Abstract
Background:
Although surgical biopsy has historically been considered to be the standard diagnostic biopsy for soft tissue and bone sarcomas, recent literature suggests that percutaneous core needle biopsy yields similar results. Therefore, an evaluation of the exact diagnostic accuracy and associated influential variables of core needle biopsy that is based on a large data set would be useful.
Methods:
We searched MEDLINE, Web of Science, and EMBASE to identify core needle biopsy studies for predicting final histological subtypes of musculoskeletal lesions. The diagnostic accuracies of core needle biopsy and of surgical biopsy were assessed and compared by using random-effect meta-analyses. The factors relevant to diagnostic accuracy were evaluated by meta-regression and subgroup analyses.
Results:
We selected 32 studies comprising 7209 musculoskeletal lesions. The pooled proportion estimate for the diagnostic accuracy of core needle biopsy was 0.84 (95% confidential interval, CI: 0.81–0.87), which indicated an approximate 84% concordance between core needle biopsy results and final histological diagnoses. The findings of meta-regression and subgroup analyses suggested that radiologists were better core needle biopsy operators than surgeons. An additional meta-analysis for direct comparison between core needle biopsy and surgical biopsy demonstrated that diagnostic accuracy was significantly lower for core needle biopsy than for surgical (pooled odds ratio: 0.39, 95% CI: 0.20–0.76).
Conclusion:
Our results suggested that core needle biopsy should be performed by expert radiologists and that surgical biopsy should be performed if diagnosis following core needle biopsy does not match the clinical presentation and radiographic findings
Keywords: bone sarcoma, core needle biopsy, meta-analysis, soft tissue sarcoma, surgical biopsy
1. Introduction
The current 2013 World Health Organization (WHO) Classification of Tumors of Soft Tissue and Bone incorporates much progress regarding the tumor classification and identification of new histological subtypes. The changes in classifying and recognizing the pathogeneses of soft tissue and bone tumors, predominantly based on newly identified genetic findings, have been particularly remarkable as compared with progress made in the pathologies of other cancers.[1,2] Sarcomas are a heterogeneous type of rare malignant soft-tissue and bone tumors and account for only approximately 1% of all malignancies in adults and 15% to 20% of pediatric malignancies. More than 50 histological subtypes have been identified, and the differential diagnosis for sarcomas is fairly extensive. Different subtypes can vary in their clinical manifestations and response to treatment. Higher-grade sarcomas exhibit more aggressive behavior and tend to hematogenously metastasize to the lungs, which is the leading cause of disease-specific death. Histological tumor grade has been identified as one of the strongest predictors of metastatic risk and patient prognosis. Despite current intensive and multimodal treatment, including surgery, radiotherapy, and chemotherapy, prognosis has plateaued since the 1990 s and remains suboptimal in many high-grade types.[3,4] Therefore, prompt and precise diagnostic procedures for these heterogeneous and refractory sarcomas are challenging.
In the management of musculoskeletal lesions, biopsy is the most critical first step in determining treatment strategy and outcomes. Unplanned biopsies can compromise reconstructive procedures and sometimes require amputation to obtain adequate surgical margins. Biopsy is principally utilized to harvest representative and viable tissue specimens for accurate diagnosis. A variety of biopsy techniques, such as fine needle aspiration (FNA), core needle biopsy (CNB), and incisional or excisional surgical biopsy (SB), are frequently used nowadays. SB has historically been the diagnostic standard; it provides large volumes of tissue sample, which facilitates accurate histological analyses and more precise estimates of patient prognoses. However, biopsy-associated complications involve hematoma, infection, and neurapraxia. A biopsy procedure can also spread tumor cells to surrounding tissue and, therefore, increase the risk of local recurrence. It is imperative that the biopsy tract be placed within the planned resection margins prior to future treatment planning involving surgical resection and radiation. On the contrary, needle biopsies are less invasive techniques, and are less time consuming, have lower costs, and have low morbidity. Because FNA only provides cytology, not true histology, it may be able to distinguish neoplasms from normal tissues, malignant from benign tumors, and high- from low-grade malignancies. CNB, which evolved as an alternative to FNA, improves the determination of the histological subtype and grade. Moreover, the advantages of CNB relative to those of SB include the low risk of contaminating adjacent tissue compartments and minimal invasiveness, which are because of the small biopsy tract and less bleeding.[5,6]
Although SB has long been considered to be the gold standard for cancer diagnosis, a recent review article suggests that percutaneous CNB yields similar results.[6] Thus, an assessment of the exact diagnostic accuracy and relevant influential factors of CNB that is based on a large data set would be useful. The purpose of this review was to provide an up-to-date and unprecedented summary of CNB in soft tissue and bone sarcomas. We conducted a systematic review and meta-analysis for assessing the diagnostic accuracy and relevant influential factors of CNB and for comparing the diagnostic accuracy of CNB with that of SB.
2. Materials and methods
This meta-analysis was reported according to the preferred reporting items for systematic reviews and meta-analyses guidelines. All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
2.1. Search strategy and selection criteria
The literature search was performed in accordance with the guidelines present in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[7] The main research question was defined using the Target Population, Index Test, Comparator Test, Outcome, and Study Design (PICOS) strategy: target population, patients with soft tissue sarcoma, bone sarcoma, or both examined by CNB; index test, results of CNB; comparator test, results of SB; outcome, definitive histological subtypes; and study design, retrospective and prospective cohort studies. Data for this systematic review and meta-analyses were identified by searches of MEDLINE, Web of Science, and EMBASE using the search terms “core needle biopsy” and “sarcoma” on February 1, 2017 without a time search limitation or language restrictions. We also hand-searched references from relevant articles. We excluded conference abstracts, clinical case series, and review articles.
The inclusion criteria were as follows: original studies reporting CNB conducted to predict final histological subtypes of musculoskeletal lesions and sufficient raw data to calculate the diagnostic accuracy of CNB. The diagnostic accuracy of CNB was determined as the proportion of lesions that showed concordance between the CNB results and final histological subtypes in the total number of lesions that were tested for CNB; a variety of histological subtypes presented.
2.2. Data analysis
The 2 orthopedic oncologists (TF and MPJ) independently screened and selected the articles. Discrepancies between the 2 reviewers were resolved by a third investigator (TK) via discussion until a consensus was reached. Articles were selected by title, by abstract, and subsequently by full text to fulfill the inclusion criteria. Next, the following information was extracted where available: author name, year of publication, study institutes, clinical characteristics of all participants and tumors, total number included in the meta-analyses, concordant rate of CNB results and the final histological diagnosis at the resection, concordant rate of results of SB and the final histological diagnosis, type of radiological guidance (ultrasound, computed tomography [CT], magnetic resonance imaging [MRI], or fluoroscope), gauge number of biopsy needle, core number of CNB specimens, type of anesthesia, and operator performing the CNB (radiologist or surgeon). The quality of study designs in the eligible articles was evaluated using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool.[8] Risk of bias concerning 4 domains (patient selection, index test, reference standard, and flow and timing) and concerns regarding the applicability of three domains (patient selection, index test, and reference standard) were rated as “low,” “high,” or “unclear.”
2.3. Statistical analysis
In the meta-analysis, a random-effects model was used to calculate a pooled proportion estimate with 95% confidential interval (CI) for the diagnostic accuracy of CNB.[9] The inconsistency index I-square (I2) test was used for assessing heterogeneity of the diagnostic accuracy of each study. Variables causing the heterogeneity were identified using the meta-regression method and subgroup analysis.
We also used a random-effect model to calculate the pooled odds ratio and 95% CI for the diagnostic accuracy between CNB and SB. All meta-analyses were conducted using Stata/SE version 14 statistical software (StataCorp LP, College Station, TX). P < .05 were considered statistical significant.
3. Results
3.1. Literature search and selection of studies
We used 3 search engines to identify 895 articles, excluding 209 because of duplication. Furthermore, 610 and 27 articles were excluded on the basis of the information in the title and abstract, respectively. We also added eight articles after reviewing references from relevant articles. Lastly, 25 articles were excluded after the review of the full text. A total of 32 studies met all the inclusion criteria for the meta-analysis regarding the diagnostic accuracy of CNB (Fig. 1).[10–41] Additionally, 5 eligible articles were selected for directly comparing the diagnostic accuracy of CNB with that of SB.[15,18,22,25,36]
Figure 1.

Flowchart of the article-selection process.
3.2. Study description and quality
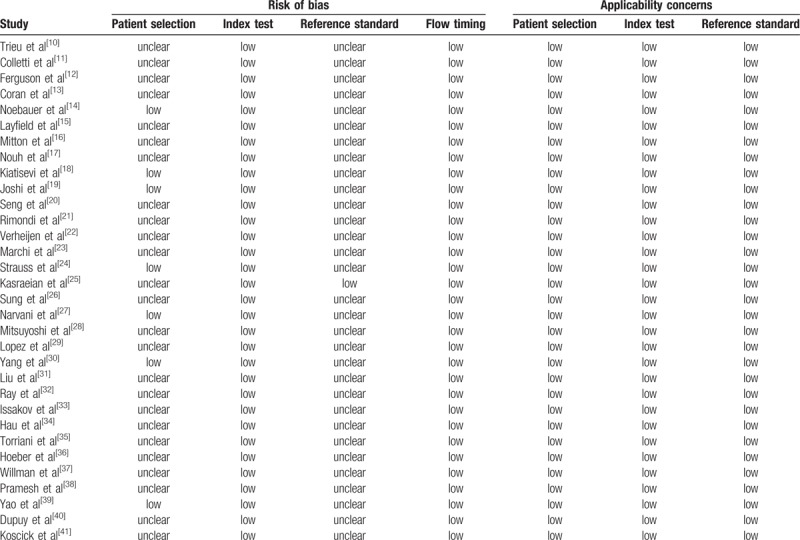
The total human population in the combined studies was 8930 individuals, with an average age of 46.1 years. There were 7209 lesions presenting histological subtypes included in the meta-analyses. The primary characteristics of the 32 studies that were included in the meta-analysis are shown in Table 1.[10–41] According to the QUADAS-2 tool for methodological quality, all studies were rated as having five or more “low” responses and no “high” response in the seven domains. Twenty-five authors did not describe the sampling methods or used inappropriate exclusions in the domain of patient selection, and 31 authors did not state blindness in the domain of reference standard (Table 2).[10–41]
Table 1.
Key characteristics of the studies included in the meta-analysis.
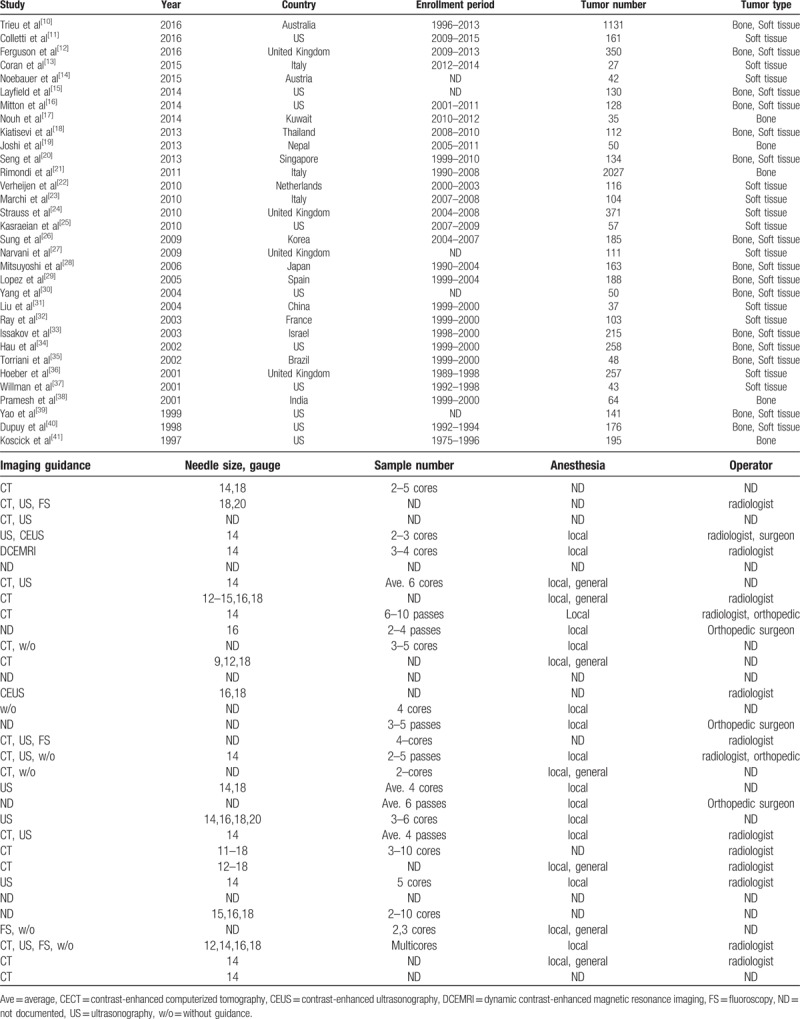
Table 2.
Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2).
3.3. Meta-analysis
Because of high heterogeneity between studies (I2 = 93.36%), we used a random-effects model for the diagnostic accuracy of CNB. Overall, the pooled proportion estimate for the diagnostic accuracy was 0.84 (95% CI: 0.81–0.87), which indicated an approximate 84% concordance between the CNB results and final histological diagnoses (Fig. 2). In the meta-regression analysis, the type of CNB operator was the only variable significantly associated with heterogeneity (P = .033). No other possible factors were significantly associated with the diagnostic accuracy (Table 3). Subgroup analysis results indicated that radiologists may be more qualified CNB examiners than surgeons for predicting the histological subtype (Fig. 3).
Figure 2.

Forest plot showing diagnostic accuracy of core needle biopsy. The square size of individual studies represents the weight of the study. Error bars indicate 95% CIs of pooled estimates. CIs = confidential intervals.
Table 3.
Meta-regression analyses.
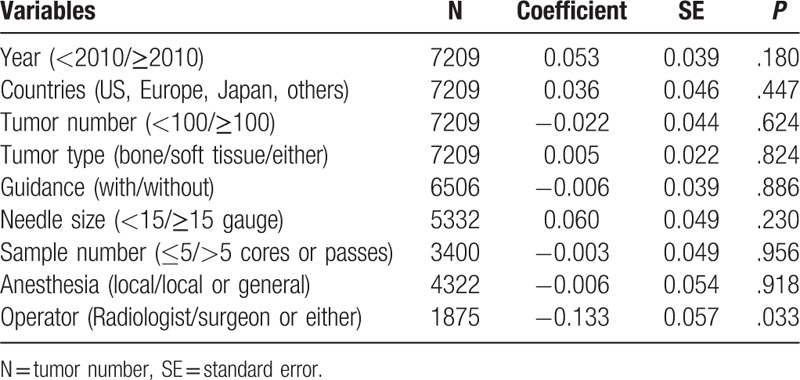
Figure 3.
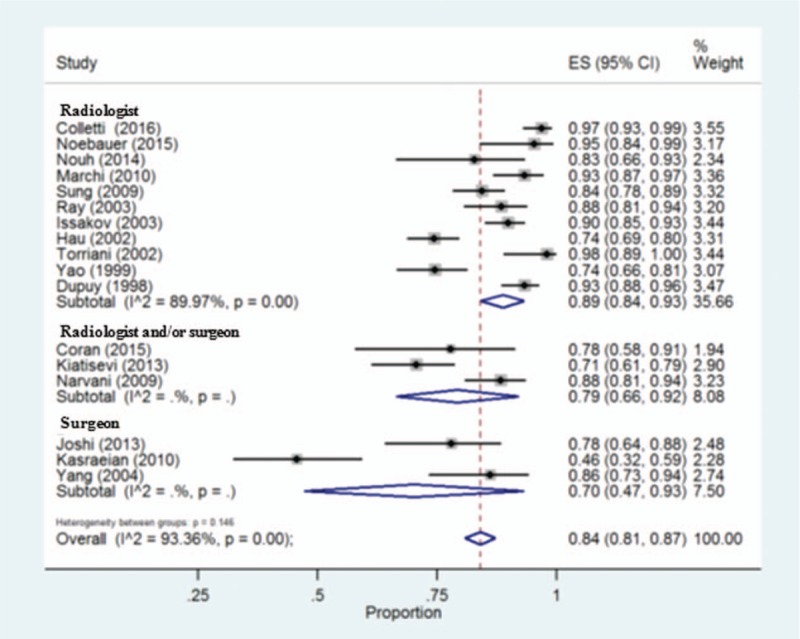
Forest plot showing diagnostic accuracy of core needle biopsy sorted by operators. The square size of individual studies represents the weight of the study. Error bars indicate 95% CIs of pooled estimates. CIs = confidential intervals.
In addition, 5 articles consisting were eligible for directly comparing the diagnostic accuracy of CNB with that of SB. Because we observed medium heterogeneity between the studies (I2 = 68.4%), we used a random-effects model for the comparison and found that the diagnostic accuracy of CNB was significantly lower than that of SB (pooled odds ratio: 0.39, 95% CI: 0.20–0.76) (Fig. 4).
Figure 4.
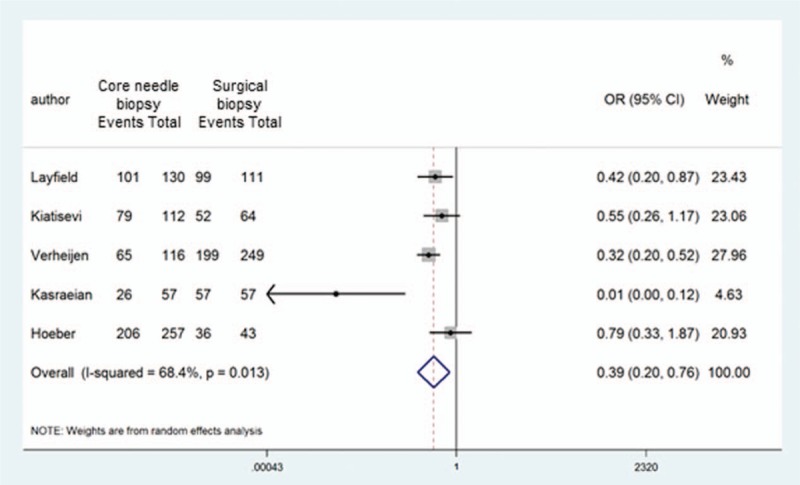
Forest plot of OR for diagnostic accuracy between core needle biopsy and surgical biopsy. The square size of individual studies represents the weight of the study. Error bars indicate 95% CIs of pooled OR. CIs = confidential intervals, OR = odds ratio.
4. Discussion
Biopsies aim to facilitate definitive pathological diagnoses while minimizing complications, limiting potential tumor seeding, and avoiding interference with subsequent therapies. A diagnosis of sarcoma or benign tumor is generally not sufficient, and the specific histological subtype should be determined from the biopsy to guide therapeutic decision-making. However, to our knowledge, the optimal biopsy procedure for the diagnosis of soft tissue and bone sarcomas is not present in current literature. SB has historically been considered to achieve diagnostic outcomes superior to those of CNB, but the difference in diagnostic accuracy between them was reportedly not significant. [5,6] CNB has been reported to provide limited sample volumes and to be less able to access deep-seated masses, which is problematic particularly for the inherent heterogeneity of sarcoma. Kasraeian et al[25] prospectively studied 57 patients with soft-tissue masses; they performed CNB, preceded by FNA and followed by SB, of the same mass. SB showed 100% diagnostic accuracy in diagnosis, however, the accuracy was only 33.3% for FNA and 45.6% for CNB. Therefore, the authors supported the usage of SB for diagnosing soft-tissue masses. On the contrary, Pohlig et al[42] retrospectively compared CNB with SB in 48 bone tumors. The diagnostic accuracies were 100% for CNB and 93.3% for SB, but the difference was not significant (P > .05). Other recent studies using image-guided percutaneous biopsy provide superior spatial localization of the tumors.[5] SB appears to be the most accurate technique, but there is not enough evidence to recommend one biopsy procedure over another. Thus far, because of the low risk of morbidity and simplicity of the percutaneous procedure, CNB appears to be more suitable as the first choice. This lack of evidence prompted us to conduct a meta-analysis to derive more robust estimates of the diagnostic yield of CNB and to directly compare the diagnostic accuracies between CNB and SB. The present meta-analysis used a large sample of data on soft tissue and bone sarcomas and showed that there was an approximate 84% concordance between the CNB results and the final histological diagnoses. We noted a significant difference in the diagnostic accuracies between SB and CNB, which suggested that SB should be performed if diagnosis following CNB does not match the clinical presentation and radiographic findings.
Certain anatomical locations and histological subtypes have been associated with the diagnostic difficulty of needle biopsies. Vertebral lesions and deep musculoskeletal tumors as well as myxoid and round-cell histologies have been associated with low-diagnostic accuracy. In addition, certain technical factors such as image-guided needle biopsy targeting representative and viable tumor regions improve the biopsy quality and diagnostic yield and reduce the complications rates. The caliber and type of biopsy needle, number of sample cores, and institute where the biopsy was performed have also been found to influence the outcomes of needle biopsy. [5,6] Therefore, we statistically performed meta-regression and subgroup analyses to assess influential parameters for diagnostic accuracy of CNB (shown in Table 3). Our analyses did not show any significant influential factors other than the operator type (surgeon or radiologist). This finding indicates that radiologists are more skillful in performing CNB than surgeons when assessed on the ability to predict the final histological diagnosis. This could be because radiologists generally have more experience in some techniques of interventional radiology, such as radiofrequency ablation, embolization, and cryosurgery under radiological guidance. In general, biopsies are technically challenging, and if not performed at a high skill level, the biopsy results can compromise patient outcomes. Therefore, biopsies should only be performed by expert radiologists at referral sarcoma centers to improve diagnostic yield and minimize complications.
Although the present study was based on thorough literature searches and careful data extraction, some limitations should be considered. First, compared with the larger sample size used for the meta-analysis of CNB diagnostic accuracy, the sample sizes of the meta-regression analysis regarding operator and the comparison of the diagnostic accuracy between CNB and SB were small. Further studies using larger sample sizes are thus required. Second, the heterogeneity of pooled estimates of diagnostic accuracy and the odds ratio between CNB and SB were high and medium, respectively. I2 represents the percentage of total variability in estimates generated from genuine between-study heterogeneity rather than by random sampling error. The observed heterogeneity may be attributable to numerous other influential factors including location, size, histological subtypes, and other technical procedures. We were unable to collect sufficient data to detect significant differences among the mentioned parameters. In addition, more studies are warranted to evaluate the differences in morbidities, cost, and procedure to determine the optimal biopsy procedure between CNB and SB. Third, bias could not be completely ruled out, although we attempted to judge as fairly as possible. The two reviewers performed this study in an independent blinded manner to minimize bias in the study selection and data extraction. Furthermore, according to the QUADAS-2 tool for methodological quality, all studies were rated as having 5 or more “low” responses and no “high” response in the 7 domains. Prospective randomized clinical trials comparing the diagnostic accuracies between CNB and SB in soft tissue and bone sarcomas would be the optimal method to completely exclude all potential biases, including selection, information, and publication bias.
5. Conclusion
In conclusion, the meta-analysis of this study indicated that the concordance rate between the CNB results and final histological diagnoses was approximate 84%, and suggested that biopsies performed by radiologists are more reliable than those performed by surgeons.
Acknowledgments
The authors would like to thank www.enago.com for the English language review.
Author contributions
TK, MO, and NA designed the analysis and had full access to the raw data. TK, FT, and MPJ collected the data and performed the statistical analysis. All authors had the opportunity to review the analysis plan and outcome, participated in writing the article, and provided final approval.
Conceptualization: Tadahiko Kubo, Mitsuo Ochi, Nobuo Adachi.
Data curation: Taisuke Furuta, Muhammad P. Johan, Tomohiko Sakuda, Nobuo Adachi.
Formal analysis: Mitsuo Ochi.
Funding acquisition: Tadahiko Kubo.
Investigation: Taisuke Furuta, Muhammad P. Johan, Tomohiko Sakuda, Mitsuo Ochi, Nobuo Adachi.
Writing – original draft: Tadahiko Kubo.
Writing – review & editing: Muhammad P. Johan, Tomohiko Sakuda, Mitsuo Ochi, Nobuo Adachi.
Footnotes
Abbreviations: CI = confidential interval, CNB = core needle biopsy, FNA = fine needle aspiration, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, QUADAS-2 = Quality Assessment of Diagnostic Accuracy Studies-2, SB = surgical biopsy, WHO = World Health Organization.
This study was supported by Japan Society for the Promotion of Science, JSPS KAKENHI Grant Number 17K10970. The authors declare no conflicts of interest.
References
- [1].Fletcher CD, Hogendoorn P, Mertens F, et al. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed.Lyon, France: IARC Press; 2013. [Google Scholar]
- [2].Doyle LA. Sarcoma classification: an update based on the 2013 World Health Organization Classification of Tumors of Soft Tissue and Bone. Cancer 2014;120:1763–74. [DOI] [PubMed] [Google Scholar]
- [3].Patel SR. Fifty years of advances in sarcoma treatment: moving the needle from conventional chemotherapy to targeted therapy. Am Soc Clin Oncol Educ Book 2014. 259–62. [DOI] [PubMed] [Google Scholar]
- [4].Ray-Coquard I, Le Cesne A. A role for maintenance therapy in managing sarcoma. Cancer Treat Rev 2012;38:368–78. [DOI] [PubMed] [Google Scholar]
- [5].Trieu J, Sinnathamby M, Di Bella C, et al. Biopsy and the diagnostic evaluation of musculoskeletal tumours: critical but often missed in the 21st century. ANZ J Surg 2016;86:133–8. [DOI] [PubMed] [Google Scholar]
- [6].Traina F, Errani C, Toscano A, et al. Current concepts in the biopsy of musculoskeletal tumors: AAOS exhibit selection. J Bone Joint Surg Am 2015;97:e7. [DOI] [PubMed] [Google Scholar]
- [7].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–36. [DOI] [PubMed] [Google Scholar]
- [9].Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health 2014;72:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Trieu J, Schlicht SM, Choong PF. Diagnosing musculoskeletal tumours: how accurate is CT-guided core needle biopsy? Eur J Surg Oncol 2016;42:1049–56. [DOI] [PubMed] [Google Scholar]
- [11].Colletti SM, Tranesh GA, Whetsell CR, et al. High diagnostic accuracy of core needle biopsy of soft tissue tumors: an institutional experience. Diagn Cytopathol 2016;44:291–8. [DOI] [PubMed] [Google Scholar]
- [12].Ferguson KB, McGlynn J, Jane M, et al. Outcome of image-guided biopsies: retrospective review of the West of Scotland musculoskeletal oncology service. Surgeon 2016;14:87–90. [DOI] [PubMed] [Google Scholar]
- [13].Coran A, Di Maggio A, Rastrelli M, et al. Core needle biopsy of soft tissue tumors, CEUS vs US guided: a pilot study. J Ultrasound 2015;18:335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Noebauer-Huhmann IM, Amann G, Krssak M, et al. Use of diagnostic dynamic contrast-enhanced (DCE)-MRI for targeting of soft tissue tumour biopsies at 3T: preliminary results. Eur Radiol 2015;25:2041–8. [DOI] [PubMed] [Google Scholar]
- [15].Layfield LJ, Schmidt RL, Sangle N, et al. Diagnostic accuracy and clinical utility of biopsy in musculoskeletal lesions: a comparison of fine-needle aspiration, core, and open biopsy techniques. Diagn Cytopathol 2014;42:476–86. [DOI] [PubMed] [Google Scholar]
- [16].Mitton B, Seeger LL, Eckardt MA, et al. Image-guided percutaneous core needle biopsy of musculoskeletal tumors in children. J Pediatr Hematol Oncol 2014;36:337–41. [DOI] [PubMed] [Google Scholar]
- [17].Nouh MR, Abu Shady HM. Initial CT-guided needle biopsy of extremity skeletal lesions: diagnostic performance and experience of a tertiary musculoskeletal center. Eur J Radiol 2014;83:360–5. [DOI] [PubMed] [Google Scholar]
- [18].Kiatisevi P, Thanakit V, Sukunthanak B, et al. Computed tomography-guided core needle biopsy versus incisional biopsy in diagnosing musculoskeletal lesions. J Orthop Surg (Hong Kong) 2013;21:204–8. [DOI] [PubMed] [Google Scholar]
- [19].Joshi A, Magar SR, Chand P, et al. Tru-cut biopsy as the initial method of tissue diagnosis in bone tumors with soft tissue extension. Indian J Orthop 2013;47:195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Seng C, Png W, Tan MH. Accuracy of core needle biopsy for musculoskeletal tumours. J Orthop Surg (Hong Kong) 2013;21:92–5. [DOI] [PubMed] [Google Scholar]
- [21].Rimondi E, Rossi G, Bartalena T, et al. Percutaneous CT-guided biopsy of the musculoskeletal system: results of 2027 cases. Eur J Radiol 2011;77:34–42. [DOI] [PubMed] [Google Scholar]
- [22].Verheijen P, Witjes H, van Gorp J, et al. Current pathology work-up of extremity soft tissue sarcomas, evaluation of the validity of different techniques. Eur J Surg Oncol 2010;36:95–9. [DOI] [PubMed] [Google Scholar]
- [23].De Marchi A, Brach del Prever EM, Linari A, et al. Accuracy of core-needle biopsy after contrast-enhanced ultrasound in soft-tissue tumours. Eur Radiol 2010;20:2740–8. [DOI] [PubMed] [Google Scholar]
- [24].Strauss DC, Qureshi YA, Hayes AJ, et al. The role of core needle biopsy in the diagnosis of suspected soft tissue tumours. J Surg Oncol 2010;102:523–9. [DOI] [PubMed] [Google Scholar]
- [25].Kasraeian S, Allison DC, Ahlmann ER, et al. A comparison of fine-needle aspiration, core biopsy, and surgical biopsy in the diagnosis of extremity soft tissue masses. Clin Orthop Relat Res 2010;468:2992–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sung KS, Seo SW, Shon MS. The diagnostic value of needle biopsy for musculoskeletal lesions. Int Orthop 2009;33:1701–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Narvani AA, Tsiridis E, Saifuddin A, et al. Does image guidance improve accuracy of core needle biopsy in diagnosis of soft tissue tumours? Acta Orthop Belg 2009;75:239–44. [PubMed] [Google Scholar]
- [28].Mitsuyoshi G, Naito N, Kawai A, et al. Accurate diagnosis of musculoskeletal lesions by core needle biopsy. J Surg Oncol 2006;94:21–7. [DOI] [PubMed] [Google Scholar]
- [29].López JI, Del Cura JL, Zabala R, et al. Usefulness and limitations of ultrasound-guided core biopsy in the diagnosis of musculoskeletal tumours. APMIS 2005;113:353–60. [DOI] [PubMed] [Google Scholar]
- [30].Yang YJ, Damron TA. Comparison of needle core biopsy and fine-needle aspiration for diagnostic accuracy in musculoskeletal lesions. Arch Pathol Lab Med 2004;128:759–64. [DOI] [PubMed] [Google Scholar]
- [31].Liu JC, Chiou HJ, Chen WM, et al. Sonographically guided core needle biopsy of soft tissue neoplasms. J Clin Ultrasound 2004;32:294–8. [DOI] [PubMed] [Google Scholar]
- [32].Ray-Coquard I, Ranchère-Vince D, Thiesse P, et al. Evaluation of core needle biopsy as a substitute to open biopsy in the diagnosis of soft-tissue masses. Eur J Cancer 2003;39:2021–5. [DOI] [PubMed] [Google Scholar]
- [33].Issakov J, Flusser G, Kollender Y, et al. Computed tomography-guided core needle biopsy for bone and soft tissue tumors. Isr Med Assoc J 2003;5:28–30. [PubMed] [Google Scholar]
- [34].Hau A, Kim I, Kattapuram S, et al. Accuracy of CT-guided biopsies in 359 patients with musculoskeletal lesions. Skeletal Radiol 2002;31:349–53. [DOI] [PubMed] [Google Scholar]
- [35].Torriani M, Etchebehere M, Amstalden E. Sonographically guided core needle biopsy of bone and soft tissue tumors. J Ultrasound Med 2002;21:275–81. [DOI] [PubMed] [Google Scholar]
- [36].Hoeber I, Spillane AJ, Fisher C, et al. Accuracy of biopsy techniques for limb and limb girdle soft tissue tumors. Ann Surg Oncol 2001;8:80–7. [DOI] [PubMed] [Google Scholar]
- [37].Willman JH, White K, Coffin CM. Pediatric core needle biopsy: strengths and limitations in evaluation of masses. Pediatr Dev Pathol 2001;4:46–52. [DOI] [PubMed] [Google Scholar]
- [38].Pramesh CS, Deshpande MS, Pardiwala DN, et al. Core needle biopsy for bone tumours. Eur J Surg Oncol 2001;27:668–71. [DOI] [PubMed] [Google Scholar]
- [39].Yao L, Nelson SD, Seeger LL, et al. Primary musculoskeletal neoplasms: effectiveness of core-needle biopsy. Radiology 1999;212:682–6. [DOI] [PubMed] [Google Scholar]
- [40].Koscick RL, Petersilge CA, Makley JT, et al. CT-guided fine needle aspiration and needle core biopsy of skeletal lesions. Complementary diagnostic techniques. Acta Cytol 1998;42:697–702. [DOI] [PubMed] [Google Scholar]
- [41].Dupuy DE, Rosenberg AE, Punyaratabandhu T, et al. Accuracy of CT-guided needle biopsy of musculoskeletal neoplasms. AJR Am J Roentgenol 1998;171:759–62. [DOI] [PubMed] [Google Scholar]
- [42].Pohlig F, Kirchhoff C, Lenze U, et al. Percutaneous core needle biopsy versus open biopsy in diagnostics of bone and soft tissue sarcoma: a retrospective study. Eur J Med Res 2012;17:29doi: 10.1186/2047-783X-17-29. [DOI] [PMC free article] [PubMed] [Google Scholar]


