Supplemental Digital Content is available in the text
Keywords: cancer, infection, methodology, transplantation death causes classification
Abstract
Correct classification of death causes is an important component of transplant trials.
We aimed to develop and validate a system to classify causes of death in hematopoietic stem cell (HSCT) and solid organ (SOT) transplant recipients.
Case record forms (CRF) of fatal cases were completed, including investigator-designated cause of death. Deaths occurring in 2010 to 2013 were used for derivation; and were validated by deaths occurring in 2013 to 2015. Underlying cause of death (referred to as recorded underlying cause) was determined through a central adjudication process involving 2 external reviewers, and subsequently compared with the Danish National Death Cause Registry.
Three hundred eighty-eight recipients died 2010 to 2015 (196 [51%] SOT and 192 [49%] HSCT). The main recorded underlying causes of death among SOT and HSCT were classified as cancer (20%, 48%), graft rejection/failure/graft-versus-host-disease (35%, 28%), and infections (20%, 11%). Kappa between the investigator-designated and the recorded underlying cause of death was 0.74 (95% CI 0.69–0.80) in derivation and comparable in the validation cohort. Death causes were concordant with the Danish National Death Cause Registry in 37.2% (95% CI 31.5–42.9) and 38.4% (95% CI 28.8–48.0) in the derivation and validation cohorts, respectively.
We developed and validated a method to systematically and reliably classify the underlying cause of death among transplant recipients. There was a high degree of discordance between this classification and that in the Danish National Death Cause Registry.
1. Introduction
During the past few decades, death following transplantation has decreased markedly. This is mainly due to better graft preservation, progress in surgical treatment, enhanced immunosuppressive regimens, and introduction of new preventive strategies towards infections. However, despite decreasing mortality following transplantation, the death rates of transplant recipients still exceed those observed in the age-matched general population.[1] This is mainly due to considerable comorbidities such as cardiovascular disease,[1–3] opportunistic infections due to immunosuppression,[2,4,5] or increased risk of de novo and secondary cancer following transplantation. While infections are important, they lead to death in substantially fewer cases than in the past.[6] This improvement in management strategies has allowed for more intensive and effective immunosuppressive treatment and had a major positive impact on the short-term mortality after transplantation.[7]
Correct classification of underlying causes of death is an important component of conducting research aimed to improve quality of care in transplant medicine. Furthermore, patterns of underlying causes of deaths may change in a field with continuing introduction of novel drugs with uncertain long term efficiency.[8] Hence, temporal surveillance of the patterns of underlying causes of deaths is required to detect potential emerging challenges in this vulnerable patient population. The merits of a uniform classification system, applicable to different countries, clinical settings, and irrespective of treatment protocols and place of death appear to be clear. However, such a classification system does not exist currently.
We therefore aimed to develop and validate a system to classify underlying causes of death in hematopoietic stem cell (HSCT) and solid organ (SOT) transplant recipients. Furthermore, we sought to evaluate if there were any specific characteristics that can facilitate the determination of the cause of death.
2. Materials and methods
2.1. Patients
All the included patients of this study were registered in the Management of Post-Transplant Infections in Collaborating Hospitals (MATCH) cohort.[9] The MATCH program was introduced at Rigshospitalet, a large tertiary transplant center in Copenhagen, Denmark in 2011, with the aim to reduce the risk of severe viral diseases in transplant recipients. MATCH constitutes a platform for collaboration between the transplantation units and the Department of Infectious Diseases, and the associated database contains data on a large cohort of consecutive transplant recipients of solid organ and hematopoietic stem cell transplantation. All recipients transplanted with a liver or lung transplants in all of Denmark since 2004 were enrolled into MATCH and furthermore all heart, kidney, and hematopoietic stem cell transplantation in the eastern region of Denmark were enrolled. Eligible patients consisted of children or adults who had received a solid organ or hematopoietic stem cell transplantation between Jan 1st, 2004 and Dec 31st, 2014, and who had died between Jan 1st, 2010 and Dec 12th, 2015. As the electronic medical system at our hospital was introduced in 2010, medical records prior to 2010 were either not included in the electronic medical system or were less complete. We, therefore, excluded patients who died prior to 2010.
2.2. Derivation of the classification systems
An expert panel consisting of specialists within the transplant field was convened to establish a consensus-based classification system. All specialists were specialized doctors and/or professors within the different included transplant fields. Based on contribution from the participants a standardized case record form (CRF) (Supplemental digital content 1), an online review form (Supplemental digital content 2), a list with pre-defined categories of death (Supplemental digital content 3), and an algorithm for defining the cause of death was proposed (Supplemental digital content 3).
The purpose of the classification system was to attribute 1 immediate, up to 5 contributing and 1 underlying cause of death to the specific pre-defined categories (14 categories in total) and to provide a certainty level to each cause of death; definite indicated a certainty of 95% to 100%; likely 80% to 95%; and possible 50% to 80%. The degrees of certainty were determined at the discretion of the external reviewers and were based on to which extent available objective parameters (such as documentation with biopsies or imaging) could prove the cause of death.
Once the CRF and classification system was drafted, a panel consisting of 2 transplant specialists reviewed 5 randomly selected cases to refine the system further.
2.3. Assessment process
After the classification system was developed, hospital medical records were retrospectively reviewed by trained investigators with medical backgrounds, for example, non-specialized physicians and research associates, to extract clinical information onto the standardized CRF. The investigators also proposed the cause of death in the CRF (investigator-designated cause of death). The CRFs then went through an assessment process as illustrated in Fig. 1. The CRF's were divided among 7 external reviewer teams. The teams consisted of 1 physician specialized within the patients’ transplant type and another specialized within another field. The 2 reviewers in each team then reviewed the CRF and independently determined the cause of death. In case of disagreement regarding the recorded underlying cause of death, an agreement was sought through an independent discussion between 2 reviewers blinded to each other. If the disagreement persisted, the underlying cause of death was determined by an expert panel consisting of 1 Professor of Internal Medicine and 1 Professor of Surgery.
Figure 1.
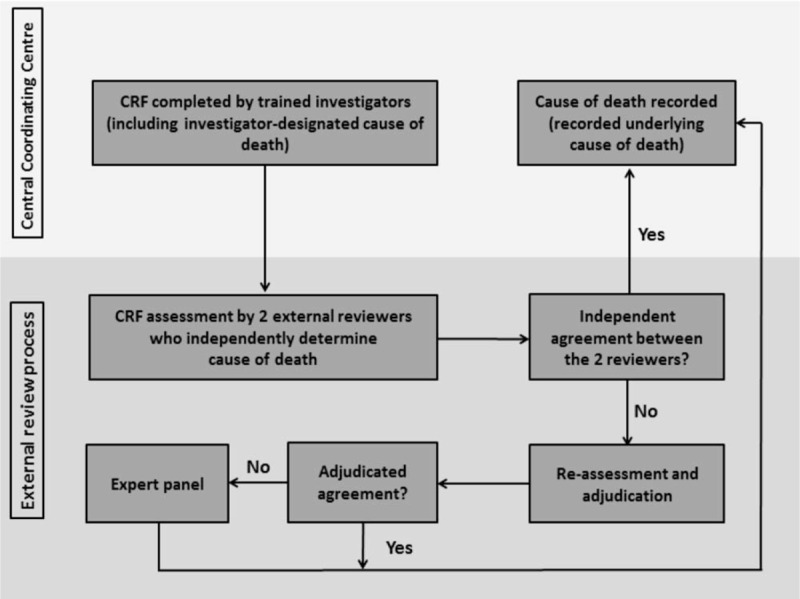
Assessment process of the CLASS project. Case record forms (CRF) were completed by trained investigators at the Central Coordinating Center. CRFs include clinical information around the time of death. Furthermore, the investigators proposed a cause of death (investigator-designated cause of death). The completed CRF's were then reviewed by 2 external reviewers who were blinded to each other and who each independently determined cause of death using the cause of death algorithm (Supplemental digital content 3). The 2 external reviewers consisted of 1 specialist within the transplant field and 1 within another field, including infectious disease specialists. Where the 2 reviewers agreed on the underlying cause of death (independent agreement), the case would be finalized and underlying cause of death recorded (recorded underlying cause of death). In case of disagreement, the underlying cause of death were resolved by an online adjudication process where the reviewers were able to see each other's recordings and correspond anonymously (Adjudicated agreement). Where the 2 external reviewers could not reach consensus on the underlying cause of death, the case would be discussed by 2 other randomly selected experts to determine the underlying cause of death.
2.4. Validation of the derived classification system
The cohort was divided in 2 groups; the classification system was derived from patients who had died between Jan 1st, 2010 and Oct 31st, 2013, and validated on those who died between Nov 1st, 2013 and Dec 12th, 2015.
2.5. Comparison of cause of death with the Danish National Death Cause Registry (DNDCR)
The recorded underlying cause of death of patients in both the derivation and validation cohorts were compared with the reported cause of listed in the Danish National Death Cause Registry. The DNDCR retrieves information from death certificates and codes cause of death according to International Classification of Diseases 10 (ICD-10).[10] For comparison, the 14 specific categories included in our classification algorithm were assigned a corresponding ICD-10 (Supplemental digital content 4).
2.6. Statistics
Agreement between the investigator-designated and recorded underlying causes of death, as well as the agreement between the 2 external reviewers was compared. Proportion of agreement was calculated and inter-rater agreement was assessed using Cohen Kappa statistics. Strength of agreement was defined as slight (0.00–0.20), fair (0.21–0.40), moderate (0.41–0.60), substantial (0.61–0.80), or almost perfect (0.81–1.00).[11]
Univariate logistics regression models were used to identify characteristics (information retrieved from the CRF) associated with agreement of the underlying cause of death. Agreement was assessed between the investigator-designated and recorded underlying cause of death (decided by the experts after adjudication) and independent agreement (before adjudication) between the 2 external reviewers. A multivariable model was constructed based on variables with P < .1 in univariate analyses. The P value of <.05 was considered as significant difference.
Characteristics independently associated with agreement between the investigator-designated and recorded underlying cause of death identified in the multivariate logistic regression model with a P-value <.1, were subsequently evaluated in different combinations by Cohen Kappa statistics in order to determine specific patterns of characteristics that led to good agreement of underlying cause of death. These patterns were identified based on the derivation data, prior to analysis of the validation data. Following the identification of these specific patterns in the derivation cohort, their reproducibility was subsequently tested in the validation cohort.
All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) version 22 (IBM, New York, NY).
2.7. Approvals
The research is conducted after approval of the National Data Protection Agency (2012-58-0004, RH-2015-67, with I-Suite number: 03787).
3. Results
3.1. Patient characteristics and recorded underlying cause of death
A total of 388 patients died between Jan 1st, 2010 and Dec 12th, 2015; of these, 286 (74%) occurred in the derivation and 102 (26%) in the validation cohort. The 2 cohorts were similar in terms of baseline characteristics (Table 1). A slightly higher proportion of recipients had a concomitant infection at time of death in the derivation cohort, compared with the validation cohort (74% vs 68%, P = .05). Conversely, the proportion with prior cerebrovascular disease and ABO compatibility (e.g A, B, O or AB blood groups compatibility) compatibility was higher in the validation cohort (12% vs 5%, P = .02 and 22% vs 5%, P < .001, respectively).
Table 1.
Characteristics of patients at time of death in the derivation and validation cohorts.
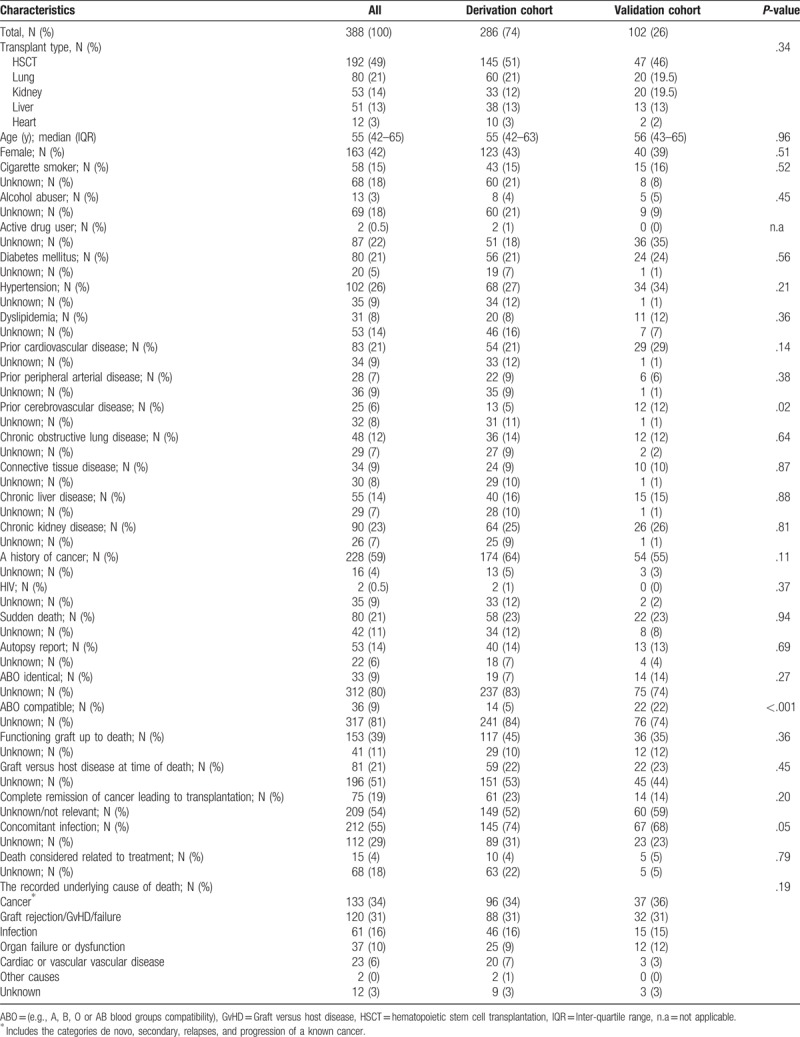
Overall, the median time from transplantation to death was 1.3 years (inter-quartile range [IQR] 0.5–3.5). However, this varied significantly between the different types of transplantation; from 40.2 (20.7–69.5) months among kidney recipients to 9.1 months (1.2–36.8) among liver recipients. The median age at death was 55 years (IQR 42–63) and 58% were men.
Almost all cases were recorded with a specific code from the list with pre-defined categories of death (Supplemental digital content 3). The 3 leading recorded underlying causes of death of the derivation and validation cohorts were cancer (34% vs 36%), graft versus host disease/graft rejection/failure (31% vs 31%), and infections (16% vs 15%). Twelve cases were recorded as “Unknown,” 1 case as “Accident” and 1 case as “Other causes.” There were no differences in the recorded underlying cause of death comparing the derivation and validation cohorts (P = .19) (Table 1). Recorded underlying cause of death according to transplant type is illustrated in supplemental digital content 5.
3.2. Comparison of investigator-designated and recorded underlying cause of death
In the derivation cohort, there was agreement between the investigator-designated and the recorded underlying cause of death in 221/286 (77%) (κ = 0.74 [95% Confidence Interval (CI) 0.69 – 0.80]). Furthermore, the corresponding numbers in the validation cohort were comparable (80/102 [78%] [κ = 0.75 [0.66–0.84]]). Best agreement was seen in the derivation cohort amongst recipients of HSCT, lung, and kidney transplantation (83%, 82%, and 79%, respectively), compared with liver and heart transplants (53% and 50%, respectively). This distribution was generally similar in the validation cohort for all transplant types except liver transplants (79%, 75%, 85%, 77%, and 50% for HSCT, lung, kidney, liver, and heart transplants, respectively (Fig. 2).
Figure 2.
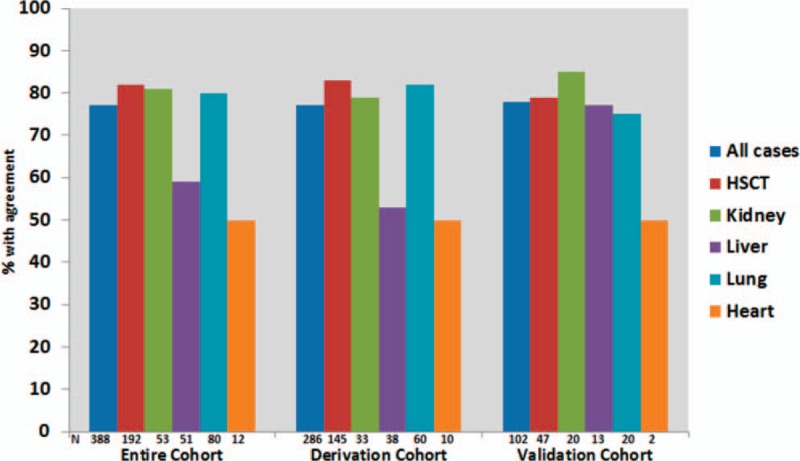
Proportion of agreement between the investigator-designated cause of death (proposed in CRF) and the recorded underlying cause of death (determined by the external reviewers after adjudication) according to transplant type. CRF = case record form.
In both cohorts, strength of inter-rater agreement was almost perfect in cases where recorded underlying cause of death was cancer (κ = 0.89 [95% CI 0.81–0.97] and κ = 0.91 [95% CI 0.79–1.03] in the derivation and validation cohorts, respectively). Strength of the inter-rater agreement was substantial in the validation cohort among those recorded as “Infection” or “Organ failure or dysfunction” whereas agreement in all other categories was moderate or less in both cohorts (Fig. 3).
Figure 3.
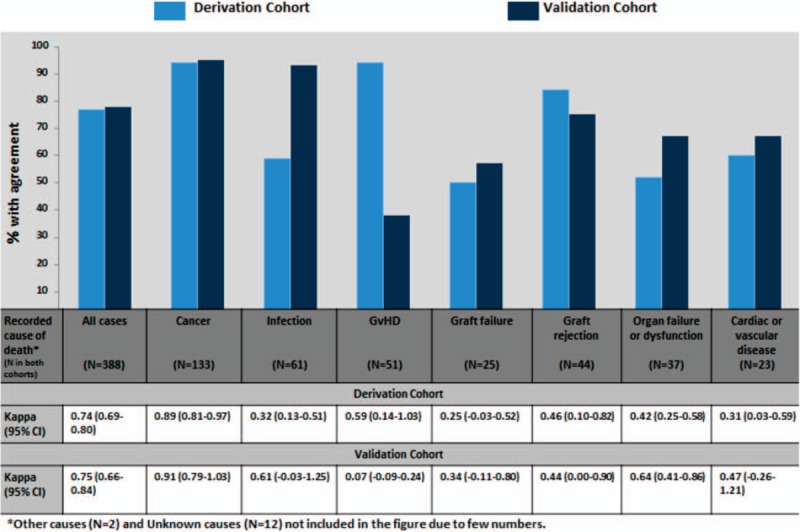
Agreement (% and Kappa) between investigator-designated cause of death (proposed in CRF) and the recorded underlying cause of death (determined by the external reviewers after adjudication) according to recorded underlying cause of death in derivation and validation cohort. CRF = case record form.
In the derivation cohort, characteristics (information retrieved from the CRF) associated with agreement after adjustment was “a functioning graft at time of death” and “a history of cancer” which both led to approximately 3 times higher odds of agreement compared with those without (adjusted Odds Ratio [aOR] 2.82 [95% CI 1.37–5.83] and aOR 3.31 [95% CI 1.32–8.32], respectively). “Cigarette smoking,” “a history of cerebrovascular disease,” “a history of liver disease,” and “use of antibiotics in the month up to death” led to lower odds of agreement (Table 2).
Table 2.
Odds and Kappa of agreement between the investigator-designated and the recorded underlying cause of death (determined by the external reviewers after adjudication or an expert panel) in derivation cohort, according to characteristics provided in the case record form.
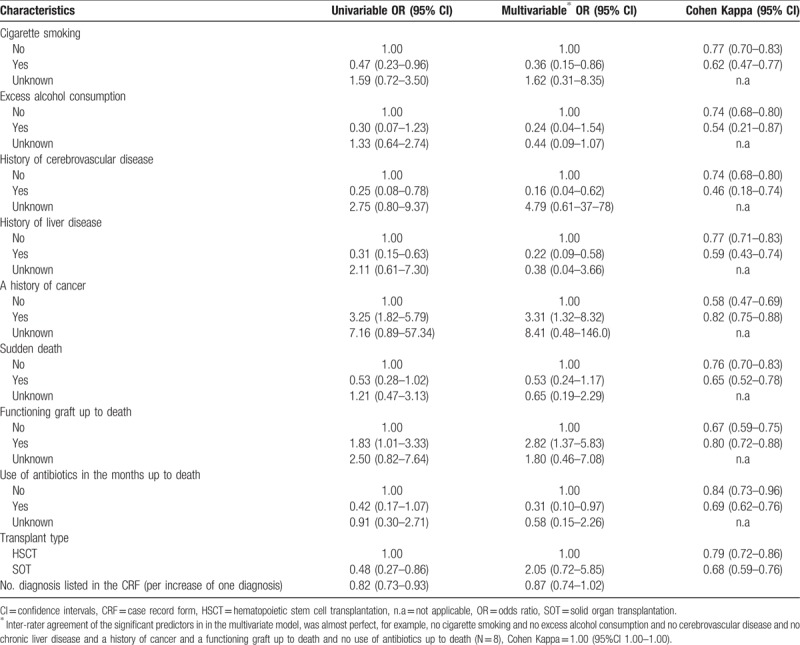
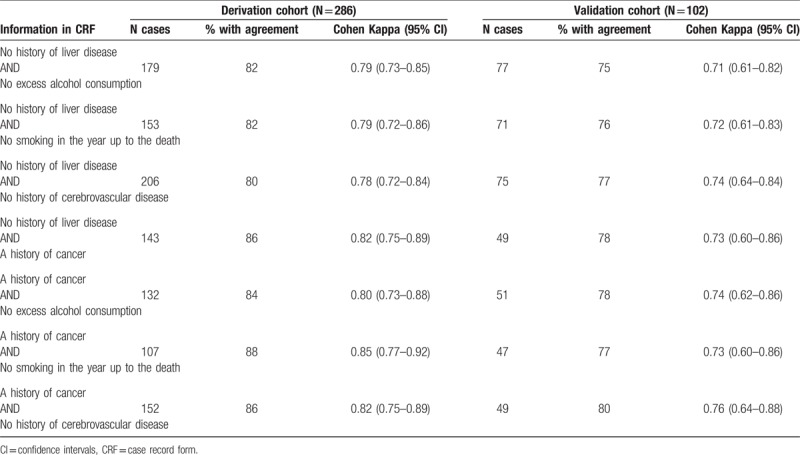
The characteristics able to significantly predict agreement between the investigator-designated and the recorded underlying cause of death in multivariate analyses were subsequently assessed in different combinations, in order to identify patterns that resulted in the highest agreement in both cohorts. The combinations of characteristics that led to κ >0.70 in both cohorts are listed in Table 3. For example, Kappa among recipients with “no history of liver disease” and “no history of cerebrovascular disease” was 0.78 (0.72–0.84) and 0.74 (0.64–0.84) in derivation (N = 206) and validation (N = 75) cohort, respectively.
Table 3.
Proportion and Kappa of agreement between the investigator-designated and the recorded underlying cause of death (determined by the external reviewers after adjudication or an expert panel), according to predictors of agreement identified in the logistic regression (derivation cohort and validation cohort).
3.3. Independent agreement of the recorded underlying cause of death between external reviewers
Independent agreement of the recorded underlying cause of death between the 2 external reviewers was obtained in 195 (68%) cases (κ = 0.64 [95% CI 0.56–0.69]) in the derivation cohort. The remaining 91 cases (32%) went through an adjudication process; which resulted in adjudicated agreement in the majority of these cases (87/91 [95%]). The remaining 4 cases were sent to an expert panel for determination of the recorded underlying cause of death.
In comparison, independent agreement of the recorded underlying cause of death was obtained in 69/102 (68%) ([κ = 0.63 [95% CI 0.52–.73]]) in the validation cohort, whereas the remaining 33 (32%) were agreed upon during the adjudication process.
Independent agreement of the recorded underlying cause of death was generally better among recipients of HSCT and lung transplantation (77% and 73%, respectively) compared with kidney, heart, and liver recipients (55%, 50%, 45%, respectively). When considering the derivation and validation cohorts separately, these proportions remained similar.
In both cohorts, the inter-rater agreement was almost perfect amongst cases where the recorded underlying cause of death was cancer (κ = 0.82 (95% CI 0.72–0.92) and κ = 0.85 (95% CI 0.70–1.00) in the derivation and validation cohorts, respectively). For the remaining recorded underlying cause of death categories, the inter-rater agreement was fair or slight in both cohorts. An exception was the “Unknown” category; for this category the kappa was 1.00 in the validation cohort. However, this group only consisted of 3 cases.
In the derivation cohort, characteristics (information retrieved from the CRF) associated with greater odds of independent agreement of the recorded underlying cause of death between the 2 external reviewers after adjustment was “a history of cancer” (aOR 3.20 [95% CI 1.45–7.06]); among 174 with this characteristics, kappa of independent agreement was 0.71 (95% CI 0.64–0.79). These findings were reproducible in the validation cohort; among 54 recipients with “a history of cancer,” kappa was 0.71 (95% CI 0.57–0.84).
3.4. Certainty of the recorded underlying cause of death
The certainty of the recorded underlying cause of death as determined by the external reviewers after adjudication in the derivation and validation cohorts was definite in 70% versus 73%, likely in 26% versus 23%, and possible in 4% versus 4%, respectively.
Among cases where independent agreement of recorded underlying cause of death was achieved in the derivation (N = 195) and validation (N = 69) cohorts, there was also independent agreement of the certainty criteria in 71% versus 62%.
3.5. Resources
Two reviewers reported the time consumption of the assessment process and spent an average of 8 minutes to review the CRF and complete the online Review Form. The assessment process was completed by all reviewers within 5 and 4 months for the derivation and validation cohorts, respectively.
3.6. Comparison of recorded underlying cause of death in this study with the national death cause registry
Comparison between the recorded underlying cause of death determined in the present study and the cause of death registered in the DNDCR was possible for 277/286 and 99/102 cases in the derivation and validation cohorts, respectively. The remaining 12 deaths had not been ascertained in the DNDCR. Concordance between the recorded underlying cause of death from our study and underlying cause of death from the DNDCR was observed in 37.2% (95% CI 31.5–42.9) and 38.4% (95% CI 28.8–48.0) in the derivation and validation cohort, respectively (Fig. 4).
Figure 4.
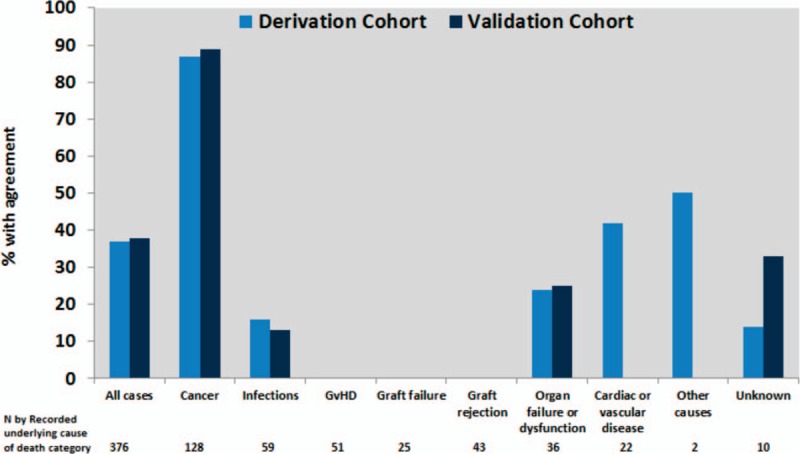
Agreement between recorded underlying cause of death (determined by the external reviewers after adjudication) in the CLASS project and the Danish National Death Cause Registry among those where this information was available (N = 376) according to recorded underlying cause of death in derivation and validation cohort.
When recorded underlying cause of death from the present study was compared with either immediate, underlying or any contributing causes of death from the DNDCR, concordance increased to 62.8% (95% CI 57.1–68.5) and 60.6% (95% CI 50.9–70.3) in the derivation and validation cohorts, respectively.
4. Discussion
We developed and validated a method which was able to systematically and reliably classify the underlying cause of death among transplant recipients. The method is flexible and is tailored to the specific needs of transplant centers, and can be applied to any cohort. The only requirement for applying this classification system is access to patient records. The electronic support structures to handle the process is depicted in Fig. 1 and all related source documents are available as part of a collaborative platform on https://www.chip.dk/MATCH. Application of this method may facilitate comparison of post-transplantation mortality in the setting of clinical trials, as well as allowing evaluation of temporal and regional trends. This is something that up until now has not been possible to do in a harmonized and consistent way.
Although this methodology may seem time consuming and complex, a similar approach has been developed for the reporting of death in HIV-infected populations, called CoDe.[12,13] The introduction of CoDe has improved international reporting of the underlying cause of death and is hence is now the standard method globally for reporting and classifying death in the HIV population.[12–14]
We were able to identify specific patterns that could help select cases that may not require the assessment of an external reviewer, since the agreement between the investigator-designated and recorded underlying cause of death was substantial or almost perfect. Thus, our results show that using a system where data were collected in a standardized manner, where the classification algorithm was clearly defined, and where investigators (i.e., clinical assistants) were trained in applying this algorithm, allowed for classification of the underlying cause of death in most cases irrespective of whether specialists or clinical assistants were responsible for the classification. Accordingly, previous studies have shown that training of participants and standardization of the classification algorithms are of importance when classifying death causes.[15–18] Thus, we recommend that cases with any characteristics listed in Table 3 should not be subjected to external review in future trials. This will reduce the resources required for application of this method to cohorts markedly.
The agreement varied between the different types of transplantation, likely reflecting the greater diversity of death causes within the groups with less agreement between the recorded underlying causes of death. Thus, the likelihood of coding the underlying cause of death differently is higher if the course leading to death is more complex and diverse which could be the case for example in deaths after a liver transplantation.
Our external reviewers independently agreed in 2/3 of the cases. The remaining cases likely represent the most difficult cases, and these were either agreed upon during the adjudication process or by the expert panel. Thus, underlying cause of death using our methodology was determined through an extensive and thorough system including assessment that involved several experts.
Conversely, we found a high degree of discordance between the recorded underlying causes of death as determined by our experts, and those listed in the DNDCR. It is important to note that this was a comparison of individual data and not aggregated data, and that <40% of the cases were concordant. The DNDCR are based on death certificates filled out by clinicians. In Denmark it is usually the youngest physician that is responsible for this. However, junior physicians may not have the necessary clinical expertise within transplant medicine to be able to determine cause of death in such a specialized setting. Furthermore, previous studies have reported significant errors associated with the completion of the death certificates[15,19,20] which is likely to also lead to errors in the determination of the underlying cause of death. In addition, autopsy reports are rarely available at time the death certificates are filled out, yet these reports may contribute to a more precise classification.[20,21] In our methodology, all available autopsy reports were part of the assessment process. Thus, the key question that is raised by the present study is whether the DNDCR can be used for classification of death in the setting of transplantation.
Conversely, reports on causes of death in transplant recipients in the literature are often based on national transplant registries.[3,19,22–25] However, causes of death in these registries are often obtained on death notification forms similar to the DNDCR.
While neither the methodology proposed here nor the different registries mentioned above can be considered the golden standard, we consider our method to be more accurate in the transplant setting. Our method involves several steps in order to get as close to the true cause of death as possible, including specific training of all participants, a standardized algorithm to determine the cause of death, and independent assessment and adjudication by specialists in case of disagreement.
Importantly, our classification system has 3 levels of certainty (possible, likely and definite cause of death) and aims to reduce the number of unknown categories. We prefer to try and anticipate a high number of cases and make use of the lower degrees of certainty, rather than having a large pool of patients who died from unknown causes. Accordingly, only 12/388 (3%) of the underlying cause of death was recorded as “Unknown.” The 3 levels of certainty also allows for sensitivity analysis of outcomes in recipients where likely and possible causes could be included or excluded, in order to test the robustness of the observations.
Our results should be seen in the light of their limitations. We acknowledge that our method relies on the accuracy and the degree of detail of the patient records; the more accurate information available, the more likely it is that we were able to determine the underlying cause of death accurately. Furthermore, this methodology may be time-consuming, although the above suggested recommendations can reduce resources significantly.
In summary, this is the first validated method to reliably classify underlying causes of death in transplant recipients. We believe that this methodology may facilitate the detection potential emerging health-threatening challenges within this vulnerable population of patients, by providing more granular and accurate information on the underlying cause of death.
Acknowledgments
The authors would like to acknowledge the work of Torben Weide and Sanne Egelund Thomsen who both helped with development of the online tool used to facilitate the assessment process as well as preparation of data for analyses.
Author contributions
Conceptualization: Neval Ete Wareham, Amanda Mocroft, Jens D. Lundgren.
Data curation: Neval Ete Wareham, Caspar Da Cunha-Bang, Álvaro H. Borges, Christina Ekenberg, Jan Gerstoft, Finn Gustafsson, Ditte Hansen, Carsten Heilmann, Marie Helleberg, Jens Hillingsøe, Paul Suno Krohn, Isabelle Paula Lodding, Thomas Kromann Lund, Louise Lundgren, Amanda Mocroft, Michael Perch, Søren Lykke Petersen, Irma Petruskevicius, Allan Rasmussen, Kasper Rossing, Andreas A. Rostved, Henrik Sengeløv, Vibeke Rømming Sørensen, Søren Schwartz Sørensen, Jens Dilling Lundgren.
Formal analysis: Neval Ete Wareham, Amanda Mocroft, Jens Dilling Lundgren.
Funding acquisition: Álvaro H. Borges, Jens Dilling Lundgren.
Investigation: Neval Ete Wareham, Caspar Da Cunha-Bang, Álvaro H. Borges, Christina Ekenberg, Jan Gerstoft, Finn Gustafsson, Ditte Hansen, Carsten Heilmann, Marie Helleberg, Jens Hillingsøe, Paul Suno Krohn, Isabelle Paula Lodding, Thomas Kromann Lund, Louise Lundgren, Amanda Mocroft, Michael Perch, Søren Lykke Petersen, Irma Petruskevicius, Allan Rasmussen, Kasper Rossing, Andreas A Rostved, Henrik Sengeløv, Vibeke Rømming Sørensen, Søren Schwartz Sørensen, Jens Dilling Lundgren.
Methodology: Neval Ete Wareham, Caspar Da Cunha-Bang, Álvaro H Borges, Christina Ekenberg, Jan Gerstoft, Finn Gustafsson, Ditte Hansen, Carsten Heilmann, Marie Helleberg, Jens Hillingsøe, Paul Suno Krohn, Isabelle Paula Lodding, Thomas Kromann Lund, Louise Lundgren, Amanda Mocroft, Michael Perch, Søren Lykke Petersen, Irma Petruskevicius, Allan Rasmussen, Kasper Rossing, Andreas A Rostved, Henrik Sengeløv, Vibeke Rømming Sørensen, Søren Schwartz Sørensen, Jens Dilling Lundgren.
Project administration: Neval Ete Wareham.
Resources: Caspar Da Cunha-Bang, Álvaro H Borges, Christina Ekenberg, Jan Gerstoft, Finn Gustafsson, Ditte Hansen, Carsten Heilmann, Marie Helleberg, Jens Hillingsøe, Paul Suno Krohn, Isabelle Paula Lodding, Thomas Kromann Lund, Louise Lundgren, Amanda Mocroft, Michael Perch, Søren Lykke Petersen, Irma Petruskevicius, Allan Rasmussen, Kasper Rossing, Andreas A Rostved, Henrik Sengeløv, Vibeke Rømming Sørensen, Søren Schwartz Sørensen, Jens Dilling Lundgren.
Software: Jens Dilling Lundgren.
Supervision: Caspar Da Cunha-Bang, Jan Gerstoft, Finn Gustafsson, Amanda Mocroft, Henrik Sengeløv, Jens Dilling Lundgren.
Validation: Neval Ete Wareham, Caspar Da Cunha-Bang, Álvaro H Borges, Christina Ekenberg, Jan Gerstoft, Finn Gustafsson, Ditte Hansen, Carsten Heilmann, Marie Helleberg, Jens Hillingsøe, Paul Suno Krohn, Isabelle Paula Lodding, Thomas Kromann Lund, Louise Lundgren, Amanda Mocroft, Michael Perch, Søren Lykke Petersen, Irma Petruskevicius, Allan Rasmussen, Kasper Rossing, Andreas A Rostved, Henrik Sengeløv, Vibeke Rømming Sørensen, Søren Schwartz Sørensen, Jens Dilling Lundgren.
Visualization: Amanda Mocroft, Jens Dilling Lundgren.
Writing – original draft: Neval Ete Wareham.
Writing – review and editing: Caspar Da Cunha-Bang, Álvaro H. Borges, Christina Ekenberg, Jan Gerstoft, Finn Gustafsson, Ditte Hansen, Carsten Heilmann, Marie Helleberg, Jens Hillingsøe, Paul Suno Krohn, Isabelle Paula Lodding, Thomas Kromann Lund, Louise Lundgren, Amanda Mocroft, Michael Perch, Søren Lykke Petersen, Irma Petruskevicius, Allan Rasmussen, Kasper Rossing, Andreas A Rostved, Henrik Sengeløv, Vibeke Rømming Sørensen, Søren Schwartz Sørensen, Jens Dilling Lundgren.
Supplementary Material
Footnotes
Abbreviations: aOR = adjusted odds ratio, CI = confidence interval, CRF = case record form, DNDCR = Danish National Death Cause Registry, GvHD = Graft versus host disease, HIV = human immunodeficiency virus, HSCT = hematopoietic stem cell transplantation, ICD = International Classification of Diseases, IQR = inter-quartile range, MATCH = Management of Post-Transplant Infections in Collaborating Hospitals, SOT = solid organ transplantation.
Sources of funding: This work was supported by Danish National Research Foundation [grant number 126] and Lundbeckfonden [grant number R219-2016-762 to AHB].
Compliance with ethical standards: Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: All relevant approval for this project was obtained from the Danish Health and Medicines Authorities according to Danish legislation on retrospective studies. For this type of study, formal consent is not required.
The authors have no conflicts of interest to declare.
Supplemental Digital Content is available for this article.
References
- [1].Johnston SD, Morris JK, Cramb R, et al. Cardiovascular morbidity and mortality after orthotopic liver transplantation. Transplantation 2002;73:901–6. [DOI] [PubMed] [Google Scholar]
- [2].Lund LH, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-second Official Adult Heart Transplantation Report--2015; Focus Theme: Early Graft Failure. J Heart Lung Transplant 2015;34:1244–54. [DOI] [PubMed] [Google Scholar]
- [3].Kahwaji J, Bunnapradist S, Hsu JW, et al. Cause of death with graft function among renal transplant recipients in an integrated healthcare system. Transplantation 2011;91:225–30. [DOI] [PubMed] [Google Scholar]
- [4].Kaplan B, Meier-Kriesche HU. Death after graft loss: an important late study endpoint in kidney transplantation. Am J Transplant 2002;2:970–4. [DOI] [PubMed] [Google Scholar]
- [5].Pascual M, Theruvath T, Kawai T, et al. Strategies to improve long-term outcomes after renal transplantation. N Engl J Med 2002;346:580–90. [DOI] [PubMed] [Google Scholar]
- [6].Hosenpud JD, Bennett LE, Keck BM, et al. The Registry of the International Society for Heart and Lung Transplantation: sixteenth official report--1999. J Heart Lung Transplant 1999;18:611–26. [DOI] [PubMed] [Google Scholar]
- [7].Martin-Gandul C, Mueller NJ, Pascual M, et al. The impact of infection on chronic allograft dysfunction and allograft survival after solid organ transplantation. Am J Transplant 2015;15:3024–40. [DOI] [PubMed] [Google Scholar]
- [8].Marty FM, Ljungman P, Chemaly RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med 2017;377:2433–44. [DOI] [PubMed] [Google Scholar]
- [9].MATCH (Management of Post-Transplant Infections in Collaborating Hospitals); 2017. Ref Type: Online Source. [Google Scholar]
- [10].Helweg-Larsen K. The Danish Register of Causes of Death. Scand J Public Health 2011;39Suppl:26–9. [DOI] [PubMed] [Google Scholar]
- [11].Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74. [PubMed] [Google Scholar]
- [12].Kowalska JD, Friis-Moller N, Kirk O, et al. The Coding Causes of Death in HIV (CoDe) Project: initial results and evaluation of methodology. Epidemiology 2011;22:516–23. [DOI] [PubMed] [Google Scholar]
- [13].Kowalska JD, Smith C, Lundgren JD. System to classify cause of deaths in HIV-positive persons: time to harmonize. AIDS 2012;26:1835–6. [DOI] [PubMed] [Google Scholar]
- [14].Kowalska JD, Mocroft A, Ledergerber B, et al. A standardized algorithm for determining the underlying cause of death in HIV infection as AIDS or non-AIDS related: results from the EuroSIDA study. HIV Clin Trials 2011;12:109–17. [DOI] [PubMed] [Google Scholar]
- [15].Cambridge B, Cina SJ. The accuracy of death certificate completion in a suburban community. Am J Forensic Med Pathol 2010;31:232–5. [DOI] [PubMed] [Google Scholar]
- [16].Alexander S, Pole JD, Gibson P, et al. Classification of treatment-related mortality in children with cancer: a systematic assessment. Lancet Oncol 2015;16:e604–10. [DOI] [PubMed] [Google Scholar]
- [17].Copelan E, Casper JT, Carter SL, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biol Blood Marrow Transplant 2007;13:1469–76. [DOI] [PubMed] [Google Scholar]
- [18].Messite J, Stellman SD. Accuracy of death certificate completion: the need for formalized physician training. JAMA 1996;275:794–6. [PubMed] [Google Scholar]
- [19].Briggs JD. Causes of death after renal transplantation. Nephrol Dial Transplant 2001;16:1545–9. [DOI] [PubMed] [Google Scholar]
- [20].Smith Sehdev AE, Hutchins GM. Problems with proper completion and accuracy of the cause-of-death statement. Arch Intern Med 2001;161:277–84. [DOI] [PubMed] [Google Scholar]
- [21].Lee PN. Comparison of autopsy, clinical and death certificate diagnosis with particular reference to lung cancer. A review of the published data. APMIS Suppl 1994;45:1–42. [PubMed] [Google Scholar]
- [22].Gratwohl A, Brand R, Frassoni F, et al. Cause of death after allogeneic haematopoietic stem cell transplantation (HSCT) in early leukaemias: an EBMT analysis of lethal infectious complications and changes over calendar time. Bone Marrow Transplant 2005;36:757–69. [DOI] [PubMed] [Google Scholar]
- [23].Pruthi J, Medkiff KA, Esrason KT, et al. Analysis of causes of death in liver transplant recipients who survived more than 3 years. Liver Transpl 2001;7:811–5. [DOI] [PubMed] [Google Scholar]
- [24].Vanderlaan RD, Manlhiot C, Edwards LB, et al. Risk factors for specific causes of death following pediatric heart transplant: an analysis of the registry of the International Society of Heart and Lung Transplantation. Pediatr Transplant 2015;19:896–905. [DOI] [PubMed] [Google Scholar]
- [25].Viecelli AK, Lim WH, Macaskill P, et al. Cancer-specific and all-cause mortality in kidney transplant recipients with and without previous cancer. Transplantation 2015;99:2586–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


