [62]. There are a few case reports on the use of iris implant devices, most of them for traumatic aniridia. Further investigation into the long-term use and safety of such devices is necessary.
Retina
The fovea and optic disc show varying degrees of hypoplasia. Most of the studies show the presence of foveal hypoplasia to be as high as 90% in these eyes [63]. Clinically, there is absence of the macular reflex and foveal pit, and presence of abnormal retinal vessels crossing the fovea (Figure 4) [12]. The presence of foveal hypoplasia has been shown by measuring increased thickness of fovea and central macula by Optical Coherence Tomography (OCT) (Figure 5) [64]. A few case reports have shown retinal detachments, exudative retinopathy, and chorioretinal degeneration in these patients [34, 58, 65]. Electroretinogram (ERG) shows a decrease in amplitude of all the waveforms, suggesting abnormality in all retinal layers [66]. Optic nerve hypoplasia is seen in 10 to 30% of these cases [40, 67]. There has been no proven correlation between the optic nerve and foveal hypoplasia, but most cases show nystagmus. Incidence of nystagmus is as high as 90%, where the majority of these eyes show horizontal pendular nystagmus [1, 63]. Vertical nystagmus as a phenotype variant with foveal hypoplasia, presenile cataract and intact irides has been recently described [68].
Figure 4. Foveal hypoplasia in aniridia.
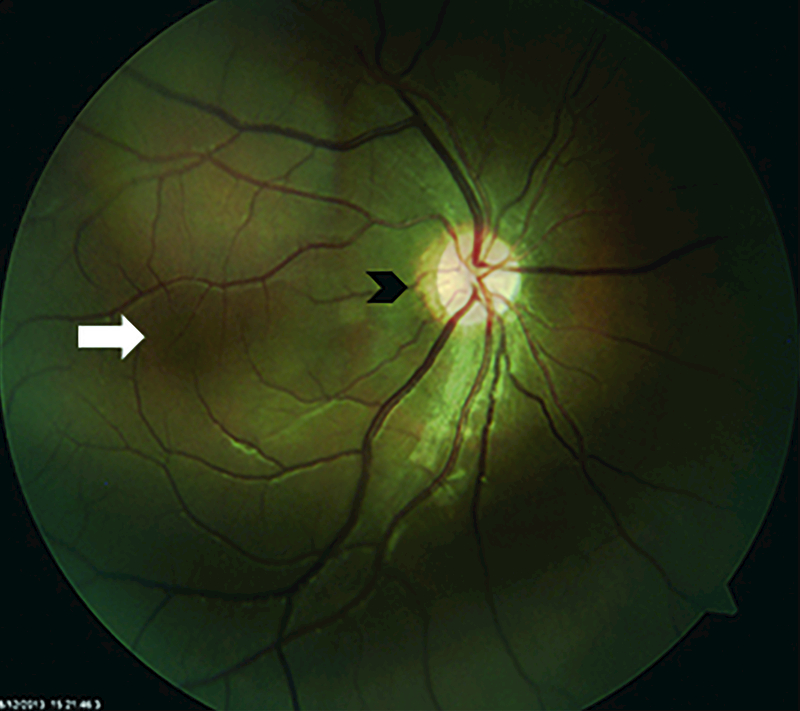
Fundus photo of a 16-year-old boy. Note the blood vessels crossing the fovea (white arrow) and optic disc hypoplasia (black arrow).
Figure 5. OCT of thickened fovea and absence of pit.
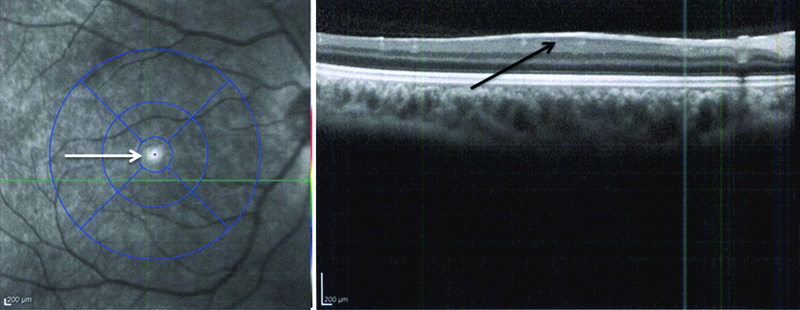
OCT images of the same eye (Figure 4) showing absence of a foveal pit and a thickened fovea (black arrow).
Other ocular manifestations
Other ocular manifestations are not typical of classic aniridia, and may include blepheroptosis, which has been reported in association with familial cases of classic aniridia [69]. Surgical management of ptosis in this subset of patients needs a careful approach according to guidelines in a report recently published for ptosis surgery in aniridic patients [70].
Systemic associations with classic aniridia
Patients with PAX6 mutations may have systemic abnormalities along with aniridia, which may not manifest in infancy. WAGR syndrome may also be present.
WAGR
Children with WAGR present early in life. Classical WAGR in older children can be diagnosed by aniridia and one of following findings: genital anomalies, Wilms’ tumor, and intellectual disability [71]. WAGR without aniridia is rare. A study done on the Dutch population has reported that patients with sporadic aniridia had 67 times higher risk of developing Wilms’ tumor (confidence interval 8.1–241) when compared to the normal population [72]. Intellectual disability is the most common neurological manifestation. The other neurological abnormalities may be olfactory problems, hypertonia, and corpus callosum agenesis. Genitourinary findings are cryptorchidism, ambiguous genitalia, and streak ovaries [71]. Obesity has also been observed in most of these patients [50, 71]. Therefore, when an infant presents with sporadic aniridia and genitourinary abnormalities, a molecular diagnosis must be made to exclude the deletion encompassing the WT1 gene, so appropriate risk management can be put in place for management of potential of Wilms’ tumor.
Clinical correlations with PAX6 mutation types in classic aniridia:
When analyzing the PAX6 mutation database (http://lsdb.hgu.mrc.ac.uk/home.php?select_db=PAX6), categorizing a type of mutation to a particular phenotype cannot be easily made. For example, when considering missense type of mutations in PAX6, a p.Ser43Pro missense mutation leads to total aniridia, a p.Ser121Pro missense causes partial aniridia, and a recently reported p.Pro76Arg missense mutation leads to intact irides. Though some reports have shown certain phenotype frequencies with a particular type of mutation [58], no definitive conclusion can be derived until further functional studies on the protein have been conducted. An exception to this is in the cases of PTC and 3’-UTR type of mutations, where the majority of occurrences lead to total aniridia.
Pax6 unrelated aniridia
There are differences in phenotypes between classic aniridia and aniridia-like associated with other etiologies (Table 4). The latter occurs in 10% of cases [73]. Therefore, if an infant presents with an absence of iris, the differences in the clinical features associated with each of the etiologies would help distinguish between classic aniridia from PAX6 mutations to the other types of aniridia-like from other etiologies. Aniridia has been previously reported in association with other conditions as described below.
Table 4.
Classic and aniridia-like phenotypes
| Aniridic genes | PAX6 (C) | FOXC1(AL) | CYP1B1 (AL) | PITX2 (AL) |
|---|---|---|---|---|
| Keratopathy | ✓ | - | - | - |
| Iris abnormality | ✓ | ✓ | ✓ | ✓ |
| Glaucoma at birth | - | - | ✓ | - |
| Cataract at birth | - | - | - | - |
| Foveal hypoplasia | ✓ | - | - | - |
| Nystagmus | ✓ | - | - | - |
| Optic disc | Hypoplasia | - | Cupping | - |
| Posterior embryotoxon | - | ✓ | - | - |
| Systemic anomalies | Kidney, brain | Facial, dental | - | Cardiac, umblical hernia |
AL: aniridia-like; C: classic aniridia
Gillespie syndrome
Children present with partial aniridia, cerebellar ataxia, and psychomotor delay. It has been reported to have an autosomal-recessive inheritance, though no gene has been consistently identified for reported cases of the disorder. Ocular findings show some amount of iris present with absent pupillary zone and there have been reports of presence of a persistent pupillary membrane along with the above findings, diagnosis of the syndrome remains clinical [74]. Complete absence of iris has been reported with Brachmann-de Lange syndrome and Omenn syndrome [75, 76].
Duane’s syndrome, megalocornea are other reported associations with aniridia. These have been single case reports without detail genetic screening [77, 78].
Future
Apart from the genes listed in the etiology section, a putative causal gene that can be added to this list is Notch1 [79]. This gene encodes the Notch1 receptor as part of the Notch pathway, and in humans resides on chromosome 9q34.3. The Notch pathway is a highly conserved signaling cascade involved in numerous differentiation processes during embryonic and postnatal development, in addition to maintenance of adult organs capable of self-renewing. It has been shown to be required for melanocyte development, and therefore pigment formation in the skin.
A recent report investigating the development of the anterior pigmented epithelium of the ciliary body indicated that the Notch pathway could play a role in iris development [79]. The notable findings were that loss of canonical Notch signaling, through Notch 2 or RBJ absence, results in normal iris development and absence of the ciliary body, while Notch1 gain-of-function result in aniridia with ciliary body hyperplasia, the latter causing glaucoma-like disease.
Treatment for aniridia has taken a dramatic step forward recently through a study examining aminoglycosides that suppress expression of premature stop codons (PTCs) in PAX6 and allow transcriptional read-through, therefore producing a functional protein. It has been determined that 72% of all PAX6-associated aniridia are caused by missense mutations, such as nonsense, splice-site, and frameshift mutations that result in PTCs [12]. However, some missense mutations cause a change of amino acid and not a PTC. A recent study has developed a postnatal nonsense mutation suppression approach for in-frame PTCs associated with aniridia [80]. In this approach, an aminoglycoside, named ataluren, promotes read-through of PTCs, because during mRNA translation a near-cognate aminoacyl tRNA is inserted into the position of the PTC. A functional PAX6 protein is therefore produced. This approach was tested on the malformation defects exhibited by the Pax6-deficient mouse model of aniridia. They found that ataluren promotes dose-dependent read-through of all three nonsense codons, with the highest read through at UGA, followed by UAG and then UAA. Read-through efficiencies were influenced by the nature of the nucleotide following the nonsense codon, where a pyrimidine (in particular cytosine, C) would increase efficiency of read-through. They demonstrated that the mammalian eye retains marked developmental plasticity into the postnatal period and molecular remodeling by reversing corneal, lens, and retinal malformation defects and restoring electrical and behavioral responses of the retina.
Expert commentary
Currently, our understanding of the mechanism of aniridia is rudimentary. Increased awareness of total/partial iris defects (total/partial aniridia) needs to be implemented so appropriate genetic testing can be done to find a molecular diagnosis rather than a clinical one. Improved genotype–phenotype correlation will likely allow reclassification of congenital aniridia according to the responsible gene. Mutation detection for PAX6 is 43.3% [81]. Even when other genes that cause aniridia like phenotype are considered, current genetic testing is not capable of finding all gene mutations. At present, treatment of aniridia-related glaucoma maybe hindered by inappropriate diagnosis, for example, while goniotomy is considered for PAX6-related aniridic glaucoma, goniotomy for FOXC1/PITX2-related glaucoma with aniridia would likely fail.
Five-year view
The classification of aniridia is likely to move to a molecular genetic one in the near future given our increased understanding of both causative mutations in different genes and the extraocular association of such mutations.
The role of other genes on 11p13, example TRIM44, on PAX6 expression will also help understand a molecular diagnosis. This will impact accurate genetic counseling and better therapeutic interventions. Specifically for PAX6-related aniridia, advances in stem cell research with early intervention may help delay the process of keratopathy, and a better understanding of iris development may allow therapies for iris growth.
Key issues.
Two types of aniridia: classic aniridia associated with mutations in PAX6 and aniridia-like associated with mutations in genes other than PAX6, such as FOXC1, PITX2, and CYP1BI.
Classic aniridia is a panocular disorder with iris hypoplasia, aniridia-associated keratopathy, cataract, foveal and optic nerve hypoplasia and nystagmus. FOXC1 aniridia-like presents usually with dental anomalies, and PITX2 aniridia-like presents with heart and umbilical hernias and CYP1B1 has no systemic manifestations but presents with glaucoma at birth.
Sporadic cases of aniridia need to be screened for WAGR syndrome and need continuous monitoring for a defined period.
All types of management in aniridic cases have better outcomes if diagnosed early.
Most interventions in aniridia management are surgical in nature
Prolonged monitoring and close compliance need to be adhered for good outcome of management.
Acknowledgments
Financial and competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Funding
We acknowledge support from NIH CORE Grant P30 EY08098 to the Department of Ophthalmology, from the Eye and Ear Foundation of Pittsburgh, and from an unrestricted grant from Research to Prevent Blindness, New York, NY.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Nelson LB, Spaeth GL, Nowinski TS, Margo CE, and Jackson L (1984). Aniridia. A review. Survey of ophthalmology 28, 621–642. [DOI] [PubMed] [Google Scholar]
- 2.Sharma V, and Mohan M (2010). Traumatic aniridia and self-sealed globe rupture following blunt trauma. Eye 24, 1526. [DOI] [PubMed] [Google Scholar]
- 3.Grant WM, and Walton DS (1974). Progressive changes in the angle in congenital aniridia, with development of glaucoma. Am J Ophthalmol 78, 842–847.** First to report the presence of an iris stump through gonioscopy in all cases of congenital aniridia, and extend findings for progressive angle changes in these patients resulting in glaucoma.
- 4.Shaham O, Menuchin Y, Farhy C, and Ashery-Padan R (2012). Pax6: a multi-level regulator of ocular development. Progress in retinal and eye research 31, 351–376. [DOI] [PubMed] [Google Scholar]
- 5.Walther C, and Gruss P (1991). Pax-6, a murine paired box gene, is expressed in the developing CNS. Development 113, 1435–1449. [DOI] [PubMed] [Google Scholar]
- 6.Chauhan BK, Reed NA, Yang Y, Cermak L, Reneker L, Duncan MK, and Cvekl A (2002). A comparative cDNA microarray analysis reveals a spectrum of genes regulated by Pax6 in mouse lens. Genes Cells 7, 1267–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chauhan BK, Reed NA, Zhang W, Duncan MK, Kilimann MW, and Cvekl A (2002). Identification of genes downstream of Pax6 in the mouse lens using cDNA microarrays. J Biol Chem 277, 11539–11548. [DOI] [PubMed] [Google Scholar]
- 8.Lakowski J, Majumder A, and Lauderdale JD (2007). Mechanisms controlling Pax6 isoform expression in the retina have been conserved between teleosts and mammals. Dev Biol 307, 498–520. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, and Lauderdale JD (2006). Analysis of Pax6 expression using a BAC transgene reveals the presence of a paired-less isoform of Pax6 in the eye and olfactory bulb. Dev Biol 292, 486–505. [DOI] [PubMed] [Google Scholar]
- 10.Grimes G HI, Williamson K and van Heyningen V (2015). Human PAX6 mutation database. [Google Scholar]
- 11.Prosser J, and van Heyningen V (1998). PAX6 mutations reviewed. Human mutation 11, 93–108. [DOI] [PubMed] [Google Scholar]
- 12.Hingorani M, Hanson I, and van Heyningen V (2012). Aniridia. European journal of human genetics : EJHG 20, 1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatia S, Bengani H, Fish M, Brown A, Divizia MT, de Marco R, Damante G, Grainger R, van Heyningen V, and Kleinjan DA (2013). Disruption of autoregulatory feedback by a mutation in a remote, ultraconserved PAX6 enhancer causes aniridia. American journal of human genetics 93, 1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Elia AV, Pellizzari L, Fabbro D, Pianta A, Divizia MT, Rinaldi R, Grammatico B, Grammatico P, Arduino C, and Damante G (2007). A deletion 3’ to the PAX6 gene in familial aniridia cases. Molecular vision 13, 1245–1250. [PubMed] [Google Scholar]
- 15.Kokotas H, and Petersen MB (2010). Clinical and molecular aspects of aniridia. Clinical genetics 77, 409–420. [DOI] [PubMed] [Google Scholar]
- 16.Tumer Z, and Bach-Holm D (2009). Axenfeld-Rieger syndrome and spectrum of PITX2 and FOXC1 mutations. European journal of human genetics : EJHG 17, 1527–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mears AJ, Jordan T, Mirzayans F, Dubois S, Kume T, Parlee M, Ritch R, Koop B, Kuo WL, Collins C, et al. (1998). Mutations of the forkhead/winged-helix gene, FKHL7, in patients with Axenfeld-Rieger anomaly. American journal of human genetics 63, 1316–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strungaru MH, Dinu I, and Walter MA (2007). Genotype-phenotype correlations in Axenfeld-Rieger malformation and glaucoma patients with FOXC1 and PITX2 mutations. Investigative ophthalmology & visual science 48, 228–237. [DOI] [PubMed] [Google Scholar]
- 19.Idrees F, Vaideanu D, Fraser SG, Sowden JC, and Khaw PT (2006). A review of anterior segment dysgeneses. Surv Ophthalmol 51, 213–231. [DOI] [PubMed] [Google Scholar]
- 20.Franco D, Christoffels VM, and Campione M (2014). Homeobox transcription factor Pitx2: The rise of an asymmetry gene in cardiogenesis and arrhythmogenesis. Trends in cardiovascular medicine 24, 23–31. [DOI] [PubMed] [Google Scholar]
- 21.L’Honore A, Drouin J, Buckingham M, and Montarras D (2014). Pitx2 and Pitx3 transcription factors: two key regulators of the redox state in adult skeletal muscle stem cells and muscle regeneration. Free radical biology & medicine 75 Suppl 1, S37. [DOI] [PubMed] [Google Scholar]
- 22.Reis LM, Tyler RC, Volkmann Kloss BA, Schilter KF, Levin AV, Lowry RB, Zwijnenburg PJ, Stroh E, Broeckel U, Murray JC, et al. (2012). PITX2 and FOXC1 spectrum of mutations in ocular syndromes. European journal of human genetics : EJHG 20, 1224–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelberman D, Islam L, Holder SE, Jacques TS, Calvas P, Hennekam RC, Nischal KK, and Sowden JC (2011). Digenic inheritance of mutations in FOXC1 and PITX2 : correlating transcription factor function and Axenfeld-Rieger disease severity. Human mutation 32, 1144–1152.* Interesting paper reporting a severe ocular phenotype from two inherited mutations in aniridic genes, one paternal mutation in FOXC1 and one maternal mutation in PITX2.
- 24.Footz T, Idrees F, Acharya M, Kozlowski K, and Walter MA (2009). Analysis of mutations of the PITX2 transcription factor found in patients with Axenfeld-Rieger syndrome. Investigative ophthalmology & visual science 50, 2599–2606. [DOI] [PubMed] [Google Scholar]
- 25.Faiq MA, Dada R, Sharma R, Saluja D, and Dada T (2014). CYP1B1: a unique gene with unique characteristics. Current drug metabolism 15, 893–914. [DOI] [PubMed] [Google Scholar]
- 26.Doshi M, Marcus C, Bejjani BA, and Edward DP (2006). Immunolocalization of CYP1B1 in normal, human, fetal and adult eyes. Exp Eye Res 82, 24–32. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Y, Sorenson CM, and Sheibani N (2015). Cytochrome P450 1B1 and Primary Congenital Glaucoma. Journal of ophthalmic & vision research 10, 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kloss BA, Reis LM, Bremond-Gignac D, Glaser T, and Semina EV (2012). Analysis of FOXD3 sequence variation in human ocular disease. Molecular vision 18, 1740–1749. [PMC free article] [PubMed] [Google Scholar]
- 29.Calloni R, Cordero EA, Henriques JA, and Bonatto D (2013). Reviewing and updating the major molecular markers for stem cells. Stem Cells Dev 22, 1455–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schunter JA, Loffler D, Wiesner T, Kovacs P, Badenhoop K, Aust G, Tonjes A, Muller P, Baber R, Simon JC, et al. (2015). A novel FoxD3 Variant Is Associated With Vitiligo and Elevated Thyroid Auto-Antibodies. The Journal of clinical endocrinology and metabolism 100, E1335–1342. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Qin G, Chen G, Li T, Gao L, Huang L, Zhang Y, Ouyang K, Wang Y, Pang Y, et al. (2015). Variants in TRIM44 Cause Aniridia by Impairing PAX6 Expression. Human mutation 36, 1164–1167.** Identifies a novel pathogenic mechanism for congenital aniridia by downregulating the expression of the classic aniridic gene PAX6 by a mutation in a ubiquitin ligase gene, TRIM44.
- 32.Khan AO, Aldahmesh MA, and Alkuraya FS (2011). Genetic and genomic analysis of classic aniridia in Saudi Arabia. Mol Vis 17, 708–714. [PMC free article] [PubMed] [Google Scholar]
- 33.Sadagopan KA, Liu GT, Capasso JE, Wuthisiri W, Keep RB, and Levin AV (2015). Anirdia-like phenotype caused by 6p25 dosage aberrations. Am J Med Genet A 167A, 524–528. [DOI] [PubMed] [Google Scholar]
- 34.Lee H, Khan R, and O’Keefe M (2008). Aniridia: current pathology and management. Acta ophthalmologica 86, 708–715. [DOI] [PubMed] [Google Scholar]
- 35.Eden U, Riise R, and Tornqvist K (2010). Corneal involvement in congenital aniridia. Cornea 29, 1096–1102. [DOI] [PubMed] [Google Scholar]
- 36.Davis J, Duncan MK, Robison WG Jr., and Piatigorsky J (2003). Requirement for Pax6 in corneal morphogenesis: a role in adhesion. Journal of cell science 116, 2157–2167. [DOI] [PubMed] [Google Scholar]
- 37.Le Q, Deng SX, and Xu J (2013). In vivo confocal microscopy of congenital aniridia-associated keratopathy. Eye 27, 763–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eden U, Fagerholm P, Danyali R, and Lagali N (2012). Pathologic epithelial and anterior corneal nerve morphology in early-stage congenital aniridic keratopathy. Ophthalmology 119, 1803–1810. [DOI] [PubMed] [Google Scholar]
- 39.Jastaneiah S, and Al-Rajhi AA (2005). Association of aniridia and dry eyes. Ophthalmology 112, 1535–1540. [DOI] [PubMed] [Google Scholar]
- 40.Schanilec P, and Biernacki R (2014). Aniridia: a comparative overview. The American orthoptic journal 64, 98–104. [DOI] [PubMed] [Google Scholar]
- 41.Lopez-Garcia JS, Garcia-Lozano I, Rivas L, and Martinez-Garchitorena J (2006). [Congenital aniridia keratopathy treatment]. Archivos de la Sociedad Espanola de Oftalmologia 81, 435–444. [DOI] [PubMed] [Google Scholar]
- 42.Lagali N, Eden U, Utheim TP, Chen X, Riise R, Dellby A, and Fagerholm P (2013). In vivo morphology of the limbal palisades of vogt correlates with progressive stem cell deficiency in aniridia-related keratopathy. Invest Ophthalmol Vis Sci 54, 5333–5342. [DOI] [PubMed] [Google Scholar]
- 43.Lopez-Garcia JS, Rivas L, Garcia-Lozano I, and Murube J (2008). Autologous serum eyedrops in the treatment of aniridic keratopathy. Ophthalmology 115, 262–267. [DOI] [PubMed] [Google Scholar]
- 44.Holland EJ, Djalilian AR, and Schwartz GS (2003). Management of aniridic keratopathy with keratolimbal allograft: a limbal stem cell transplantation technique. Ophthalmology 110, 125–130. [DOI] [PubMed] [Google Scholar]
- 45.Skeens HM, Brooks BP, and Holland EJ (2011). Congenital aniridia variant: minimally abnormal irides with severe limbal stem cell deficiency. Ophthalmology 118, 1260–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ang AY, Chan CC, Biber JM, and Holland EJ (2013). Ocular surface stem cell transplantation rejection: incidence, characteristics, and outcomes. Cornea 32, 229–236. [DOI] [PubMed] [Google Scholar]
- 47.Hassanaly SI, Talajic JC, and Harissi-Dagher M (2014). Outcomes following Boston type 1 keratoprosthesis implantation in aniridia patients at the University of Montreal. American journal of ophthalmology 158, 270–276 e271. [DOI] [PubMed] [Google Scholar]
- 48.Akpek EK, Harissi-Dagher M, Petrarca R, Butrus SI, Pineda R 2nd, Aquavella JV, and Dohlman CH (2007). Outcomes of Boston keratoprosthesis in aniridia: a retrospective multicenter study. American journal of ophthalmology 144, 227–231. [DOI] [PubMed] [Google Scholar]
- 49.Tsai JH, Freeman JM, Chan CC, Schwartz GS, Derby EA, Petersen MR, and Holland EJ (2005). A progressive anterior fibrosis syndrome in patients with postsurgical congenital aniridia. American journal of ophthalmology 140, 1075–1079. [DOI] [PubMed] [Google Scholar]
- 50.Netland PA, Scott ML, Boyle J.W.t., and Lauderdale JD (2011). Ocular and systemic findings in a survey of aniridia subjects. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus / American Association for Pediatric Ophthalmology and Strabismus 15, 562–566. [DOI] [PubMed] [Google Scholar]
- 51.Okamoto F, Nakano S, Okamoto C, Hommura S, and Oshika T (2004). Ultrasound biomicroscopic findings in aniridia. American journal of ophthalmology 137, 858–862. [DOI] [PubMed] [Google Scholar]
- 52.Walton DS (1986). Aniridic glaucoma: the results of gonio-surgery to prevent and treat this problem. Transactions of the American Ophthalmological Society 84, 59–70. [PMC free article] [PubMed] [Google Scholar]
- 53.Swanner JC, Walton DS, and Chen TC (2004). Prevention of aniridic glaucoma with goniosurgery. International ophthalmology clinics 44, 67–71. [PubMed] [Google Scholar]
- 54.Adachi M, Dickens CJ, Hetherington J Jr., Hoskins HD, Iwach AG, Wong PC, Nguyen N, and Ma AS (1997). Clinical experience of trabeculotomy for the surgical treatment of aniridic glaucoma. Ophthalmology 104, 2121–2125. [DOI] [PubMed] [Google Scholar]
- 55.Almousa R, and Lake DB (2014). Intraocular pressure control with Ahmed glaucoma drainage device in patients with cicatricial ocular surface disease-associated or aniridia-related glaucoma. International ophthalmology 34, 753–760. [DOI] [PubMed] [Google Scholar]
- 56.Jain A, Gupta S, James MK, Dutta P, and Gupta V (2015). Aniridic Glaucoma: Long-term Visual Outcomes and Phenotypic Associations. J Glaucoma 24, 539–542.* Largest series of aniridic glaucoma pateints ,although a retroseptive review gives varied reason for poor outcome.
- 57.Shiple D, Finklea B, Lauderdale JD, and Netland PA (2015). Keratopathy, cataract, and dry eye in a survey of aniridia subjects. Clin Ophthalmol 9, 291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hingorani M., Williamson KA, Moore AT, and van Heyningen V. (2009). Detailed ophthalmologic evaluation of 43 individuals with PAX6 mutations. Investigative ophthalmology & visual science 50, 2581–2590.** An important study stating genotype-phenotype correlations in classic aniridia.
- 59.Eden U, Lagali N, Dellby A, Utheim TP, Riise R, Chen X, and Fagerholm P (2014). Cataract development in Norwegian patients with congenital aniridia. Acta Ophthalmol 92, e165–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan CC, and Holland EJ (2011). Cataract surgery after keratolimbal allograft surgery in patients with congenital aniridia. Journal of cataract and refractive surgery 37, 786–787. [DOI] [PubMed] [Google Scholar]
- 61.Schneider S, Osher RH, Burk SE, Lutz TB, and Montione R (2003). Thinning of the anterior capsule associated with congenital aniridia. Journal of cataract and refractive surgery 29, 523–525. [DOI] [PubMed] [Google Scholar]
- 62.Aslam SA, Wong SC, Ficker LA, and MacLaren RE (2008). Implantation of the black diaphragm intraocular lens in congenital and traumatic aniridia. Ophthalmology 115, 1705–1712. [DOI] [PubMed] [Google Scholar]
- 63.Chang JW, Kim JH, Kim SJ, and Yu YS (2014). Congenital aniridia: long-term clinical course, visual outcome, and prognostic factors. Korean J Ophthalmol 28, 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Holmstrom G, Eriksson U, Hellgren K, and Larsson E (2010). Optical coherence tomography is helpful in the diagnosis of foveal hypoplasia. Acta Ophthalmol 88, 439–442. [DOI] [PubMed] [Google Scholar]
- 65.Aggarwal S, Jinda W, Limwongse C, Atchaneeyasakul LO, and Phadke SR (2011). Run-on mutation in the PAX6 gene and chorioretinal degeneration in autosomal dominant aniridia. Molecular vision 17, 1305–1309. [PMC free article] [PubMed] [Google Scholar]
- 66.Tremblay F, Gupta SK, De Becker I, Guernsey DL, and Neumann PE (1998). Effects of PAX6 mutations on retinal function: an electroretinographic study. American journal of ophthalmology 126, 211–218. [DOI] [PubMed] [Google Scholar]
- 67.Lee H, Meyers K, Lanigan B, and O’Keefe M (2010). Complications and visual prognosis in children with aniridia. Journal of pediatric ophthalmology and strabismus 47, 205–210; quiz 211–202. [DOI] [PubMed] [Google Scholar]
- 68.Thomas S, Thomas MG, Andrews C, Chan WM, Proudlock FA, McLean RJ, Pradeep A, Engle EC, and Gottlob I (2014). Autosomal-dominant nystagmus, foveal hypoplasia and presenile cataract associated with a novel PAX6 mutation. European journal of human genetics : EJHG 22, 344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peter NM, Leyland M, Mudhar HS, Lowndes J, Owen KR, and Stewart H (2013). PAX6 mutation in association with ptosis, cataract, iris hypoplasia, corneal opacification and diabetes: a new variant of familial aniridia? Clinical & experimental ophthalmology 41, 835–841. [DOI] [PubMed] [Google Scholar]
- 70.Peralta RJ, Kersten RC, Holland EJ, Snyder ME, and Nerad JA (2015). The clinical characterization and surgical correction of blepharoptosis associated with congenital aniridia. Ophthalmic plastic and reconstructive surgery 31, 38–42. [DOI] [PubMed] [Google Scholar]
- 71.Fischbach BV, Trout KL, Lewis J, Luis CA, and Sika M (2005). WAGR syndrome: a clinical review of 54 cases. Pediatrics 116, 984–988. [DOI] [PubMed] [Google Scholar]
- 72.Gronskov K, Olsen JH, Sand A, Pedersen W, Carlsen N, Bak Jylling AM, Lyngbye T, Brondum-Nielsen K, and Rosenberg T (2001). Population-based risk estimates of Wilms tumor in sporadic aniridia. A comprehensive mutation screening procedure of PAX6 identifies 80% of mutations in aniridia. Human genetics 109, 11–18. [DOI] [PubMed] [Google Scholar]
- 73.Lim HT, Seo EJ, Kim GH, Ahn H, Lee HJ, Shin KH, Lee JK, and Yoo HW (2012). Comparison between aniridia with and without PAX6 mutations: clinical and molecular analysis in 14 Korean patients with aniridia. Ophthalmology 119, 1258–1264. [DOI] [PubMed] [Google Scholar]
- 74.Donald KA, Grotte R, Crutchley AC, and Wilmshurst JM (2006). Gillespie syndrome: two further cases. Journal of child neurology 21, 337–340. [DOI] [PubMed] [Google Scholar]
- 75.Lee WB, Brandt JD, Mannis MJ, Huang CQ, and Rabin GJ (2003). Aniridia and Brachmann-de Lange syndrome: a review of ocular surface and anterior segment findings. Cornea 22, 178–180. [DOI] [PubMed] [Google Scholar]
- 76.Sheehan WJ, Delmonte OM, Miller DT, Roberts AE, Bonilla FA, Morra M, Giliani S, Pai SY, Notarangelo LD, and Oettgen HC (2009). Novel presentation of Omenn syndrome in association with aniridia. The Journal of allergy and clinical immunology 123, 966–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khan AO, and Aldahmesh M (2006). Bilateral Duane syndrome and bilateral aniridia. J AAPOS 10, 273–274. [DOI] [PubMed] [Google Scholar]
- 78.Lipsky SN, and Salim S (2011). Concurrent presentation of aniridia and megalocornea without glaucoma. J AAPOS 15, 297–298. [DOI] [PubMed] [Google Scholar]
- 79.Sarode B, Nowell CS, Ihm J, Kostic C, Arsenijevic Y, Moulin AP, Schorderet DF, Beermann F, and Radtke F (2014). Notch signaling in the pigmented epithelium of the anterior eye segment promotes ciliary body development at the expense of iris formation. Pigment cell & melanoma research 27, 580–589. [DOI] [PubMed] [Google Scholar]
- 80.Gregory-Evans CY, Wang X, Wasan KM, Zhao J, Metcalfe AL, and Gregory-Evans K (2014). Postnatal manipulation of Pax6 dosage reverses congenital tissue malformation defects. The Journal of clinical investigation 124, 111–116.** Important study in the development of an eyedrop that postnatally reverses symptoms of classic aniridia by transcript read-through of a PTC in PAX6.
- 81.Dubey SK, Mahalaxmi N, Vijayalakshmi P, and Sundaresan P (2015). Mutational analysis and genotype-phenotype correlations in southern Indian patients with sporadic and familial aniridia. Mol Vis 21, 88–97.** An essential study giving an updated analysis of PAX6 mutations and its correlation with phenotype, further suggesting that factors other than PAX6 may influence the phenotype.
- 82.Kidson SH, Kume T, Deng K, Winfrey V, and Hogan BL (1999). The forkhead/winged-helix gene, Mf1, is necessary for the normal development of the cornea and formation of the anterior chamber in the mouse eye. Developmental biology 211, 306–322. [DOI] [PubMed] [Google Scholar]
- 83.Hjalt TA, Semina EV, Amendt BA, and Murray JC (2000). The Pitx2 protein in mouse development. Dev Dyn 218, 195–200. [DOI] [PubMed] [Google Scholar]
- 84.Berry FB, Lines MA, Oas JM, Footz T, Underhill DA, Gage PJ, and Walter MA (2006). Functional interactions between FOXC1 and PITX2 underlie the sensitivity to FOXC1 gene dose in Axenfeld-Rieger syndrome and anterior segment dysgenesis. Human molecular genetics 15, 905–919. [DOI] [PubMed] [Google Scholar]
- 85.Nishina S, Kohsaka S, Yamaguchi Y, Handa H, Kawakami A, Fujisawa H, and Azuma N (1999). PAX6 expression in the developing human eye. The British journal of ophthalmology 83, 723–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Furimsky M, and Wallace VA (2006). Complementary Gli activity mediates early patterning of the mouse visual system. Developmental dynamics : an official publication of the American Association of Anatomists 235, 594–605. [DOI] [PubMed] [Google Scholar]
- 87.Markintantova Iu V, Firsova NV, Smirnova Iu A, Panova IG, Sukhikh GT, Zinov’eva RD, and Mitashov VI (2008). [Localization of the PITX2 gene expression in human eye cells in the course of prenatal development]. Izvestiia Akademii nauk. Seriia biologicheskaia / Rossiiskaia akademiia nauk, 139–145. [PubMed] [Google Scholar]
- 88.Khan AO, Aldahmesh MA, Mohamed JY, and Alkuraya FS (2013). Congenital glaucoma with acquired peripheral circumferential iris degeneration. J AAPOS 17, 105–107. [DOI] [PubMed] [Google Scholar]
- 89.Perveen R, Lloyd IC, Clayton-Smith J, Churchill A, van Heyningen V, Hanson I, Taylor D, McKeown C, Super M, Kerr B, et al. (2000). Phenotypic variability and asymmetry of Rieger syndrome associated with PITX2 mutations. Investigative ophthalmology & visual science 41, 2456–2460. [PubMed] [Google Scholar]
- 90.Alzuhairy S, Abu-Amero KK, Al-Shahwan S, and Edward DP (2015). A novel CYP1B1 mutation with congenital glaucoma and total aniridia. Ophthalmic genetics 36, 89–91. [DOI] [PubMed] [Google Scholar]
- 91.Singh B, Mohamed A, Chaurasia S, Ramappa M, Mandal AK, Jalali S, and Sangwan VS (2014). Clinical manifestations of congenital aniridia. Journal of pediatric ophthalmology and strabismus 51, 59–62. [DOI] [PubMed] [Google Scholar]


