Abstract
Disruption of liver immune tolerance allows for the development autoimmune hepatitis (AIH) and hepatocellular carcinoma (HCC). AIH rarely progresses to HCC but the diseases similarly induce the production of IL-18 and matrix metalloproteinases. These molecules have distinct effects on the immune response, including the Programmed cell-death 1 (PD-1) axis. In this review, differences in PD-1 function and possible cell signals in AIH and HCC are highlighted.
Keywords: Autoimmune hepatitis, hepatocellular carcinoma, Programmed cell-death 1 (PD-1), matrix metalloproteinases, IL-18
1. Introduction
Antibodies to programmed cell-death 1 (PD-1/CD279) and its ligand (PD-L1/CD274) are increasingly being examined in cancer models and experimental trials. Several of these agents have been approved for use in various human malignancies and are also being evaluated in patients with hepatocellular carcinoma (HCC) in a number of ongoing clinical trials. The promise of these agents is coupled with a risk of immune-related hepatitis, which is a known adverse event with immuno-oncology agents of this class. The pathogenesis of autoimmune hepatitis (AIH) is thought to be comparable to immune-related hepatitis, which can occur as an adverse event with PD-1 and PD-L1 agents. Although basic immunology research has revealed some aspects of PD-1 and PD-L1-mediated functions in the host response, additional study is needed to understand the immunobiology of the PD-1/PD-L1 pathway and evaluate potential adverse events of PD-1/PD-L1 antagonists in the clinical setting. The relevant immunobiology of the PD-1/PD-L1 pathway in AIH and HCC may help provide insight into newer and safer treatments for both conditions.
The liver receives a constant influx of immune cells via arterial blood (hepatic artery), venous blood (portal vein) and lymphatic vessels that coordinately mediate immunity with resident Kupffer cells (KCs), pit cells (natural killer cells, also known as NK cells), hepatocytes, endothelial, epithelial, and Ito (perisinusoidal stellate) cells [1]. In general, the liver is considered to be an immune tolerant organ, with AIH representing a significant departure from the natural state [2]. Data indicating a low incidence of HCC in patients with AIH suggests that the distinct immunobiology of AIH may prevent HCC development unlike the tumor-inducing inflammation associated with chronic viral hepatitis C [3].
The aim of this review is to examine PD-1 immunobiology in AIH versus HCC in order to identify potential pathways in disease etiology and treatment. Identification of the disparate and common cell signals between HCC and AIH may aid in understanding the mechanisms promote and inhibit disease.
2. AIH
The most recent reports of AIH in western countries suggest an approximate incidence of 2–3 per 100,000 people [4]. AIH affects women 3.6 times more than men [5]. Characterized serum antibodies have been identified suggesting 3 subtypes of disease. AIH-1 is positive for anti-nuclear (ANA) and/or anti-smooth muscle antibodies (SMAs) with identified peaks between 10–20 and 45–70 years of age. AIH-2 is defined by the presence of anti-liver kidney microsomal antibody type 1 (LKM-1) and/or anti-liver cytosol type 1 (LC-1) antibodies and generally presents between ages 2 and 4 years. Lastly, AIH-3 is positive for soluble liver antigen/liver-pancreas (SLA/LP) antibodies and is clinically indistinguishable from AIH-1 [6, 7].
Although the etiology of AIH is not clearly known, susceptibility to AIH has been linked to deviant alleles within the human leukocyte antigen (HLA)-DRB1 locus[4]. Aberrant antigen presentation and concomitant T cell recognition are therefore initial links to a disease where immune cell plasticity, binding interactions, and the extracellular microenvironment (ECM) likely have integral roles. This disruption of immune homeostasis results in the clinical signs of AIH which includes increased levels of immunoglobulin G (IgG) and an influx of immune cells that induce erosion of the hepatic parenchyma and elevate transaminase levels.
In the liver, PD-1 has been identified on KCs, B and T cells whereas PD-1 ligands are reported on KCs, hepatocytes, dendritic cells (DCs), liver sinusoidal epithelial cells (LSECs), and Ito cells [8–11]. PD-1 mRNA was not detected in liver biopsies of healthy subjects but the mRNA was identified in AIH patients [9]. In addition, soluble PD-1 (sPD-1) has been found to be significantly elevated in AIH-1 patients with active disease compared to healthy controls [12]. The cells releasing sPD-1 and the exact mechanism of PD-1 release are not clearly known. However, increased levels of matrix metalloproteinases (MMPs) are characterized in autoimmunity (MMP-9) and liver injury [13, 14]. MMPs may therefore cleave PD-1 from the cell surface whereby MMP inhibitors could possibly modulate the AIH immune response.
One cell type, that may be responsible for the increased presence of sPD-1, is the KC. In PD-1-deficient septic mice, KCs displayed markedly enhanced phagocytosis compared to septic KCs from wild-type mice. PD-1 gene deficiency also decreased LPS-induced apoptosis of ex-vivo septic KCs [11]. The receptor tyrosine kinases Tyro3, Axl and Mer (TAM) have a functional role in macrophage clearance of apoptotic cells. Mice deficient in TAM receptor tyrosine kinases (RTKs) develop persistent inflammatory liver damage resembling AIH. This phenotype was rescued upon transplantation of wild-type bone marrow into TAM receptor deficient mice [15]. Because the expression of TAMs or PD-1 deficiency positively influences KC clearance of apoptotic cells [11, 15], cell signals in these pathways may potentially interact. This correlates with the idea that TAMs are inhibitors of toll-like receptors (TLR) cell signals [16] and both PD-1 [17] and PD-L1 [18] are modulated by TLR activation.
Additionally, increased levels of PD-1+ KCs in septic mice occur in association with increased PD-L1 expression on LSECs that also exhibit increased vascular permeability, reduced expression of vascular endothelial growth factor receptor (VEGFR)-2, and lower levels of Ki67. This study also revealed that the interaction of PD-1+ KCs and PD-L1 LSECs inhibits angiogenesis [19]. However, immunohistological examination of AIH biopsy specimens revealed increased expression of PD-L1 and PD-L2 on KCs and LSECs within the sinusoids and increased PD-1 on T cells within the portal tracts compared to healthy control [20]. The distinct functions of PD-1 molecules in KCs and LSECs in AIH are therefore likely linked to pathogenicity, and possibly similar to the murine sepsis model [19], aspects of angiogenesis.
Murine models have also been useful in examining the role of PD-1 in autoimmunity. For example, the transfer of murine PD-1−/− hematopoietic stem cells (HSCs) or PD-1−/− lymphocytes into Rag−/− (lymphopenic) mice revealed an induction of lethal autoimmunity in the transfer of HSCs but not lymphocytes. PD-1 therefore seems to be required in the generation of T cell tolerance from HSCs. However, the transfer of PD-1−/− HSCs into Rag−/−γc−/− (lymphopenic and lymph node deficient) mice did not generate lethal autoimmunity [21]. Thus, the functions of PD-1 appear to be highly important in regulating immune responses within lymphoid stroma.
Further study has indicated that PD-1 is not required for the suppressive functions of T regulatory cells (Tregs) but has an integral role in the differentiation of conventional CD4+ T cells into peripherally induced Tregs [22]. This is highlighted in a murine model where a neonatal thymectomy of PD-1−/− mice generates a concurrent loss of Tregs and PD-1-mediated signaling that then leads to fatal AIH [23]. Studies in AIH-1 patients have also identified functional impairments in Treg populations [24]. Age-related thymic involution and reduced thymopoeisis may be factors in AIH etiology, but given the wide age range (2 years – 70 years) in AIH onset [7], additional processes, possibly involving PD-1 cell signals, are likely.
Common to the activation of B cells, follicular helper T cells (Tfh/CD4+CXCR5+), are significantly increased in AIH-1 patients compared to age-matched controls [25]. Through the production of antibodies characterized in the 3 types of AIH, B cells are pivotal to AIH development. Data has also revealed that serum samples retrieved from 52 AIH-1 patients were positive (63%) for anti-PD-1 antibodies compared to 62 healthy volunteers (3%) [26]. Because B cells also express PD-1, increased production of PD-1 antibodies may allow for increased antibody recognition of B cells unless B cells are themselves releasing sPD-1. B cell PD-1 is indicated to cluster with the BCR after B cell activation and appears to be a negative regulator of TLR9-induced CD27+IgM memory B cell proliferation in vitro [27]. Peripheral blood mononuclear cells (PBMCs) from 17 AIH-1 patients were found to have reduced numbers of CD27+IL-10+ B cells compared to 17 healthy controls which may suggest a deficiency in a regulatory B cell subgroup [25]. Depletion of B cells with anti-CD20 antibodies in a murine model of AIH, significantly reduced T cell proliferation in association with reduced liver inflammation and alanine aminotransferase levels [28], indicating that AIH inflammation is heavily dependent on B and T cell interactions.
Additional study in PD-1−/− mice given a neonatal thymectomy revealed increased levels of serum IL-18 and CXCL9 which were respectively produced by DCs and KCs. These mediators were involved in the activation and recruitment of Th1 CD8+ and CD4+ cells required for the fatal progression of AIH [29]. Elevated serum levels of IL-18 and CXCL9 have also been reported in AIH patient studies [30, 31]. IL-18 activated NK cells are known to produce MMPs (e.g.: membrane type 1 (MT1)-MMP and MMP-2) [32]. NK cells may therefore play a role in the release of sPD-1 from lymphocytes.
Moreover, IL-18 in the presence of IL-12 or IL-15 is well characterized in inducing the production of IFN-γ, particularly from NK cells [33]. The expression of PD-1, PD-L1, and PD-L2 have been identified on human NK cells where the increased expression of PD-1 is correlated with IFN-γ production [34]. Pediatric AIH-1 liver biopsies notably express elevated levels of mRNA for IFN-γ and the Vα24 receptor of invariant natural killer T cells (iNKT) compared to healthy controls [35]. In adults, NK cell activity, identified by genomic assessment of the killer immunoglobulin-like receptors (KIR) gene KIR2DS1 in PBMCs, has also been found to be higher in AIH-1 patients compared to healthy controls [36]. These findings may be linked to a disrupted regulatory function of NK cells as has been identified in additional autoimmune diseases (e.g.: SLE, multiple sclerosis, rheumatoid arthritis (RA), scleroderma, type I diabetes) [37]. In addition, IFN-γ is known to induce myeloid production of the IL-18 decoy receptor, IL-18 binding protein (IL-18BP) [33, 38]. KC or DC production of IL-18BP may be disrupted in AIH as has been previously reported in the autoimmune disorders of SLE[39] and primary Sjögren’s syndrome (pSS) [40].
3. HCC
HCC affects more than 500,000 people worldwide each year, and in the U.S. the disease affects men 2.8 times more than women. Ethnicity coupled with an age >45 increases the risk from 3.7 per 100,000 people in whites to 10.3 and 8.2 for Asians and Africans respectively [41]. The development of HCC is generally precluded by liver fibrosis and cirrhosis which are induced by genetic mutations (hereditary haemochromatosis), metabolic disorders (diabetes, obesity, non-alcoholic fatty liver disease), viruses (hepatitis B or C virus), infectious pathogens (brucellosis, syphilis, echinococcosis, schistosomiases), lifestyle (alcoholism), and autoimmune disorders (primary biliary cirrhosis) [42].
Myeloid cells are thought to significantly influence immune suppression in HCC [43]. This is highlighted by the increased presence of PD-L1+ KCs and PD-1+ CD8 T cells in tumor tissue compared to the surrounding non-tumor tissue from liver resections of HCC patients [44]. Although TAM receptors have been linked to the potential functions of leukocytes in AIH [15], TAM receptor activity in the HCC immune response has not been significantly explored. The TAM receptor Axl is however identified in murine hepatocarcinoma cell lines (Hca-F and Hca-P) and is an identified factor in transforming growth factor (TGF)- β production, tumor cell proliferation, migration, and invasion in vitro [45, 46]. In vivo, Axl enhances the production of tumor growth and the metastatic potential of tumor cell lines [45, 46] and in HCC patients, histopathological analysis has revealed that Axl expression is a marker for aggressive tumor invasiveness and a poor prognostic indicator for HCC patients [47].
LSECs function along with DCs and KCs in the clearance and presentation of antigens and the maintenance of tolerance [48]. LSECs express PD-L1, but their precise role in HCC has not been elucidated [19]. DC subsets in HCC are identified with varied phenotypic properties and generally produce IL-10, which is characteristic of tolerogenic DCs [49]. Murine PD-1-deficient DCs increased CD8+ T cell effector functions and reduced tumorigenesis in vivo. Immunohistochemical staining has identified CD3-CD11c+PD-1+ myeloid cells in tumor tissue extracted from HCC patients [50]. These immunosuppressive cells may encourage the development of a microenvironment that produces lower levels of Tfh/CXCR5+CD4+ cells, higher numbers of Tregs (CD25-FOXP3-PD-1+) and the increased production of PD-1hi B cells that additionally suppress tumor specific T cell immunity in HCC disease progression [51–53]. In examining 112 HCC patients retrospectively via immunohistochemical stains, clusters of CD20+ B cells in close proximity to CD3+ T cells were correlated with a better prognosis. A possible mechanism may involve B cell activation of T cells as was supported in a murine model involving transplanted hepatoma cells that progressed in growth upon B cell depletion. In this same study, the expression of PD-1 was significantly increased on CD8+ T cells in the absence of B cells [54]. Thus, B cell interactions with T cells appear to be important to the anti-tumor response.
With respect to NK cells, increased activity is identified in the early stages of hepatitis B virus or hepatitis C virus infection that then declines with tumorigenesis [55]. Increased PD-1 expression on HCC patient NK cells (CD56dimCD16+ and CD56brightCD16−) from peripheral blood and tumor-infiltrating NK cells compared respectively to healthy controls and paired adjacent non-cancerous tissues is noted [56]. In an additional study, CD56dimCD16+ NK cells are reduced and generate less IFN-γ when isolated from HCC tumor tissue or HCC PBMCs compared to non-tumor tissue and healthy controls respectively [57]. NK cells from healthy donor PBMCs exert significant cytotoxicity against HCC cell lines and engrafted HepB3 cells in mice deficient of lymphocytes and NK cells (NOD/scid IL2RGnull) are significantly rejected by i.p. administered NK cells in an NKG2D-dependent manner [58]. NK cells are therefore integral to the anti-tumor response. As a result, a cytokine characterized in activating NK cells, IL-18, would be anticipated to have an antitumor response in HCC. However, elevated serum levels of IL-18 are identified in HCC where the cytokine is also a poor prognostic indicator [59].
IL-18 is generated in a pro-form and cleaved to an activated molecule by inflammasome-induced activation of caspase-1 in various cell types (KCs, DCs, epithelial cells) [60, 61]. Inflammasome activation occurs in response to damage or pathogen associated molecular patterns (DAMPs or PAMPs) which may include components of viruses (RNA, DNA), bacteria (LPS, CpG oligonucleotides), or tissue damage (high mobility group box-1 (HMGB-1), ATP) [62]. The IL-18 receptor (IL-18R) has been identified in immortal hepatic (HL-7702) and hepatoma (HepG2 and HepG2.2.15) cell lines as well as HCC tissue specimens where IL-18 may promote tumorigenesis via cell signals that prevent tumor cell apoptosis and/or encourage migration/metastases through NF-κB activation [63, 64].
Activation of the IL-18 receptor may be modulated by another cytokine with a similar structure, IL-37. IL-18 and IL-37 are both members of the IL-1 family and both cytokines are produced in a precursor form that is cleaved by inflammasome-induced caspase-1 activity [65]. Unlike IL-18, IL-37 is an anti-inflammatory cytokine that binds to the alpha chain of the IL-18R and thereby recruits Toll/IL-1R (TIR)-8 in antagonizing NF-κB activation [66]. In analyzing tumor tissue from 163 patients, high expression of IL-37 was associated with better overall survival, disease-free survival and an increased presence of tumor-infiltrating CD57+ NK cells [67]. Possibly, differential responses in these patients may be due to the presence of IL-18BP which can bind and affect the activity of both IL-18 and IL-37 [66].
IL-18 also induces the production of MMP-2, MMP-3, and MMP-9 in hepatoma cell lines. These same MMPs are elevated in liver cancer tissue specimens compared to normal tissue at the mRNA level [64]. Ito cells [68] and hepatocytes [69] produce MMPs that may cleave PD-1 molecules from cell surfaces. In patients infected with the hepatitis B virus, high viral load and sPD-1 production has been associated with a 6-fold increase in HCC risk [70]. An additional study found that the overall survival of patients with hepatitis B virus-associated HCC was reduced in individuals that expressed high levels of sPD-1 (>10 ng/mL) compared to patients with low sPD-1 levels (≤10 ng/mL) [71]. However, serum sPD-L1 levels have also been positively correlated with the stages of HCC as well as increased mortality risk [72]. Possibly, the difference between the soluble ligands identified in hepatitis B versus HCC is linked to fibrosis given that sPD-L1 is an identified cleavage product of fibroblasts in vitro [73].
4. Conclusions
Liver immune tolerance is a complex dynamic between the parenchymal cells, immune subsets, and the ECM. The development and persistence of disease disrupts the immune homeostatic balance, generates shifts in cell function, and physically alters the microenvironment (see Figure 1). AIH occurs most frequently in women and is characterized by the heightened activation of DC, NK, B, Th1, and Tfh cells but reduced Treg function. HCC occurs most frequently in men and exhibits reduced activation of NK, Th1, and Tfh cells but increased suppressor functions involving Tregs, B cells, and DCs. Despite the disparate immune subsets identified, IL-18 is prevalent in both AIH and HCC. A distinction between these diseases may be found in IL-37 cell signals which may antagonize HCC progression and is currently not characterized in AIH. Because both PD-1 and PD-L1 are induced by TLR-mediated IL-1 cell signals, IL-18 and IL-37 might regulate the expression of these checkpoints in HCC and AIH. IL-18 and IL-37 are also implicated in the production of MMPs that cleave PD-1 molecules from the cell surface. sPD-1 and sPD-L1 may be respective biomarkers of inflammation and immune suppression in liver disease. Monitoring the production trends of these molecules in patients undergoing PD-1 therapeutic treatments may identify subgroups that are susceptible immune-related hepatitis, tumor regression, or tumor progression. The dynamics of PD-1 molecules, IL-18, and MMPs in the context of the liver microenvironment deserves future exploration.
Figure 1. AIH versus HCC.
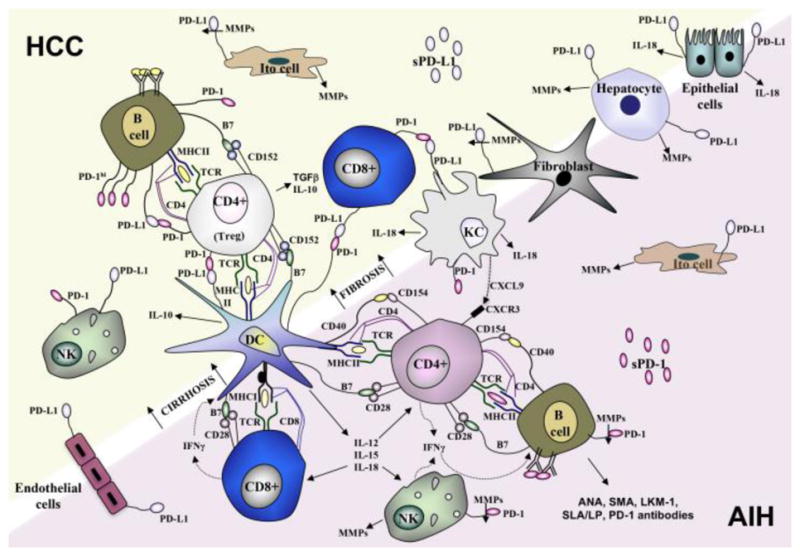
AIH is an autoimmune disease depicted by the production of antibodies, the increased activation of DC, NK, B and Tfh cells and the production of cytokines (IL-12, IL-15, IL-18, IFN-γ) that promote the recruitment (CXCL9) and activation of Th1 CD4+ cells. In some cases of AIH, sPD-1 is produced which may be cleaved from the surface of lymphocytes by MMPs produced from Ito cells, NK cells or hepatocytes. Fibrosis and cirrhosis are precursors and risk factors in the development of HCC. IL-18 is a significant factor in AIH and HCC but in HCC the immune response is dampened by the immunosuppressive functions of DCs, B cells, and Tregs as well as the production of certain cytokines (IL-10, TGF-β). The release of sPD-L1 by MMPs in HCC may be a distinct biomarker of fibrotic disease and in identifying immune suppression in comparison to inflammation and sPD-1 release.
Footnotes
DECLARATIONS OF INTEREST: NONE
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heuman DM, Mills AS, McGuire HH. Gastroenterology. W.B. Saunders Co; Philadelphia: 1997. [Google Scholar]
- 2.Horst AK, Neumann K, Diehl L, Tiegs G. Modulation of liver tolerance by conventional and nonconventional antigen-presenting cells and regulatory immune cells. Cell Mol Immunol. 2016;13(3):277–92. doi: 10.1038/cmi.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teufel A, Weinmann A, Centner C, Piendl A, Lohse AW, Galle PR, Kanzler S. Hepatocellular carcinoma in patients with autoimmune hepatitis. World J Gastroenterol. 2009;15(5):578–82. doi: 10.3748/wjg.15.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liberal R, Grant CR, Mieli-Vergani G, Vergani D. Autoimmune hepatitis: a comprehensive review. J Autoimmun. 2013;41:126–39. doi: 10.1016/j.jaut.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson LA. Autoimmune hepatitis: a noninfectious killer. J Am Assoc Nurse Pract. 2014;26(1):13–8. doi: 10.1002/2327-6924.12055. [DOI] [PubMed] [Google Scholar]
- 6.Liberal R, Grant CR, Longhi MS, Mieli-Vergani G, Vergani D. Diagnostic criteria of autoimmune hepatitis. Autoimmun Rev. 2014;13(4–5):435–40. doi: 10.1016/j.autrev.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Washington MK. Autoimmune liver disease: overlap and outliers. Mod Pathol. 2007;20(Suppl 1):S15–30. doi: 10.1038/modpathol.3800684. [DOI] [PubMed] [Google Scholar]
- 8.Wang BJ, Bao JJ, Wang JZ, Wang Y, Jiang M, Xing MY, Zhang WG, Qi JY, Roggendorf M, Lu MJ, Yang DL. Immunostaining of PD-1/PD-Ls in liver tissues of patients with hepatitis and hepatocellular carcinoma. World J Gastroenterol. 2011;17(28):3322–9. doi: 10.3748/wjg.v17.i28.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mataki N, Kikuchi K, Kawai T, Higashiyama M, Okada Y, Kurihara C, Hokari R, Kawaguchi A, Nagao S, Kondo T, Itoh K, Miyakawa H, Miura S. Expression of PD-1, PD-L1, and PD-L2 in the liver in autoimmune liver diseases. Am J Gastroenterol. 2007;102(2):302–12. doi: 10.1111/j.1572-0241.2006.00948.x. [DOI] [PubMed] [Google Scholar]
- 10.Yu MC, Chen CH, Liang X, Wang L, Gandhi CR, Fung JJ, Lu L, Qian S. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology. 2004;40(6):1312–21. doi: 10.1002/hep.20488. [DOI] [PubMed] [Google Scholar]
- 11.Wang F, Huang X, Chung CS, Chen Y, Hutchins NA, Ayala A. Contribution of programmed cell death receptor (PD)-1 to Kupffer cell dysfunction in murine polymicrobial sepsis. Am J Physiol Gastrointest Liver Physiol. 2016;311(2):G237–45. doi: 10.1152/ajpgi.00371.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aarslev K, Dige A, Greisen SR, Kreutzfeldt M, Jessen N, Vilstrup H, Deleuran B, Gronbaek H. Soluble Programmed Death-1 levels are associated with disease activity and treatment response in patients with autoimmune hepatitis. Scand J Gastroenterol. 2016:1–24. doi: 10.1080/00365521.2016.1233576. [DOI] [PubMed] [Google Scholar]
- 13.Ram M, Sherer Y, Shoenfeld Y. Matrix metalloproteinase-9 and autoimmune diseases. J Clin Immunol. 2006;26(4):299–307. doi: 10.1007/s10875-006-9022-6. [DOI] [PubMed] [Google Scholar]
- 14.Duarte S, Baber J, Fujii T, Coito AJ. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol. 2015;44–46:147–56. doi: 10.1016/j.matbio.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi N, Liu P, Zhang Y, Wu H, Chen Y, Han D. Development of a spontaneous liver disease resembling autoimmune hepatitis in mice lacking tyro3, axl and mer receptor tyrosine kinases. PLoS One. 2013;8(6):e66604. doi: 10.1371/journal.pone.0066604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131(6):1124–36. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 17.Bally AP, Lu P, Tang Y, Austin JW, Scharer CD, Ahmed R, Boss JM. NF-kappaB regulates PD-1 expression in macrophages. J Immunol. 2015;194(9):4545–54. doi: 10.4049/jimmunol.1402550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, Saudemont A, Quesnel B. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110(1):296–304. doi: 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]
- 19.Hutchins NA, Wang F, Wang Y, Chung CS, Ayala A. Kupffer cells potentiate liver sinusoidal endothelial cell injury in sepsis by ligating programmed cell death ligand-1. J Leukoc Biol. 2013;94(5):963–70. doi: 10.1189/jlb.0113051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oikawa T, Takahashi H, Ishikawa T, Hokari A, Otsuki N, Azuma M, Zeniya M, Tajiri H. Intrahepatic expression of the co-stimulatory molecules programmed death-1, and its ligands in autoimmune liver disease. Pathol Int. 2007;57(8):485–92. doi: 10.1111/j.1440-1827.2007.02129.x. [DOI] [PubMed] [Google Scholar]
- 21.Thangavelu G, Parkman JC, Ewen CL, Uwiera RR, Baldwin TA, Anderson CC. Programmed death-1 is required for systemic self-tolerance in newly generated T cells during the establishment of immune homeostasis. J Autoimmun. 2011;36(3–4):301–12. doi: 10.1016/j.jaut.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Fosco D, Kline DE, Meng L, Nishi S, Savage PA, Kline J. PD-1 regulates extrathymic regulatory T-cell differentiation. Eur J Immunol. 2014;44(9):2603–16. doi: 10.1002/eji.201344423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kido M, Watanabe N, Okazaki T, Akamatsu T, Tanaka J, Saga K, Nishio A, Honjo T, Chiba T. Fatal autoimmune hepatitis induced by concurrent loss of naturally arising regulatory T cells and PD-1-mediated signaling. Gastroenterology. 2008;135(4):1333–43. doi: 10.1053/j.gastro.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 24.Longhi MS, Ma Y, Grant CR, Samyn M, Gordon P, Mieli-Vergani G, Vergani D. T-regs in autoimmune hepatitis-systemic lupus erythematosus/mixed connective tissue disease overlap syndrome are functionally defective and display a Th1 cytokine profile. J Autoimmun. 2013;41:146–51. doi: 10.1016/j.jaut.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Ma L, Qin J, Ji H, Zhao P, Jiang Y. Tfh and plasma cells are correlated with hypergammaglobulinaemia in patients with autoimmune hepatitis. Liver Int. 2014;34(3):405–15. doi: 10.1111/liv.12245. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto K, Miyake Y, Matsushita H, Ohnishi A, Ikeda F, Shiraha H, Takaki A, Nouso K, Yamamoto K. Anti-programmed cell death-1 antibody as a new serological marker for type 1 autoimmune hepatitis. J Gastroenterol Hepatol. 2014;29(1):110–5. doi: 10.1111/jgh.12340. [DOI] [PubMed] [Google Scholar]
- 27.Buermann A, Romermann D, Baars W, Hundrieser J, Klempnauer J, Schwinzer R. Inhibition of B-cell activation and antibody production by triggering inhibitory signals via the PD-1/PD-ligand pathway. Xenotransplantation. 2016;23(5):347–56. doi: 10.1111/xen.12261. [DOI] [PubMed] [Google Scholar]
- 28.Beland K, Marceau G, Labardy A, Bourbonnais S, Alvarez F. Depletion of B cells induces remission of autoimmune hepatitis in mice through reduced antigen presentation and help to T cells. Hepatology. 2015;62(5):1511–23. doi: 10.1002/hep.27991. [DOI] [PubMed] [Google Scholar]
- 29.Ikeda A, Aoki N, Kido M, Iwamoto S, Nishiura H, Maruoka R, Chiba T, Watanabe N. Progression of autoimmune hepatitis is mediated by IL-18-producing dendritic cells and hepatic CXCL9 expression in mice. Hepatology. 2014;60(1):224–36. doi: 10.1002/hep.27087. [DOI] [PubMed] [Google Scholar]
- 30.Yamano T, Higashi T, Nouso K, Nakatsukasa H, Kariyama K, Yumoto E, Kobayashi Y, Yamamoto K, Iwagaki H, Yagi T, Tanimoto T, Kurimoto M, Tanaka N, Tsuji T. Serum interferon-gamma-inducing factor/IL-18 levels in primary biliary cirrhosis. Clin Exp Immunol. 2000;122(2):227–31. doi: 10.1046/j.1365-2249.2000.01356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abe K, Takahashi A, Imaizumi H, Hayashi M, Okai K, Kanno Y, Watanabe H, Ohira H. Interleukin-21 plays a critical role in the pathogenesis and severity of type I autoimmune hepatitis. Springerplus. 2016;5(1):777. doi: 10.1186/s40064-016-2512-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishida Y, Migita K, Izumi Y, Nakao K, Ida H, Kawakami A, Abiru S, Ishibashi H, Eguchi K, Ishii N. The role of IL-18 in the modulation of matrix metalloproteinases and migration of human natural killer (NK) cells. FEBS Lett. 2004;569(1–3):156–60. doi: 10.1016/j.febslet.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 33.Novick D, Kim S, Kaplanski G, Dinarello CA. Interleukin-18, more than a Th1 cytokine. Semin Immunol. 2013;25(6):439–48. doi: 10.1016/j.smim.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Alvarez IB, Pasquinelli V, Jurado JO, Abbate E, Musella RM, de la Barrera SS, Garcia VE. Role played by the programmed death-1-programmed death ligand pathway during innate immunity against Mycobacterium tuberculosis. J Infect Dis. 2010;202(4):524–32. doi: 10.1086/654932. [DOI] [PubMed] [Google Scholar]
- 35.Ferreyra Solari NE, Galoppo C, Cuarterolo M, Goni J, Fernandez-Salazar L, Arranz LE, Garrote JA, Chernavsky AC. The simultaneous high expression of Valpha24, IFN-gamma and FoxP3 characterizes the liver of children with type I autoimmune hepatitis. Clin Immunol. 2010;137(3):396–405. doi: 10.1016/j.clim.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Littera R, Chessa L, Onali S, Figorilli F, Lai S, Secci L, La Nasa G, Caocci G, Arras M, Melis M, Cappellini S, Balestrieri C, Serra G, Conti M, Zolfino T, Casale M, Casu S, Pasetto MC, Barca L, Salustro C, Matta L, Scioscia R, Zamboni F, Faa G, Orru S, Carcassi C. Exploring the Role of Killer Cell Immunoglobulin-Like Receptors and Their HLA Class I Ligands in Autoimmune Hepatitis. PLoS One. 2016;11(1):e0146086. doi: 10.1371/journal.pone.0146086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian Z, Gershwin ME, Zhang C. Regulatory NK cells in autoimmune disease. J Autoimmun. 2012;39(3):206–15. doi: 10.1016/j.jaut.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 38.Fabbi M, Carbotti G, Ferrini S. Context-dependent role of IL-18 in cancer biology and counter-regulation by IL-18BP. J Leukoc Biol. 2015;97(4):665–75. doi: 10.1189/jlb.5RU0714-360RR. [DOI] [PubMed] [Google Scholar]
- 39.Migliorini P, Anzilotti C, Pratesi F, Quattroni P, Bargagna M, Dinarello CA, Boraschi D. Serum and urinary levels of IL-18 and its inhibitor IL-18BP in systemic lupus erythematosus. Eur Cytokine Netw. 2010;21(4):264–71. doi: 10.1684/ecn.2010.0210. [DOI] [PubMed] [Google Scholar]
- 40.Liuqing W, Liping X, Hui S, Jing L. Elevated IL-37, IL-18 and IL-18BP serum concentrations in patients with primary Sjogren’s syndrome. J Investig Med. 2017 doi: 10.1136/jim-2016-000301. [DOI] [PubMed] [Google Scholar]
- 41.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47(Suppl):S2–6. doi: 10.1097/MCG.0b013e3182872f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol. 2008;14(27):4300–8. doi: 10.3748/wjg.14.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wan S, Kuo N, Kryczek I, Zou W, Welling TH. Myeloid cells in hepatocellular carcinoma. Hepatology. 2015;62(4):1304–12. doi: 10.1002/hep.27867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu K, Kryczek I, Chen L, Zou W, Welling TH. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 2009;69(20):8067–75. doi: 10.1158/0008-5472.CAN-09-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He L, Zhang J, Jiang L, Jin C, Zhao Y, Yang G, Jia L. Differential expression of Axl in hepatocellular carcinoma and correlation with tumor lymphatic metastasis. Mol Carcinog. 2010;49(10):882–91. doi: 10.1002/mc.20664. [DOI] [PubMed] [Google Scholar]
- 46.Reichl P, Dengler M, van Zijl F, Huber H, Fuhrlinger G, Reichel C, Sieghart W, Peck-Radosavljevic M, Grubinger M, Mikulits W. Axl activates autocrine transforming growth factor-beta signaling in hepatocellular carcinoma. Hepatology. 2015;61(3):930–41. doi: 10.1002/hep.27492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J, Wang K, Yan Z, Xia Y, Li J, Shi L, Zou Q, Wan X, Jiao B, Wang H, Wu M, Zhang Y, Shen F. Axl Expression Stratifies Patients with Poor Prognosis after Hepatectomy for Hepatocellular Carcinoma. PLoS One. 2016;11(5):e0154767. doi: 10.1371/journal.pone.0154767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10(11):753–66. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- 49.Tanoue S, Kaplan DE. CD14(+) regulatory dendritic cells in patients with hepatocellular carcinoma and cirrhosis. Hepatology. 2016;63(4):1391–2. doi: 10.1002/hep.28419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim TS, Chew V, Sieow JL, Goh S, Yeong JP, Soon AL, Ricciardi-Castagnoli P. PD-1 expression on dendritic cells suppresses CD8+ T cell function and antitumor immunity. Oncoimmunology. 2016;5(3):e1085146. doi: 10.1080/2162402X.2015.1085146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jia Y, Zeng Z, Li Y, Li Z, Jin L, Zhang Z, Wang L, Wang FS. Impaired function of CD4+ T follicular helper (Tfh) cells associated with hepatocellular carcinoma progression. PLoS One. 2015;10(2):e0117458. doi: 10.1371/journal.pone.0117458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao X, Lao XM, Chen MM, Liu RX, Wei Y, Ouyang FZ, Chen DP, Zhao XY, Zhao Q, Li XF, Liu CL, Zheng L, Kuang DM. PD-1hi Identifies a Novel Regulatory B-cell Population in Human Hepatoma That Promotes Disease Progression. Cancer Discov. 2016;6(5):546–59. doi: 10.1158/2159-8290.CD-15-1408. [DOI] [PubMed] [Google Scholar]
- 53.Kakita N, Kanto T, Itose I, Kuroda S, Inoue M, Matsubara T, Higashitani K, Miyazaki M, Sakakibara M, Hiramatsu N, Takehara T, Kasahara A, Hayashi N. Comparative analyses of regulatory T cell subsets in patients with hepatocellular carcinoma: a crucial role of CD25(−) FOXP3(−) T cells. Int J Cancer. 2012;131(11):2573–83. doi: 10.1002/ijc.27535. [DOI] [PubMed] [Google Scholar]
- 54.Garnelo M, Tan A, Her Z, Yeong J, Lim CJ, Chen J, Lim KH, Weber A, Chow P, Chung A, Ooi LL, Toh HC, Heikenwalder M, Ng IO, Nardin A, Chen Q, Abastado JP, Chew V. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut. 2017;66(2):342–351. doi: 10.1136/gutjnl-2015-310814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun C, Sun HY, Xiao WH, Zhang C, Tian ZG. Natural killer cell dysfunction in hepatocellular carcinoma and NK cell-based immunotherapy. Acta Pharmacol Sin. 2015;36(10):1191–9. doi: 10.1038/aps.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y, Cheng Y, Xu Y, Wang Z, Du X, Li C, Peng J, Gao L, Liang X, Ma C. Increased expression of programmed cell death protein 1 on NK cells inhibits NK-cell-mediated anti-tumor function and indicates poor prognosis in digestive cancers. Oncogene. 2017 doi: 10.1038/onc.2017.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai L, Zhang Z, Zhou L, Wang H, Fu J, Zhang S, Shi M, Zhang H, Yang Y, Wu H, Tien P, Wang FS. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol. 2008;129(3):428–37. doi: 10.1016/j.clim.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 58.Kamiya T, Chang YH, Campana D. Expanded and Activated Natural Killer Cells for Immunotherapy of Hepatocellular Carcinoma. Cancer Immunol Res. 2016;4(7):574–81. doi: 10.1158/2326-6066.CIR-15-0229. [DOI] [PubMed] [Google Scholar]
- 59.Tangkijvanich P, Thong-Ngam D, Mahachai V, Theamboonlers A, Poovorawan Y. Role of serum interleukin-18 as a prognostic factor in patients with hepatocellular carcinoma. World J Gastroenterol. 2007;13(32):4345–9. doi: 10.3748/wjg.v13.i32.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zannetti C, Roblot G, Charrier E, Ainouze M, Tout I, Briat F, Isorce N, Faure-Dupuy S, Michelet M, Marotel M, Kati S, Schulz TF, Rivoire M, Traverse-Glehen A, Luangsay S, Alatiff O, Henry T, Walzer T, Durantel D, Hasan U. Characterization of the Inflammasome in Human Kupffer Cells in Response to Synthetic Agonists and Pathogens. J Immunol. 2016;197(1):356–67. doi: 10.4049/jimmunol.1502301. [DOI] [PubMed] [Google Scholar]
- 61.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–74. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 62.Szabo G, Petrasek J. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol. 2015;12(7):387–400. doi: 10.1038/nrgastro.2015.94. [DOI] [PubMed] [Google Scholar]
- 63.Asakawa M, Kono H, Amemiya H, Matsuda M, Suzuki T, Maki A, Fujii H. Role of interleukin-18 and its receptor in hepatocellular carcinoma associated with hepatitis C virus infection. Int J Cancer. 2006;118(3):564–70. doi: 10.1002/ijc.21367. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y, Li Y, Ma Y, Liu S, She Y, Zhao P, Jing M, Han T, Yan C, Wu Z, Gao J, Ye L. Dual effects of interleukin-18: inhibiting hepatitis B virus replication in HepG2.2.15 cells and promoting hepatoma cells metastasis. Am J Physiol Gastrointest Liver Physiol. 2011;301(3):G565–73. doi: 10.1152/ajpgi.00058.2011. [DOI] [PubMed] [Google Scholar]
- 65.Bulau AM, Nold MF, Li S, Nold-Petry CA, Fink M, Mansell A, Schwerd T, Hong J, Rubartelli A, Dinarello CA, Bufler P. Role of caspase-1 in nuclear translocation of IL-37, release of the cytokine, and IL-37 inhibition of innate immune responses. Proc Natl Acad Sci U S A. 2014;111(7):2650–5. doi: 10.1073/pnas.1324140111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abulkhir A, Samarani S, Amre D, Duval M, Haddad E, Sinnett D, Leclerc JM, Diorio C, Ahmad A. A protective role of IL-37 in cancer: a new hope for cancer patients. J Leukoc Biol. 2017;101(2):395–406. doi: 10.1189/jlb.5RU0816-341R. [DOI] [PubMed] [Google Scholar]
- 67.Zhao JJ, Pan QZ, Pan K, Weng DS, Wang QJ, Li JJ, Lv L, Wang DD, Zheng HX, Jiang SS, Zhang XF, Xia JC. Interleukin-37 mediates the antitumor activity in hepatocellular carcinoma: role for CD57+ NK cells. Sci Rep. 2014;4:5177. doi: 10.1038/srep05177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han YP, Yan C, Zhou L, Qin L, Tsukamoto H. A matrix metalloproteinase-9 activation cascade by hepatic stellate cells in trans-differentiation in the three-dimensional extracellular matrix. J Biol Chem. 2007;282(17):12928–39. doi: 10.1074/jbc.M700554200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Calabro SR, Maczurek AE, Morgan AJ, Tu T, Wen VW, Yee C, Mridha A, Lee M, d’Avigdor W, Locarnini SA, McCaughan GW, Warner FJ, McLennan SV, Shackel NA. Hepatocyte produced matrix metalloproteinases are regulated by CD147 in liver fibrogenesis. PLoS One. 2014;9(7):e90571. doi: 10.1371/journal.pone.0090571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheng HY, Kang PJ, Chuang YH, Wang YH, Jan MC, Wu CF, Lin CL, Liu CJ, Liaw YF, Lin SM, Chen PJ, Lee SD, Yu MW. Circulating programmed death-1 as a marker for sustained high hepatitis B viral load and risk of hepatocellular carcinoma. PLoS One. 2014;9(11):e95870. doi: 10.1371/journal.pone.0095870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li N, Zhou Z, Li F, Sang J, Han Q, Lv Y, Zhao W, Li C, Liu Z. Circulating soluble programmed death-1 levels may differentiate immune-tolerant phase from other phases and hepatocellular carcinoma from other clinical diseases in chronic hepatitis B virus infection. Oncotarget. 2017;8(28):46020–46033. doi: 10.18632/oncotarget.17546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Finkelmeier F, Canli O, Tal A, Pleli T, Trojan J, Schmidt M, Kronenberger B, Zeuzem S, Piiper A, Greten FR, Waidmann O. High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur J Cancer. 2016;59:152–9. doi: 10.1016/j.ejca.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 73.Dezutter-Dambuyant C, Durand I, Alberti L, Bendriss-Vermare N, Valladeau-Guilemond J, Duc A, Magron A, Morel AP, Sisirak V, Rodriguez C, Cox D, Olive D, Caux C. A novel regulation of PD-1 ligands on mesenchymal stromal cells through MMP-mediated proteolytic cleavage. Oncoimmunology. 2016;5(3):e1091146. doi: 10.1080/2162402X.2015.1091146. [DOI] [PMC free article] [PubMed] [Google Scholar]


