Abstract
There is little information regarding the predictive ability of albumin-bilirubin grades (ALBI) plus platelet-to-lymphocyte ratio (PLR) in patients with hepatocellular carcinoma (HCC) following liver resection. In this study, we aimed to evaluate the prognostic power of the ALBI-PLR score in patients with hepatitis B virus-related (HBV-related) HCC within Barcelona Clinic Liver Cancer (BCLC) stage A after liver resection.
Around 475 patients were included in this study. Patients with preoperative ALBI grades 1, 2, or 3 were allocated a score of 0, 1, or 2, respectively. Patients with preoperative PLR >150 or ≤150 were allocated a score of 0 or 1, respectively. The ALBI-PLR score was the summary of the ALBI and PLR scores.
During the follow-up period, 256 patients experienced recurrence, and 150 patients died. Multivariate analysis revealed tumor size, multiple tumors, positive HBV-DNA load, cirrhosis, and ALBI-PLR score as being independently associated with postoperative recurrence, whereas tumor size, high preoperative α-fetoprotein level, and ALBI-PLR score were independent risk factors for postoperative mortality. HCC patients with high ALBI-PLR score had poor recurrence-free and overall survival.
The preoperative ALBI-PLR score is a surrogate marker for predicting HBV-related HCC patient's prognosis after liver resection. A high ALBI-PLR score is associated with a high incidence of postoperative recurrence and mortality.
Keywords: albumin-bilirubin grades, hepatocellular carcinoma, platelet-to-lymphocyte ratio
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most frequent and aggressive malignancies and is the third leading cause of cancer-associated mortality in the world.[1] Every year, more than 700,000 new HCC patients are diagnosed, and almost as many HCC patients die because of the poor biological behavior of this disease.[2] Chronic viral infections, including hepatitis B and C viral (HBV and HCV) infection, are 2 predominant etiologies of HCC.[3] HBV infection has a high prevalence in China. A seroepidemiological survey performed in 2006 revealed that hepatitis B surface antigen was detected in approximately 7.18% of the overall Chinese population.[4] Due to this high infection rate, each year, over 50% of new diagnoses of and deaths from HCC worldwide occur in China.[2] Partial liver resection is widely accepted as a potential curative treatment for HCC patients with well-compensated liver function. However, the long-term outcomes of HCC patients after liver resection remain unsatisfactory because of the high incidence of postoperative recurrence. Previous investigations have reported that the 5-year recurrence rate of HCC patients following liver resection may be up to 50% to 70%, even for those in early stage.[5,6]
A number of studies have suggested that poor liver function is associated with a high surgical risk and poor long-term survival for patients with HCC after liver resection.[7,8] In 2015, Johnson et al[9] developed the albumin-bilirubin (ALBI) grade to assess patient's liver function. Compared with the Child–Pugh score, the ALBI grade only involves 2 objective parameters, the total bilirubin and albumin; the Child–Pugh score has subjective parameters, including ascites and encephalopathy. Some investigations have confirmed the ALBI grade as a useful tool to predict HCC patient's prognosis after liver resection, transarterial chemoembolization and sorafenib.[7,10–12] Moreover, several studies have suggested that a patient's systemic inflammation response contributed to the prognosis of HCC following liver transplantation, liver resection, radiofrequency ablation, and transarterial chemoembolization.[13–16] The platelet-to-lymphocyte ratio (PLR) is one of the most widely used inflammation based markers to predict HCC patient's outcomes after liver resection. The PLR only includes platelet and lymphocyte counts, which are 2 easily obtained laboratory parameters. It is unclear if the ALBI grade incorporating the PLR could strengthen the predictive ability for patients with HCC after liver resection. To clarify this issue, we performed this study.
2. Patients and methods
The data of patients with HCC who underwent liver resection at our center between 2010 and 2017 were reviewed. The exclusion criteria included re-resection, ruptured HCC, no history of HBV infection, coinfection with hepatitis C virus, receiving preoperative antitumor treatment, a positive surgical margin, and the presence of other types of tumors. HCC was confirmed by postoperative pathological examination. The hepatitis B surface antigen was detected in all patients. This study was approved by the ethics committee of West China Hospital. All the study participants provided informed consent. The study was conducted in accordance with the ethical principles stated in the Declaration of Helsinki. All methods were performed in accordance with the relevant guidelines and regulations
2.1. Follow-up
All preoperative blood tests were performed 2 days before the operation. After liver resection, patients were regularly followed-up every 3 months. Before and after the operation, antiviral drugs (entecavir or lamivudine) were conventionally administered to patients with positive hepatitis B virus-DNA (HBV-DNA) load. During follow-up, blood cell tests, liver function tests, serum alpha-fetoprotein (AFP) measurement, HBV-DNA tests, visceral ultrasonography, computed tomography or magnetic resonance imaging and chest radiography were performed for all patients. Bone scintigraphy was performed whenever HCC recurrence was suspected. Postoperative recurrence was defined as positive imaging findings compared with the preoperative examination values or as confirmation by biopsy or resection.[17]
2.2. Definitions
The ABLI grade was calculated using the following formula: ALBI = (log10 bilirubin (μmol/L) × 0.66) + (albumin (g/L) × −0.085).[9] ALBI values were divided into 3 grades as follows: grade 1 (<−2.60), grade 2 (between −2.60 and −1.39) and grade 3 (above −1.39).[9] The neutrophil-to-lymphocyte ratio (NLR) was defined as the neutrophil counts divided by the lymphocyte counts.[18] A NLR ≥ 3 was considered as a high NLR.[18] The PLR was defined as the platelet counts divided by the lymphocyte counts.[18] A PLR ≥ 150 was considered as a high PLR, whereas a PLR < 150 was considered as a low PLR.[18] Patients with ALBI grades 1, 2, or 3 were allocated a score of 0, 1, or 2, respectively. Patients with high preoperative PLR or low PLR were allocated a score of 1 or 0, respectively. The combination of the ALBI and PLR (ALBI-PLR) scores were the summation of the ALBI and PLR scores. The ALBI-PLR scores ranged from 0 to 3. A high preoperative AFP was defined as an AFP level greater than 400 ng/mL.[17]
2.3. Statistical analysis
All statistical analyses were performed using SPSS 21.0 (SPSS Company, Chicago, IL) for Windows. All continuous variables were expressed as the means ± standard deviation and analyzed using one-way analysis of variance. Categorical variables were compared using the χ2 test or Fisher's exact test. To assess the ability of different models to predict postoperative prognosis, our analysis was performed using the c-statistic equivalent to the area under the receiver operating curves (AUC). The recurrence-free survival (RFS) and overall survival (OS) were determined using the Kaplan–Meier method, and comparisons were determined using the log-rank test. Independent risk factors for the RFS and OS were identified using Cox regression analysis. All variables found to be significant (P < .05) in the univariate analysis were included in the multivariate analysis. A P-value of < .05 was considered statistically significant.
3. Results
A total of 475 patients were included in this study, including 401 male patients and 74 female patients. The mean age was 51.2 ± 11.1 years. The mean tumor size was 6.3 ± 4.0 cm. A high preoperative AFP was found in 184 patients. Microvascular invasion (MVI) was detected in 136 patients, and 197 patients had a positive HBV-DNA load. Multiple tumors were observed in 39 patients. Cirrhosis was detected in 397 patients. Around 312 were in TNM stage I, whereas 163 patients were in TNM stage II. In this study, 276 patients fulfilled the Milan criteria, and 199 patients were beyond the Milan criteria. In this study, 336 patients were in ALBI grade 1, and 139 patients were in ALBI grade 2. There were no patients in ALBI grade 3. A preoperative high PLR was observed in 194 patients. A high NLR was detected in 111 patients. The ALBI-PLR scores ranged from 0 to 2. A total of 209 patients had an ALBI-PLR score 0, 199 patients had an ALBI-PLR score 1, and 67 patients had an ALBI-PLR score 2.
With a mean of 36.4 ± 19.1 months follow-up, 256 patients suffered from recurrence, and 150 patients died. The 1-, 3-, 5-year RFS were 87.6%, 53.2%, and 35.0%, respectively (Fig. 1A). The 1-, 3-, 5-year OS were 94.5%, 71.0%, and 50.4%, respectively (Fig. 1B).
Figure 1.
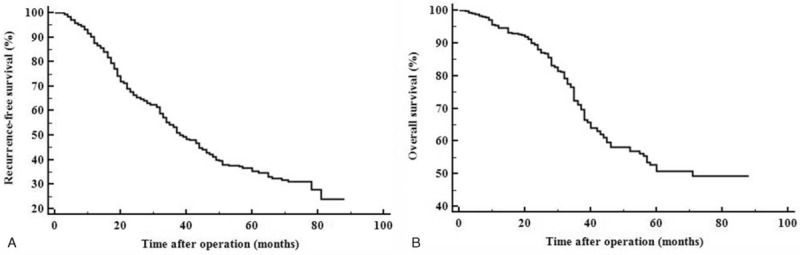
The recurrence-free (A) and overall (B) survival curves of this study.
3.1. Univariate and multivariate analysis for RFS
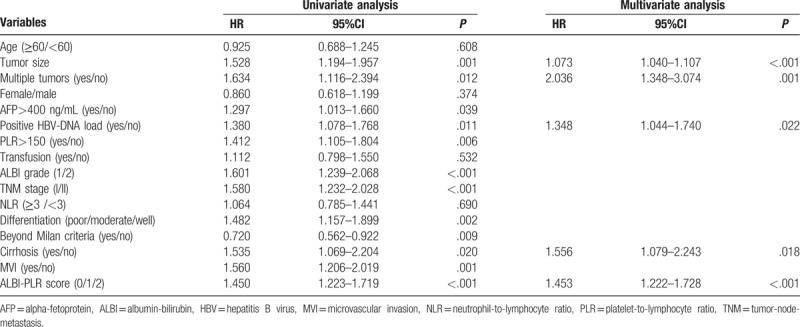
As shown in Table 1, in the univariate analyses, tumor size, multiple tumors, high AFP level, positive HBV-DNA load, differentiation, high preoperative PLR, ALBI grade, presence of MVI, cirrhosis, TNM stage, beyond Milan criteria and ALBI-PLR score were potentially associated with postoperative recurrence. However, in the multivariate analysis, only tumor size (HR = 1.073, 95%CI = 1.040–1.107, P<.001), multiple tumors (HR = 2.036, 95%CI = 1.348–3.074, P = .001), positive HBV-DNA load (HR = 1.348, 95%CI = 1.044–1.740, P = .022), cirrhosis (HR = 1.556, 95%CI = 1.079–2.243, P = .018) and ALBI-PLR score (HR = 1.453, 95%CI = 1.222–1.728, P<.001) were independent risk factors for postoperative recurrence.
Table 1.
Univariate and multivariate analyses of prognostic factors for recurrence-free survival.
3.2. Univariate and multivariate analysis for OS
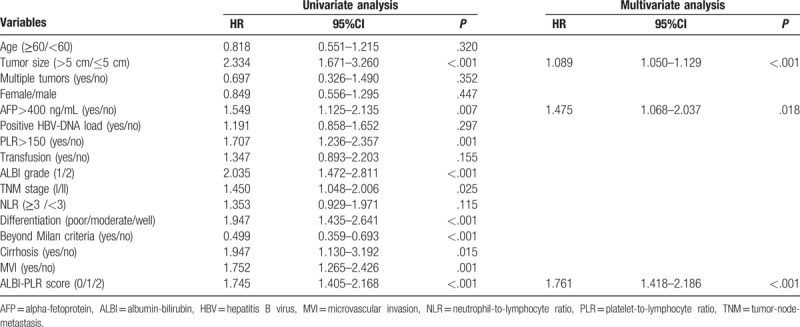
As shown in Table 2, tumor size, high AFP level, high preoperative PLR, ALBI grade, cirrhosis, presence of MVI, TNM stage, beyond Milan criteria, and ALBI-PLR score showed potential predictive ability for OS in the univariate analysis. However, only tumor size (HR = 1.089, 95%CI = 1.050–1.129, P<.001), high preoperative AFP level (HR = 1.475, 95%CI = 1.068–2.037, P = .018) and ALBI-PLR score (HR = 1.761, 95%CI = 1.418–2.186, P<.001) were independently associated with postoperative mortality in the Cox regression.
Table 2.
Univariate and multivariate analyses of prognostic factors for overall survival.
3.3. Comparison of patients with different ALBI-PLR scores
As shown in Figure 2A, the 1-, 3-, 5-year RFS rates were, respectively, 90.4%, 63.7%, and 49.4% for patients with ALBI-PLR score 0; 85.2%, 49.0%, and 28.9% for patients with ALBI-PLR score 1, and 86.8%, 36.4%, and 17.0% for patients with ALBI-PLR score 2. A significant difference was observed (P<.001). As shown in Figure 2B, the 1-, 3-, 5-year OS rates for patients with ALBI-PLR score 0 were, respectively, 97.0%, 78.0%, and 67.8%; for patients with ALBI-PLR score 1 were 92.4%, 68.3%, and 48.8%; and for patients with ALBI-PLR score 2 were 94.1%, 60.9% and 17.8%, respectively (P<.001). We compared the clinicopathological characteristics of patients with different ALBI-PLR scores. Patients with high ALBI-PLR scores had a higher incidence of MVI and TNM stage II (Table 3).
Figure 2.
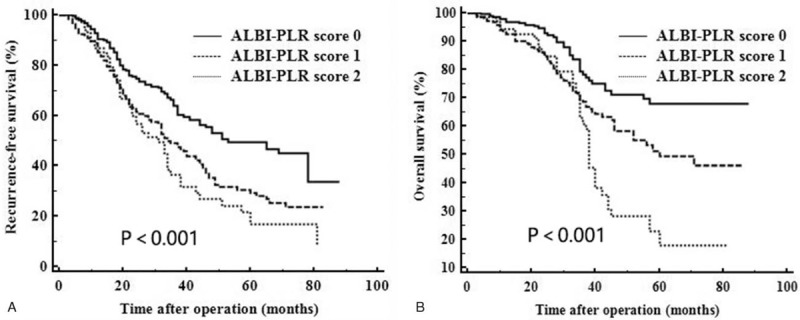
Comparison of recurrence-free (A) and overall (B) survival of patients with different ALBI-PLR scores. ALBI = albumin-bilirubin, PLR = platelet-to-lymphocyte ratio.
Table 3.
Comparison of clinicopathological characteristics of patients with different ALBI-PLR scores.
3.4. Comparison of the predictive ability of ALBI-PLR, ALBI and PLR
We also compared the AUC of ALBI-PLR, ALBI, and PLR. For predicting postoperative recurrence (Fig. 3A), the ALBI-PLR had the highest AUC (0.617), followed by PLR (0.583, PALBI-PLRvs.PLR = .031) and ALBI (0.568, PALBI-PLRvs.ALBI = .006). For predicting postoperative survival (Fig. 3B), the ALBI-PLR also had the highest AUC (0.646), followed by ALBI (0.605, PALBI-PLRvs.ALBI = .035) and PLR (0.594, PALBI-PLRvs.PLR = .004).
Figure 3.
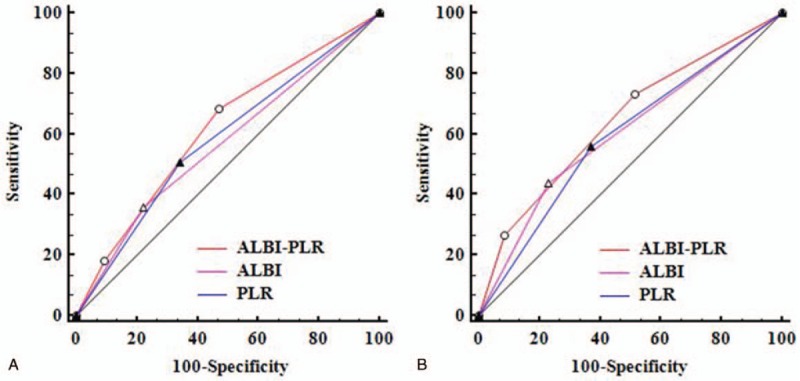
Comparison of the AUC of ALBI-PLR, PLR, and ALBI in predicting postoperative recurrence (A) and survival (B). ALBI = albumin-bilirubin, AUC = area under the receiver operating characteristic curve, PLR = platelet-to-lymphocyte ratio.
4. Discussion
Liver resection is promisingly recommended to patients with BCLC stage 0/A HCC.[19] However, the outcomes are still not satisfactory because of the high recurrence rate after liver resection.[19] In this study, we identified the ALBI-PLR score as a prognostic predictor for patients with HCC following liver resection.
The ALBI grade has been validated as a simple, evidence-based and objective tool to evaluate a patient's liver function.[9,10,20] Compared with the Child–Pugh score, the ALBI grade only incorporates objective factors.[9] Some investigations have suggested that poor liver function, which is assessed by the ALBI grade, is associated with a high incidence of tumor recurrence and poor OS of patients with HCC after liver resection.[7,10,21] Toyoda et al[10] confirmed that the OS of HCC patients with ALBI grade 1 was approximately twofold higher than that of patients with ALBI grade 2 after liver resection. Li et al[22] confirmed that the ALBI grade is superior to the Child–Pugh score in predicting HCC patient outcomes after liver resection. Other studies have also suggested that worse liver function contributes to worse outcomes of patients with HCC after liver resection, although these studies used the Child–Pugh score to evaluate the patients’ liver function. For example, Okajima et al[23] confirmed that both OS and disease-free survival of HCC patients with Child–Pugh scores of 5 were much better than those with Child–Pugh scores of 6, although all patients were in Child–Pugh class A. Another potential explanation for why a high ALBI grade contributed to poor outcomes is the anti-tumor role of albumin. According to the formula of ALBI, a low preoperative albumin is associated with a high ALBI grade. A previous study confirmed that albumin can directly inhibit the growth of HCC via the modulation of AFP or through its actions on growth-controlling kinases.[24] Recently, Carr et al's study indicated that HCC patients with lower serum albumin levels had significantly larger tumor size, more portal vein thrombus, increased tumor multifocality, and higher AFP levels than those with higher albumin levels.[25] Moreover, patients with high ALBI grade have a high risk of postoperative liver failure which could negatively influence patient's prognosis following liver resection.[26] Some investigators suggested laparoscopic liver resection results in less postoperative complications comparable with open surgery.[27] However, whether laparoscopic liver resection could improve patient's outcomes needs a further study.
Recently, many studies have suggested that the systemic inflammation response adversely impacted the prognosis of patients with HCC after liver resection.[15,28] The PLR is a commonly used inflammation based marker. Song et al[29] suggested that an elevated PLR is associated with a high recurrence rate and poor OS for patients with HCC after liver resection. Goh et al[30] confirmed that the PLR is useful for predicting early mortality for patients with huge HCC after liver resection. Recently, a meta-analysis conducted by Zhao et al[28] also revealed that the PLR is an unfavorable indicator of OS in HCC patients. Platelets play crucial roles in the regulation of HCC's progression. Zhang et al[31] indicated that activated platelets could inhibit the differentiation of HCC cells and promote tumor progression through platelet-tumor cell binding. Carr et al[32] suggested that increasing platelet counts are associated with increasing tumor size and portal vein invasion. Platelets could enhance the growth, migration and invasion of HCC cells.[32] He et al[33] confirmed that platelet releasates could accelerate the proliferation of HCC cells via suppressing the expression of KLF6. Morimoto et al[34] even showed that high platelet counts are correlated with extra-hepatic metastasis of patients who underwent noncurative treatments. Some well-designed clinical studies also confirmed antiplatelet treatment could reduce the incidence of recurrence of HCC patients after liver resection.[35,36] Moreover, patients with a high PLR might have high platelet counts and/or low lymphocyte counts. However, lymphocytes play an antitumor role. Patients with low lymphocytes might have an impaired ability of antitumor.
There are some published investigations revealing that either the ALBI grade or the PLR could predict patient outcomes after liver resection.[10,37] Our study suggested that the combination of the ALBI grade and PLR could strengthen the prognostic power. The ALBI grade is used to assess a patient's liver function, whereas the PLR is a marker of systemic inflammation response. We believe the combination of the ALBI grade and PLR could more exactly elucidate a patient's general condition than using the ALBI grade or PLR alone. For example, patients with ALBI grade 1 might have a high PLR. Our study suggested that the outcomes of such patients were poorer than those with ALBI grade 1 and low PLR. Accordingly, using the ALBI-PLR score could better stratify HCC patients. Our study revealed patients with high ALBI-PLR scores had more MVI and advanced TNM stage. Many studies have confirmed that both MVI and advanced TNM stage are independently associated with recurrence and mortality of patients with HCC after liver resection.[38,39] This finding might be a potential explanation for why patients with high ALBI-PLR scores had poor outcomes after liver resection.
There are some limitations of this study. This was a single center retrospective study. Moreover, because HBV infection is the predominant cause of HCC in China, this study only included HBV-related HCC. Whether this conclusion is suitable for other cause-related HCC needs further study.
Our study confirmed that the ALBI-PLR score is a simple and useful prognostic factor for predicting the prognosis of patients with HCC after liver resection. This result suggests that we should pay attention to the patient's liver function and systemic inflammation response.
Author contributions
Li C: Proposed this study; collected and analyzed data.
Zhang XY collected and analyzed data;
Peng W collected data;
Wen TF proposed this study;
Yan LN collected data;
Li B collected data;
Yang JY collected data;
Wang WT collected data;
Xu MQ collected data.
Chen LP proposed this study
Conceptualization: Chuan Li, Li Ping Chen.
Data curation: Chuan Li, Xiao Yun Zhang, Wei Peng.
Formal analysis: Chuan Li.
Funding acquisition: Tian Fu Wen.
Investigation: Chuan Li.
Methodology: Chuan Li, Xiao Yun Zhang, Wei Peng, Tian Fu Wen, Lu Nan Yan, Bo Li, Jia Yin Yang, Wen Tao Wang, Ming Qing Xu.
Resources: Li Ping Chen.
Writing – original draft: Chuan Li.
Writing – review & editing: Li Ping Chen.
Footnotes
Abbreviations: AFP = alpha-fetoprotein, ALBI = albumin-bilirubin, AUC = area under the receiver operating characteristic curve, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, MVI = microvascular invasion, NLR = neutrophil-to-lymphocyte ratio, OS = overall survival, PLR = platelet-to-lymphocyte ratio, RFS = recurrence-free survival, TNM = tumor-node-metastasis.
Funding: This study was supported by grants from Science and Technological Supports Project of Sichuan Province (2016SZ0025 and 2015SZ0049) as well as Health and Family Planning Commission of Sichuan Province (17PJ393).
The authors have no conflicts of interest to disclose.
References
- [1].Hong SK, Lee KW, Kim HS, et al. Living donor liver transplantation for hepatocellular carcinoma in Seoul National University Hepatobiliary. Surg Nutr 2016;5:453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [3].Brown CR, MacLachlan JH, Cowie BC. Addressing the increasing global burden of viral hepatitis hepatobiliary. Surg Nutr 2017;6:274–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cui Y, Jia J. Update on epidemiology of hepatitis B and C in China. J Gastroenterol Hepatol 2013;28suppl 1:7–10. [DOI] [PubMed] [Google Scholar]
- [5].Liu PH, Hsu CY, Hsia CY, et al. Surgical resection versus radiofrequency ablation for single hepatocellular carcinoma </= 2 cm in a propensity. Score Model Ann Surg 2016;263:538–45. [DOI] [PubMed] [Google Scholar]
- [6].Poon RT, Fan ST, Lo CM, et al. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg 2002;235:373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang YY, Zhong JH, Su ZY, et al. Albumin-bilirubin versus Child–Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg 2016;103:725–34. [DOI] [PubMed] [Google Scholar]
- [8].Zhou SJ, Zhang EL, Liang BY, et al. Morphologic severity of cirrhosis determines the extent of liver resection in patients with hepatocellular carcinoma and Child–Pugh grade A cirrhosis. J Surg Res 2016;200:444–51. [DOI] [PubMed] [Google Scholar]
- [9].Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 2015;33:550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Toyoda H, Lai PB, O’Beirne J, et al. Long-term impact of liver function on curative therapy for hepatocellular carcinoma: application of the ALBI grade. Br J Cancer 2016;114:744–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hiraoka A, Kumada T, Nouso K, et al. Proposed new sub-grouping for intermediate-stage hepatocellular carcinoma using albumin-bilirubin grade. Oncology 2016;91:153–61. [DOI] [PubMed] [Google Scholar]
- [12].Pinato DJ, Sharma R, Allara E, et al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J Hepatol 2017;66:338–46. [DOI] [PubMed] [Google Scholar]
- [13].Li X, Han Z, Cheng Z, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of recurrence following thermal ablation for recurrent hepatocellular carcinoma: a retrospective analysis. PLoS One 2014;9:e110546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhou D, Liang J, Xu LI, et al. Derived neutrophil to lymphocyte ratio predicts prognosis for patients with HBV-associated hepatocellular carcinoma following transarterial chemoembolization. Oncol Lett 2016;11:2987–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fu SJ, Ji F, Han M, et al. Prognostic value of combined preoperative fibrinogen and neutrophil-lymphocyte ratio in patients with hepatocellular carcinoma after liver transplantation. Oncotarget 2017;8:4301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Huang GQ, Zhu GQ, Liu YL, et al. Stratified neutrophil-to-lymphocyte ratio accurately predict mortality risk in hepatocellular carcinoma patients following curative liver resection. Oncotarget 2016;7:5429–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li C, Wen TF, Yan LN, et al. Postoperative neutrophil-to-lymphocyte ratio plus platelet-to-lymphocyte ratio predicts the outcomes of hepatocellular carcinoma. J Surg Res 2015;198:73–9. [DOI] [PubMed] [Google Scholar]
- [18].Yamamura K, Sugimoto H, Kanda M, et al. Comparison of inflammation-based prognostic scores as predictors of tumor recurrence in patients with hepatocellular carcinoma after curative resection. J Hepatobiliary Pancreat Sci 2014;21:682–8. [DOI] [PubMed] [Google Scholar]
- [19].Heimbach J, Kulik LM, Finn R, et al. Aasld guidelines for the treatment of hepatocellular carcinoma. Hepatology 2017;67:358–80. [DOI] [PubMed] [Google Scholar]
- [20].Liu PH, Hsu CY, Hsia CY, et al. ALBI and PALBI grade predict survival for HCC across treatment modalities and BCLC stages in the MELD Era. J Gastroenterol Hepatol 2017;32:879–86. [DOI] [PubMed] [Google Scholar]
- [21].Hiraoka A, Kumada T, Michitaka K, et al. Usefulness of albumin-bilirubin grade for evaluation of prognosis of 2584 Japanese patients with hepatocellular carcinoma. J Gastroenterol Hepatol 2016;31:1031–6. [DOI] [PubMed] [Google Scholar]
- [22].Li MX, Zhao H, Bi XY, et al. Prognostic value of the albumin-bilirubin grade in patients with hepatocellular carcinoma: validation in a Chinese cohort. Hepatol Res 2017;47:731–41. [DOI] [PubMed] [Google Scholar]
- [23].Okajima C, Arii S, Tanaka S, et al. Prognostic role of Child–Pugh score 5 and 6 in hepatocellular carcinoma patients who underwent curative hepatic resection. Am J Surg 2015;209:199–205. [DOI] [PubMed] [Google Scholar]
- [24].Bagirsakci E, Sahin E, Atabey N, et al. Role of albumin in growth inhibition in hepatocellular carcinoma. Oncology 2017;93:136–42. [DOI] [PubMed] [Google Scholar]
- [25].Carr BI. Guerra V serum albumin levels in relation to tumor parameters in hepatocellular carcinoma patients. Int J Biol Markers 2017;32:e391–6. [DOI] [PubMed] [Google Scholar]
- [26].Zou H, Yang X, Li QL, et al. A comparative study of albumin-bilirubin score with Child–Pugh score, model for end-stage liver disease score and indocyanine green R15 in predicting posthepatectomy liver failure for hepatocellular carcinoma. Patients Dig Dis 2018;36:236–43. [DOI] [PubMed] [Google Scholar]
- [27].Komatsu S, Brustia R, Goumard C, et al. Laparoscopic versus open major hepatectomy for hepatocellular carcinoma: a matched pair analysis. Surg Endosc 2016;30:1965–74. [DOI] [PubMed] [Google Scholar]
- [28].Zhao Y, Si G, Zhu F, et al. Prognostic role of platelet to lymphocyte ratio in hepatocellular carcinoma: a systematic review and meta-analysis. Oncotarget 2017;8:22854–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Song W, Wang K, Zhong FP, et al. Clinicopathological and prognostic significance of platelet-to-lymphocyte ratio in patients with hepatocellular carcinoma. Oncotarget 2016;7:81830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Goh BK, Kam JH, Lee SY, et al. Significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and prognostic nutrition index as preoperative predictors of early mortality after liver resection for huge (>/=10 cm) hepatocellular carcinoma. J Surg Oncol 2016;113:621–7. [DOI] [PubMed] [Google Scholar]
- [31].Zhang R, Guo H, Xu J, et al. Activated platelets inhibit hepatocellular carcinoma cell differentiation and promote tumor progression via platelet-tumor cell binding. Oncotarget 2016;7:60609–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Carr BI, Cavallini A, D’Alessandro R, et al. Platelet extracts induce growth, migration and invasion in human hepatocellular carcinoma in vitro. BMC Cancer 2014;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].He AD, Xie W, Song W, et al. Platelet releasates promote the proliferation of hepatocellular carcinoma cells by suppressing the expression of KLF6. Sci Rep 2017;7:3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Morimoto Y, Nouso K, Wada N, et al. Involvement of platelets in extrahepatic metastasis of hepatocellular carcinoma. Hepatol Res 2014;44:E353–359. [DOI] [PubMed] [Google Scholar]
- [35].Lee PC, Yeh CM, Hu YW, et al. Antiplatelet therapy is associated with a better prognosis for patients with hepatitis B virus-related hepatocellular carcinoma after liver resection. Ann Surg Oncol 2016;23:874–83. [DOI] [PubMed] [Google Scholar]
- [36].Lee M, Chung GE, Lee JH, et al. Antiplatelet therapy and the risk of hepatocellular carcinoma in chronic hepatitis B patients on antiviral treatment. Hepatology 2017;66:1556–69. [DOI] [PubMed] [Google Scholar]
- [37].Chen Q, Dai Z, Yin D, et al. Negative impact of preoperative platelet-lymphocyte ratio on outcome after hepatic resection for intrahepatic cholangiocarcinoma. Medicine (Baltimore) 2015;94:e574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang K, Zhang H, Xia Y, et al. Surgical options for intrahepatic cholangiocarcinoma hepatobiliary. Surg Nutr 2017;6:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhao H, Hua Y, Lu Z, et al. Prognostic value and preoperative predictors of microvascular invasion in solitary hepatocellular carcinoma </= 5 cm without macrovascular invasion. Oncotarget 2017;8:61203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]


