Abstract
Objective
The role of adjuvant therapy in small bowel adenocarcinoma (SBA), a rare malignancy with a poor prognosis, is controversial. The purpose of this article is to investigate the impact of adjuvant therapy on the survival of patients with SBA in a meta-analysis.
Methods
We performed a comprehensive search of PubMed, EMBASE and the Cochrane Library database between 2010 and 2017. Hazard ratios (HR) with 95% confidence intervals (95%CI) were used to assess the effect of adjuvant chemotherapy and/or radiation treatment after curative surgery in patients with SBA. Moreover, impact of age, sex, stage, differentiation, lymph node involvement, and margin status was also evaluated.
Results
We included 15 studies to evaluate the effect of adjuvant therapy on the survival of patients with SBA. The pooled HR of overall survival (OS) involving 5986 patients showed that adjuvant therapy did not have a statistically significant effect on the survival of patients with SBA (pooled HR = 0.89, 95% CI = 0.73–1.09, p = 0.25). Further, 607 patients with duodenal adenocarcinoma (DA) had similar results (pooled HR = 0.96, 95% CI = 0.75–1.23, p = 0.77). Similarly, adjuvant treatment vs. non-adjuvant treatment in terms of disease-free survival (DFS) or relapse-free survival (RFS) showed the same results (pooled HR = 0.89, 95% CI = 0.64–1.23, p = 0.48). However, we found that adjuvant therapy resulted in favorable postoperative survival in Europe according to the subgroup analysis (pooled HR = 0.63, 95% CI = 0.5–0.8, p = 0.0002). In addition, the pooled HR shows that stage, differentiation, lymph node involvement, and margin status were related to the OS of patients with SBA.
Conclusion
Patients with SBA who received adjuvant therapy after surgery did not receive a significant survival benefit. Adjuvant therapy may be more useful in advanced cancer or metastatic patients.
Introduction
Small bowel adenocarcinoma (SBA) is a malignant tumor of the small intestine mucosa that is mostly located around the duodenal papilla. SBA as a rare cancer is the most common intestinal malignancy and accounts for about 40% of small bowel tumors. The most SBA arise in the duodenum, which is duodenal adenocarcinoma [1, 2]. In recent years, the global incidence of intestinal adenocarcinoma has increased [3, 4]. At present, the diagnosis and treatment of SBA requires improvement, which has also led to a poor prognosis in small bowel cancer; the 5-year survival rate is about 30% [5, 6]. Therefore, it is urgently needed for general surgeons to explore new treatment modalities in patients with SBA.
In recent years, a large number of adjuvant treatments, including chemotherapy and radiotherapy, have been used in a variety of cancers. In addition, the favorable effect of adjuvant treatments on long-term survival and recurrence has been well acknowledged in non-small cell lung cancer, gastric cancer, breast cancer, colon cancer, and ampulla of Vater cancer [7–11]. Currently, surgical resection is still the main treatment for the patients with SBA. However, more and more adjuvant therapies are being used in the treatment of patients with SBA because of its poor prognosis and high risk of relapse [12, 13]. Chemotherapy is the main therapeutic strategy in patients with SBA, colon adenocarcinoma, or upper gastrointestinal tumors. Globally, the combinations of 5-fluorouracil (5-FU) with either cisplatin, oxaliplatin, or irinotecan are frequently used in the more common gastrointestinal tract tumors; these combinations have been tried in SBA with varying degrees of success [14, 15]. In the meantime, more and more studies were performed to explore the impact of adjuvant therapy on the survival of patients with SBA. However, these studies have not shown consistent results regarding the impact of adjuvant therapy. The effect of adjuvant therapy on the survival of patients with SBA is still not completely clear. Thus, we need to investigate the effect of AT on the survival of patients with SBA in a meta-analysis.
In this meta-analysis, we explore the effect of adjuvant chemotherapy on the OS and DFS/RFS of SBA patients. In addition, we evaluate the impact of other related metrics on the OS of SBA patients.
Materials and methods
Search strategy
The PubMed, Embase, and Cochrane Library database articles published in English were searched for eligible studies from January 1, 2010 to December 5, 2017(No significant changes in SBA treatment since 2010). The search was performed with the following terms and their combinations “operation or surgical or surgery,” “adjuvant or chemotherapy or chemoradiation or radiotherapy,”“overall survival or progression-free survival or disease-free survival or relapse-free survival or recurrence-free survival,” “small bowel adenocarcinoma or small bowel tumor or duodenal adenocarcinoma.” References of the acquired articles were manually searched for additional studies.
Study selection
Related articles were independently reviewed by two authors and selected studies met all of the following inclusion criteria: 1) must be published in English; 2) all included patients had SBA or DA; 3) studies must explore the effect of AC on the OS and DFS/RFS of SBA patients; 4) patients receiving postoperative adjuvant therapy served as the experimental group and patients only undergoing surgery were the control group; 5) log-hazard ratios (HR) and 95% confidence intervals (CI) could be extracted directly or indirectly from articles. The exclusion criteria were as follows: 1) inappropriate article types, including review articles, letters, case reports, editorials, and conference abstracts; 2) tumor research on non-small bowel adenocarcinoma; 3)no set experimental group and control group that met the standards; 4) unable to obtain relevant HR and CI from the data in the article.
Data extraction and quality assessment
Data including the name of the first author, publication year, country, tumor type, number of patients, tumor stage, tumor differentiation, margin status, statistical method, lymph node involvement, and adjuvant treatment details and the related HR and 95% CI were extracted by two independent reviewers. The study quality was assessed independently according to the Newcastle–Ottawa Quality assessment scale (case-control studies). A higher score indicates a higher quality and the maximum score is 9 points.
Statistical analysis
We used HRs and their 95% CIs as the effect size to analyze the impact of adjuvant therapy on the survival of patients with SBA. There are two ways to obtain HRs and their 95% CIs; one is obtained directly by the article. The other is to use available data, number of events, and the log-rank statistic to calculate the effect size as described by Tierney et al., or to obtain data from survival curves (data are extracted using Engauge Digitizer software) [16]. We believe that the effect size of direct extraction is more accurate than that indirectly obtained. The I2 statistic and Chi-squared tests are used to evaluate statistical heterogeneity between the included studies. Substantial heterogeneity was found when I2 >50% or p<0.05. The statistical model selection was based on whether or not there was heterogeneity. A random effects model was used to eliminate the effects of heterogeneity and a fixed effect model was applied when heterogeneity does not exist. Subgroup analysis was further performed to explore the sources of heterogeneity. Subgroup analyses were carried out according to the following categories: country, scale of study, adjuvant therapy methods, and statistical methods. In addition, sensitivity analysis was conducted to evaluate the stability of the results by the successive omission of individual studies. Results were considered statistically significant when the corresponding 95% CI did not by more than 1 and the P values were less than 0.05. Publication bias was assessed using funnel plots with Egger’s test and Begg’s test. We determined that no publication bias existed when the test P value was>0.05 or when there was funnel diagram symmetry. We used Review Manager (RevMan) Version 5.3 and Stata12.0 software for all statistical analyses in this meta-analysis.
Results
Included studies
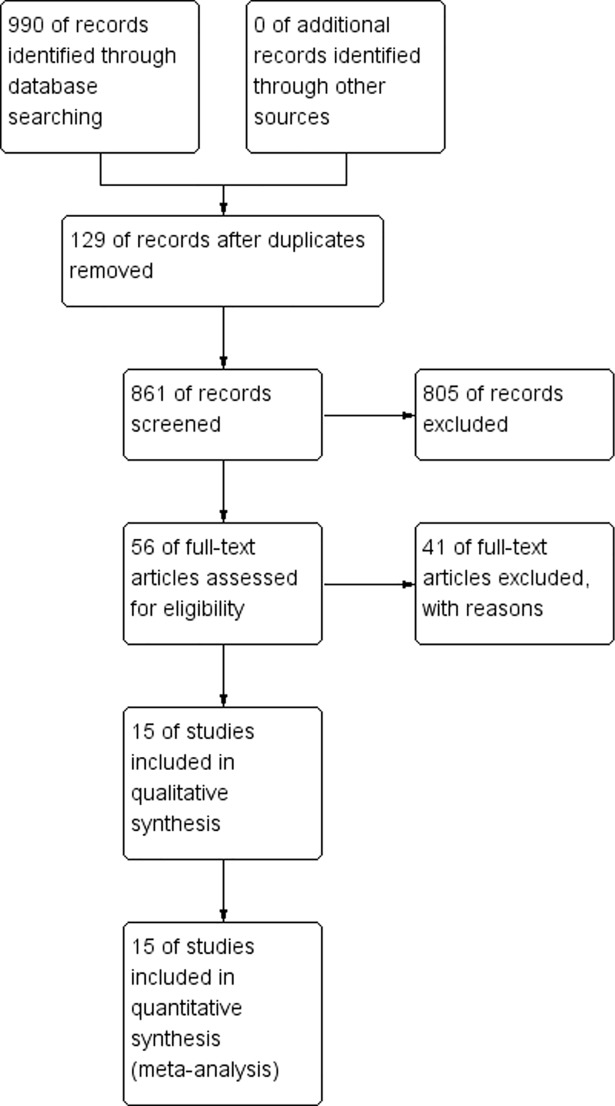
A total of 990 relevant studies were identified, including 129 duplicates, using the first search in PubMed, Embase, and Cochrane Library database. Eight hundred and five studies were additionally excluded after screening the titles and abstracts. Fifteen studies [17–31] were retained after reading the full text. Two studies [31, 22] described the same data analysis, so we only included the later study [31] in the subgroup analysis of DA. The article retrieval flow diagram is shown in Fig 1.
Fig 1. Meta-analysis flow diagram.
Characteristics of included studies
Table 1 shows the main characteristics of the selected 15 eligible studies. In this table, a total of 15 studies involved 6242 patients who come from China, the United States, South Korea, UK, Turkey, Austria, the Netherlands, and France. Ten articles involving 5635 patients with SBA and 5 articles involving 5635 patients with SBA and 607 patients with DA were included in this analysis. The data of 4 studies were from the national database and the rest came from institutional data. There were 11 studies of adjuvant chemotherapy and 4 studies of adjuvant chemoradiation. Eight studies only provided the effect of adjuvant therapy on the OS of patients and 7 studies used OS and RFS/DFS as the indicator to assess the effect of adjuvant therapy. In statistical methods, HRs and their 95% CIs of 5 studies came from multivariate (MU) analysis and the rest used univariate (UV) analysis in their calculations. We obtained the HR and 95% CI directly from the article in 11 studies and there were only 4 studies from which we needed to calculate the HR and 95% CI indirectly using the available data or survival curves; this adds credibility to our analysis. All of the selected eligible studies scored above 6 points in the quality assessment, which means that the quality of the articles is high.
Table 1. Characteristics of included studies.
| Reference | Study period | Country | Tumor type | Data Sources | Sample size | Stage | Method | Analysis | Outcome | data | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wu et al[17] | 1999–2008 | China | DA | Institutional | 141 | I-IV | CR | UV | OS | indirect | 9 |
| Schwameis et al[18] | 1994–2012 | Austria | SBA | Institutional | 26 | NR | CR | UV | OS | indirect | 6 |
| Guo et al[19] | 2000–2011 | China | SBA | Institutional | 149 | NR | CR | UV | OS | indirect | 8 |
| Young et al[20] | 1992–2010 | United States | SBA | National | 1644 | I-IV | CR | MU | OS | direct | 9 |
| Kanhan et al[21] | 1996–2011 | Britain | SBA | Institutional | 48 | ES | CR | UV | OS/RFS | direct | 8 |
| Ecker et al[22] | 1998–2011 | United States | SBA | National | 2297 | I-IV | CR | UV | OS | direct | 8 |
| Legue et al[23] | 1999–2013 | Netherlands | SBA | National | 1194 | I-IV | CR | MU | OS | direct | 8 |
| Fu et al[24] | 1997–2009 | United States | DA | Institutional | 64 | III | CRT | MU | OS/DFS | direct | 7 |
| Aydin et al[25] | 2003–2013 | Turkey | SBA | Institutional | 78 | I-IV | CR | UV | OS/DFS | direct | 9 |
| Kim et al[26] | 1991–2002 | Korea | DA | Institutional | 24 | I-IV | CRT | UV | OS/RFS | indirect | 8 |
| Koo et al[27] | 1989–2009 | Korea | SBA | Institutional | 52 | I-IV | CR | MU | OS/DFS | direct | 7 |
| Overman et al[28] | 1990–2006 | United States | SBA | Institutional | 54 | I-IV | CRT | MU | OS/DFS | direct | 8 |
| Poultsides et al[29] | 1984–2006 | United States | DA | Institutional | 122 | I-IV | CRT | MU | OS | direct | 9 |
| Zaanan et al[30] | 1996–2008 | France | SBA | Institutional | 93 | NR | CR | UV | OS/RFS | direct | 9 |
| Ecker et al[31] | 1998–2011 | United States | DA | National | 256 | NR | CR | UV | OS | direct | 9 |
SBA: small bowel adenocarcinoma; DA: duodenal adenocarcinoma; NR: not reported; ES: Early stage; CR: Chemotherapy; CRT: Chemoradiation; UV: univariate; MU: multivariate; OS: overall survival; RFS: recurrence-free survival; DFS: disease-free survival; NOS: Newcastle-Ottawa Scale. NOS: Newcastle-Ottawa Scale.
Main results
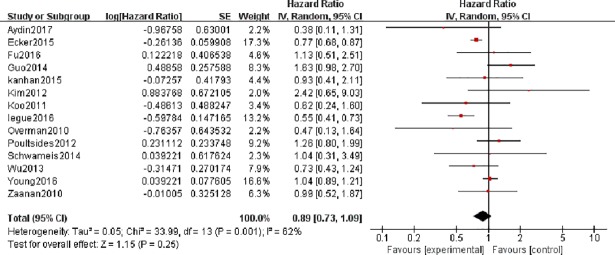
The forest plot of 14 studies involving 5986 patients shows that adjuvant therapy did not have a statistically significant effect on the OS of patients with SBA (pooled HR = 0.89, 95% CI = 0.73–1.09, p = 0.25) (Fig 2). Because of the heterogeneity, we used a random effect model to pool effect size (I2 = 62% and P = 0.001). We also conducted subgroup analyses and sensitivity analysis to explore the sources of heterogeneity.
Fig 2. Forest plots to assess the effect of adjuvant therapy on the overall survival of patients with small bowel adenocarcinoma.
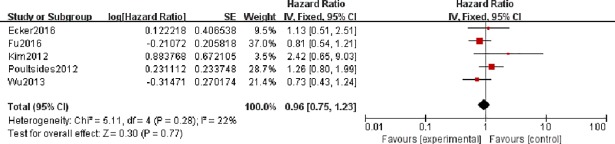
In addition, 5 studies involving 607 patients with HRs and 95% CIs of OS were selected for aggregated survival analysis, which showed similar results (pooled HR = 0.96, 95% CI = 0.75–1.23, p = 0.77) in patients with DA. We did not find heterogeneity in this analysis (I2 = 22% and P = 0.28).
We hypothesized that RFS/DFS can be used as an indicator of relapse. Thus, the results of 7 relevant studies were combined to show that adjuvant therapy did not have a statistically significant effect on SBA recurrence after surgery (pooled HR = 0.89, 95% CI = 0.64–1.23, p = 0.48). The results did not change in the subgroup analysis of RFS and DFS (pooled HR = 1.19, 95% CI = 0.76–1.85, p = 0.44 and pooled HR = 0.62, 95% CI = 0.38–1.23, p = 0.06). No heterogeneity was observed in these three analyses (I2 = 11% and P = 0.48, I2 = 0% and P = 0.8, I2 = 0% and P = 0.47) (Fig 3).
Fig 3. Forest plots to assess the effect of adjuvant therapy on the recurrence of patients with small bowel adenocarcinoma.
Subgroup analysis
We conducted a subgroup analysis for the results of pooled HRs of OS in patients with SBA according to country, scale of study, the method of adjuvant therapy, and statistical methods. The combined effect size showed that adjuvant therapy had a statistically significant effect on the OS of patients with SBA in Europe (pooled HR = 0.63, 95% CI = 0.5–0.8, p = 0.0002). However, no statistical significance was observed in Asia and America (pooled HR = 1.09, 95% CI = 0.61–1.94, p = 0.78 and pooled HR = 0.94, 95% CI = 0.74–1.2, p = 0.62, respectively). The result of the national database consolidation was pooled HR = 0.78, 95% CI = 0.58–1.04, p = 0.78 and pooled HR = 1.04, 95% CI = 0.84–1.29, p = 0.78 in the institution data subgroup. Nine univariate analysis studies showed that adjuvant therapy had a statistically significant effect on the IOS of patients with SBA (pooled HR = 0.81, 95% CI = 0.73–0.9, p = 0.0001). However, 5 multivariate analyses did not show similar results in this subgroup analysis (pooled HR = 0.81, 95% CI = 0.54–1.20, p = 0.29). Finally, the results of the AC and ACR subgroups were pooled HR = 0.84, 95% CI = 0.68–1.04, p = 0.11 and pooled HR = 1.19, 95% CI = 0.83–1.72, p = 0.35. Detailed pooled HRs and CIs of the subgroup analysis are displayed in Table 2.
Table 2. Results of pooled hazard ratios for overall survival according to subgroup analysis.
| Subgroup | No. of patients | No. of studies | Combined results | Heterogeneity | Statistical Method | ||||
|---|---|---|---|---|---|---|---|---|---|
| HR(95%CI) | p value | I2 (%) | P value | ||||||
| Overall survival | 5986 | 14 | 0.89[0.73,1.09] | 0.25 | 62 | 0.001 | Random model | ||
| Country | |||||||||
| Asia | 366 | 4 | 1.09 [0.61, 1.94] | 0.78 | 60 | 0.06 | Random model | ||
| Europe | 1439 | 5 | 0.63 [0.5, 0.8] | 0.0002 | 19 | 0.29 | Fixed model | ||
| America | 4241 | 5 | 0.94 [0.74, 1.20] | 0.62 | 70 | 0.01 | Random model | ||
| Analysis type | |||||||||
| MU | 3066 | 5 | 0.81 [0.54, 1.20] | 0.29 | 78 | 0.001 | Random model | ||
| UV | 2920 | 9 | 0.81 [0.73, 0.90] | 0.0001 | 41 | 0.09 | Fixed model | ||
| Treatment method | |||||||||
| Chemotherapy | 5722 | 10 | 0.84 [0.68, 1.04] | 0.11 | 67 | 0.001 | Random model | ||
| Chemoradiation | 264 | 4 | 1.18 [0.79, 1.77] | 0.35 | 9 | 0.35 | Fixed model | ||
| Data Sources | |||||||||
| National | 5135 | 3 | 0.78 [0.58, 1.04] | 0.09 | 89 | 0.0001 | Random model | ||
| Institutional | 851 | 11 | 1.04 [0.84, 1.29] | 0.91 | 19 | 0.26 | Fixed model | ||
MU: multivariate; UV: univariate; HR: Hazard Ratio.
Relevant clinicopathological parameters
In order to further study the impact of clinical parameters on postoperative survival, we conducted a meta-analysis of six clinical parameters, which were age, sex, stage, differentiation, lymph node involvement, and margin status. The pooled HR shows that stage, differentiation, lymph node involvement and margin status were related to the OS of patients with SBA. However, age and sex had no effect on postoperative survival in patients with SBA. Detailed pooled HRs and CIs are displayed on Table 3.
Table 3. Results of pooled hazard ratios for overall survival according to clinicopathological parameters.
| Outcome | No. of patients | No. of studies | Combined results | Heterogeneity | Statistical Method | |||
|---|---|---|---|---|---|---|---|---|
| HR(95%CI) | p value | I2 (%) | P value | |||||
| Age (>60 years) | 142 | 2 | 1.04 [0.30, 3.61] | 0.95 | 74 | 0.05 | Random model | |
| Gender | 3559 | 6 | 1.03 [0.94, 1.13] | 0.51 | 34 | 0.18 | Fixed model | |
| PD | 7157 | 6 | 2.19 [1.29, 3.70] | 0.003 | 93 | <0.00001 | Random model | |
| positive margins | 4936 | 3 | 1.96 [1.71,2.24] | < .00001 | 0 | 0.54 | Fixed model | |
| HTS | 317 | 3 | 1.72 [1.16, 2.57] | 0.007 | 0 | 0.6 | Fixed model | |
| NLNI | 130 | 2 | 0.09 [0.04, 0.22] | < .00001 | 35 | 0.21 | Fixed model | |
PD: poorly differentiation; HTS: high tumor stage; NLNI: no lymph nodes involved; HR: Hazard Ratio.
Sensitivity analysis
Sensitivity analysis was performed by the successive omission of individual studies to observe changes in heterogeneity. In this sensitivity analysis, we did not observe a great change in heterogeneity, which proved that no single study had significant heterogeneity and indicating that our analysis results are robust. Thus, heterogeneity in this study does not come from a single article.
Publication bias
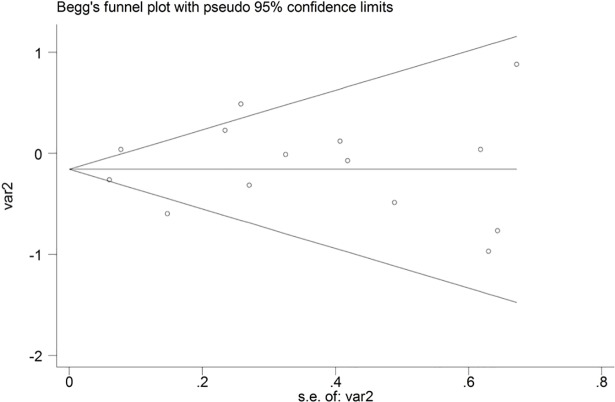
We performed publication bias analysis in 14 studies because 2 studies (Ecker2016 and Ecker2015) contained the same data analysis. Begg’s funnel plots did not reveal obvious asymmetry and the results were Begg’s test P = 0.584; Egger’s test P = 0.693. Therefore, there was no obvious publication bias in this meta-analysis (Fig 4).
Fig 4. Begg`’s funnel plots to evaluate publication bias in related studies.
Discussion
Although SBA is a very rare malignancy, the incidence is gradually increasing. DA is the most common type of SBA [32]. Because of the increasingly poor prognosis, more postoperative treatment is required in patients with SBA. The most commonly used treatments are adjuvant chemotherapy and adjuvant chemoradiation in postoperative patients. Adjuvant therapy for SBA is a controversial issue, which requires further investigation by a general surgeon. The result of our meta-analysis has demonstrated that no associated survival benefit was conferred by the use of adjuvant therapy in patients with SBA. However, individual studies have also shown that adjuvant therapy has a significant survival benefit in patients with advanced or metastatic SBA [33, 34]. Unfortunately, because of the lack of high-quality research, especially randomized controlled trials (RCT), more research is needed to prove this conclusion.
In our review of the included studies, we found postoperative adjuvant chemotherapy may be better than adjuvant chemoradiation in SBA. Subgroup analysis of chemotherapy and chemoradiation also confirmed this idea (pooled HR = 0.84, 95% CI = 0.68–1.04, p = 0.11 vs. pooled HR = 1.19, 95% CI = 0.83–1.72, p = 0.35). {Postoperative chemotherapy may be more useful than chemoradiation. However, only 4 articles involving 264 patients on adjuvant chemoradiation were included in this subgroup study, so we need to perform more postoperative chemoradiation studies to confirm this conclusion. We found an interesting phenomenon that adjuvant therapy has a survival benefit for SBA patients in Europe, which may be because the included articles did not include adjuvant chemoradiation research. Although the American subgroup is not statistically significant, it still has the same trend as in Europe and the overall medical level in Asia may affect the outcome of Asian subgroups. In addition, we believe that multivariate analysis can eliminate the impact of related clinical factors and the results of multivariate analysis may be more credible. Thus, subgroup analysis and multivariate analysis may provide more accurate conclusions. In the relevant clinical parameters, poorly differentiated tumors, positive margins, and high tumor stage have a significant adverse effect on survival (pooled HR = 2.19, 95% CI = 1.29–3.7, p = 0.003, pooled HR = 1.96, 95% CI = 1.71–2.24, p<0.00001, pooled HR = 1.72, 95% CI = 1.16–2.57, p = 0.007), and no lymph node involvement has an associated survival benefit in patients with SBA (pooled HR = 0.09, 95% CI = 0.04–0.22, p<0.00001). We believe that these clinical parameters can be used as predictors of OS in patients with SBA. In addition, these clinical parameters can also be used as indicator of the effect of adjuvant therapy.
Related research shows that most adjuvant therapies are based on fluorouracil and FOLFOX (5-FU and oxaliplatin) is the most commonly used treatment, which is based on the treatment of other colorectal cancers. In addition, individual studies show no significant difference between the treatment regimens [18, 25]. However, related research does not provide enough evidence, so more studies should be performed to find the best treatment programs. The side effects of chemotherapy or radiotherapy should also be noted. Most of the side effects occur in the blood system and the most common hematological toxicity was neutropenia. Other toxicities include neurotoxicity, nephrotoxicity, and allergic reactions [25, 30]. Thus, we believe that postoperative adjuvant therapy should be used with caution when combined with the results of this analysis. Adjuvant radiotherapy is rarely used and related research is also rare. Therefore, no relevant analysis has been carried out in this article. The most effective chemotherapeutic regimen is still being explored and the commonly used chemotherapy regimen did not significantly improve postoperative survival [30]. In addition, studies have shown that the FOLFIRI regimen is an effective treatment for patients with advanced small bowel adenocarcinoma who are refractory to platinum-based chemotherapy [35]. So, need to explore more effective chemotherapy.
There are still some limitations in this meta-analysis. First of all, the quality of the included studies limited our research because they were all retrospective analyses. In general, the results of randomized controlled trials are more credible and the results of a meta-analysis consisting of RCTs are the most reliable. Second, subgroup analyses did not completely eliminate the effects of heterogeneity and sensitivity analysis also did not explain the source of heterogeneity. This shows that not all heterogeneity sources were considered in this meta-analysis. Although the random effects model was used to eliminate the heterogeneity, it also affected the results. In addition, we did not evaluate the role of adjuvant radiotherapy and performed limited research on adjuvant chemoradiation. Finally, we obtained the effect size according to the survival curves in 4 studies, which may have an impact on the results of our analysis.
Conclusions
In this meta-analysis, we discovered that adjuvant treatment after surgery does not improve OS compared with only surgery in patients with SBA. In addition, poorly differentiated disease, positive margins, high tumor stage, and lymph node involvement had a marginally significant effect on survival in patients with SBA. Exploration of new adjuvant therapies and postoperative management is necessary.
Supporting information
(DOC)
Abbreviations
- SBA
small bowel adenocarcinoma
- HR
Hazard ratio
- OS
overall survival
- DA
duodenal adenocarcinoma
- DFS
disease-free survival
- RFS
relapse-free survival
- 5-FU
5-fluorouracil
- AT
adjuvant therapy
- ACR
adjuvant chemoradiation
- AC
adjuvant chemotherapy
- MU
multivariate
- UV
univariate
- RCT
randomized controlled trials
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
This study was supported by the Graduate Student Innovation Special Fund Project of Nanchang University (CX2016063) to XJY. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Haselkorn T, Whittemore AS, Lilienfeld DE (2005) Incidence of small bowel cancer in the United States and worldwide: geographic, temporal, and racial differences. Cancer Causes Control 16: 781–787. 10.1007/s10552-005-3635-6 [DOI] [PubMed] [Google Scholar]
- 2.Ecker BL, McMillan MT, Datta J, Lee MK, Karakousis GC, Vollmer C J, et al. (2017) Adjuvant chemotherapy versus chemoradiotherapy in the management of patients with surgically resected duodenal adenocarcinoma: A propensity score-matched analysis of a nationwide clinical oncology database. Cancer 123: 967–976. 10.1002/cncr.30439 [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al. (2014) SEER Cancer Statistics Review, 1975–2011. Bethesda, MD: National Cancer Institute. [Google Scholar]
- 4.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. (2008) Cancer statistics, 2008. CA Cancer J Clin 58: 71–96. 10.3322/CA.2007.0010 [DOI] [PubMed] [Google Scholar]
- 5.Aparicio T, Zaanan A, Svrcek M, Laurent-Puig P, Carrere N, Manfredi S, et al. (2014) Small bowel adenocarcinoma: epidemiology, risk factors, diagnosis and treatment. Dig Liver Dis 46: 97–104. 10.1016/j.dld.2013.04.013 [DOI] [PubMed] [Google Scholar]
- 6.Bilimoria K.Y., Bentrem D.J., Wayne J.D., Ko C.Y., Bennett C.L., Talamonti M.S. (2009). Small bowel cancer in the United States: changes in epidemiology, treatment, and Survival over the last 20 years. Annals of Surgery, 249:63–71. 10.1097/SLA.0b013e31818e4641 [DOI] [PubMed] [Google Scholar]
- 7.Ahmad U, Crabtree TD, Patel AP, Morgensztern D, Robinson CG, Krupnick AS, et al. (2017) Adjuvant Chemotherapy Is Associated With Improved Survival in Locally Invasive Node Negative Non-Small Cell Lung Cancer. Ann Thorac Surg 104: 303–307. 10.1016/j.athoracsur.2017.01.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Datta J, McMillan MT, Ecker BL, Karakousis GC, Mamtani R, Plastaras JP, et al. (2016) Implications of Lymph Node Staging on Selection of Adjuvant Therapy for Gastric Cancer in the United States: A Propensity Score-matched Analysis. Ann Surg 263: 298–305. 10.1097/SLA.0000000000001360 [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Yang Z, Fan J, He J, Zhang B, Yang H, et al. (2017) A nation-wide multicenter 10-year (1999–2008) retrospective study of chemotherapy in Chinese breast cancer patients. Oncotarget 8: 75864–75873. 10.18632/oncotarget.16439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andre T, de Gramont A, Vernerey D, Chibaudel B, Bonnetain F, Tijeras-Raballand A, et al. (2015) Adjuvant Fluorouracil, Leucovorin, and Oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF Mutation and Mismatch Repair Status of the MOSAIC Study. J Clin Oncol 33: 4176–4187. 10.1200/JCO.2015.63.4238 [DOI] [PubMed] [Google Scholar]
- 11.Kwon J, Kim BH, Kim K, Chie EK, Ha SW (2015) Survival Benefit of Adjuvant Chemoradiotherapy in Patients With Ampulla of Vater Cancer: A Systematic Review and Meta-analysis. Ann Surg 262: 47–52. 10.1097/SLA.0000000000001182 [DOI] [PubMed] [Google Scholar]
- 12.Shenoy S (2014) Primary small-bowel malignancy: update in tumor biology, markers, and management strategies. J Gastrointest Cancer 45: 421–430. 10.1007/s12029-014-9658-z [DOI] [PubMed] [Google Scholar]
- 13.Han SL, Cheng J, Zhou HZ, Guo SC, Jia ZR, Wang PF. (2010) Surgically treated primary malignant tumor of small bowel: a clinical analysis. World J Gastroenterol 16: 1527–1532. 10.3748/wjg.v16.i12.1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onkendi EO, Boostrom SY, Sarr MG, Farnell MB, Nagorney DM, Donohue JH, et al. (2012) 15-year experience with surgical treatment of duodenal carcinoma: a comparison of periampullary and extra-ampullary duodenal carcinomas. J Gastrointest Surg 16: 682–691. 10.1007/s11605-011-1808-z [DOI] [PubMed] [Google Scholar]
- 15.Koo DH, Yun SC, Hong YS, Ryu MH, Lee JL, Chang H, et al. (2011) Systemic chemotherapy for treatment of advanced small bowel adenocarcinoma with prognostic factor analysis: Retrospective study. BMC Cancer 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR (2013) Response to: Practical methods for incorporating summary time-to-event data into meta. Authors' reply. Trials 14: 391. [PubMed] [Google Scholar]
- 17.Wu XY, Chen J, Cao QH, Dong M, Lin Q, Fan XJ, et al. (2013) Beclin 1 activation enhances chemosensitivity and predicts a favorable outcome for primary duodenal adenocarcinoma. Tumour Biol 34: 713–722. 10.1007/s13277-012-0599-5 [DOI] [PubMed] [Google Scholar]
- 18.Schwameis K, Schoppmann SF, Stift J, Schwameis M, Stift A (2014) Small bowel adenocarcinoma—terra incognita: A demand for cross-national pooling of data. Oncol Lett 7: 1613–1617. 10.3892/ol.2014.1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo X, Mao Z, Su D, Jiang Z, Bai L (2014) The clinical pathological features, diagnosis, treatment and prognosis of small intestine primary malignant tumors. Med Oncol 31: 913 10.1007/s12032-014-0913-8 [DOI] [PubMed] [Google Scholar]
- 20.Young JI, Mongoue-Tchokote S, Wieghard N, Mori M, Vaccaro GM, Sheppard BC, et al. (2016) Treatment and Survival of Small-bowel Adenocarcinoma in the United States: A Comparison With Colon Cancer. Dis Colon Rectum 59: 306–315. 10.1097/DCR.0000000000000562 [DOI] [PubMed] [Google Scholar]
- 21.Khan K, Peckitt C, Sclafani F, Watkins D, Rao S, Starling N, et al. (2015) Prognostic factors and treatment outcomes in patients with Small Bowel Adenocarcinoma (SBA): The Royal Marsden Hospital (RMH) experience. BMC Cancer 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ecker BL, McMillan MT, Datta J, Mamtani R, Giantonio BJ, Dempsey DT, et al. (2016) Efficacy of adjuvant chemotherapy for small bowel adenocarcinoma: A propensity score-matched analysis. Cancer 122: 693–701. 10.1002/cncr.29840 [DOI] [PubMed] [Google Scholar]
- 23.Legue LM, Bernards N, Gerritse SL, van Oudheusden TR, de Hingh IH, Creemers GM, et al. (2016) Trends in incidence, treatment and survival of small bowel adenocarcinomas between 1999 and 2013: a population-based study in The Netherlands. Acta Oncol 55: 1183–1189. 10.1080/0284186X.2016.1182211 [DOI] [PubMed] [Google Scholar]
- 24.Fu T, Sharmab A, Xie F, Liu Y, Li K, Wan W, et al. (2016) Methylation of MGMT Is Associated with Poor Prognosis in Patients with Stage III Duodenal Adenocarcinoma. PLoS One 11: e162929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aydin D, Sendur MA, Kefeli U, Unal OU, Tastekin D, Akyol M, et al. (2017) Evaluation of Prognostic Factors and Adjuvant Chemotherapy in Patients With Small Bowel Adenocarcinoma Who Underwent Curative Resection. Clin Colorectal Cancer 16: 220–227. 10.1016/j.clcc.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 26.Kim K, Chie EK, Jang JY, Kim SW, Oh DY, Im SA, et al. (2012) Role of adjuvant chemoradiotherapy for duodenal cancer: a single center experience. Am J Clin Oncol 35: 533–536. 10.1097/COC.0b013e31821dee31 [DOI] [PubMed] [Google Scholar]
- 27.Koo DH, Yun SC, Hong YS, Ryu MH, Lee JL, Chang HM, et al. (2011) Adjuvant chemotherapy for small bowel adenocarcinoma after curative surgery. Oncology 80: 208–213. 10.1159/000328506 [DOI] [PubMed] [Google Scholar]
- 28.Overman MJ, Kopetz S, Lin E, Abbruzzese JL, Wolff RA (2010) Is there a role for adjuvant therapy in resected adenocarcinoma of the small intestine. Acta Oncol 49: 474–479. 10.3109/02841860903490051 [DOI] [PubMed] [Google Scholar]
- 29.Poultsides GA, Huang LC, Cameron JL, Tuli R, Lan L, Hruban RH, et al. (2012) Duodenal adenocarcinoma: clinicopathologic analysis and implications for treatment. Ann Surg Oncol 19: 1928–1935. 10.1245/s10434-011-2168-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaanan A, Costes L, Gauthier M, Malka D, Locher C, Mitry E, et al. (2010) Chemotherapy of advanced small-bowel adenocarcinoma: A multicenter AGEO study. Annals of Oncology 21: 1786–1793. 10.1093/annonc/mdq038 [DOI] [PubMed] [Google Scholar]
- 31.Ecker BL, McMillan MT, Datta J, Dempsey DT, Karakousis GC, Fraker DL, et al. (2016) Lymph node evaluation and survival after curative-intent resection of duodenal adenocarcinoma: A matched cohort study. Eur J Cancer 69: 135–141. 10.1016/j.ejca.2016.09.027 [DOI] [PubMed] [Google Scholar]
- 32.Ecker BL, McMillan MT, Datta J, Lee MK, Karakousis GC, Vollmer CJ, et al. (2017) Adjuvant chemotherapy versus chemoradiotherapy in the management of patients with surgically resected duodenal adenocarcinoma: A propensity score-matched analysis of a nationwide clinical oncology database. Cancer 123: 967–976. 10.1002/cncr.30439 [DOI] [PubMed] [Google Scholar]
- 33.Legue LM, Simkens GA, Creemers GM, Lemmens V, de Hingh I (2017) Synchronous peritoneal metastases of small bowel adenocarcinoma: Insights into an underexposed clinical phenomenon. Eur J Cancer 87: 84–91. 10.1016/j.ejca.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 34.Yamada I, Ozaka M, Ishii H, Sasahira N, Takano K, Matsuyama M, et al. (2015) A retrospective study of FOLFOX in the treatment of patients with advanced duodenal adenocarcinoma: A Japanese single-center experience. Journal of Clinical Oncology 33. [Google Scholar]
- 35.Zaanan A, Gauthier M, Malka D, Locher C, Gornet J M, Thirot-Bidault A, et al. (2011) Second-line chemotherapy with fluorouracil, leucovorin, and irinotecan (FOLFIRI regimen) in patients with advanced small bowel adenocarcinoma after failure of first-line platinum-based chemotherapy: a multicenter AGEO study. Cancer 117: 1422–1428. 10.1002/cncr.25614 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.