Abstract
Objective:
Several published articles have investigated the association between the −174 G/C (rs1800795) polymorphism in the interleukin-6 (IL-6) gene and the risk of polycystic ovary syndrome (PCOS). However, the results were inconsistent. In the present study, a meta-analysis was performed to resolve this inconsistency.
Methods:
Eligible studies reporting an association between the IL-6 rs1800795 polymorphism and PCOS susceptibility were included from PubMed, Embase, Web of Science, and the Cochrane Library up to December 1, 2017. The odds ratio (OR) with the 95% confidence interval (CI) was used to calculate the strength of the associations. Publication bias detection was conducted using Begg test. We used STATA 11.0 software to perform the statistical analyses.
Results:
Six articles detailing case-control studies were included, reporting a total of 512 cases and 606 controls. The meta-analysis results indicated that rs1800795 was associated with decreased PCOS susceptibility in the overall population under the allelic model (G vs. C, OR = 0.59, 95% CI: 0.41–0.85, P = .005), the homozygous model (GG vs. CC, OR = 0.45, 95% CI: 0.28–0.73, P = .001), heterozygous model (GG vs. CG, OR = 0.50, 95% CI: 0.26–0.95, P = .036), and the dominant model (GC + CC vs. GG, OR = 0.48, 95% CI: 0.26–0.89, P = .020). However, a threshold P value of .05 was found under the recessive model (CC vs. GG + CG, OR = 0.62, 95% CI: 0.39–1.00).
Conclusions:
This meta-analysis concluded that IL-6 rs1800795 polymorphism has a decreased association with PCOS risk among all populations studied. The results suggested that the IL-6 rs1800795 polymorphism is a protective factor for PCOS susceptibility. Given the limited ethnic groups and sample size, further studies are required to validate the association.
Keywords: interleukin-6, meta-analysis, polycystic ovary syndrome, polymorphism, rs1800795
1. Introduction
Polycystic ovary syndrome (PCOS) is a common and complex gynecological endocrine disorder that affects approximately 6% to 10% of women of their reproductive age.[1,2] PCOS is characterized by oligoanovulation or anovulation, hyperandrogenismhyper and insulinemia, menstrual irregularity, and consequent subfertility.[3] PCOS is also associated with dyslipidemia, atherosclerosis, obesity, hirsutism, insulin resistance, and an increased incidence of type 2 diabetes mellitus.[4–6] Recent studies reported that 15% to 55% of the PCOS patients were diagnosed as having nonalcoholic fatty liver disease (NAFLD). In addition, 32.7% to 44.6% of patients with PCOS suffer from metabolic syndrome. Metabolic syndrome occurs with high prevalence in PCOS patients complicated with NAFLD.[7,8] The exact etiological mechanism of PCOS is still under debate; however, there is evidence that genetic factors play a vital role.[9,10]
Interleukin 6 (IL-6), an important proinflammatory and immunomodulatory pleiotropic cytokine, plays an important role in reproductive physiology. IL-6 is involved in various human diseases and pathophysiological processes, such as the development of atherosclerosis, ovarian steroid production, fertilization and implantation, coronary heart disease, osteoporosis, and allergic reactions.[11–15] The IL-6 gene is located at chromosome 7p21–24 locus with a 303 bp promoter.[16] A common single-nucleotide polymorphism (SNP), which results in variation of G>C −174 in the promoter region of IL-6, has been proven to alter the gene's transcription rate.[17] Studies involving the relationship between IL–6 –174 G/C and PCOS susceptibility have been performed.[18–26] However, the results remain inconclusive and controversial, probably because of the varied populations and limited sample sizes. Therefore, we employed a meta-analysis to investigate the relationship between IL–6 –174 G/C (rs1800795) and PCOS susceptibility.
2. Materials and methods
2.1. Search strategy
The meta-analysis was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.[27] A comprehensive strategy was carried out to search for potential publications concerning the association between IL-6 –174 G/C (rs1800795) and PCOS susceptibility in the following databases: Web of Science, Embase, PubMed, and the Cochrane Library, from inception to December 1, 2017. The search terms included “interleukin-6,” OR “IL-6,” OR “IL6,” AND “Polycystic ovary syndrome,” OR “PCOS,” AND“polymorphism,” OR “mutations,” OR “single nucleotide polymorphism,” OR “SNP,” OR “variant,” OR “genotype,” OR “Alleles,” without any limitation imposed. Publications listed in the references were also hand-searched carefully to obtain potentially relevant articles. Disagreements concerning the inclusion of a particular study were resolved by consensus. The study protocol was approved by the ethics committee of the Hangzhou Women's Hospital.
2.2. Inclusion and exclusion criteria
Studies were included if they met all the following criteria: research designed as a case-control study with healthy populations as controls and patients with PCOS cases; studies assessing the relationship between the IL-6 –174 G/C (rs1800795) polymorphism and PCOS susceptibility; availability of data regarding the total number of cases and controls, number of cases and controls with G/G, G/C, and C/C genotypes; and at least 2 comparison groups (cancer group and control group). Studies were excluded if: the studies had no control group, or were case reports, review articles, or comments; the studies did not disclose the raw data regarding the IL-6 –174 G/C (rs1800795) polymorphism; and animal studies.
2.3. Data extraction
Two experienced investigators independently extracted the data according to a preset data extraction form. The following information was extracted: first author's surname; country in which the study was performed; publication year; nation or ethnicity; genotyping method; total number in the case and control groups, as well as the number of cases and controls with G/G, G/C, and C/C genotypes; and the P value used to calculate the Hardy-Weinberg equilibrium (HWE).
2.4. Quality assessment
Two authors independently performed a quality assessment of each study based on the 9-star Newcastle-Ottawa Scale (NOS),[28] which is a widely used rating system to assessing the quality of observational studies in a meta-analysis. The NOS involves 3 broad aspects: case and control selection, comparability, and exposure. Each aspect consists of 4, 2, and 3 items respectively. This scale contains 9 items and each item scores a value of 1. An NOS score of 6 stars or above would indicate a high-quality study.
2.5. Statistical analysis
The association strength between IL-6 –174 G/C (rs1800795) polymorphism and PCOS susceptibility was assessed by calculating the pooled odds ratio (OR) along with the 95% confidence interval (CI). The pooled ORs were measured using the Z test. The HWE for each SNP was tested using the χ2 test. The ORs were combined using either the random-effects model or the fixed-effects model, as previously described.[29,30] A P value <.05 was regarded as a statistically significant difference. Heterogeneity across the studies was determined using the Q test and the I2 statistical tests, with I2 < 50% indicating little difference. To explore the influence of a single study on the overall results, sensitivity analysis was also performed to test the stability of the results under all genetic models. We used Begg funnel plot test to assess possible publication bias, with P < .05 being considered to present statistical significance. All statistical analyses were conducted with STATA 11.0 software (StataCorp, College Station, TX).
3. Results
3.1. Characteristics of the included studies
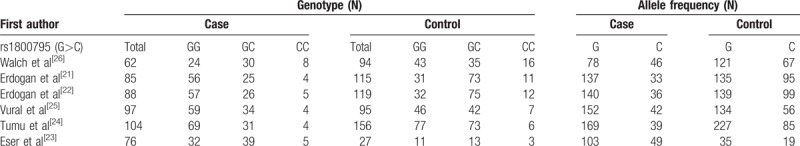
The initial search of electronic databases yielded 47 references. The flow chart of the study selection process is shown in Figure 1. Finally, 6 original articles were identified that met the inclusion criteria for the meta-analysis. The detailed characteristics of the 6 included studies are shown in Table 1.[21–26] Four studies were performed in Turkey,[21–23,25] one in Austria,[26] and another in India.[24] The frequencies of each genotype and allele, with their HWE values, are presented in Table 2. The analyzed SNP was within the HWE in 3 studies,[23,25,26] whereas in the other 3 studies, the polymorphism was not consistent with the HWE.[21,22,24] The NOS results indicated that the studies had scores ranging from 6 to 8, with an average of 6.33, which indicated that the methodological quality of the 6 selected studies was generally reliable (Table 1).
Figure 1.
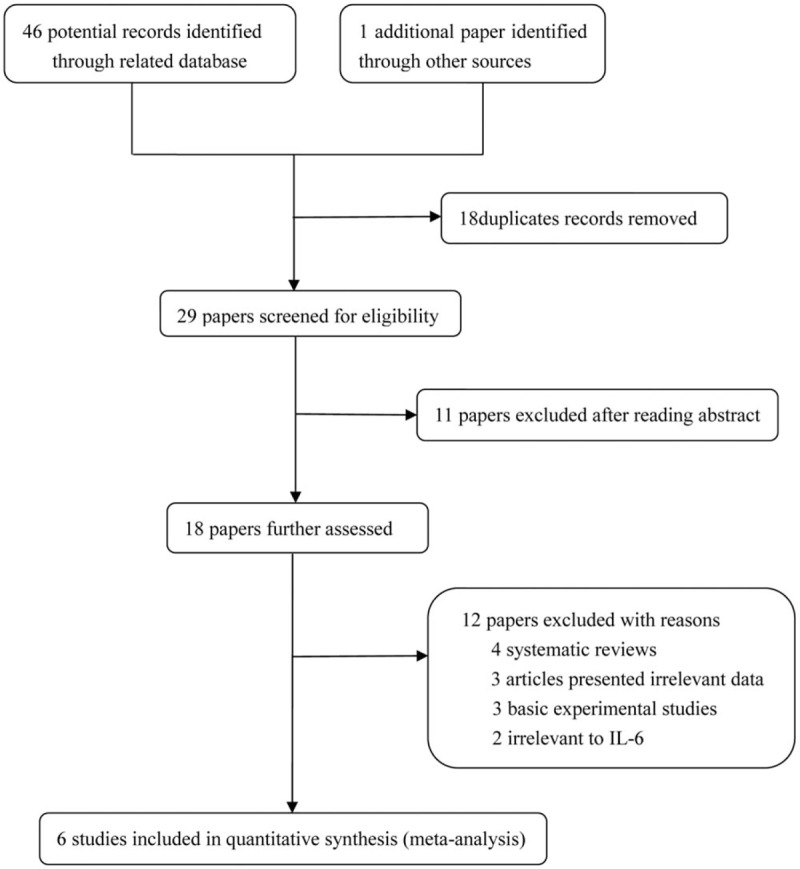
The detailed procedures for the literature search.
Table 1.
Characteristics of the studies included in the meta-analysis.

Table 2.
Polymorphisms genotype distribution and allele frequency in cases and controls.
3.2. Meta-analysis results
The 6 studies involved a total of 512 patients with PCOS and 606 controls. Overall, there was a decreased association between rs1800795 and PCOS susceptibility in the overall population under the allelic model (G vs. C, OR = 0.59, 95% CI: 0.41–0.85, P = .005), the homozygous model (GG vs. CC, OR = 0.45, 95% CI: 0.28–0.73, P = .001), the heterozygous model (GG vs. CG, OR = 0.50, 95% CI: 0.26–0.95, P = .036), and the dominant model (GC + CC vs. GG, OR = 0.48, 95% CI: 0.26–0.89, P = .020). However, a threshold P value of .05 was found under the recessive model (CC vs. GG + CG, OR = 0.62, 95% CI: 0.39–1.00). The main results of the meta-analysis were listed in Table 3. The forest plot of the pooled ORs of the association of rs1800795 with PCOS susceptibility under the homozygous model is shown in Figure 2.
Table 3.
Meta-analysis of the association between IL-6 rs1800795 polymorphism and PCOS susceptibility.
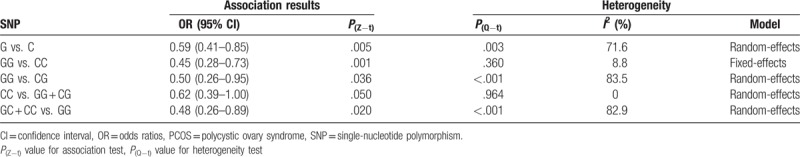
Figure 2.
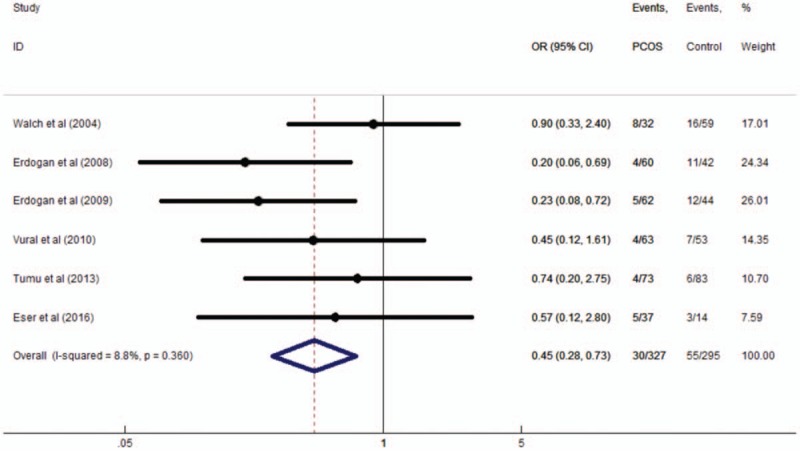
The forest plot of pooled odds ratios of the association of IL-6 rs1800795 polymorphism with polycystic ovary syndrome susceptibility under the homozygous model.
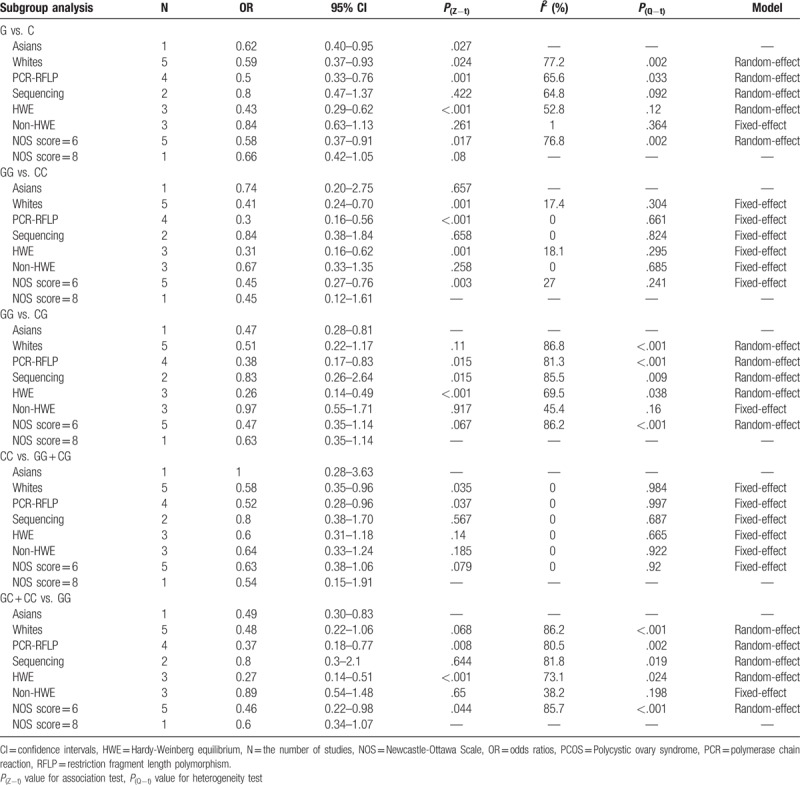
3.3. Subgroup analysis
Subgroup analysis was available for the genotyping method, ethnicity, NOS score, and whether the selected studies conformed to the HWE. The results of subgroup analysis of the association of rs1800795 and PCOS susceptibility under the 5 models are shown in Table 4. However, some findings should be interpreted with caution because of the limited studies in certain subgroup.
Table 4.
Subgroup analysis of the association of rs1800795 polymorphism and PCOS susceptibility under 5 models.
3.4. Sensitivity analysis
Sequential omission of each single study was used to perform sensitivity analysis in the 5 models. The pooled ORs and 95% CIs showed no materially differences, indicating that the pooled results of the meta-analysis were robust and reliable. Sensitivity analysis of the association between rs1800795 and PCOS susceptibility under the recessive model is shown Figure 3.
Figure 3.
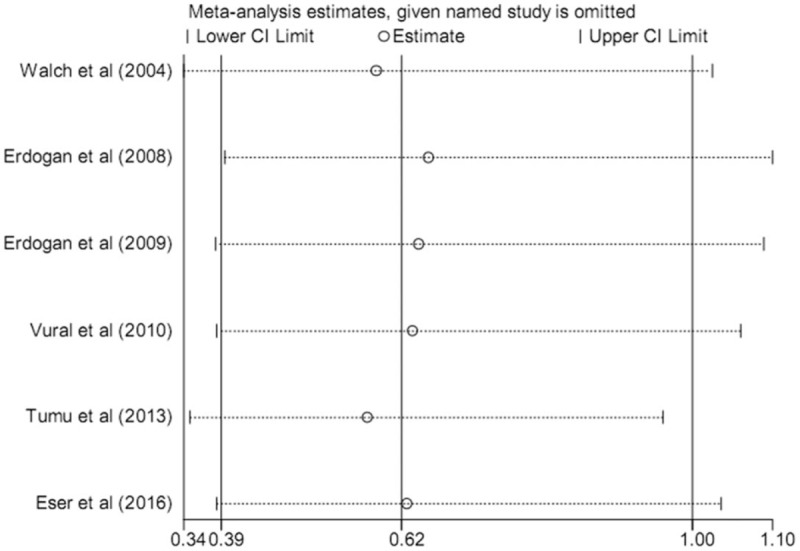
Sensitivity analysis of the association between IL-6 rs1800795 polymorphism and the risk of polycystic ovary syndrome under the recessive model.
3.5. Publication bias
We estimated the potential publication bias using a Begg funnel plot test. No obvious publication bias was found for the association between rs1800795 and PCOS susceptibility under any models. Publication bias under the dominant model suggested that the result is statistically robust (P = .531, Fig. 4).
Figure 4.
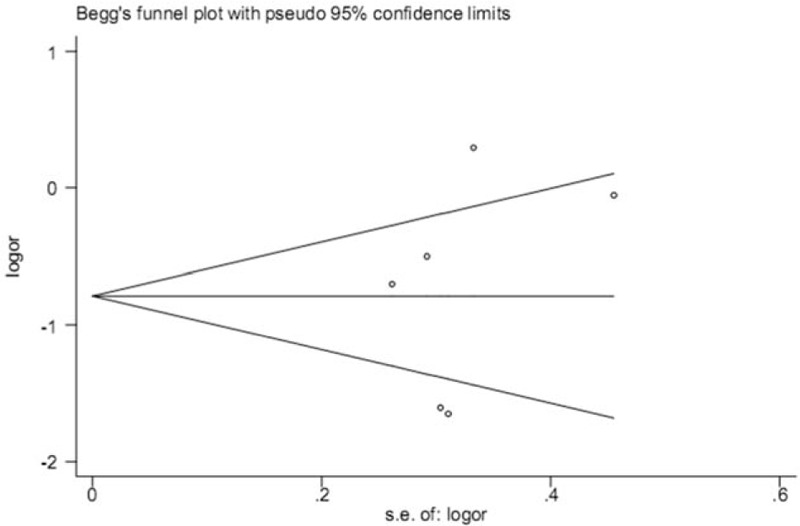
Publication bias detection between IL-6 rs1800795 polymorphism and polycystic ovary syndrome susceptibility under the dominant model.
4. Discussion
PCOS is a common endocrine disorder disease involving multiple genetic and epigenetic alterations. Cytokine genes polymorphisms may play an important role in the etiology of PCOS.[25] As a common multifunctional cytokines, IL-6, has been proven to influence the processes of fertilization, implantation, and ovulation, which are also affected in women with PCOS.[31] Many genetic studies have been performed to investigate the associations between IL−6 rs1800795 polymorphism and PCOS risk; however, the results were inconclusive.[21–26]
A single study lacks sufficient statistical power to confirm the relationship between rs1800795 and the risk of PCOS because of the small sample size. To explore the uncertain association between these 2 entities, we performed a meta-analysis in a large sample size (512 patients with PCOS and 606 controls) to verify the associations between PCOS susceptibility and the IL-6 −174 G/C (rs1800795) functional polymorphism. The results demonstrated that IL-6 rs1800795 was associated with a decreased risk of PCOS among all populations studied. The results suggested that the IL-6 rs1800795 polymorphism is a protective factor for PCOS susceptibility.
A previous meta-analysis with only 4 studies, containing 351 cases and 464control participants, failed to confirm an association between these 2 entities because of the small sample size.[20] In addition, they failed to show a correlation between IL-6 rs1800795 polymorphism and PCOS susceptibility in subgroup analysis among the studies that conformed to the HWE under the allele model, recessive model, and dominant model. However, in our study, we found a statistically significant correlation among studies that conformed to the HWE under the allele model and dominant model. Another meta-analysis found positive associations between the IL-6 rs1800795 polymorphisms and the risk of PCOS under the allelic model, homozygous model, heterozygous model, and dominant model; however, the results were inaccurate because some original data were wrong when they were extracted from the included studies.[19] For example, the number of G/C genotypes in healthy women was 35, whereas in the study mentioned above, the corresponding number was 25.[19] The number of C/C genotypes in patients with PCOS was 56, whereas in the study mentioned above, the number was 36.[19] We believed that these mistakes made the results unreliable. Considering the relative small sample size, no definite consensus could be reached. The objective of the present study was to present accurate evidence for the association between IL−6 rs1800795 polymorphisms and PCOS risk. The results suggested that IL-6 polymorphism rs1800795 likely has a protective effect against PCOS, even though the P value under the recessive model was marginal. One possible reason for this association is that the C allele results in the reduction of IL-6 production, which helps to normalize ovarian function.[18]
However, some limitations should be considered in interpreting the results. First, only 6 studies were included in our meta-analysis, which limited further analyses because certain original data were unavailable. Second, because of the limited studies in certain subgroups, some conclusions should be interpreted with caution in the subgroup analysis. In addition, we were unable to adjust for potential confounding effects such as environmental factors, lifestyle, sex, medication consumption, and other exposure factors because of the limited data. Third, 3 studies did not conform to the HWE, even though the combined ORs were not materially affected in the sensitivity analysis. Furthermore, Begg funnel plot showed no potential publication bias; however, 3 of the 6 studies are outliers. Deviation from an asymmetric distribution of the funnel plot can still indicate publication bias. We failed to verify the results at the level of molecular mechanism. Data from large sample size multicenter epidemiological studies are still needed to clarify the associations.
5. Conclusion
In summary, our meta-analysis based on 6 case-control studies concluded that IL-6 −174G/C (rs1800795) polymorphism was associated with PCOS susceptibility. The rs1800795 polymorphism is a protective factor for PCOS susceptibility. Given the limited number of ethnic groups and sample size, more evidence from larger epidemiological studies is needed to validate these results.
Author contributions
Linjie Chen designed this study; Zhifen Zhang and Jian Huang collected and collated data; Minjuan Jin and Zhifen Zhang extracted and confirmed the data; Linjie Chen and Minjuan Jin analyzed data; Linjie Chen wrote the manuscript; Zhifen Zhang and Minjuan Jin edited the manuscript.
Author contributions
Conceptualization: Linjie Chen, Minjuan Jin.
Formal analysis: Zhifen Zhang, Minjuan Jin.
Investigation: Zhifen Zhang.
Resources: Jian Huang.
Software: Zhifen Zhang, Jian Huang.
Supervision: Jian Huang.
Validation: Jian Huang.
Writing – original draft: Linjie Chen, Minjuan Jin.
Writing – review & editing: Zhifen Zhang, Minjuan Jin.
Footnotes
Abbreviations: CI = confidence interval, HWE = Hardy-Weinberg equilibrium, IL-6 = interleukin-6, NOS = Newcastle-Ottawa Scale, OR = Odds ratio, PCOS = polycystic ovary syndrome, SNP = single-nucleotide polymorphism.
This work was supported by Hangzhou science and technology bureau project (No: 20170533B56); National health and family planning commission scientific research fund and Zhejiang medical technology project (No: WKj 2013-2-024); Zhejiang science and technology department program (No: 2014C03044-1).
The authors report no conflicts of interests.
References
- [1].Azziz R, Woods KS, Reyna R, et al. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 2004;89:2745–9. [DOI] [PubMed] [Google Scholar]
- [2].Asuncion M, Calvo RM, San Millan JL, et al. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab 2000;85:2434–8. [DOI] [PubMed] [Google Scholar]
- [3].Zacur HA. Polycystic ovary syndrome, hyperandrogenism, and insulin resistance. Obstet Gynecol Clin North Am 2001;28:21–33. [DOI] [PubMed] [Google Scholar]
- [4].Mukherjee S, Maitra A. Molecular & genetic factors contributing to insulin resistance in polycystic ovary syndrome. Indian J Med Res 2010;131:743–60. [PubMed] [Google Scholar]
- [5].Conn JJ, Jacobs HS, Conway GS. The prevalence of polycystic ovaries in women with type 2 diabetes mellitus. Clin Endocrinol 2000;52:81–6. [DOI] [PubMed] [Google Scholar]
- [6].Kandaraki E, Christakou C, Diamanti-Kandarakis E. Metabolic syndrome and polycystic ovary syndrome... and vice versa. Arq Bras Endocrinol Metabol 2009;53:227–37. [DOI] [PubMed] [Google Scholar]
- [7].Romanowski MD, Parolin MB, Freitas AC, et al. Prevalence of non-alcoholic fatty liver disease in women with polycystic ovary syndrome and its correlation with metabolic syndrome. Arq Gastroenterol 2015;52:117–23. [DOI] [PubMed] [Google Scholar]
- [8].Kelley CE, Brown AJ, Diehl AM, et al. Review of nonalcoholic fatty liver disease in women with polycystic ovary syndrome. World J Gastroenterol 2014;20:14172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Franks S, Gharani N, Waterworth D, et al. The genetic basis of polycystic ovary syndrome. Hum Reprod (Oxford, England) 1997;12:2641–8. [DOI] [PubMed] [Google Scholar]
- [10].Taskin MI, Eser B, Adali E, et al. NUCB2 gene polymorphism and its relationship with nesfatin-1 levels in polycystic ovary syndrome. Gynecol Endocrinol 2016;32:46–50. [DOI] [PubMed] [Google Scholar]
- [11].Fernandez-Real JM, Vayreda M, Richart C, et al. Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women. J Clin Endocrinol Metab 2001;86:1154–9. [DOI] [PubMed] [Google Scholar]
- [12].Stephens JW, Humphries SE. The molecular genetics of cardiovascular disease: clinical implications. J Intern Med 2003;253:120–7. [DOI] [PubMed] [Google Scholar]
- [13].Sukhikh GT, Vanko LV. Interrelationships between immune and reproductive systems in human. Russ J Immunol 1999;4:312–4. [PubMed] [Google Scholar]
- [14].Westphal GA, Schnuch A, Moessner R, et al. Cytokine gene polymorphisms in allergic contact dermatitis. Contact dermatitis 2003;48:93–8. [DOI] [PubMed] [Google Scholar]
- [15].Ferrari SL, Ahn-Luong L, Garnero P, et al. Two promoter polymorphisms regulating interleukin-6 gene expression are associated with circulating levels of C-reactive protein and markers of bone resorption in postmenopausal women. J Clin Endocrinol Metab 2003;88:255–9. [DOI] [PubMed] [Google Scholar]
- [16].Motro B, Itin A, Sachs L, et al. Pattern of interleukin 6 gene expression in vivo suggests a role for this cytokine in angiogenesis. Proc Natl Acad Sci U S A 1990;87:3092–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fishman D, Faulds G, Jeffery R, et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest 1998;102:1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Guo R, Zheng Y, Yang J, et al. Association of TNF-alpha, IL-6 and IL-1beta gene polymorphisms with polycystic ovary syndrome: a meta-analysis. BMC Genet 2015;16:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wu H, Yu K, Yang Z. Associations between TNF-alpha and interleukin gene polymorphisms with polycystic ovary syndrome risk: a systematic review and meta-analysis. J Assist Reprod Genet 2015;32:625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang Q, Tong X, Ji Y, et al. Meta-analysis of the correlation between IL-6-174 G/C polymorphism and polycystic ovarian syndrome. J Obstet Gynaecol Res 2015;41:1087–92. [DOI] [PubMed] [Google Scholar]
- [21].Erdogan M, Karadeniz M, Berdeli A, et al. The relationship of the interleukin-6-174 G>C gene polymorphism with oxidative stress markers in Turkish polycystic ovary syndrome patients. J Endocrinol Invest 2008;31:624–9. [DOI] [PubMed] [Google Scholar]
- [22].Erdogan M, Karadeniz M, Berdeli A, et al. The relationship of the interleukin-6-174 G>C gene polymorphism with cardiovascular risk factors in Turkish polycystic ovary syndrome patients. Int J Immunogenet 2009;36:283–8. [DOI] [PubMed] [Google Scholar]
- [23].Eser B, Islimye Taskin M, Hismiogullari AA, et al. The effects of IL-1A and IL-6 genes polymorphisms on gene expressions, hormonal and biochemical parameters in polycystic ovary syndrome. J Obstet Gynaecol 2017;37:358–62. [DOI] [PubMed] [Google Scholar]
- [24].Tumu VR, Govatati S, Guruvaiah P, et al. An interleukin-6 gene promoter polymorphism is associated with polycystic ovary syndrome in South Indian women. J Assist Reprod Genet 2013;30:1541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vural P, Degirmencioglu S, Saral NY, et al. Tumor necrosis factor alpha (-308), interleukin-6 (-174) and interleukin-10 (-1082) gene polymorphisms in polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 2010;150:61–5. [DOI] [PubMed] [Google Scholar]
- [26].Walch K, Grimm C, Zeillinger R, et al. A common interleukin-6 gene promoter polymorphism influences the clinical characteristics of women with polycystic ovary syndrome. Fertil Steril 2004;81:1638–41. [DOI] [PubMed] [Google Scholar]
- [27].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical research ed) 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [29].DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trial 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [30].Chen S, Zhao E, Li W, et al. Association between dipeptidyl peptidase-4 inhibitor drugs and risk of acute pancreatitis: a meta-analysis. Medicine 2017;96:e8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gonzalez F. Inflammation in polycystic ovary syndrome: underpinning of insulin resistance and ovarian dysfunction. Steroids 2012;77:300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


