Abstract
Campylobacter coli is a bacterial species that is a major cause of diarrheal disease worldwide, and Campylobacter spp. are among the top 5 foodborne pathogens in the United States. During food production organic acids (OAs) are often used to remove bacteria from animal carcasses. The interactions of six OAs with 111 C. coli strains obtained from swine and retail pork chops were studied by determining the molar minimum inhibitory concentrations (MICMs) of the C. coli strains, and the pH at the MICMs. The Henderson-Hasselbalch equation was used to calculate the concentrations of the undissociated and dissociated OAs at the MICMs of the C. coli strains. The results for the 111 different C. coli strains obtained from different locations were treated as a single group for each OA since many of the C. coli strains behaved similarly to each different OA. Inhibition of C. coli was not dependent on pH or on the undissociated OA species, but C. coli inhibition correlated with the dissociated OA species. Therefore, if the concentration of the dissociated OAs decreases from optimum, one may then expect that C. coli bacteria would escape disinfection. The concentration of the dissociated OA should be carefully controlled in a carcass wash. We suggest maintaining a concentration of the dissociated acetic, butyric, citric, formic, lactic and propionic acids at 29, 23, 11, 35, 22 and 25 mM, respectively, when using a carcass wash with these OAs to remove C. coli bacteria. However, due to C. coli utilization of acetate, formate, lactate and propionate, these four OAs may not be the best choice to use for a carcass wash to remove C. coli contamination. Of the six OAs, citric acid was the most efficient at inhibiting C. coli.
Introduction
Campylobacter spp. are Gram-negative, non-spore forming bacterial rods [1,2] that are a major cause of diarrheal disease in the United States [3] and throughout the world [1,4–8]. The Centers for Disease Control and Prevention (CDC) has estimated that each year there are 9.4 million domestically acquired foodborne illnesses, 55,961 hospitalizations, and 1,351 deaths due to 31 major pathogens in the United States [3,9]. Campylobacter spp. are among the top 5 foodborne pathogens in the United States, and they are estimated to be responsible for 845,024 illnesses, 8,463 hospitalizations, and 76 deaths each year [3,9]. Campylobacter jejuni and C. coli are the two main species most often associated with human foodborne illness in this genus [10–13], and they have a high % of DNA homology [14] and possess identical or highly related antigens [15]. In 2016, the CDC reported that Campylobacter and Salmonella caused the most reported bacterial foodborne illnesses in the United States [16]. In England during 2002 C. jejuni accounted for 93% of the reported cases and C. coli accounted for 7% of the reported cases [4]. Campylobacteriosis was the most often reported zoonosis in the European Union (E.U.) in 2015 [7]. The type and number of organisms in the E.U. illnesses during 2015 caused by Campylobacter spp. were primarily divided between C. jejuni and C. coli at 81.0% and 8.4%, respectively [7], although in France C. coli had a higher percentage of cases at 15.25% [13]. Therefore, C. jejuni, which is commonly found in poultry and poultry products, causes the most campylobacteriosis, and low levels of C. jejuni are also found in swine [17]. However, in some areas of the world the percentage of campylobacteriosis caused by C. coli may be as high as 35–40% [18]. Campylobacter coli is the predominant Campylobacter species found in the intestines of pigs and on pork products [19,20]. The impact of C. coli on infectious intestinal disease in humans has largely been ignored, even though C. coli is the second most common cause of human campylobacteriosis [21]. Most likely C. coli have been neglected as a human pathogen because of the predominance of C. jejuni campylobacteriosis [21]. Trace back investigations of C. coli foodborne outbreaks in Belgium (1995) [22], in Poland (2006) [23] and in Alaska (2013) [24] have all resulted in not determining the source of contamination. Epidemiologic and microbiologic data compiled by the Great Britain Public Health Laboratory Service (PHLS) Communicable Disease Surveillance Centre determined that risk factors for transmission of C. coli to humans are different compared to those for C. jejuni [4]. Therefore, this data shows a need to carry out species-specific studies, and develop separate strategies for control of these different organisms [21].
Comprehensive strategies to control foodborne pathogens throughout the food chain from the farm to the table are important [25]. A critical step in processing animals into food products is to wash the animal carcasses with organic acids (OAs) to remove surface bacteria. The OAs often used are acetic [26–28], citric [26], formic [27], lactic [26–31] and propionic acids [27,28]. Bacteria that are not removed from the carcass during the acid wash may later be found on the processed meat. Therefore, the efficacy of the acid wash step should be carefully evaluated.
It is believed that bacterial inhibition by OAs is dependent on pH or the undissociated acid species [32–35]; however, the specific mechanisms by which pH and OAs inhibit bacteria are not understood [36]. In our previous studies, molar values have been used for minimum inhibitory concentrations (MICMs) when comparing pH, undissociated or dissociated acid forms because it allows an equivalent comparison of MIC results for acids with different molecular weights [37]. Previous studies evaluated Escherichia coli O157:H7 [37], Pseudomonas aeruginosa [38], non-O157 Shiga toxin-producing E. coli (non-O157 STECs) [39] and Salmonella enterica serovars [40] against OAs and clearly show that pH and levels of undissociated acids do not correlate with the MICMs. However, levels of dissociated acids do closely correlate with the MICMs. Also, a fully dissociable acid has been shown to cause the disintegration of the bacterial LPS layer [41]. During our previous studies it was observed that a decrease in the concentration of the dissociated acids may result in a large number of bacteria escaping disinfection [37–40].
In this present study, we describe the interactions of six different OAs with 111 C. coli strains, which were obtained in earlier studies that evaluated the pathogens in market age pigs [42], and food animals and retail meat [43]. Susceptibility studies of 111 C. coli strains to the OAs, acetic, butyric, citric, formic, lactic and propionic acids were conducted here. Comparisons are shown of the pH, undissociated acid species and dissociated acid species at the MICMs of the C. coli strains.
Materials and methods
Ethics statement
No animals were utilized in this study. All C. coli strains were obtained from frozen stocks in glycerol as prepared by researchers in previous studies.
Campylobacter coli and media
Previously, C. coli was isolated from cecal contents (n = 7), rectal swabs (n = 51) and feces (n = 5) of market age pigs [42], and C. coli also was previously isolated from cecal contents (n = 16) of market age pigs, from cecal contents of sows (n = 20) and from retail pork chops (n = 12) [43]. The above 111 C. coli strains were grown in our laboratory for 48 hours at 42°C on trypticase soy agar w/5% sheep blood BBL Stacker Plates (Becton, Dickinson and Company, Sparks, MD, USA) in a microaerobic atmosphere of 10% CO2, 5% O2, and 85% N2. For cryopreservation, the 111 C. coli strains were transferred from the BBL Stacker Plates and placed in FBP medium [44]. Briefly, FBP medium was made with Nutrient Broth (234000, Difco, Franklin Lakes, NJ, USA), Bacto™ Agar (214010, BD, Franklin Lakes, NJ, USA) at a final concentration of 0.12% (w/v), glycerol (49769, Fluka, Sigma-Aldrich, St. Louis, MO, USA) at a final concentration of 15% (v/v), and Bacto™ Yeast Extract (212750, BC, Franklin Lakes, NJ, USA) at a final concentration of 0.1% (w/v). The prepared FBP mixture was then autoclaved for 15 min at 121°C and 15 PSI and allowed to cool to 50°C in a water bath. Per label directions, Campylobacter Growth Supplement (SR0232E, Oxoid, Basingstoke, United Kingdom) was added to the cooled mixture. The prepared medium (1 ml) was added to each sterile cryogenic vial (5000–0020, Thermo Fisher Scientific, Houston, TX, USA). Campylobacter cells were added to the FBP medium at a turbidity of McFarland 3 to 4. The cells were then placed in a –80°C freezer for long term storage.
Organic acid susceptibility testing
The OA MICs against the C. coli strains were determined by broth microdilution testing of fastidious bacteria according to the Clinical and Laboratory Standards Institute (CLSI) [45], and the methods presented by TREK Diagnostic Systems for susceptibility using Campylobacter sensititre plates [46]. Briefly, The C. coli strains were grown for 48 hours at 42°C, as described earlier. All Campylobacter susceptibility studies required incubation for 48 hours at 42°C either on trypticase soy agar w/5% sheep blood or in 96-well plates (U-bottom microplate, Greiner bio-one North America Inc., Monroe, North Carolina, USA) for broth microdilution testing because there were some strains that did not grow a sufficient amount in 24 hours to run the test. Several C. coli colonies were selected from the trypticase soy agar plates and diluted in 5 ml of Sensititre™ cation adjusted Mueller-Hinton broth w/TES (Remel Lenexa, KS, USA) to a 0.5 McFarland standard in a Nephelometer (TREK Diagnostic Systems Ltd., East Grinsted, UK). Since our experiments have a final total liquid volume of 100 μl in each well, to maintain a consistent bacterial concentration as suggested by the TREK Diagnostic Systems sensititre susceptibility test for Campylobacter, 200 μl of the 0.5 McFarland suspension was placed in tubes containing 11 ml of Sensititre™ cation adjusted Mueller-Hinton broth w/TES w/Lysed horse blood to provide 1 × 106 CFU/ml. Following the proper dilution of OAs to 50 μl in each well of the 96-well plates [40], 50 μl of the lysed horse blood diluted bacteria was layered in all 96-wells of the microplate. Briefly, the OA dilutions consisted of 50 μl of each OA solution placed in wells 1 and 2, and the well 2 solution was diluted 1:2 across a 96-well U-bottom Greiner bio-one microplate through column 11, and column 12 was used as the positive control [40]. The bacteria filled microplates were covered with a perforated plastic adhesive cover sheet (YG522EA, Remel, Lenexa, KS, USA) and placed in a BD GasPak™ EZ standard or small incubation container (BD #260671 or BD #260002, respectively, Becton, Dickinson and Company, Sparks, MD, USA). BD GasPak™ EZ Campy Container System Sachets (BD #260680, Becton, Dickinson and Company, Sparks, MD, USA) were placed inside the incubation containers and the sealed containers were allowed to incubate for 48 hours at 42°C. MICs were determined as the lowest concentration of a compound that showed no visible growth of the organism [47] on a SensiTouch imaging system (TREK Diagnostic Systems Ltd., East Grinsted, UK). Campylobacter jejuni ATCC 33560 was used as a control organism for the OA susceptibility testing in the microaerobic atmosphere. These results were compared with results obtained from testing Escherichia coli ATCC 25922 in aerobic conditions, as ATCC 25922 was previously used as the control organism during aerobic OA testing [37–40].
Acetic acid was obtained from EM Science (Gibbstown, NY, USA). Butyric, citric, formic and propionic acids were obtained from Sigma-Aldrich (Milwaukee, WI, USA). Lactic acid was obtained from Alfa Aesar (Wad Hill, MA, USA). To make working solutions, the OAs were diluted with reverse osmosis water and then filter-sterilized using a 0.2 μm × 25 mm syringe filter (No. 431224, Corning Inc., Corning, NY, USA). The following concentrations of OAs were tested: acetic acid, 32–32,768 μg/ml; butyric acid, 16–16,384 μg/ml; citric acid, 16–16,384 μg/ml; formic acid, 16–16,384 μg/ml; lactic acid, 8–8,192 μg/ml; and propionic acid, 32–32,768 μg/ml.
Determination of solution pH in 96-well plates at the C. coli MICs
Determination of pH was conducted as previously described [40]. Briefly, the pH was determined in three separate samples at each MIC for each OA, and then the means and standard deviations were determined. The solutions from 16-wells (1,600 μl) at the same MIC value for each OA were combined in a sterile 5 ml microtube (Argos Technologies, Inc., Vernon Hills, IL, USA). An Orion 3 STAR benchtop pH meter was used to measure the pH with a ROSS Ultra, glass combination pH electrode (Thermo Fisher Scientific, Chelmsford, MA, USA). Each pH determination at each MIC was conducted in triplicate.
Calculation of the ratio of undissociated to dissociated acids
The Henderson-Hasselbalch equation can be used to calculate the concentration of conjugate base and undissociated weak acid [48]:
| (1) |
Where the pKa is–log10 of the acid dissociation constant (Ka), [A–] is the molar concentration of the conjugate base (or dissociated weak acid), and [HA] is the molar concentration of the undissociated weak acid [48]. The Henderson-Hasselbalch equation can be rearranged to provide the ratio of undissociated to dissociated acid [33]:
| (2) |
Therefore, when the pKa of a particular acid and the pH of the solution are known, then the ratio of the undissociated to dissociated acid can be calculated. The pKa for acetic, butyric, citric, formic, lactic and propionic acid is 4.75, 4.82, 3.14, 3.75, 3.86 and 4.87, respectively. If the molar concentration of the acid is known, then the concentrations of the undissociated and dissociated acid species can be calculated from the ratio [37–40].
Statistics
A contingency table association analysis was conducted on the data in Table 1 between the MICM values and sources. A Fishers Exact test (due to the small sample size) was used to assess for patterns requiring greater OA concentrations for control of C. coli strains from different sources.
Table 1. Organic acid MICs and MICMsa for 111 Campylobacter coli strains isolated from cecal contents, feces and rectal swabs of market age pigs, cecal contents of sows and from retail pork chops.
| MIC (μg/mL) |
MICM (mM) |
Number of Bacteria from Swine | ||||
|---|---|---|---|---|---|---|
| Market Age Pigs | ||||||
| Cecal | Feces | Rectal Swabs | Cecal (sows) | Pork Chops | ||
| Acetic Acid | ||||||
| 4096 | 68.2 | –b | – | – | – | 1 |
| 2048 | 34.1 | 19 | 4 | 40 | 14 | 5 |
| 1024 | 17.05 | 4 | 1 | 11 | 6 | 6 |
| Butyric Acid | ||||||
| 2048 | 23.24 | 22 | 5 | 48 | 15 | 10 |
| 1024 | 11.62 | 1 | – | 3 | 5 | 2 |
| Citric Acid | ||||||
| 2048 | 10.66 | 14 | 2 | 27 | 14 | 10 |
| 1024 | 5.33 | 9 | 3 | 24 | 6 | 2 |
| Formic Acid | ||||||
| 2048 | 44.5 | – | 4 | 24 | 3 | – |
| 1024 | 22.25 | 23 | 1 | 26 | 17 | 12 |
| 512 | 11.12 | – | – | 1 | – | – |
| Lactic Acid | ||||||
| 4096 | 45.47 | 1 | – | 1 | 3 | 4 |
| 2048 | 22.74 | 4 | 3 | 17 | 8 | 5 |
| 1024 | 11.37 | 18 | 2 | 32 | 9 | 3 |
| 512 | 5.68 | – | – | 1 | – | – |
| Propionic Acid | ||||||
| 2048 | 27.65 | 16 | 5 | 36 | 13 | 8 |
| 1024 | 13.82 | 7 | – | 13 | 7 | 4 |
| 512 | 6.91 | – | – | 1 | – | – |
| 256 | 3.45 | – | – | 1 | – | – |
aMICMs = Molar MICs.
b’–‘ = No observed MIC at this acid concentration.
Results
The MICs and MICMs obtained for C. coli strains against the OAs tested here are shown in Table 1. The C. coli MICMs for acetic, butyric, citric, formic, lactic and propionic acids are similar for each individual acid whether the bacterial strains were obtained from market age pigs, sows or pork chops. Campylobacter coli strains from feces and rectal swabs of market age pigs required differential levels of OAs for control. The highest level of formic acid (44.5 mM) was required for inhibition of 50% of the feces and rectal swab strains. But a citric acid level of only 10.66 mM inhibited these same C. coli strains, which also was a lower acid concentration than the other OAs, acetic, butyric, formic, lactic, and propionic acids, except for lactic and propionic acids which inhibited 1 and 2 strains at levels of 5.68 and 6.91 mM, respectively. The highest level of an OA required for control of C. coli strains was for retail pork chop samples, which required 45.47 mM of lactic acid, and one strain required 68.2 mM acetic acid for inhibition. The lowest OA levels required for control of all strains was for citric acid (10.66 mM).
Interplay of the six organic acids with respect to differential association for inhibition of Campylobacter coli from different isolation sources
Using Fishers Exact test, acetic and butyric acids have a weak differential association with respect to the control of C. coli strains from the different isolation sources, P = 0.107 and P = 0.097, respectively. Citric acid has no differential association with respect to the control of C. coli from the different isolation sources, P = 0.24.
Formic acid has differential control of C. coli strains from different isolation sources, P = 0.0001. Eighty percent of the strains from fecal samples required the highest formic acid concentrations (44.5 mM) for control, and 77.4% of the strains from rectal swab samples from market aged pigs required the highest formic acid concentration (44.5 mM) for control (Table 1).
Lactic acid also has differential control of C. coli strains from different isolation sources, P = 0.012. Thirty-three percent of the C. coli strains from retail pork chops required the highest lactic acid concentration (45.47 mM) for bacterial control (Table 1). Also, 41.7% of the C. coli strains from retail pork chops and 40% of the C. coli strains from cecal sow samples required the 2nd highest concentration of lactic acid (22.74 mM) for bacterial control (Table 1). While 78.3% of the C. coli strains from cecal samples of market age pigs were controlled at 11.37 mM lactic acid (Table 1). Propionic acid showed no differential control of C. coli from different sources, P = 0.91, but required 27.65 mM to inhibit 73.2% of the C. coli strains from fecal and rectal swab samples (Table 1).
Table 2 presents the median, mode, range and 90th percentile of the C. coli MICs and MICMs for each OA.
Table 2. Central Tendency of the MICs and MICMsa for the 111 Campylobacter coli strains from cecal contents, feces and rectal swabs of market age pigs, cecal contents of sows and from retail pork chops against six organic acids.
| Organic Acid | Median | Mode | Range | 90th Percentile |
|---|---|---|---|---|
| Acetic Acid | ||||
| MIC (μg/mL) | 2048 | 2048 | 1024–4096 | 2048 |
| MICM (mM) | 34.1 | 34.1 | 17.05–68.1 | 34.1 |
| Butyric Acid | ||||
| MIC (μg/mL) | 2048 | 2048 | 1024–2048 | 2048 |
| MICM (mM) | 23.24 | 23.24 | 11.62–23.24 | 23.24 |
| Citric Acid | ||||
| MIC (μg/mL) | 2048 | 2048 | 1024–2048 | 2048 |
| MICM (mM) | 10.66 | 10.66 | 5.33–10.66 | 10.66 |
| Formic Acid | ||||
| MIC (μg/mL) | 1024 | 1024 | 512–2048 | 2048 |
| MICM (mM) | 22.25 | 22.25 | 11.12–44.5 | 44.5 |
| Lactic Acid | ||||
| MIC (μg/mL) | 1024 | 1024 | 512–4096 | 2048 |
| MICM (mM) | 11.37 | 11.37 | 5.68–45.47 | 22.74 |
| Propionic Acid | ||||
| MIC (μg/mL) | 2048 | 2048 | 256–2048 | 2048 |
| MICM (mM) | 27.65 | 27.65 | 3.45–27.65 | 27.65 |
aMICMs = Molar MICs.
Measured pH at the MICs of the Campylobacter coli against organic acids
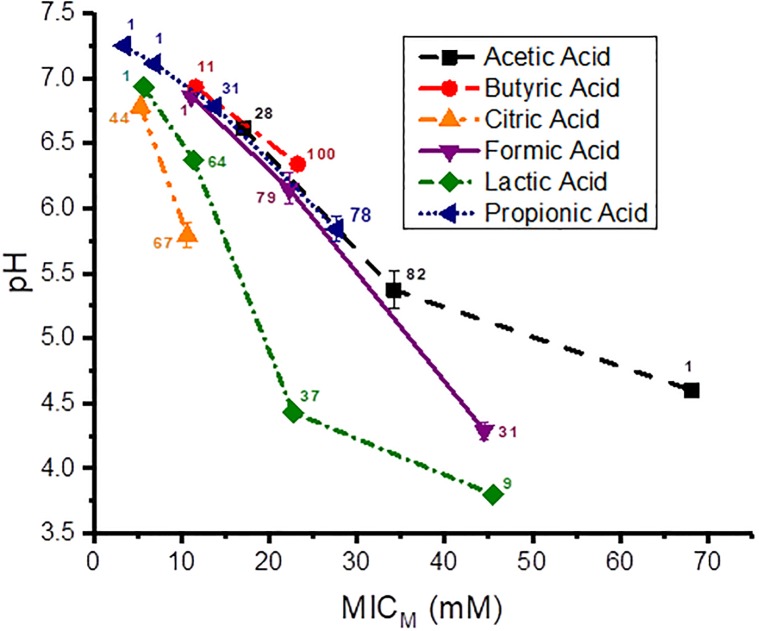
Since the C. coli strains behaved similarly against many of the individual different OAs, the pH determined at the C. coli MICMs for all strains (n = 111) against each individual OA were combined into a single group for each OA. The pH values obtained at the C. coli MICMs for the six OAs are graphically presented in Fig 1. Each data point is the mean and standard deviation of triplicate samples, and next to each data point on the graph is depicted the number of strains at each MICM. The pH at the MICM for 100% of the strains against butyric, citric and propionic acids was 6.34, 5.79 and 5.84, respectively, an average pH of 5.99 ± 0.304. But the pH at the MICM for 100% of the strains against acetic, formic and lactic acids was 4.60, 4.29 and 3.80, respectively, an average pH of 4.23 ± 0.403. The pH difference for 100% of the C. coli strains against these two groups of acids is on average 1.76 pH units.
Fig 1. pH at the MICMs of acetic, butyric, citric, formic, lactic and propionic acids for the 111 Campylobacter coli strains.
The number of strains is shown next to each data point. Each data point is the mean and standard deviation of triplicate samples.
Graphical presentations showing the pH at the MICMs of the C. coli strains isolated from the individual sources, cecal contents, feces and rectal swabs of market age pigs, cecal contents of sows, and from retail pork chops against the six OAs are shown for each source in S1–S5 Figs, respectively.
Undissociated organic acid concentrations calculated at the C. coli MICMs
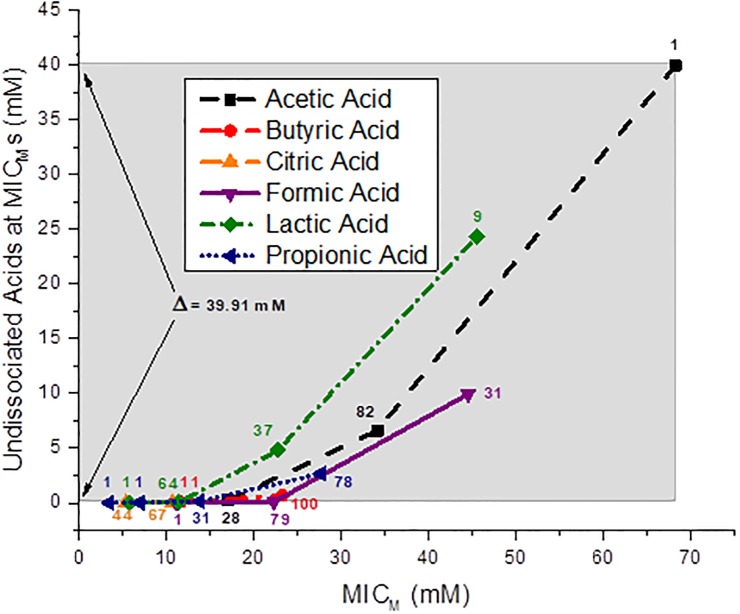
The results calculated by the Henderson-Hasselbalch calculation for the undissociated OA concentrations of acetic, butyric, citric, formic, lactic and propionic acids at the MICMs of 111 C. coli strains are shown in Fig 2. The undissociated acetic, formic and lactic acid concentrations at the MICM for 100% of the C. coli strains tested was 39.93, 9.96 and 24.3 mM, respectively. The undissociated butyric, citric and propionic acid concentrations at the MICM for 100% of the C. coli strains tested was 0.68, 0.024 and 2.68 mM, respectively. The MICM of all 111 strains occurred at an undissociated citric acid level of 0.024 mM. The MICM of all 111 C. coli strains occurred at an undissociated acetic acid concentration of 39.93 mM. A concentration of undissociated butyric and citric acids of 0.68 and 0.024 mM was observed at 100% of the C. coli at their MICMs. A difference of Δ = 39.91 mM OA levels between the MICM of 100% of the strains against acetic and citric acids is shown by the shaded band in Fig 2.
Fig 2. Concentration (mM) of the undissociated acids at the MICMs of the 111 Campylobacter coli strains.
The shaded band depicts the difference between the undissociated acetic and citric acid concentrations required for disinfection of 100% of the strains; Δ = 39.91 mM. The number of strains is shown next to each data point.
Graphical presentations showing the undissociated acid species at the MICMs of the 111 C. coli strains isolated from the individual sources, cecal contents, feces and rectal swabs of market age pigs, cecal contents of sows, and from retail pork chops against the six OAs are shown for each individual source in S6–S10 Figs, respectively.
Dissociated organic acid concentrations calculated at the C. coli MICMs
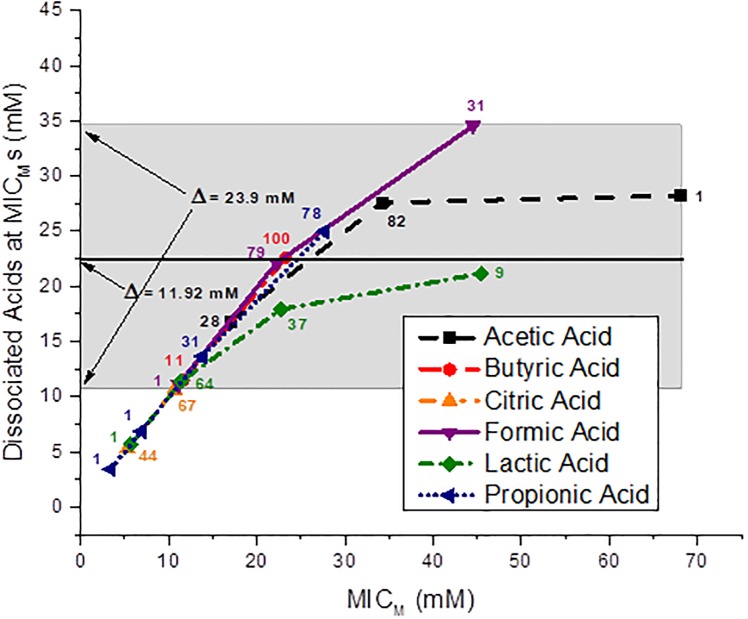
The calculated concentrations of the dissociated OAs, acetic, butyric, citric, formic, lactic and propionic acids at the MICMs of the 111 C. coli strains are shown in Fig 3. The molar dissociated OA concentrations required to produce MICMs for 100% of the 111 C. coli strains by all six OAs are shown by the shaded band in Fig 3. The shaded band shows a Δ = 23.9 mM difference between the MICM of 100% of the 111 C. coli strains inhibited by citric acid and 100% of the 111 strains inhibited by the other five OAs. The MICM for 100% of the 111 strains occurs at a dissociated acid level of 10.64 mM citrate. The MICM for 100% of the 111 strains for all dissociated acids occurs at a level of 34.54 mM formate. However, only the results for the dissociated butyric and citric acids may not be affected by C. coli utilization. The concentration difference of these two dissociated acids for inhibition of 100% of the 111 C. coli results in a Δ = 11.92 mM.
Fig 3. Concentration (mM) of the dissociated acids at the MICMs of the 111 Campylobacter coli strains.
The shaded band depicts the difference between the dissociated formic and citric acid concentrations required for disinfection of 100% of the strains, Δ = 23.9 mM; and the line through the 100 strain data point for butyric acid and the 67 strain data point for citric acid shows the difference in concentration for inhibition of 100% of the strains for these two acids, Δ = 11.92 mM. The number of strains is shown next to each data point.
Graphical presentations of the dissociated acid species at the MICMs of the 111 C. coli strains isolated from the individual sources, cecal contents, feces and rectal swabs of market age pigs, cecal contents of sows, and from retail pork chops against the six OAs is shown for each individual source in S11–S15 Figs, respectively.
Discussion
Organic acids are regularly used to decontaminate meat surfaces. But many bacterial food pathogens have the ability to adapt to varying pH environments, and decontamination strategies are often based on pH [49]. We studied six different OAs, acetic, butyric, citric, formic, lactic and propionic acids against 111 C. coli strains to evaluate the effect that pH, the undissociated and dissociated acid species had on these bacteria at their MICMs.
The median MICM for acetic and propionic acids required for disinfection of the same strains are the highest and the median MICM for inhibition of the C. coli strains by butyric and formic acids have an intermediate value, while the median MICM for inhibition by citric and lactic acids have the lowest values. However, acetic, formic and lactic acids have the highest MICM values for the range of disinfection of all six OAs, and 33.3% of C. coli from retail pork chops required the highest level of lactic acid for bacterial control. While the citric acid MICMs demonstrate the lowest range, and the lowest 90th percentile value of 10.66 mM for inhibition of all the 111 C. coli strains. This suggests that citric acid may be the best OA for inhibiting C. coli. This is also confirmed by showing that citric acid has no differential association with respect to the control of C. coli from different isolation sources, P = 0.24. Conversely, citric acid has a common inhibition effect and lowest concentration required on C. coli no matter where the bacteria are isolated from.
Interestingly, it only took a pH of 6.34, 5.79 and 5.84 to inhibit 100% of these bacteria with butyric, citric and propionic acids, respectively. But with acetic, formic and lactic acids it required a pH of 4.60, 4.29 and 3.80, respectively, to inhibit the same 111 C. coli strains. This is an average of 1.76 pH unit difference between the pH required for these two groups of acids to inhibit the same 111 C. coli strains. We have reported pH differences between OAs against other Gram-negative strains, but not this large a difference. Approximately 98% of 175 P. aeruginosa strains showed a 0.98 pH unit difference when inhibited by different OAs [38]. A 0.56 pH unit difference was observed between the inhibition by different OAs for 98% of 344 E. coli O157:H7 strains [37], a 0.99 pH unit difference between different OAs was required to inhibit 100% of 138 non-O157 STEC strains [39], and a 1.1 pH unit difference was observed between four different OAs for inhibition of 95 to 100% of the same 145 Salmonella strains [40]. These data show that the inhibition of C. coli or the other Gram-negative bacteria are not primarily dependent on the pH of the acids, as has been suggested by others [33], but rather inhibition must be dependent on some other aspect of these acids. If indeed pH were the primary factor in bacterial inhibition, then one would expect that the MICMs for the same bacteria for all the different OAs would be at the same pH value; but that is not the case. Also, we saw more acid-tolerance in E. coli O157:H7 strains [37], since they have glutamate and arginine–dependent acid-resistance systems for protection against acid stress [50].
The inhibition range for 100% of the 111 C. coli strains by all six undissociated OAs, acetic, butyric, citric, formic, lactic and propionic acids extended from 0.024 mM citric acid to 39.93 mM acetic acid, which is an undissociated acid difference of 39.91 mM across the six different OA species for the same 111 strains. Also, undissociated citric acid shows an inhibition of C. coli strains at a very dilute acid concentration of 1 μM. There appears to be no correlation as to concentration of the undissociated OAs with the MICMs for the 111 C. coli strains. These results are in agreement with the four other Gram-negative foodborne pathogens we have previously studied. In 175 P. aeruginosa strains the difference between undissociated citric acid (2.53 mM) and acetic acid (21.65 mM) for inhibition of 100% of the strains at the MICMs was 19.12 mM [38]. In 344 E. coli O157:H7 the difference between undissociated citric acid (2.86 mM) and acetic acid (50.63) for inhibition of 98.3% of the strains at the MICMs was 47.77 mM [37]. In 138 non-O157 STECs the difference between undissociated citric acid (2.2 mM) and acetic acid (49.11 mM) for inhibition of 100% of the strains at the MICMs was 46.91 mM [39], and in 145 Salmonella strains the difference between undissociated citric acid (2.29 mM) and acetic acid (19.0 mM) for inhibition of 100% of the strains at the MICMs was 16.71 mM [40]. In all of these cases, the undissociated acid concentrations did not correlate with the MICMs. Higher undissociated acid values were observed for E. coli O157:H7 and non-O157 STECs, but most likely this was a result of the glutamate and arginine–dependent acid-resistance systems inherent to those bacteria and used to protect themselves from extreme acid stress [50,51].
The inhibition of 100% of the 111 C. coli strains by the dissociated OAs was definitely a much smaller concentration range than that observed for the undissociated acids. But the inhibition concentration range shown for all six dissociated acids against C. coli is still large when compared to the dissociated OA concentration ranges against the other four Gram-negative foodborne pathogens that we previously studied. The inhibition of approximately 98% of 175 P. aeruginosa strains by dissociated citric acid (10.24 mM) and acetic acid (9.98 mM) had a concentration difference of 0.26 mM [38]. The inhibition of 98.3% of 344 E. coli O157:H7 strains by dissociated lactic acid (19.36 mM) and dissociated propionic acid (13.825 mM) had a concentration difference of 5.54 mM [37]. The inhibition of 100% of 138 non-O157 STEC strains by dissociated citric acid (19.12 mM) and lactic acid (12.93 mM) had a concentration difference of 6.19 mM [39], and the inhibition of 100% of 145 Salmonella strains by dissociated citric acid (19.03 mM) and propionic acid (13.67 mM) had a concentration difference of 5.36 mM [40]. The overall difference in dissociated acids required for inhibition of these four Gram-negative bacteria was from 0.26 mM to 6.19 mM. However with P. aeruginosa, we saw a large increase in the dissociated lactic acid concentration required for inhibition [38]. It is known that P. aeruginosa utilizes lactate [52,53], and the high inhibition concentration obtained for dissociated lactic acid could be expected [38]. Lactic acid is not an appropriate OA to use against P. aeruginosa [38].
Most C. coli strains from swine do not utilize citrate [54], and we see in this study the inhibition concentration for dissociated citric acid remains low, ≤ 10.64 mM. Also, C. coli were shown not to utilize butyrate [54]. This study corroborates earlier observations by demonstrating levels of dissociated butyric acid needed for inhibition of C. coli not widely different from the levels of other dissociated OAs against Gram-negative pathogens [37,39,40]. However, C. coli are known to utilize formate, lactate and propionate [55], and in a previous study approximately 13.5% of the C. coli strains utilized acetate [54]. The authors also noted the source of C. coli strains utilizing acetate was restricted to hogs [54]. Since all 111 strains are inhibited by both citric and butyric acid by ≤ 22.56 mM (knowing that C. coli does not utilize citrate or butyrate [54]), it is very interesting that 31 strains are not inhibited by dissociated formic acid until nearly 35 mM, 78 strains are not inhibited by dissociated propionic acid until about 25 mM, and 83 strains are not inhibited by dissociated acetic acid until about 28 mM. Based on our data for the dissociated acid species at the MICMs of 111 C. coli strains from swine, perhaps as much as 83/111 strains (75%) of the C. coli analyzed from swine or swine products may utilize acetate.
Conclusion
Inhibition of Campylobacter coli strains in this study was not primarily dependent on pH or on the concentration of undissociated OAs. The concentration of dissociated OA, butyric, citric, formic, lactic and propionic acids correlated with the MICMs of 100% of the 111 C. coli strains. However, some C. coli can utilize acetate, formate, lactate and propionate, which most likely resulted in increased levels of these acids at the MICs in our studies. One may expect that a large number of bacteria could escape disinfection as a result of only a small drop in the concentration of a dissociated OA. Therefore, an OA carcass wash may not provide the expected elimination of surface bacteria if the concentration levels of the dissociated OA used is not carefully controlled. A concentration of dissociated acetic, butyric, citric, formic, lactic and propionic acids of 29, 23, 11, 35, 22 and 25 mM, respectively, should be maintained when disinfecting the C. coli strains studied here. However, due to the utilization of acetate, formate, lactate and propionate by C. coli, these four OAs would probably not be the best choice for control of C. coli. If these 4 acids are used for disinfection of C. coli bacteria the concentrations of these dissociated organic acids must be held at high enough levels to facilitate complete inhibition of the bacteria. Of the six OAs, citric acid is the most efficient at inhibiting C. coli.
Supporting information
The number of strains is shown next to each data point. Each data point is the mean and standard deviation of triplicate samples.
(TIF)
The number of strains is shown next to each data point. Each data point is the mean and standard deviation of triplicate samples.
(TIF)
The number of strains is shown next to each data point. Each data point is the mean and standard deviation of triplicate samples.
(TIF)
The number of strains is shown next to each data point. Each data point is the mean and standard deviation of triplicate samples.
(TIF)
The number of strains is shown next to each data point. Each data point is the mean and standard deviation of triplicate samples.
(TIF)
The shaded band depicts the difference between the undissociated lactic and citric acid concentrations required for disinfection of 100% of the strains; Δ = 24.3 mM. The number of strains is shown next to each data point.
(TIF)
The shaded band depicts the difference between the undissociated formic and citric acid concentrations required for disinfection of 100% of the strains; Δ = 9.96 mM. The number of strains is shown next to each data point.
(TIF)
The shaded band depicts the difference between the undissociated lactic and citric acid concentrations required for disinfection of 100% of the strains; Δ = 24.3 mM. The number of strains is shown next to each data point.
(TIF)
The shaded band depicts the difference between the undissociated lactic and citric acid concentrations required for disinfection of 100% of the strains; Δ = 24.3 mM. The number of strains is shown next to each data point.
(TIF)
The shaded band depicts the difference between the undissociated acetic and citric acid concentrations required for disinfection of 100% of the strains; Δ = 39.86 mM. The number of strains is shown next to each data point.
(TIF)
The shaded band depicts the difference between the dissociated formic and citric acid concentrations required for disinfection of 100% of the strains; Δ = 16.96 mM. The number of strains is shown next to each data point.
(TIF)
The shaded band depicts the difference between the dissociated formic and citric acid concentrations required for disinfection of 100% of the strains; Δ = 23.9 mM. The number of strains is shown next to each data point.
(TIF)
The shaded band depicts the difference between the dissociated formic and citric acid concentrations required for disinfection of 100% of the strains; Δ = 23.9 mM. The number of strains is shown next to each data point.
(TIF)
The shaded band depicts the difference between the dissociated formic and citric acid concentrations required for disinfection of 100% of the strains; Δ = 23.9 mM. The number of strains is shown next to each data point.
(TIF)
The shaded band depicts the difference between the dissociated acetic and citric acid concentrations required for disinfection of 100% of the strains; Δ = 17.59 mM. The number of strains is shown next to each data point.
(TIF)
Acknowledgments
This work was funded by the USDA, Agricultural Research Service. Mention of trade names, proprietary products or specific equipment is solely for the purpose of providing specific information and does not constitute a guarantee, warranty or endorsement by the U.S. Department of Agriculture or by the U.S. Food and Drug Administration and does not imply its approval to the exclusion of other products that may be suitable. Additionally, the views expressed in this article are those of the authors and do not necessarily reflect the official policy of the U.S. Department of Agriculture, the U.S. Food and Drug Administration or the U.S. Government.
Data Availability
All relevant data are within the paper and Supporting information.
Funding Statement
This study was funded by the USDA, Agricultural Research Service.
References
- 1.WHO. Media centre Campylobacter: fact sheet. 2016. http://www.who.int/mediacentre/factsheets/fs255/en/ (Accessed: 19 July 2017).
- 2.Penner JL. The Genus Campylobacter: A decade of progress. Clin Microbiol Rev. 1988; 1:157–172. 10.1128/CMR.1.2.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC). Burden of foodborne illness: Findings. 2011. https://www.cdc.gov/foodborneburden/2011-foodborne-estimates.html (Accessed: 19 July 2017).
- 4.Gillespie IA, O’Brien SJ, Frost JA, Adak GK, Horby P, Swan AV, et al. A case-case comparison of Campylobacter coli and Campylobacter jejuni infection: A tool for generating hypotheses. Emerg Infect Dis. 2002; 8(9):937–942. https://pdfs.semanticscholar.org/c17e/4c0027b423e3b7e2eab58d799b24b2a1c93d.pdf?_ga=2.195374702.392223553.1505748840-707578097.1505748840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillespie IA, O’Bien SJ, Bolton FJ. Age patterns of persons with Campylobacteriosis, England and Wales, 1990–2007. Emerg Infect Dis. 2009; 15(12):2046–2048. 10.3201/eid1512.090280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukherjee P, Ramamurthy T, Bhattacharya MK, Rajendran K, Mukhopadhyay AK. Campylobacter Jejuni in hospitalized patients with diarrhea, Kolkata, India. Emerg Infect Dis. 2013; 19(7):1155–1156. 10.3201/eid1907.121278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Food Safety Authority and European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2015. EFSA J. 2016; 14:4634, 231 pp. 10.2903/j.efsa.2016.4634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kempf I, Kerouanton A, Bougeard S, Nagard B, Rose V, Mourand G, et al. Campylobacter coli in organic and conventional pig production in France and Sweden: Prevalence and antimicrobial resistance. Front Microbiol. 2017; 8:955, 9 pp. 10.3389/fmicb.2017.00955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson M-A, Roy SL, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011; 17(1):7–15. 10.3201/eid1701.P11101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nachamkin I, Szymanski CM, Blaser MJ. Campylobacter. 3rd ed Washington DC: ASM Press; 2008. [Google Scholar]
- 11.Epps SVR, Harvey RB, Hume ME, Phillips TD, Anderson RC, Nisbet DJ. Foodborne Campylobacter: Infections, metabolism, pathogenesis and reservoirs. Int J Environ Res Public Health. 2013; 10(12):6292–6304. 10.3390/ijerph10126292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baer AA, Miller MJ, Dilger AC. Pathogens of interest to the pork industry: A review of research on interventions to assure food safety. Compr Rev Food Sci F. 2013; 12(2):183–217. 10.1111/1541-4337.12001 [DOI] [Google Scholar]
- 13.Sifré E, Salha BA, Ducournau A, Floch P, Chardon H, Mégraud F, et al. EUCAST recommendations for antimicrobial susceptibility testing applied to the three main Campylobacter species isolated in humans. J Microbiol Methods. 2015; 119:206–213. 10.1016/j.mimet.2015.10.018 [DOI] [PubMed] [Google Scholar]
- 14.Roop RM II, Smibert RM, Johnson JL, Krieg NR. Differential characteristics of catalase-positive campylobacters correlated with DNA homology groups. Can J Microbiol. 1984; 30(7):938–951. 10.1139/m84-147 [DOI] [PubMed] [Google Scholar]
- 15.Hébert GA, Edmonds P, Brenner DJ. DNA relatedness among strains of Campylobacter jejuni and Campylobacter coli with divergent serogroup and hippurate reactions. J Clin Microbiol. 1984; 20(1):138–140. http://jcm.asm.org/content/20/1/138.full.pdf+html [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC). Campylobacter, Salmonella led bacterial foodborne illnesses in 2016. 2017. https://www.cdc.gov/media/releases/2017/p0420-campylobacter-salmonella.html (Accessed: 14 September 2017). [Google Scholar]
- 17.Schuppers ME, Stephan R, Ledergerber U, Danuser J, Bissig-Choisat B, Stärk KDC, et al. Clinical herd health, farm management and antimicrobial resistance in Campylovacter coli on finishing pig farms in Switzerland. Prev Vet Med. 2005; 69(3–4):189–202. 10.1016/j.prevetmed.2005.02.004 [DOI] [PubMed] [Google Scholar]
- 18.Blackburn CdeW, McClure PJ. Foodborne Pathogens: Hazards, Risk Analysis and Control, 2nd ed Sawston, England: Woodhead Publishing; 2009. p. 720. [Google Scholar]
- 19.Oporto B, Esteban JI, Aduriz G, Juste RA, Hurtado A. Prevalence and strain diversity of thermophilic campylobacters in cattle, sheep, and swine farms. J Appl Microbiol. 2007; 103(4):977–984. 10.1111/j.1365-2672.2007.03328.x [DOI] [PubMed] [Google Scholar]
- 20.Animal and Plant Health Inspection Services (APHIS), United States Department of Agriculture. Campylobacter on U.S. swine sites—antimicrobial susceptibility. December, 2008. https://www.aphis.usda.gov/animal_health/nahms/swine/downloads/swine2006/Swine2006_is_campy.pdf (Accessed: 19 July 2017).
- 21.Tam CC, O’Brien SJ, Adak GK, Meakins SM, Frost JA. Campylobacter coli–an important foodborne pathogen. J Infect. 2003; 47(1):28–32. http://www.journalofinfection.com/article/S0163-4453(03)00042-2/pdf [DOI] [PubMed] [Google Scholar]
- 22.Ronveaux O, Quoilin S, Van Loock F, Lheureux P, Struelens M, Butzler J-P. A Campylobacter coli foodborne outbreak in Belgium. Acta Clin Belg. 2000; 55(6):307–311. 10.1080/17843286.2000.11754317 [DOI] [PubMed] [Google Scholar]
- 23.Wardak S, Szych J, Sadkowska-Todys M. The first report on Campylobacter coli family outbreak detected in Poland in 2006. Eurosurveillance. 2008; 13(1–3):1–3. http://eurosurveillance.org/images/dynamic/EQ/V13N01/V13N01.pdf#page=47 [PubMed] [Google Scholar]
- 24.Clark M. Foodborne Illness Outbreak Database. Campylobacter outbreak associated with consumption of raw milk, Alaska. January 2013. Final Report May 1, 2013. http://outbreakdatabase.com/details/campylobacter-outbreak-associated-with-consumption-of-raw-milk-alaska-2013/?outbreak=Campylobacter+coli&organism=Campylobacter (Accessed: 10 July 2018).
- 25.Wachsmuth IK, Sparling PH, Barrett TJ, Potter ME. Enterohemorrhagic Escherichia coli in the United States. FEMS Immunol Med Microbiol. 1997; 18(4):233–239. 10.1111/j.1574-695X.1997.tb01051.x [DOI] [PubMed] [Google Scholar]
- 26.Departments of Pennsylvania State University, Texas Tech University, and Washington State University. Antimicrobial spray treatments for red meat carcasses processed in very small meat establishments, 2005. http://meathaccp.wisc.edu/validation/assets/acid_spray_intervention_booklet_from_penn_state_2005.pdf (Accessed: 12 September 2017).
- 27.Raftari M, Jalilian FA, Abdulamir AS, Son R, Sekawi Z, Fatimah AB. Effect of organic acids on Escherichia coli O157:H7 and Staphylococcus aureus contaminated meat. Microbiol J. 2009; 3:121–127. 10.2174/1874285800903010121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raftari M, Jalilian FA, Abdulamir AS, Ghafurian S, Radu S, Sekawi Z, et al. Optimized antibacterial measures against Escherichia coli O157:H7 and Staphylococcus aureus. Afr J Microbiol Res. 2011; 5(20):3113–3121. 10.5897/AJMR10.099 [DOI] [Google Scholar]
- 29.Epling LK, Carpenter JA, Blankenship LC. Prevalence of Campylobacter spp. and Salmonella spp. on pork carcasses and the reduction effected by spraying with lactic acid. J Food Protect. 1993; 56(6):536–537. 10.4315/0362-028X-56.6.536 [DOI] [PubMed] [Google Scholar]
- 30.Castillo A, Lucia LM, Roberson DB, Stevenson TH, Mercado I, Acuff GR. Lactic acid sprays reduce bacterial pathogens on cold beef carcass surfaces and in subsequently produced ground beef. J Food Protect. 2001; 64(1):58–62. 10.4315/0362-028X-64.1.58 [DOI] [PubMed] [Google Scholar]
- 31.Reynolds AE, Jr. Utilization of spray wash with organic acids (peroxyacetic acid and lactic acid) and chlorinated wash in combination, utilizing direct application methods, for pathogen reduction on pork and beef carcasses in small and very small meat processing plants, 2005. http://www.fsis.usda.gov/wps/wcm/connect/acc8bddb-eb64-4ea2-82eb-565cf337691a/New_Technology_C29_Summary_FY2003.pdf?MOD=AJPERES (Accessed: 12 September 2017).
- 32.Sofos JN, Busta FF. Antimicrobial activity of sorbate. J Food Protect. 1981; 44(8):614–622. 10.4315/0362-028X-44.8.614 [DOI] [PubMed] [Google Scholar]
- 33.Blocher JC, Busta FF, Sofos JN. Influence of potassium sorbate and pH on ten strains of type A and B Clostridium botulinum. J Food Sci. 1982; 47(6):2028–2032. 10.1111/j.1365-2621.1982.tb12938.x [DOI] [Google Scholar]
- 34.Ray B, Sandine WE. Acetic, propionic, and lactic acids of starter culture bacteria as biopreservatives In: Ray B, Daeschel M, editors. Food biopreservatives of microbial origin. Boca Raton, FL: CRC Press, Inc.; 1992. pp. 103–136. [Google Scholar]
- 35.Leeson S. Balancing science versus societal issues in poultry nutrition. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources. 2007; 2(71), 6 pp. [Google Scholar]
- 36.Presser KA, Ross T, Ratkowsky DA. Modelling the growth limits (growth/no growth interface) of Escherichia coli as a function of temperature, pH, lactic acid concentration, and water activity. Appl Environ Microbiol. 1998; 64(5):1773–1779. http://aem.asm.org/content/64/5/1773.full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beier RC, Poole TL, Brichta-Harhay DM, Anderson RC, Bischoff KM, Hernandez CA, et al. Disinfectant and antibiotic susceptibility profiles of Escherichia coli O157:H7 strains from cattle carcasses, feces, and hides and ground beef from the United States. J Food Protect. 2013; 76(1):6–17. 10.4315/0362-028X.JFP-12-253 [DOI] [PubMed] [Google Scholar]
- 38.Beier RC, Foley SL, Davidson MK, White DG, McDermott PF, Bodeis-Jones S, et al. 2014. Characterization of antibiotic and disinfectant susceptibility profiles among Pseudomonas aeruginosa veterinary isolates recovered during 1994–2003. J Appl Microbiol. 2014; 118(2):326–342. 10.1111/jam.12707 [DOI] [PubMed] [Google Scholar]
- 39.Beier RC, Franz E, Bono JL, Mandrell RE, Fratamico PM, Callaway TR, et al. Disinfectant and antimicrobial susceptibility profiles of the big six non-O157 Shiga toxin-producing Escherichia coli strains from food animals and humans. J Food Protect. 2016; 79(8):1355–1370. 10.4315/0362-028X.JFP-15-600 [DOI] [PubMed] [Google Scholar]
- 40.Beier RC, Callaway TR, Andrews K, Poole TL, Crippen TL, Anderson RC, et al. Interactions of organic acids with Salmonella strains from feedlot water-sprinkled cattle. J Food Chem Nanotechnol. 2017; 3(2):60–66. 10.17756/jfcn.2017-038 [DOI] [Google Scholar]
- 41.Alakomi H-L, Skyttä E, Saarela M, Mattila-Sandholm T, Latva-Kala K, Helander IM. Lactic acid permeabilizes Gram-negative bacteria by disrupting the outer membrane. Appl Environ Microbiol. 2000; 66(5):2001–2005. 10.1128/AEM.66.5.2001-2005.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harvey RB, Anderson RC, Young CR, Hume ME, Genovese KJ, Ziprin RL, et al. Prevalence of Campylobacter, Salmonella, and Arcobacter species at slaughter in market age pigs, Chapter 25 In: Paul PS, Francis DH, editors. Mechanisms in the pathogenesis of enteric diseases 2. New York, NY: Kluwer Academic/Plenum Publishers; 1999. pp. 237–239. 10.1007/978-1-4615-4143-1_25 [DOI] [PubMed] [Google Scholar]
- 43.National Antimicrobial Resistance Monitoring System (NARMS). NARMS isolates of Campylobacter coli from the FDA (retail meats) and USDA (food animals). 2015. https://www.fda.gov/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/default.htm (Accessed: 20 September 2017).
- 44.Gorman R, Adley CC. An evaluation of five preservation techniques and conventional freezing temperatures of –20°C and –85°C for long-term preservation of Campylobacter jejuni. Lett Appl Microbiol. 2004; 38(4):306–310. 10.1111/j.1472-765X.2004.01490.x [DOI] [PubMed] [Google Scholar]
- 45.Clinical and Laboratory Standards Institute (CLSI). Methods for dilution antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria; Approved Guideline—2nd ed. M45-A2, Vol. 30, No 18. Wayne, PA: Clinical and Laboratory Standards Institute; 2010.
- 46.TREK Diagnostic Systems. TREK materials and methods for sensititre susceptibility plates for Campylobacter. http://www.uniscience.co.kr/data/trds/sensi_manuals/Campylobacter_panel.pdf (Accessed: 12 September 2017).
- 47.Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001; 48(Suppl. S1):5–16. 10.1093/jac/48.suppl_1.5 [DOI] [PubMed] [Google Scholar]
- 48.Helmenstine AM. Henderson Hasselbalch equation and example, 2014. http://chemistry.about.com/od/acidsbase1/a/hendersonhasselbalch.htm (Accessed: 07 September 2017).
- 49.Shaheen BW, Miller ME, Oyarzabal OA. In vitro survival at low pH and acid adaptation response of Campylobacter jejuni and Campylobacter coli. J Food Saf. 2007; 27(3):326–343. 10.1111/j.1745-4565.2007.00083.x [DOI] [Google Scholar]
- 50.Bearson BL, Lee IS, Casey TA. Escherichia coli O157:H7 glutamate- and arginine-dependent acid-resistance systems protect against oxidative stress during extreme acid challenge. Microbiology. 2009; 155(3):805–812. 10.1099/mic.0.022905-0 [DOI] [PubMed] [Google Scholar]
- 51.Large TM, Walk ST, Whittam TS. Variation in acid resistance among Shiga toxin-producing clones of pathogenic Escherichia coli. Appl Environ Microbiol. 2005; 71(5):2493–2500. 10.1128/AEM.71.5.2493-2500.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao C, Hu C, Zheng Z, Ma C, Jiang T, Dou P, et al. Lactate utilization is regulated by the FadR-type regulator LldR in Pseudomonas aeruginosa. J Bacteriol. 2012; 194(10):2687–2692. 10.1128/JB.06579-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao C, Hu C, Ma C, Su F, Yu H, Jiang T, et al. 2012. Genome sequence of the lactate-utilizing Pseudomonas aeruginosa strain XMG. J Bacteriol. 2012; 194(17):4751–4752. 10.1128/JB.00943-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elharrif Z, Mégraud F. Characterization of thermophilic Campylobacter: I. Carbon-substrate utilization tests. Curr Microbiol. 1986; 13(3):117–122. 10.1007/BF01568505 [DOI] [Google Scholar]
- 55.Wagley S, Newcombe J, Laing E, Yusuf E, Sambles CM, Studholme DJ, et al. Differences in carbon source utilization distinguish Campylobacter jejuni from Campylobacter coli. BMC Microbiol. 2014; 14:262, 10 pp. 10.1186/s12866-014-0262-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The number of strains is shown next to each data point. Each data point is the mean and standard deviation of triplicate samples.
(TIF)
The number of strains is shown next to each data point. Each data point is the mean and standard deviation of triplicate samples.
(TIF)
The number of strains is shown next to each data point. Each data point is the mean and standard deviation of triplicate samples.
(TIF)
The number of strains is shown next to each data point. Each data point is the mean and standard deviation of triplicate samples.
(TIF)
The number of strains is shown next to each data point. Each data point is the mean and standard deviation of triplicate samples.
(TIF)
The shaded band depicts the difference between the undissociated lactic and citric acid concentrations required for disinfection of 100% of the strains; Δ = 24.3 mM. The number of strains is shown next to each data point.
(TIF)
The shaded band depicts the difference between the undissociated formic and citric acid concentrations required for disinfection of 100% of the strains; Δ = 9.96 mM. The number of strains is shown next to each data point.
(TIF)
The shaded band depicts the difference between the undissociated lactic and citric acid concentrations required for disinfection of 100% of the strains; Δ = 24.3 mM. The number of strains is shown next to each data point.
(TIF)
The shaded band depicts the difference between the undissociated lactic and citric acid concentrations required for disinfection of 100% of the strains; Δ = 24.3 mM. The number of strains is shown next to each data point.
(TIF)
The shaded band depicts the difference between the undissociated acetic and citric acid concentrations required for disinfection of 100% of the strains; Δ = 39.86 mM. The number of strains is shown next to each data point.
(TIF)
The shaded band depicts the difference between the dissociated formic and citric acid concentrations required for disinfection of 100% of the strains; Δ = 16.96 mM. The number of strains is shown next to each data point.
(TIF)
The shaded band depicts the difference between the dissociated formic and citric acid concentrations required for disinfection of 100% of the strains; Δ = 23.9 mM. The number of strains is shown next to each data point.
(TIF)
The shaded band depicts the difference between the dissociated formic and citric acid concentrations required for disinfection of 100% of the strains; Δ = 23.9 mM. The number of strains is shown next to each data point.
(TIF)
The shaded band depicts the difference between the dissociated formic and citric acid concentrations required for disinfection of 100% of the strains; Δ = 23.9 mM. The number of strains is shown next to each data point.
(TIF)
The shaded band depicts the difference between the dissociated acetic and citric acid concentrations required for disinfection of 100% of the strains; Δ = 17.59 mM. The number of strains is shown next to each data point.
(TIF)
Data Availability Statement
All relevant data are within the paper and Supporting information.