Abstract
Heart-healthy dietary recommendations include decreasing the intake of saturated fatty acids (SFA). However, the relative benefit of replacing SFA with monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), or carbohydrates (CARB) is still being debated. We have used two mouse models of atherosclerosis, low density lipoprotein receptor-deficient (LDLRKO) and apolipoprotein E-deficient (apoEKO) mice to measure the effects of four isocaloric diets enriched with either SFA, MUFA, PUFA, or CARB on atherosclerotic lesion area and lipoprotein levels. In LDLRKO mice, compared with the SFA diet, the MUFA and CARB diets significantly increased atherosclerosis in both sexes, but the PUFA diet had no effect. The MUFA and CARB diets also increased very low density lipoprotein-cholesterol (VLDL-C) and LDL-cholesterol (LDL-C) in males and VLDL-C levels in females. Analysis of data from LDLRKO mice on all diets showed that atherosclerotic lesion area correlated positively with VLDL-C levels (males: r = 0.47, P < 0.005; females: r = 0.52, P < 0.001). In contrast, in apoEKO mice there were no significant dietary effects on atherosclerosis in either sex. Compared with the SFA diet, the CARB diet significantly decreased VLDL-C in males and the MUFA, PUFA, and CARB diets decreased VLDL-C and the CARB diet decreased LDL-C in females. In summary, in LDLRKO mice the replacement of dietary SFA by either MUFA or CARB causes a proportionate increase in both atherosclerotic lesion area and VLDL-C. There were no significant dietary effects on atherosclerotic lesion area in apoEKO mice. These results are surprising and suggest that, depending on the underlying genotype, dietary MUFA and CARB can actually increase atherosclerosis susceptibility, probably by raising VLDL-C levels through a non-LDL receptor, apoE-dependent pathway.
Increased levels of low density lipoprotein (LDL) and cholesterol-enriched very low density lipoprotein (VLDL) have been shown to increase atherosclerosis susceptibility and lead to clinical sequelae such as coronary heart disease and stroke, the major causes of morbidity and mortality in much of the world today (1). In humans it has been shown that dietary cholesterol and saturated fatty acids (SFA) increase the levels of these atherogenic lipoproteins, and current recommendations include decreasing their intake as part of a heart-healthy diet (2). However, the best replacement for the calories supplied by dietary SFA is a matter of controversy. Some argue that a low-fat diet should be used, essentially replacing dietary SFA with carbohydrates (CARB, ref. 3), whereas others promote replacing SFA with monounsaturated fatty acids (MUFA, ref. 4). The arguments largely center around epidemiological studies of diet and coronary heart disease and metabolic studies of dietary effects on lipoprotein patterns (5, 6). Asians typically eat a diet low in fat and high in CARB and have less coronary heart disease than Westerners, who eat a high-fat diet containing large amounts of SFA (1). However, within Westernized populations' lower rates of coronary heart disease are found in Mediterranean countries that ingest diets enriched in MUFA (7). Therefore, epidemiological studies suggest both high-CARB and high-MUFA diets are healthful, but this type of study merely provides correlations and cannot be used to prove a cause-and-effect relationship, nor do epidemiological studies help us distinguish between the diets.
In metabolic studies, replacing SFA with CARB lowers LDL cholesterol (LDL-C) and high density lipoprotein cholesterol (HDL-C) and increases triglycerides (TG) and very low density lipoprotein-cholesterol (VLDL-C). Replacing SFA with MUFA lowers LDL-C, but maintains HDL-C and does not increase TG and VLDL-C (6). These observations have led some to suggest that a high MUFA diet is superior to a high CARB diet (4). However, metabolic studies show only diet-lipoprotein relationships and do not prove that choosing a high-MUFA rather than a high-CARB diet would result in less atherosclerosis. Although this issue is quite important for public health recommendations, because of logistical issues such as the required size of the trial, duration of follow-up, impossibility of blinding, problems with adherence, and expense, it is unlikely to be settled by a randomized clinical trial. The definitive answer to whether it is better to feed MUFA or CARB instead of SFA with atherosclerotic disease as an end point most likely will not be forthcoming from human studies.
Although animal models are only an approximation of the human condition, they can be used to examine the direct effect of diet on atherosclerosis susceptibility because they allow lesion quantitation at the end of the study. To this end, four diets enriched with either SFA, MUFA, polyunsaturated fatty acids (PUFA), or CARB were fed to two induced mutant mouse models of atherosclerosis, LDL receptor-deficient (LDLRKO) and apolipoprotein E-deficient (apoEKO) mice. At 16 weeks of age, mice were killed for measurements of atherosclerotic lesion area and lipoprotein levels. In the LDLRKO mice, compared with SFA-fed mice, lesion area was greatest in MUFA- and CARB-fed mice, which correlated with the diet-induced increase in VLDL-C levels. In the apoEKO mice there was no dietary effect on atherosclerotic lesion area. This study showed that in both LDLRKO and apoEKO mice neither MUFA nor CARB, when substituted for SFA, protected against atherosclerosis. Surprisingly, both MUFA and CARB diets increased atherosclerosis susceptibility apparently by increasing VLDL-C levels via a non-LDL receptor, apo E-dependent pathway. This study also highlights how host genes, such as the LDL receptor and the apo E genes, can influence the effects of diet on atherosclerosis susceptibility.
Methods
Diets.
Four diets were designed enriched with SFA, MUFA, PUFA, or CARB (see Table 1 for composition). The caloric density of each diet was 4.0 kcal/g, and Harlan Teklad, Madison, WI, produced the diets as pellets.
Table 1.
Composition of the four diets
| Diet | SFA | MUFA | PUFA | CARB |
|---|---|---|---|---|
| Energy sources (% cal) | ||||
| Protein | 18 | 18 | 18 | 18 |
| Fat | 42 | 42 | 42 | 20 |
| SFA | 30 | 8 | 8 | 8 |
| MUFA | 8 | 30 | 8 | 8 |
| PUFA | 4 | 4 | 26 | 4 |
| CARB | 40 | 40 | 40 | 62 |
| Components (g/kg) | ||||
| Coconut oil | 144 | 13 | 19 | 33 |
| Olive oil | 23 | 168 | — | 33 |
| Corn oil | 17 | 3 | 102 | 21 |
| Safflower oil | — | — | 64 | — |
| Corn starch | 123 | 123 | 123 | 245 |
| Sucrose | 182 | 182 | 182 | 292 |
| Cellulose | 146 | 146 | 146 | 12 |
Four synthetic diets were designed with 78% identical calories (18% protein, 40% CARB, 8% saturated, 8% monounsaturated, and 4% polyunsaturated fat as well as other essential components). Twenty two percent of calories were derived from SFA, MUFA, PUFA, or CARB. All diets (g/kg): Casein (206), maltodextrin (100), cholesterol (2.0), methionine (3.0), vitamin mix (10), choline bitartrate (2.3), ethoxyquin (0.037), mineral mix (37), calcium phosphate (4.2).
Mice.
C57BL/6J wild-type, LDLRKO (8), and apoEKO (9) mice were purchased from The Jackson Laboratory. Both LDLRKO and apoEKO mice had been back-crossed 10 generations onto the C57BL/6 background. Groups of male and female mice were placed on the different diets after weaning at 4 weeks of age. Food was changed weekly. Food, water intake, and weight were monitored. At 16 weeks of age, mice were anesthetized and, after the right atrium was nicked, they were exsanguinated by perfusion through the left ventricle and tissues were excised.
Atherosclerotic Lesion Size.
The hearts were fixed in phosphate-buffered 10% formalin, embedded in gelatin, cut into 12-μm sections starting at the aortic sinus, and stained with oil red O and hematoxylin/eosin. From these sections, atherosclerotic lesion size was determined as described (9).
Plasma Lipid and Lipoprotein Analysis.
At 16 weeks of age blood samples were taken by cardiac puncture after 8 h of daytime fasting. Plasma cholesterol and TG were determined by using commercial kits adapted for 96-well microtiter plates. Lipoprotein fractions from individual mice were separated by sequential ultracentrifugation (10). In addition, a plasma pool (200 μl) was subjected to gel filtration chromatography over two Superose 6 columns (Amersham Pharmacia) as confirmation of the lipoprotein pattern and for estimation of relative lipoprotein sizes (9).
Tissue Lipid Determination.
Total lipids were extracted from homogenized livers and adipose tissues (11). Liver total and free cholesterol concentrations were determined by GLC with coprostanol as the standard (12). Liver cholesterol esters (CE) and TG were separated by TLC, and their respective fractions were methylated with 5% methanolic HCl. The fatty acid profile from 12:0 to 22:6n-3 was determined by GLC (13). Adipose tissue and dietary fatty acid composition (8:0 to 22:6n-3) was determined by GLC after transmethylation (Meth Prep II, Alltech, Applied Science, Deerfield, IL). Modified temperature programming and the application of appropriate area response correction factors were applied to confirm very low incorporation of dietary medium chain fatty acids (8:0 and 10:0) in adipose tissue.
Statistical Analysis.
Results are given as mean ±SD. Statistical significance was tested by using ANOVA, and if the ANOVA P for a group was <0.05 a Tukey-Kramer posttest was performed (prism computer program, GraphPad, San Diego).
Results
Diet and Diet Composition.
As shown in Table 1, all four diets contained 18% of calories as protein. The SFA, MUFA, and PUFA diets contained 42% of calories as fat and 40% as CARB, whereas the CARB diet contained 20% of calories as fat and 62% as CARB. All four diets contained 0.2% cholesterol. They were well tolerated without significant differences between the diet groups in body weight or food and water intake.
Atherosclerosis.
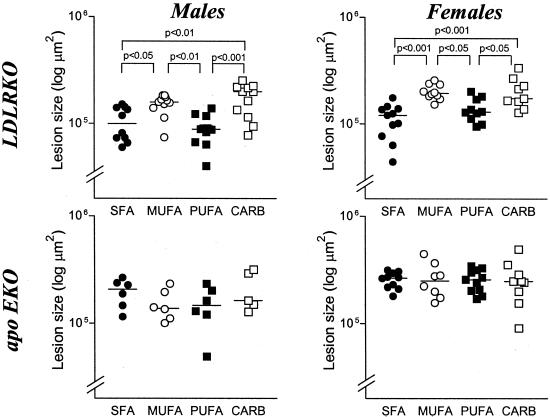
Aortic root atherosclerotic lesion area was measured in 16-week-old LDLRKO and apoEKO mice, and the results are shown in Fig. 1. In LDLRKO mice diet had a significant effect on lesion area in both sexes (P ANOVA <0.0001). In males the lesion areas were CARB 172,438 ± 58,937 μm2, MUFA 151,230 ± 32,576 μm2, SFA 103,756 ± 36,276 μm2, and PUFA 89,639 ± 26,793 μm2, and the Tukey's posttest indicated CARB = MUFA>SFA = PUFA. In females the lesion areas were CARB 201,861 ± 67,538 μm2, MUFA 200,678 ± 32,982 μm2, PUFA 137,933 ± 35,199 μm2, and SFA 111,028 ± 38,817 μm2, and the Tukey's posttest indicated CARB = MUFA>PUFA = SFA. In apoEKO mice there was no significant dietary effect on lesion area. In males the lesion areas were CARB 210,051 ± 87,772 μm2, SFA 195,829 ± 57,595 μm2, MUFA 152,166 ± 51,404 μm2, and PUFA 149,184 ± 64,911 μm2. In females the lesion areas were MUFA 264,020 ± 98,956 μm2, CARB 256,994 ± 115,493 μm2, PUFA 255,206 ± 60,905 μm2, and SFA 254,526 ± 44,290 μm2.
Figure 1.
Atherosclerotic lesion size in LDLRKO and apoEKO mice. Male LDLRKO mice: ANOVA, P < 0.0001; female LDLRKO mice: ANOVA, P < 0.0001; male apoEKO mice: ANOVA, not significant; female apoEKO mice: ANOVA, not significant. P values are the results of posthoc Tukey-Kramer test when the ANOVA was significant. Lesion area data were log-transformed; the horizontal bars represent the mean for each group.
Plasma Lipoproteins.
Before death, blood was drawn after daytime fast and lipid and lipoprotein levels were measured, as shown in Table 2. In LDLRKO mice dietary effects with P ANOVA < 0.0001 were seen on total TG, total cholesterol, VLDL-C, and LDL-C in male mice and total TG, total cholesterol, and VLDL-C in female mice. In both males and females much of the diet-induced difference in total cholesterol derived from differences in VLDL-C. For example, in males VLDL-C levels in mg/dl were MUFA 831 ± 261, CARB 672 ± 147, SFA 373 ± 154, and PUFA 242 ± 137, and the Tukey's posttest indicated MUFA = CARB>SFA = PUFA. In female mice VLDL-C levels in mg/dl were MUFA 813 ± 240, CARB 730 ± 228, SFA 406 ± 87, and PUFA 257 ± 53, and the Tukey's posttest indicated MUFA = CARB>SFA = PUFA.
Table 2.
Plasma triglyceride, cholesterol, and lipoprotein levels in LDLRKO and apoEKO mice
| n | TG | TC | VLDL-C | LDL-C | HDL-C | |
|---|---|---|---|---|---|---|
| LDLRKO male | ||||||
| SFA | 12 | 221 ± 63 | 1014 ± 183 | 373 ± 154 | 608 ± 101 | 90 ± 7.2 |
| MUFA | 12 | 346 ± 110 | 1834 ± 298 | 831 ± 261 | 788 ± 113 | 84 ± 5.7 |
| PUFA | 12 | 101 ± 45 | 801 ± 92 | 242 ± 137 | 526 ± 82 | 89 ± 11 |
| CARB | 11 | 178 ± 69 | 1518 ± 318 | 672 ± 147 | 744 ± 151 | 93 ± 8.1 |
| ANOVA | <0.0001 | <0.0001 | <0.0001 | <0.0001 | NS | |
| Tukey-Kramer | M>C⩵S>P | M>C>S⩵P | M⩵C>S⩵P | M⩵C>S⩵P | ||
| LDLRKO female | ||||||
| SFA | 12 | 171 ± 26 | 933 ± 78 | 406 ± 87 | 481 ± 52 | 69 ± 6.6 |
| MUFA | 11 | 194 ± 56 | 1358 ± 313 | 813 ± 240 | 426 ± 133 | 56 ± 12 |
| PUFA | 12 | 73 ± 18 | 703 ± 71 | 257 ± 53 | 414 ± 64 | 64 ± 9.9 |
| CARB | 10 | 108 ± 17 | 1199 ± 205 | 730 ± 228 | 455 ± 37 | 60 ± 10 |
| ANOVA | <0.0001 | <0.0001 | <0.0001 | NS | <0.05 | |
| Tukey-Kramer | M⩵S>C⩵P | M⩵C>S>P | M⩵C>S⩵P | S>M | ||
| apoEKO male | ||||||
| SFA | 6 | 170 ± 27 | 1842 ± 330 | 1480 ± 332 | 252 ± 187 | 35 ± 5.0 |
| MUFA | 6 | 127 ± 28 | 1706 ± 248 | 1257 ± 306 | 175 ± 46 | 24 ± 2.0 |
| PUFA | 6 | 159 ± 47 | 1141 ± 307 | 1059 ± 274 | 150 ± 74 | 26 ± 3.5 |
| CARB | 6 | 107 ± 13 | 1171 ± 185 | 999 ± 188 | 189 ± 95 | 22 ± 3.7 |
| ANOVA | <0.01 | <0.0005 | <0.05 | NS | <0.0001 | |
| Tukey-Kramer | S⩵P>C | S⩵M>C⩵P | S>C | S>P⩵M⩵C | ||
| apoEKO female | ||||||
| SFA | 10 | 125 ± 18 | 1426 ± 280 | 1220 ± 285 | 174 ± 27 | 25 ± 5.3 |
| MUFA | 10 | 98 ± 13 | 1085 ± 203 | 944 ± 203 | 165 ± 39 | 14 ± 2.8 |
| PUFA | 11 | 102 ± 5.6 | 975 ± 126 | 739 ± 126 | 190 ± 38 | 16 ± 3.5 |
| CARB | 10 | 107 ± 8.5 | 856 ± 130 | 728 ± 131 | 123 ± 30 | 19 ± 2.4 |
| ANOVA | <0.0001 | <0.0001 | <0.0001 | <0.0005 | <0.0001 | |
| Tukey-Kramer | S>C⩵P⩵M | S>M⩵P, M>C | S>M⩵P⩵C | P>M⩵C, S>C | S>C⩵P, S>M, C>M |
Data are given as mean ± SD in mg/dl. When the overall ANOVA was significant, Tukey-Kramer test was performed to identify differences between individual diets. NS, not significant; M, MUFA; C, CARB; S, SFA; P, PUFA.
In apoEKO mice the effect of diet on lipid and lipoprotein levels was markedly different from in LDLRKO mice. In apoEKO mice the strongest dietary effects (P ANOVA <0.0001) were seen mainly in female mice and occurred for total TG, total cholesterol, VLDL-C, and HDL-C, whereas in male mice this was found only for HDL-C. In female mice much of the diet-induced difference in total cholesterol was because of differences in VLDL-C. In female mice VLDL-C levels in mg/dl were SFA 1220 ± 285, MUFA 944 ± 203, PUFA 739 ± 126, and CARB 728 ± 131, and the Tukey's posttest indicated SFA>MUFA = PUFA = CARB. In female mice HDL-C levels were highest on the SFA diet, and the Tukey's posttest indicated SFA>CARB = PUFA = MUFA, and CARB>MUFA.
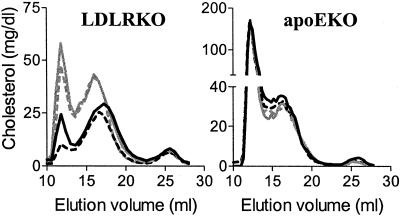
Lipoprotein patterns on the different diets were also assessed by FPLC and basically confirmed the results from the sequential ultracentrifugation analysis. As shown in Fig. 2, in the male LDLRKO mice on the MUFA and CARB diets the VLDL, intermediate density lipoprotein, and LDL portions of the elution profile are all increased relative to the SFA and PUFA diets. ApoEKO mice on all four diets show a similar FPLC elution profile.
Figure 2.
Plasma lipoprotein profile by FPLC from male LDLRKO and apoEKO mice. Plain black line, SFA; plain gray line, MUFA; dotted line, PUFA; dotted gray line, CARB. FPLC from female mice showed similar results.
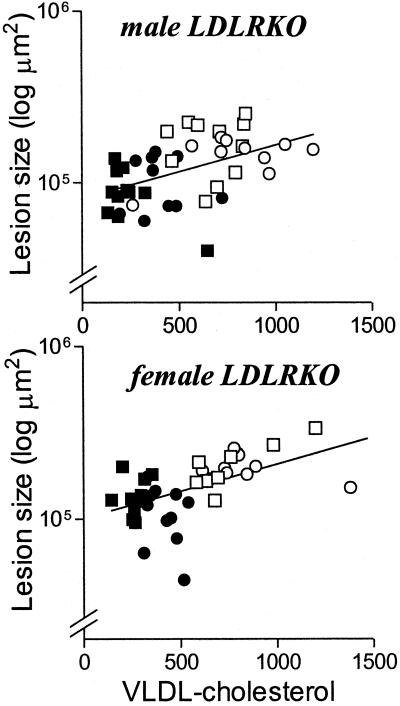
In the LDLRKO mice it appears by group analysis that atherosclerosis lesion area on a particular diet correlates with total and particularly VLDL-C levels. This was also true when atherosclerotic lesion area was correlated with total and VLDL-C levels in all LDLRKO mice (total cholesterol: males r = 0.470, P < 0.005; females r = 0.457, P < 0.005; VLDL-C: males r = 0.473, P < 0.005; females r = 0.521, P < 0.001, see Fig. 3). In LDLRKO mice atherosclerotic lesion area did not correlate with TG, LDL-C, or HDL-C levels. Within diet groups there was no correlation between the atherosclerotic lesion area and any of the lipid and lipoprotein parameters studied. In apoEKO mice, there was no correlation between atherosclerotic lesion area and any of the lipid and lipoprotein variables measured.
Figure 3.
Correlation between atherosclerotic lesion area and VLDL-C levels in LDLRKO mice. Male LDLRKO mice: r = 0.473, P < 0.005; female LDLRKO mice: r = 0.521, P < 0.001. No correlation was found in apoEKO mice. Lesion areas were log-transformed. The shapes of the data points are as in Fig. 1.
Liver Cholesterol and CE Concentrations.
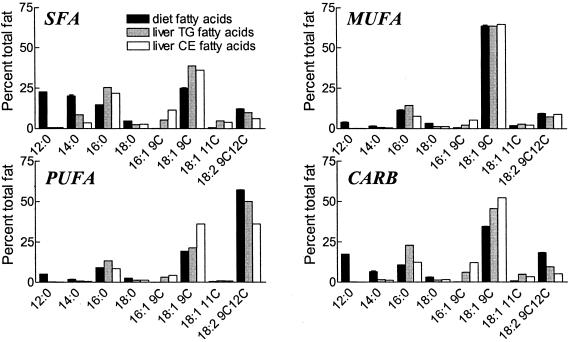
Because liver is usually the major site of origin of plasma lipoproteins, dietary effects on liver free cholesterol and CE concentrations were measured in 16-week-old female mice at the time of death (Table 3). In LDLRKO mice, compared with those fed the SFA diet, animals fed the MUFA diet had higher liver free cholesterol and CE concentrations and mice fed the CARB diet had higher liver CE concentration. In apoEKO mice, compared with those fed the SFA diet, animals fed the MUFA diet had higher liver CE concentration. Thus within the LDLRKO mouse group the MUFA and CARB diets that resulted in the highest liver CE concentration also had the largest atherosclerosis lesion areas and the highest VLDL-C levels. In the apoEKO mice, diet did not correlate with atherosclerotic lesion area, and MUFA diet-fed mice with the highest liver CE concentrations in fact showed a trend to lower atherosclerotic lesion area and VLDL-C levels compared with SFA diet-fed mice. The fatty acid compositions of liver CE and TG for mice fed the different diets were measured in LDLRKO mice (Fig. 4). The expected dietary effects on fatty acid composition were observed, such as increased 16:0 on the SFA diet, increased 18:1 on the MUFA and CARB diets, and increased 18:2 on the PUFA diet. In each case the effect of a particular diet on liver cholesterol and TG fatty acids was similar.
Table 3.
Liver free cholesterol and CE composition in female LDLRKO and apoEKO mice
| n | TC | FC | CE | |
|---|---|---|---|---|
| LDLRKO female | ||||
| SFA | 6 | 10 ± 3 | 2.7 ± 0.4 | 7 ± 3 |
| MUFA | 6 | 20 ± 5 | 3.6 ± 0.6 | 16 ± 5 |
| PUFA | 6 | 12 ± 1 | 2.2 ± 0.4 | 9 ± 1 |
| CARB | 4 | 24 ± 2 | 3.0 ± 0.2 | 21 ± 2 |
| ANOVA | <0.0001 | <0.0005 | <0.0001 | |
| Tukey-Kramer | C⩵M>P⩵S | M⩵C>P, M>S | C⩵M>P⩵S | |
| apoEKO female | ||||
| SFA | 4 | 19 ± 3.3 | 2.5 ± 0.3 | 17 ± 3.1 |
| MUFA | 5 | 27 ± 3.7 | 2.2 ± 0.8 | 25 ± 3.4 |
| PUFA | 5 | 16 ± 3.7 | 3.0 ± 0.8 | 13 ± 3.6 |
| CARB | 5 | 13 ± 4.6 | 2.7 ± 0.8 | 10 ± 4.5 |
| ANOVA | <0.0005 | NS | <0.0005 | |
| Tukey-Kramer | M>S⩵P⩵C | M>S⩵P⩵C |
Data are given as mg/g wet liver tissue ± SD. When the overall ANOVA was significant, Tukey-Kramer test was performed to identify differences between individual diets. TC, total cholesterol; FC, free cholesterol; NS, not significant; M, MUFA; C, CARB; S, SFA; P, PUFA.
Figure 4.
Fatty acid composition of liver TG and CE in LDLRKO female mice compared with the four diets. Data are given in percent of total fatty acids. Diet composition: mean ±SD of two samples.
Adipose Tissue Fatty Acid Composition.
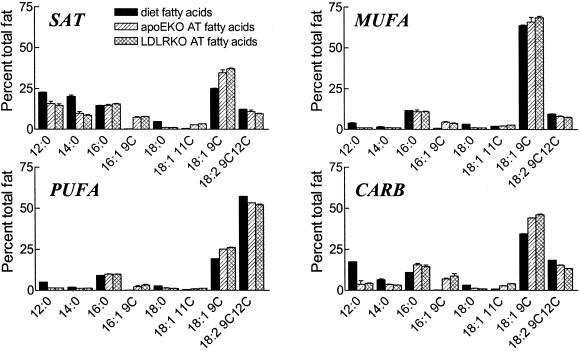
Dietary and adipose tissue fatty acid composition were determined in male LDLRKO and apoEKO mice and shown in Fig. 5. As expected, adipose tissue fatty acid concentrations reflected dietary fatty acid composition. Compared to those on the SFA diet, mice fed a MUFA diet had lower C12, C14, and C16, higher C18:1 and unchanged C18:2 levels, whereas mice fed the PUFA diet had decreased C12, C14, and C16 levels, but unchanged 18:1 and increased 18:2 levels. Mice on the CARB diet had decreased C12 and C14, unchanged C16, increased C18:1, and slightly increased C18:2 levels. On a given diet LDLRK0 and apoEKO had very similar adipose tissue fatty acid composition.
Figure 5.
Fatty acid composition of the four diets and the adipose tissues (AT) of male LDLRKO and apoEKO mice on these diets. Data are given in percent of total fatty acids in mean ±SD (diet composition: two samples; AT composition: two groups, three animals per group).
Discussion
The present study was designed to investigate the impact on atherosclerosis of replacing SFA in the diet with MUFA, PUFA, or CARB by using two induced mutant mouse models of atherosclerosis, LDLRKO and apoEKO mice. In LDLRKO mice, compared with the SFA diet the MUFA and the CARB diets increased atherosclerosis, whereas the PUFA diet was neutral. In the apoEKO mouse, replacing SFA with MUFA, PUFA, or CARB had no effect on atherosclerotic lesion area. These results do not help resolve the public health quandary as to whether to replace SFA in the diet with MUFA or CARB. The results with LDLRKO mice even suggest that under some circumstances dietary MUFA or CARB may be harmful compared with dietary SFA. However, the results of this study provide strong evidence that the genotype of the host can markedly influence the effect of diet on atherosclerosis. Although complete LDL receptor deficiency occurs in only one in a million of the population, these results question the value of decreasing SFA in favor of MUFA and/or CARB in those individuals. A few case reports of complete apo E deficiency have appeared (14) and in those humans the fatty acid content of the diet might not play such an important role in atherosclerosis susceptibility.
Although the current study was not designed to explore the mechanism whereby diet affects atherosclerosis susceptibility, the correlation between atherosclerotic lesion area and VLDL-C levels in LDLRKO mice suggests dietary factors influencing production or catabolism of VLDL-C are important. VLDL production is in part regulated by the availability of liver CE and TG (15). In LDLRKO mice, animals fed the MUFA and CARB diets had higher liver CE content and VLDL-C levels than mice fed the SFA diet. CE production in the liver is regulated by acetyl cholesterol ester transferase (ACAT), and MUFA may be better substrates than SFA for both ACAT1 and ACAT2 (16, 17). In MUFA-fed mice, exogenous fatty acid provides the substrate for liver ACAT2, whereas in CARB-fed mice hepatic fatty acid synthesis and stearoyl coA desaturase are up-regulated, providing endogenously synthesized MUFA for ACAT2 to convert to CE (18). In MUFA- and CARB-fed mice C18:1 is also the preferred substrate for macrophage ACAT1 and this may potentiate foam cell formation and increase lesion size.
In apoEKO female mice, compared with SFA-fed mice, MUFA-fed animals had higher liver CE content but lower VLDL-C levels and in CARB-fed mice there was no significant change in liver CE content but lower VLDL-C levels. In apoEKO male mice there were no dietary effects on atherosclerotic lesion area or VLDL-C levels and liver CE content was not measured. How can we explain the differences observed between LDLRKO and apoEKO mice? Although never formally proven, it is thought that most of the VLDL-C in LDLRKO mice is from the liver, whereas in apoEKO mice it is derived mainly from chylomicron remnants that are of intestinal origin (19). In this case one would not expect a positive correlation in apoEKO mice between hepatic CE content and VLDL-C levels. Alternatively, if a significant fraction of the VLDL-C in apoEKO mice is liver-derived it is possible that the lack of apo E prevents proper intracellular trafficking of liver cholesterol/CE involved in VLDL production. In other studies, we have shown that cholesterol feeding of apoEKO mice increases hepatic cholesterol but fails to elevate biliary cholesterol as it does in normal mice, suggesting a role for apo E in hepatic intracellular trafficking of cholesterol (20). Finally, because hepatic apo E production has been found to increase VLDL assembly and secretion (21), it is possible that the lack of apo E prevents the normal diet-induced increase in VLDL production.
It is also possible that in the absence of LDL receptors VLDL clearance is inhibited by diets enriched in MUFA or CARB, and that in the presence of LDL receptors this does not occur or is less important. In the absence of LDL receptors, receptor-mediated lipoprotein clearance by the liver is mainly through the LDL receptor-related protein (LRP, ref. 22). Apo E is a major ligand for this receptor (23). It is possible that MUFA and CARB diets inhibit LRP-mediated removal of lipoproteins from plasma. Several mechanisms are possible including altering lipoprotein particle or hepatic membrane composition in a manner that decreases ligand-receptor affinity, decreasing LRP synthesis or processing resulting in fewer receptors on the hepatocyte membrane, or stimulating the production of a receptor antagonist, receptor-associated protein (24). Any decrease in the function of LRP induced by MUFA and CARB diets might be compensated for by the presence of LDL receptors. This might explain why MUFA and CARB diets did not increase VLDL-C levels and atherosclerotic lesion area in the apoEKO mice. It is also possible that MUFA and CARB diets inhibit the VLDL receptor in peripheral tissues and in the absence of LDL receptors this becomes physiologically significant and raises VLDL-C levels.
Rudel and colleagues (25, 26) have recently used two animal models to compare the effects of various dietary fats on atherosclerosis. In their first study they fed adult male African green monkeys for 5 years with experimental diets containing 35% of calories as fat enriched in palm oil (SFA), oleic acid-enriched safflower oil (MUFA), or safflower oil (PUFA) and ≈0.4% (wt/wt) cholesterol. The monkeys fed SFA and MUFA developed equivalent amounts of coronary atherosclerosis and the ones fed PUFA developed less. Although SFA- and MUFA-fed monkeys had the same atherosclerosis, the SFA-fed animals had higher total cholesterol and LDL-C and did not differ in HDL-C. Therefore, the authors suggested that the LDL in the MUFA-fed monkeys had increased atherogenic properties, which they attributed to their large size and enrichment with cholesterol oleate.
In a second study they fed 8- to 9-week-old male and female out-bred human apo B transgenic LDL receptor knockout (HuBtg/LDLR0, ref. 27) mice for 16 weeks with experimental diets containing various fats as 10% of calories and 0.005% cholesterol (28). Of the six diets used, three can be designated as enriched in SFA, MUFA, and PUFA as they contained 4% palm oil, oleic acid-rich safflower oil, and safflower oil, respectively. Atherosclerosis as measured by aortic CE content was equivalent between the SFA and MUFA diets and both were greater than the PUFA diet. The SFA and MUFA groups had higher cholesterol mainly because of VLDL-C levels compared with the PUFA group. LDL-C concentration, and LDL protein, phospholipid, free and esterified cholesterol mass percentages did not differ between the groups. Thus, in studies of two species with differing lipoprotein patterns, Rudel's group failed to find that MUFA protects against atherosclerosis compared with SFA. In the current study we have used inbred LDLRKO mice without the HuBtg, diets containing a higher percentage of fat, and a different SFA (coconut versus palm) and have shown that a MUFA diet can actually increase atherosclerosis, cholesterol, and VLDL-C levels compared with SFA. Thus our results agree in suggesting that MUFA in the LDLRKO mouse model does not protect against atherosclerosis and under some circumstances can actually be worse. Rudel et al. did not study the effect of a high-CARB diet on atherosclerosis in their experiments.
Calleja et al. (29) have published a study in which they fed 8-week-old male and female out-bred apoEKO mice for 10 weeks with chow diets containing various fats added at 10%. Of the diets used two were high in SFA (palm and coconut oil), three were high in MUFA (two types of olive oil and oleic acid-rich safflower oil), and one high in PUFA (safflower oil). In female mice there were no significant dietary effects on atherosclerotic lesion area. In male mice lesion size was significantly less on one of the three MUFA diets (oleic acid-rich safflower oil) and the PUFA diet compared with one of the two SFA diets (palm oil) (P < 0.05). The failure to see the diet effect uniformly by class of diet (i.e., not all MUFAs and SFAs showed the difference) and its presence only in males at marginal significance suggests this is not a major effect in apoEKO mice. This study essentially confirms the results we obtained in apoEKO mice.
In conclusion, it is hard to extrapolate from animal models to human dietary recommendations. However, because a direct test of dietary fat effects on atherosclerosis in humans may never be realized, animal models may be necessary to obtain evidence for the safest fats in the diet. The current study could not distinguish whether substituting MUFA or CARB for SFA were safer. However, in the LDLRKO model PUFA were safer than either MUFA or CARB, and in Rudel et al.'s studies PUFA were safer than SFA or MUFA. Thus, even though PUFA diets enrich lipoproteins with polyunsaturates and make them more susceptible to oxidation, their net effect appears to be anti-atherogenic. The animal work thus far suggests that one should pursue replacing SFA in the diets with PUFA rather than MUFA. The current paper also emphasizes the importance of host genotype in determining responsiveness to diet and more information on this topic will need to be gathered before a dietary prescription can be comfortably made.
Acknowledgments
We thank J. D. Smith and E. Sehayek for scientific discussions and I. S. Cho, K. Haddock, and K. Mao for their excellent technical assistance. This study was supported by National Institutes of Health Grant HL 54591 (to J.L.B.) and Educational Grant Me 1507/1–1 from the Deutsche Forschungsgemeinschaft (to M.M.).
Abbreviations
- SFA
saturated fatty acids
- MUFA
monounsaturated fatty acids, PUFA, polyunsaturated fatty acids, CARB, carbohydrates
- apo E
apolipoprotein E
- apoEKO
apo E-deficient
- LDL
low density lipoprotein
- VLDL
very low density lipoprotein
- LDLRKO
LDL receptor-deficient
- TG
triglycerides
- CE
cholesterol esters
- VLDL-C
VLDL cholesterol
- LDL-C
LDL cholesterol
- HDL-C
high density lipoprotein cholesterol
- ACAT
acetyl cholesterol ester transferase
References
- 1.American Heart Association. Heart and Stroke Statistical Update. Dallas: American Heart Association; 2000. [Google Scholar]
- 2.Krauss R M, Eckel R H, Howard B, Appel L J, Daniels S R, Deckelbaum R J, Erdman J W, Jr, Kris-Etherton P, Goldberg I J, Kotchen T A, et al. Circulation. 2000;102:2284–2299. doi: 10.1161/01.cir.102.18.2284. [DOI] [PubMed] [Google Scholar]
- 3.Connor W E, Connor S L. N Engl J Med. 1997;337:562–563. doi: 10.1056/NEJM199708213370811. ; discussion 566–567. [DOI] [PubMed] [Google Scholar]
- 4.Katan M B, Grundy S M, Willett W C. N Engl J Med. 1997;337:563–566. [PubMed] [Google Scholar]
- 5.Hu F B, Stampfer M J, Manson J E, Rimm E, Colditz G A, Rosner B A, Hennekens C H, Willett W C. N Engl J Med. 1997;337:1491–1499. doi: 10.1056/NEJM199711203372102. [DOI] [PubMed] [Google Scholar]
- 6.Mensink R P, Katan M B. Arterioscler Thromb. 1992;12:911–919. doi: 10.1161/01.atv.12.8.911. [DOI] [PubMed] [Google Scholar]
- 7.Keys A. Seven Countries: A Multivariate Analysis of Death and Coronary Heart Disease. Cambridge, MA: Harvard Univ. Press; 1980. [Google Scholar]
- 8.Ishibashi S, Brown M S, Goldstein J L, Gerard R D, Hammer R E, Herz J. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plump A S, Smith J D, Hayek T, Aalto-Setala K, Walsh A, Verstuyft J G, Rubin E M, Breslow J L. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 10.Havel R J, Eder H A, Bragdon J H. J Clin Invest. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folch J, Lees M, Sloane-Stanley G H. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 12.Plump A S, Azrolan N, Odaka H, Wu L, Jiang X, Tall A, Eisenberg S, Breslow J L. J Lipid Res. 1997;38:1033–1047. [PubMed] [Google Scholar]
- 13.Hudgins L C, Hellerstein M, Seidman C, Neese R, Diakun J, Hirsch J. J Clin Invest. 1996;97:2081–2091. doi: 10.1172/JCI118645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feussner G. J Clin Pathol. 1996;49:985–989. doi: 10.1136/jcp.49.12.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis G F. Curr Opin Lipidol. 1997;8:146–153. doi: 10.1097/00041433-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Seo T, Oelkers P M, Giattina M R, Worgall T S, Sturley S L, Deckelbaum R J. Biochemistry. 2001;40:4756–4762. doi: 10.1021/bi0022947. [DOI] [PubMed] [Google Scholar]
- 17.Lee R G, Willingham M C, Davis M A, Skinner K A, Rudel L L. J Lipid Res. 2000;41:1991–2001. [PubMed] [Google Scholar]
- 18.Miyazaki M, Kim Y C, Ntambi J M. J Lipid Res. 2001;42:1018–1024. [PubMed] [Google Scholar]
- 19.Breslow J L. Science. 1996;272:685–688. doi: 10.1126/science.272.5262.685. [DOI] [PubMed] [Google Scholar]
- 20.Sehayek E, Shefer S, Nguyen L B, Ono J G, Merkel M, Breslow J L. Proc Natl Acad Sci USA. 2000;97:3433–3437. doi: 10.1073/pnas.050016197. . (First Published March 21, 2000; 10.1073/pnas.050016197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Y, Ji Z S, Brecht W J, Rall S C, Jr, Taylor J M, Mahley R W. Arterioscler Thromb Vasc Biol. 1999;19:2952–2959. doi: 10.1161/01.atv.19.12.2952. [DOI] [PubMed] [Google Scholar]
- 22.Willnow T E, Sheng Z, Ishibashi S, Herz J. Science. 1994;264:1471–1474. doi: 10.1126/science.7515194. [DOI] [PubMed] [Google Scholar]
- 23.Beisiegel U. Curr Opin Lipidol. 1995;6:117–122. doi: 10.1097/00041433-199506000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Herz J, Goldstein J L, Strickland D K, Ho Y K, Brown M S. J Biol Chem. 1991;266:21232–21238. [PubMed] [Google Scholar]
- 25.Rudel L L, Johnson F L, Sawyer J K, Wilson M S, Parks J S. Am J Clin Nutr. 1995;62:463S–470S. doi: 10.1093/ajcn/62.2.463S. [DOI] [PubMed] [Google Scholar]
- 26.Rudel L L, Haines J, Sawyer J K, Shah R, Wilson M S, Carr T P. J Clin Invest. 1997;100:74–83. doi: 10.1172/JCI119524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanan D A, Newland D L, Tao R, Marcovina S, Wang J, Mooser V, Hammer R E, Hobbs H H. Proc Natl Acad Sci USA. 1998;95:4544–4549. doi: 10.1073/pnas.95.8.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudel L L, Kelley K, Sawyer J K, Shah R, Wilson M S. Arterioscler Thromb Vasc Biol. 1998;18:1818–1827. doi: 10.1161/01.atv.18.11.1818. [DOI] [PubMed] [Google Scholar]
- 29.Calleja L, Paris M A, Paul A, Vilella E, Joven J, Jimenez A, Beltrán G, Uceda M, Maeda N, Osada J. Arterioscler Thromb Vasc Biol. 1999;19:2368–2375. doi: 10.1161/01.atv.19.10.2368. [DOI] [PubMed] [Google Scholar]