Abstract
Gamma-glutamyl transferase (GGT) is involved in the pathogenesis of atherosclerosis and has been associated with adverse cardiovascular outcomes in patients with ischemic heart disease. However, the association between GGT and long-term mortality has not been studied in patients with acute myocardial infarction (AMI).
A total of 2239 AMI patients for whom serum GGT values were available and who underwent percutaneous coronary intervention (PCI) were enrolled in the COREA-AMI (CardiOvascular Risk and idEntificAtion of potential high-risk population in Korean patients with AMI) registry. Patients with acute liver injury were excluded. Patients were classified into 2 groups according to normal (n = 1983) or elevated (n = 256) levels of serum GGT. The primary clinical outcome was all-cause mortality. The secondary outcome was cardiac death and recurrent non-fatal myocardial infarction (MI).
The median follow-up period was 3.7 years, and both groups had similar characteristics. Patients with elevated GGT had significantly higher all-cause mortality compared to patients with normal GGT (21.9% vs. 14.4%, P = .001). The multivariate Cox proportional hazards model showed that elevated serum GGT level was independently correlated with mortality (hazard ratio 2.12[1.44–3.11]; P < .001). Although elevated serum GGT was independently associated with long-term mortality after 30 days after PCI, there was no association within 30 days after PCI. Elevated GGT was also associated with death of cardiac causes with statistical significance. In the subgroup analysis, stronger associations were observed in the young and female patients and in patients who had ST-segment elevation MI and preserved left ventricular ejection fraction at the first echocardiography after the indexed PCI.
Elevated serum GGT is an independent predictor of long-term mortality in AMI patients.
Keywords: gamma-glutamyl transferase, long-term mortality, myocardial infarction
1. Introduction
The enzyme gamma-glutamyl transferase (GGT) is present in the serum and on the surface of various cell membranes. GGT is considered a marker of liver or biliary tract diseases and alcohol consumption. However, GGT has recently been identified as a novel indicator of the development and prognosis of cardiovascular diseases. Although the exact mechanism has not been elucidated, the abundance of GGT in atheroma and its function in blood vessels may play a role. GGT catalyzes the first step in the extracellular degradation of glutathione. During the process of GGT-mediated glutathione degradation, low-density lipoprotein (LDL) is oxidized and accumulates in the arterial wall; this process is involved in the pathogenesis of atherosclerosis.[1] Moreover, degradation of the antioxidant glutathione results in formation of peroxide free radicals and, consequently, oxidative stress. Thus, the combination of abundant oxidized LDL and GGT in atherosclerotic plaques causes oxidative stress in the endothelium, which can affect plaque evolution and rupture.[2] Furthermore, many studies have reported an association between serum GGT levels and various established cardiovascular disease risk factors, such as hypertension, diabetes, metabolic syndrome, and coronary artery disease (CAD).[3–6] In addition, increased GGT levels in established CAD patients have been associated with an increase in secondary events, including myocardial infarction (MI), stroke, and cardiovascular death.[7,8] However, studies of the association between serum GGT levels and long-term clinical outcomes in patients with acute MI (AMI) have included only a few patients with ST-segment elevation MI (STEMI) and yielded inconsistent results.[9,10] Therefore, we investigated whether higher serum GGT levels can predict short-term and long-term mortality in patients with AMI.
2. Methods
This study used data from the CardiOvascular Risk and idEntificAtion of potential high-risk population in Korean patients with AMI (COREA-AMI) registry, which was designed to evaluate real-world outcomes in “all-comers” with AMI. The COREA-AMI, a large, observational registry included clinical, angiographic, short-term and long-term outcome data for AMI patients who underwent percutaneous coronary intervention (PCI) at 9 major cardiac centers in Korea between January 2004 and December 2009.[11]
Initially, our study sample included a total of 4748 patients. Among these, 2281 patients who had serum GGT values were available were enrolled. To avoid the confounding effects of unknown underlying active liver disease on the prognosis, patients who had a serum alanine aminotransferase (ALT) level >3 times the upper limit of normal (ULN) and the ALT greater than the level of aspartate aminotransferase (AST) were excluded (n = 28).[12] To avoid unreasonable deviation of GGT level, the data were trimmed with exclusion of extreme values. The value of 5 patients (who were excluded because of lower than measurable range) was recorded near to zero; these cases were deleted for the possibility of data collection errors. We screened the accessible electrical medical records of the patients from the highest GGT level. The number of patients with GGT level over 2× ULN), 3× ULN, and 5× ULN were 47 (2.1%), 14 (0.6%), and 9 (0.4%), respectively. We excluded the only 9 patients of over 5× ULN because 3 of them had obvious hepatobiliary problems; one died from GB cancer, another had recurrent cholangitis, and the other had pancreatic disease. We could not find clear reason of GGT elevation among the others. Finally, a total 2239 of patients were included in this analysis (Fig. 1).
Figure 1.
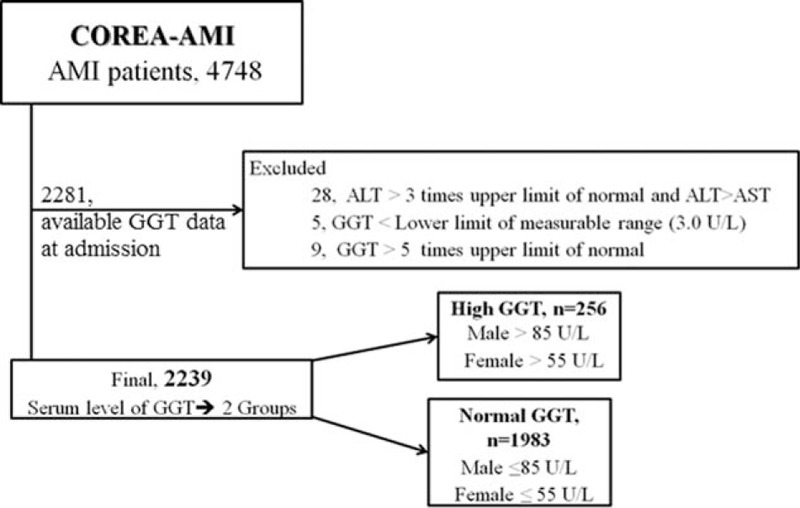
Study flow chart. Inclusion and exclusion criteria of study population. ALT = alanine aminotransferase, AMI = acute myocardial infarction, AST = aspartate aminotransferase, GGT = gamma-glutamyl transferase.
AMI was diagnosed based on characteristic clinical symptoms, serial changes on electrocardiograms (ECGs) consistent with infarction, and increased cardiac enzyme values. The diagnosis was confirmed by coronary angiography in all patients. We excluded patients who were not indicated for PCI based on coronary angiography to strengthen the homogeneity of the study population. All patients received standard medical treatment during PCI and hospitalization. The study protocol was approved by the institutional review board at each participating center and is in accordance with the Declaration of Helsinki. All patients provided written informed consent at the time of admission to enrollment in the registry and the use of their clinical data in future retrospective analyses.
We recorded demographic data, cardiovascular risk factors, and laboratory data for all patients. Cardiovascular risk factors included smoking status, previously diagnosed diabetes mellitus, hypertension, chronic kidney disease, and history of familial CAD. Data on other risk factors were reported by the patients themselves or extracted from medical records. Blood samples were drawn within 24 hours of the initial visit and used for a standard battery of hematological and biochemical tests. Serum GGT levels were measured using the enzymatic colorimetric test at 37°C, and l-g-glutamyl-3-carboxy-4-nitroanilide was used as the substrate at each cardiac center under identical conditions.[10] Patients were categorized into 2 groups based on elevated or normal serum GGT levels compared to the upper limit of the clinical reference range. The normal reference range was 9 to 85 U/L for males and 5 to 55 U/L for females.[13]
All procedures were performed according to current standard guidelines. The specific drug-eluting stent used in the procedures was chosen by the operator. The operator assessed the type of lesion according to American College of Cardiology/American Heart Association guidelines. After the procedure, aspirin was prescribed indefinitely, and clopidogrel was prescribed for at least 6 months. Immediate post-procedural and in-hospital events were recorded. Patient follow-up was conducted during office visits or through telephone interviews at 1, 6, and 12 months and annually thereafter. Echocardiography was performed within 3 days of the PCI, and a quantitative assessment of the left ventricular systolic function was performed using the modified biplane Simpson method to calculate the left ventricular ejection fraction (LVEF).
The primary objective of this study was to evaluate the association between GGT level and all-cause mortality during clinical follow-up post intervention. The secondary objectives were to evaluate the association between high GGT levels and cardiac death and recurrent non-fatal MI. Cardiac death was defined as death from CAD, heart failure, or arrhythmia, and death was attributed to cardiac events unless non-cardiac death could be clearly identified.[14] Recurrent MI was defined as the presence of recurrent symptoms and new ECG changes that considered to be MI or cardiac markers that were at least twice the normal limit. Medical records were thoroughly reviewed by an independent research nurse. Telephone interviews were conducted to collect data on the occurrence of adverse events following PCI. Clinical outcomes of interest were confirmed by source documents and centrally adjudicated by a local events committee at the Cardiovascular Center of Seoul St. Mary's Hospital and an independent group of clinicians who were unaware of patient status. To verify the accuracy of mortality data, we matched our data to official national data collected by the National Statistical Office from death certificates, which previous studies have shown to be reliable.[11]
We classified patients into two groups according to normal or high GGT levels and used these 2 categories in the subsequent analyses. Differences between groups of continuous variables were evaluated using an independent t-test or the Mann-Whitney U test. Differences in discrete variables were analyzed using a chi-square or Fisher's exact test and expressed as counts and percentages. Landmark analyses were performed to evaluate the impact of high serum GGT on short-term and long-term mortality. The landmark method of survival analysis uses a fixed time after PCI. In this study, the cut-off for early mortality was the 30th day after PCI. We constructed Kaplan-Meier curves to the end points for patients with normal GGT or high GGT, and differences between the groups were assessed by the log-rank test. Cox proportional hazard models were applied to calculate estimated hazard ratios (HRs) for each end-point. We selected covariates that differed significantly between the groups at baseline and that previous studies have related to GGT level or cardiovascular outcomes after PCI. [15,16] The HRs were adjusted for important covariates that had significant effects (p < 0.05) on clinical outcomes in the univariate analysis. All analyses were two-tailed, and clinical significance was defined as p < 0.05. The same process was used for subgroup analyses to evaluate differences according to age, gender, STEMI or Non ST-segment elevation MI (NSTEMI), body mass index (BMI), high or low levels of LDL cholesterol (LDL-C), high or low levels of high-density lipoprotein (HDL) cholesterol (HDL-C), hypertriglyceridemia (triglyceride >150), glycated hemoglobin(HbA1c), and LVEF ≥50% at the first echocardiography after the indexed PCI.
To analyze the association between GGT levels and the mortality in our study group of AMI patients, we computed receiver-operating characteristic (ROC) curves, tested for equality of the areas under the curves (AUCs), and calculated 95% confidence intervals (CIs) for GGT. Statistical analyses were performed using the statistical package SPSS V.20.0 (SPSS Inc., Chicago, IL) and MedCalc V.12.7 (MedCalc Software, Mariakerke, Belgium).
3. Results
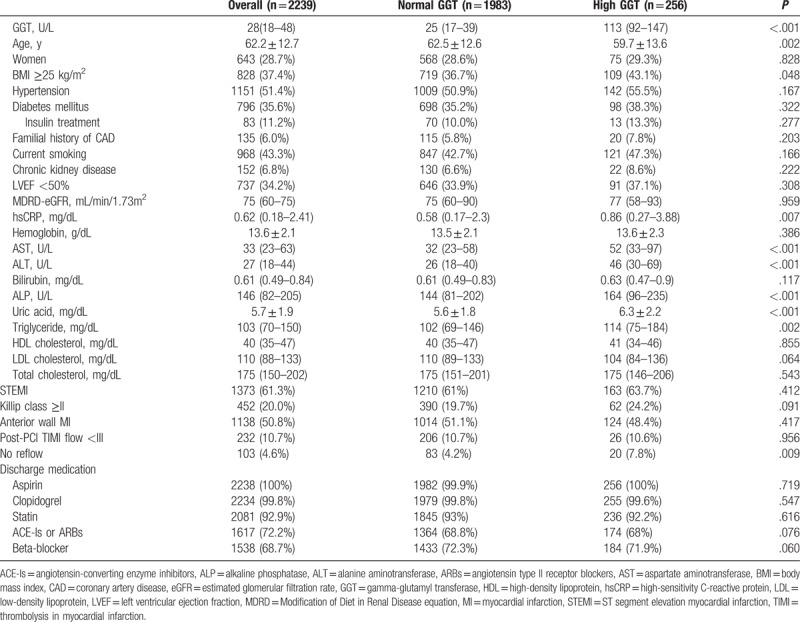
Patient GGT levels were non-normally distributed, and a high GGT level, defined as above the normal range, was observed in 256 patients (11.4%). The median GGT level was 31 ( interquartile range [IQR] 20–55; mean 46 ± 44.6) U/L in males and 21 (IQR 13–34; mean 29.3 ± 26.4) U/L in females, and the percentages of male and female patients with high GGT levels were 11.3% and 11.7%, respectively. The baseline characteristics of the GGT groups are summarized in Table 1. At baseline, patients in the high serum GGT group had more conventional cardiovascular risk factors compared to the normal serum GGT group. Patients in the high GGT group were younger than those in the normal serum GGT group (mean age of 59.7 ± 13.6 vs. 62.5 ± 12.6 years, respectively). Compared with the normal GGT group, more patients in the high GGT group were obese (BMI ≥25 kg/m2; 36.7% vs. 43.1%, P = .048). High-sensitive C-reactive protein (hsCRP), uric acid, and serum triglyceride were positively associated with high serum GGT levels, and the difference of them was also significant. No reflow phenomenon after PCI was also more frequently observed in high GGT group (7.8% vs. 4.2%, P = .009). Biomarkers associated with liver disease, including AST, ALT, and alkaline phosphatase (ALP), were higher in the high GGT group. No other differences were observed between the two groups.
Table 1.
Baseline characteristics of normal GGT group and high GGT group.
A total of 341 deaths (15.2%) were recorded during a median follow-up time of 3.7 years (IQR: 2.4–5.0 years). The number of cardiac death and noncardiac death of high GGT versus normal GGT group was 31 (12.1%) vs. 153 (7.7%) and 19 (7.4%) vs.123 (6.2%), respectively. The proportion of unrevealed cause of death was 2.3% (n = 6) vs. 0.9% (n = 18). All-cause mortality during the entire follow-up period was significantly higher in the high GGT group than the normal GGT group (21.9% vs. 14.4%, P = .001 by the log-rank test). Early mortality at day 30 following PCI (5.9% vs. 3.2%, P = .03) and late mortality from day 30 to the end of follow-up (17.1% vs. 11.5%, P = .013) were also higher in the high GGT group than the normal GGT group. The Kaplan-Meier curves for all-cause mortality are presented in Figure 2. Compared with the normal GGT group, the high GGT group had an age and sex adjusted HR for death of 1.97 (P < .0001, Model 1). Additional adjustment for differences at baseline (Model 2: BMI ≥25 kg/m2, ALT, AST, ALP, uric acid, hsCRP, hypertriglyceridemia (triglyceride >150), and presence of no-reflow phenomenon after PCI) and other cardiovascular risk factors (Model 3: hypertension, diabetes, chronic kidney disease, current smoking, high LDL-C (LDL ≥100 mg/dL), Killip class ≥ II at admission and final TIMI flow <III after PCI) could not attenuated this relationship. Elevated GGT remained an independent risk factor for all-cause death (multivariable-adjusted HR 2.12, P < .001) (Table 2). In landmark analysis, the effect of high GGT group on the long-term mortality after 30 days after indexed PCI was consistent with the result of overall period death (adjusted HR 1.81, P = .009), but there was no difference in early 30 days mortality between 2 groups. When we analyzed cardiac death separately, the association of GGT level was consistent with the former result (multivariable-adjusted HR 1.86 P = .037) (Fig. 3). The total number of patients who suffered nonfatal MI was 43, the difference between high and normal GGT group was insignificant (n = 9 [3.5%] vs. n = 34 [1.8%], P = .107).
Figure 2.
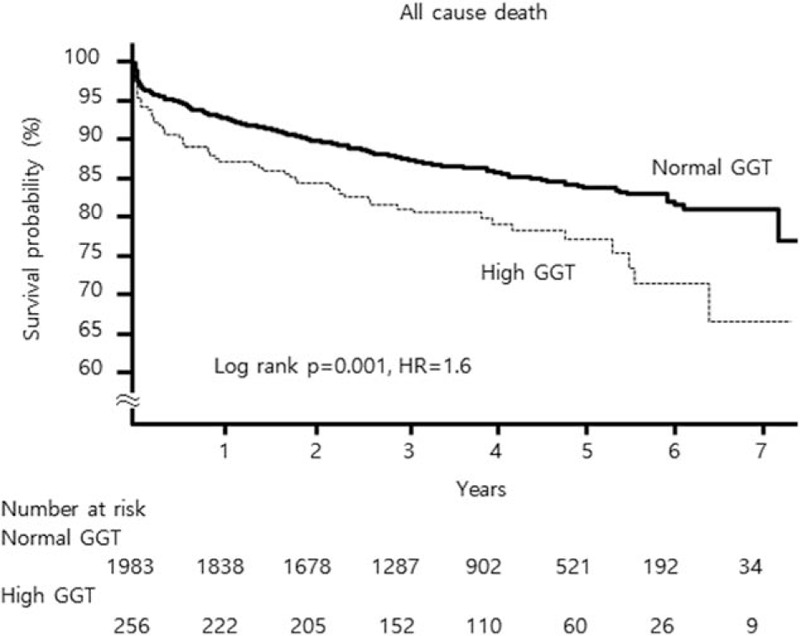
Kaplan-Meier survival curves. All-cause mortality of patients with acute myocardial infarction according to normal or high serum GGT level. GGT = gamma-glutamyl transferase.
Table 2.
Hazard ratios and 95% confidence intervals for mortality according to the 2 groups of serum GGT.
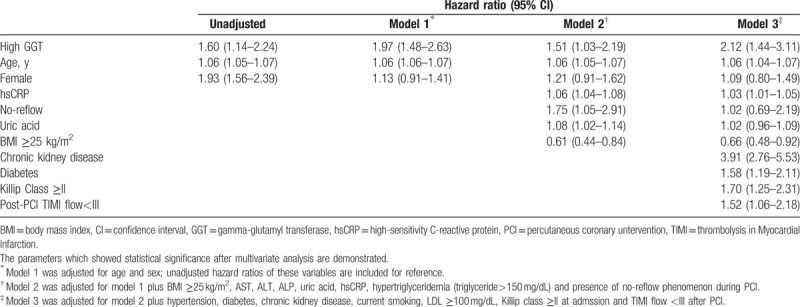
Figure 3.
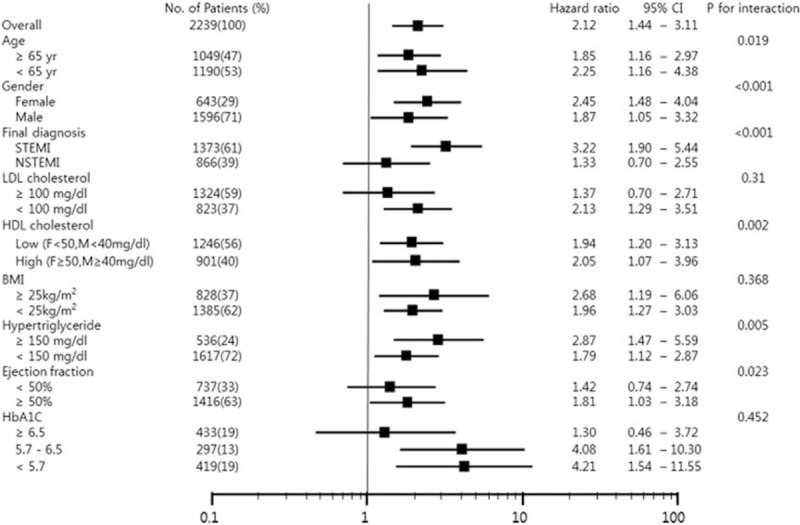
Subgroup analysis. The association between serum GGT level and all-cause mortality differed among the specific groups according to age, sex, final diagnosis, LDL cholesterol, HDL cholesterol, body mass index, hypertriglyceride, ejection fraction and HbA1c. HRs were adjusted for age, sex, hypertension, diabetes, chronic kidney disease, current smoking, BMI ≥25 kg/m2, AST, ALT, ALP, uric acid, hsCRP, hypertriglyceridemia (triglyceride >150 mg/dL), high LDL cholesterol (LDL ≥100 mg/dL), Killip class ≥II at admssion, presence of no-reflow phenomenon during PCI, and TIMI flow <III after PCI. ALP = alkaline phosphatase, ALT = alanine aminotransferase, AMI = acute myocardial infarction, AST = aspartate aminotransferase, BMI = body mass index, GGT = gamma-glutamyl transferase, HDL = high-density lipoprotein, HR = hazard ratio, hsCRP = high-sensitivity C-reactive protein, LDL = low-density lipoprotein, PCI = percutaneous coronary intervention, TIMI = thrombolysis in myocardial infarction.
The results of the subgroup analysis are presented in Figure 3. The association between serum GGT level and all-cause mortality differed among the specific groups. After stratifying by sex, an association between serum GGT levels and all-cause death was observed in both female and male patients. However, the association was stronger in female patients than in male patients (P for interaction, <.001). When stratified by the presentation of MI depending on the ST-segment change, the association between serum GGT levels and all-cause mortality was only observed among STEMI patients (P for interaction, <0.001). Also, the association between high serum GGT levels and all-cause mortality was significant in who had preserved LVEF at admission (LVEF ≥50%, P for interaction, .023). The association between high GGT levels and all-cause mortality was stronger in patients under the age of 65 years (P for interaction, .019) and in those had high HDL-C (P for interaction, .002), but the HR value of each subgroup showed just a slight difference. Additionally, the lower the HbA1c level of subgroups, the association tended to be stronger, but there were too many missing values (n = 1090) to show statistical significance (P for trend, <.452). The difference between obese and nonobese patients was not clear. Whether the value of GGT was more meaningful in low LDL-C (<100 mg/dL, HR = 2.13, P = .003) was not obvious (P for interaction = .31).
The baseline difference of male and female subgroup is presented in Table 3. Male and female AMI patients have very different cardiovascular risk factors, respectively. Men tend to be obese and have more history of current smoking. Male AMI patients’ uric acid level is higher than women's and HDL-C is lower. Female AMI patients are much older and have more hypertension and diabetes. Estimated glomerular filtration rate and hemoglobin are significantly lower in these patients, and total and LDL-C level is higher. Furthermore, more females presented as worse Killip class and NSTEMI. As a result, the prognosis of female AMI patients was shown much worse than male patients (mortality 22.4% vs.12.3%). On this basis, we repeated the same survival analysis on each sex group. Figure 4 is a summary of the association of high GGT and mortality in according to sex. After stratifying by sex, a stronger association between serum GGT level and all-cause death was observed in female patient. Especially in long-term mortality after 30th day, the association was observed only in females. In the aspect of cardiac death, the association was significant only in females, but the difference between 2 sex groups turned to be uncertain after multivariable adjustment.
Table 3.
The baseline characteristics of male and female AMI patients.
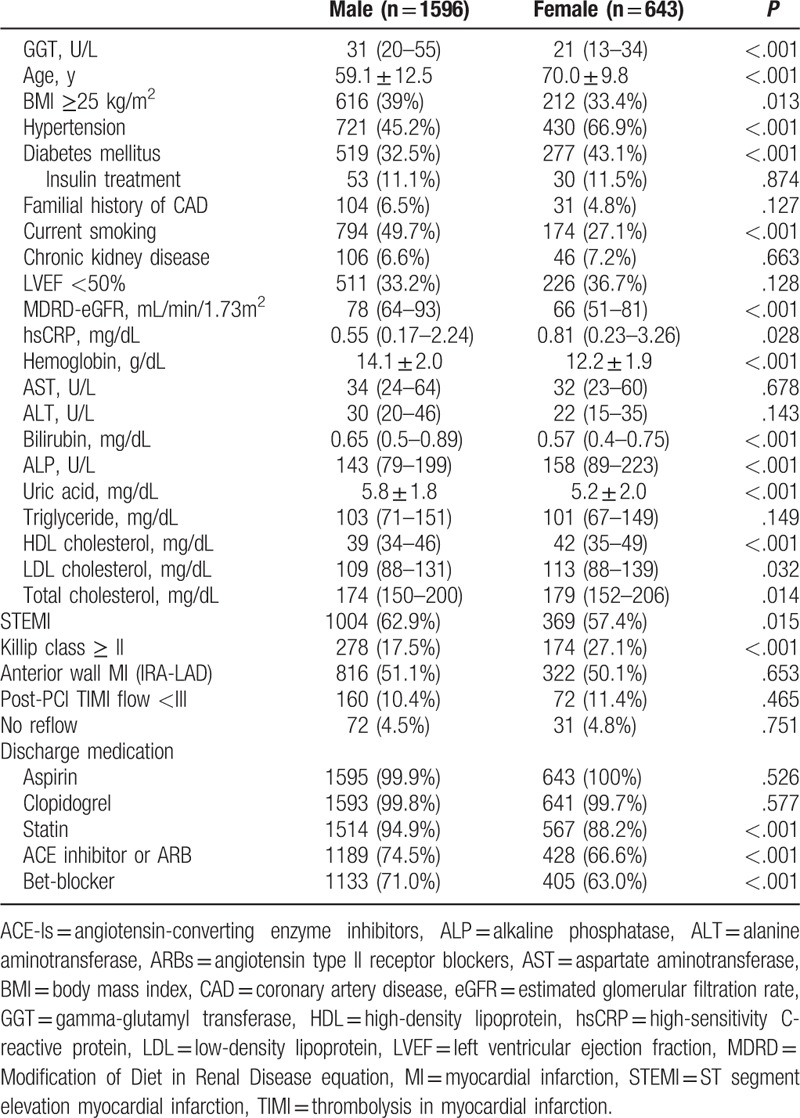
Figure 4.
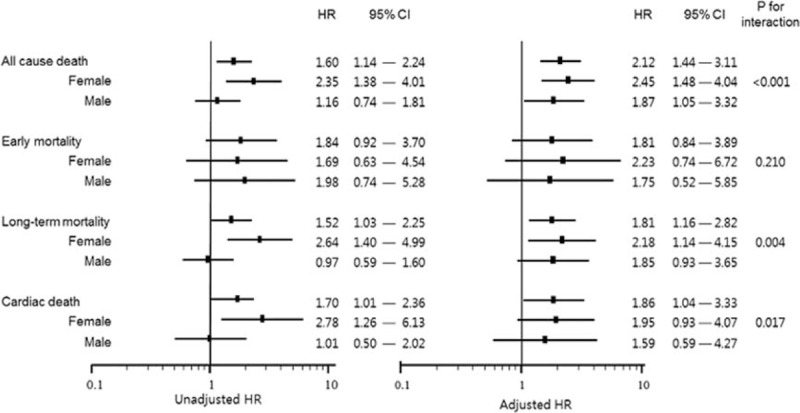
Univariate- and multivariate-adjusted time-to-death curves of high gamma-glutamyl transferase (GGT) on normal GGT group about all-cause mortality (early within 30 days and long term) and cardiac mortality in according to female and male. HRs were adjusted for age, hypertension, diabetes, chronic kidney disease, current smoking, body mass index ≥25 kg/m2, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, uric acid, high-sensitivity C-reactive protein, hypertriglyceridemia (triglyceride >150 mg/dL), high low-density lipoprotein (LDL) cholesterol (LDL ≥100 mg/dL), Killip class ≥ II at admssion, presence of no-reflow phenomenon during percutaneous coronary intervention (PCI) and thrombolysis in myocardial infarction flow <III after PCI. CI = confidence interval, HR = hazard ratio.
ROC curve analyses were done to find out the cutoff value. It demonstrated that cutoff values of the serum GGT for predicting all-cause mortality could not be determined (AUC = 0.501, P = .94). In female group, we could find statistical significant value (AUC = 0.58, P = .003), but AUC showed very low discrimination accuracy. The cutoff value of GGT in female group was 58 U/L with 21.5% sensitivity and 91.6% specificity. It was very close to the minimum value of in female high GGT group in our study (57 U/L) (Fig. 5).
Figure 5.
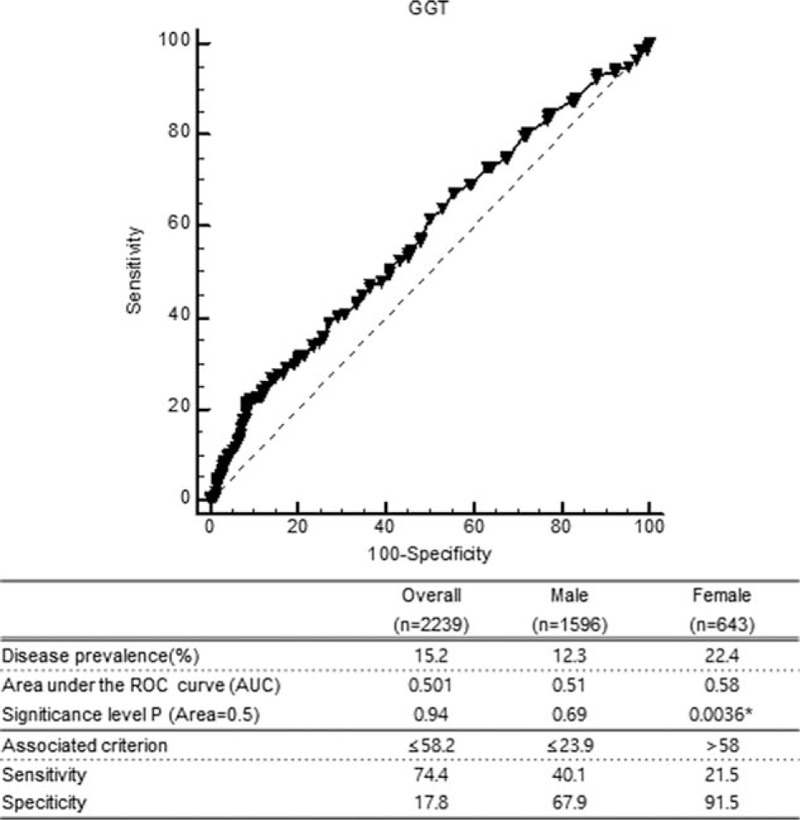
ROC curve analyses of serum GGT level and all-cause mortality in female acute myocardial infarction patients. AUC = area under the curve, GGT = gamma-glutamyl transferase, ROC = receiver-operating characteristic.
4. Discussion
In this study, we observed a significant association of serum GGT levels with all-cause mortality and identified serum GGT as a reliable, independent predictor of long-term mortality and cardiac mortality following AMI over a median follow-up period of 3.7 years. Interestingly, high GGT exhibited worse clinical outcomes in the female and in patients with STEMI and normal LVEF. These results are consistent with those of previous studies. In addition, this study is stronger than previous studies because it involved a larger AMI sample in which all subjects received PCI with drug eluting stent and had a longer follow-up period.
These findings may be explained by changes in lipid metabolism in the setting of AMI, in association with an acute-phase reaction and inflammatory response.[17] The patterns of alteration in the composition of lipid profile were wide in various previous reports, but a consensus about active inflammatory changes after AMI was developed. As previously explained, GGT is an enzyme associated with lipid metabolism and oxidative stress; therefore, the level of serum GGT at admission may reflect the degree of baseline change in lipid metabolism and inflammation after AMI, which may have implications for prognosis. In a small cohort study suggest the alteration of lipid metabolism might be result of secondary liver failure because of extensive and severe MI,[18] but the impact of high GGT on mortality was not altered after adjustment of liver enzyme and lipid profiles. It suggests the independent role of GGT on inflammation process apart from the level of lipids and liver enzymes.
The differences in baseline characteristics between the 2 GGT groups observed in this study are also consistent with explanation. The inflammatory markers hsCRP[19] and uric acid[20] were significantly higher in the high GGT group. Uric acid is considered a metabolic product of inflammation and is elevated in various inflammatory conditions such as CAD and heart failure.[21,22]
In general, LDL-C is the most important determinants of atherosclerotic events in the lipid profile.[17] It is interesting that there were some trends toward lower LDL-C levels in patients with high GGT. In our multivariable analysis, the high LDL-C tended to be preventive for mortality (≥100 mg/dL; HR = 0.81 P = .139) or LDL (as continuous variable, mg/dL; HR 1.0, P = .309), but it was not statistically significant. The use of statins could be a confounding factor, but unfortunately there was no information about either the usage of statin at the time of blood sampling or the level of LDL-C at the time of discharge in our registry. These might be one of the limitations of retrospective data from real world practice. Other lipid profiles including HDL-C and triglyceride have been previously reported in association with GGT, but the results were conflicting as our study.
Subgroup analyses revealed that the association between serum GGT level and all-cause mortality were more significant in STEMI patients. This association may reflect difference in nature of coronary obstruction in STEMI and NSTEMI. STEMI is primarily attributed to acute plaque rupture owing to inflammation, suggesting a role for GGT. This discrepancy may also be because of the inclusion of an insufficient number of NSTEMI patients.
Adverse outcomes may also be attributed to the association between serum GGT level and metabolic syndrome. Obesity and hypertriglyceridemia were more frequently observed in the high GGT group. In this study, high GGT was more significant in patients who were younger than 65 years or who had normal left ventricular function. Such patients are usually expected to have a favorable prognosis but may be negligent in their long-term health care. In this regard, GGT levels may serve as a guide for these patients because they may receive the greatest benefit from being aware of metabolic disturbances. Although there was no statistical significance, the lower the HbA1c level of subgroups, the association tended to be stronger with GGT that could be an implication in the same vein.
To determine whether male and female patients with AMI differed with baseline risk factors and treatment, we repeated analysis of baseline characteristics after re-grouping. Age at the time of MI showed big difference as previous studies,[23] women were much older than men. So, the greater risk of mortality and morbidity towing to age may attenuate the other risk difference of females. But in our study, after adjustment for age the meaning of high GGT was not changed. Our study reported a higher percentage of hypertension, diabetes mellitus and higher killip classes in women, whereas current smoking, obesity, familial history of CAD, and presentation as STEMI tended to be more in men. The distribution of risk factors was different between them and these findings are consistent with previous reports,[23] then we can find out that we should assess differently and separately of each sex group. Interestingly, there was significant difference of discharge medication between 2 groups. In females, statins were less prescribed in spite of higher total and LDL-C, and angiotensin-converting enzyme-inhibitors, angiotensin type II receptor blockers, and beta-blockers were not prescribed either to more females those tended to present lower LVEF. These factors may explain the higher long-term mortality of women with AMI.
A previous STEMI study suggested that the differences in microvascular reperfusion after PCI between men and women may attribute to higher risks in female.[24] In the study, TIMI myocardial perfusion grade and incomplete ST segment resolution were used as a parameter of microvascular dysfunction, and females had lower TIMI grade and more incomplete ST segment resolution. In our study, the percentage of lower TIMI flow grade after PCI and presence of no-reflow phenomenon during PCI were compared; no significant difference was observed between both sex groups. But presence of no-reflow was significantly different between high GGT and normal GGT group. Furthermore, no-reflow and TIMI flow after PCI were also important co-variables with clinical significance in multivariable analysis of all-cause mortality in association with GGT. This might be an implication of the association of GGT and myocardial microvascular dysfunction, but more concrete investigation will be needed.
We made 3 interesting observations concerning the association between elevated serum GGT levels and clinical outcome in patients with AMI. First, elevated serum GGT levels are a useful marker for easily and reliably predicting long-term clinical outcomes in patients with AMI. GGT levels are reportedly higher in patients with CAD than in the general population, and GGT levels are higher in patients with NSTEMI and STEMI than in patients with unstable angina.[19] In our AMI population, the proportion of patients with high GGT levels who did not have overt liver disease exceeded 11%, and the results were statistically significant after adjusting for liver markers such as AST, ALT, and ALP. Therefore, this method is not only acceptable but also likely easy to apply in clinical practice. Second, elevated GGT levels were more strongly associated with clinical outcome in female patients than in male patients. No previous studies have examined sex differences in the association between GGT level and outcomes in MI.[23] Studies of the association of GGT and other cardiovascular risk factors have observed heterogeneity in sex differences. In 2 studies of coronary calcification and hypertension in Korea, GGT level was an independent predictor in men but not women.[25,26] However, one study of the association between GGT and vascular events observed a significant positive association between GGT levels and cardiovascular disease in women.[27] The sex differences observed in our study may be because of the differences in alcohol consumption between men and women. More Korean men drink alcohol than women.[26] As an alternative explanation, BMI differed significantly between the normal and high GGT groups and between men and women. There was a striking 34% to 39% reduction in mortality in patients with BMI ≥25 kg/m2 at Table 2 and the patients with BMI ≥25 kg/m2 were more in men than women. It is mostly understood that patients with overweight or mild obesity (BMI of 25–30 kg/m2) might have relatively better outcomes, so this might affect the result consequently. Severe obesity (BMI >30 kg/m2) is commonly considered as a predictor of worse outcomes, and the mortality of overt obese (BMI >30 kg/m2; n = 97, 11.3%) group tended to be higher than that of overweight (BMI of 25–30 kg/m2; n = 731, 9.7%) group in our AMI patients, but it was not statistically significant (HR = 1.21, Log rank, P = .557).
Third, our study population is unique in that we included both STEMI and NSTEMI patients and all patients underwent PCI with drug eluting stent. Previous studies have included either STEMI patients or non-ST segment elevation-ACS patients. According to current guidelines on long-term management following ACS, the treatment strategy depends primarily on whether the clinical outcome is MI.[28–30] Therefore, the overall outcome following AMI is also crucial. The all-cause mortality rates in our study population and in the high GGT level group were slightly higher and much higher, respectively, than those in the HORIZONS AMI trial in patients with STEMI (approximately 6%–7%).[31] When the early mortality rate was assessed, it was similar to the result that obtained using the long-term outcome. This result suggests that the initial GGT level might reflect not only the acute phase of inflammation, but also the chronic systemic metabolic status of an individual, which can affect the long-term outcome.
5. Limitations
This study has several limitations. First, this was an observational study and may be subject to bias and confounding. Specifically, information on alcohol consumption, which can influence the level of GGT, was not included in this study and 52% of patients did not have a GGT serum level recorded. Moreover, the exclusion of liver disease in patients was not confirmed by imaging or serology for viral hepatitis.[27,32] Isolated GGT elevation has not been significantly associated with adverse outcomes in liver disease; therefore, the relevance of GGT elevation in MI should not to be attenuated.
Second, there were a limited number of adverse events, and we were unable to specify the various subtypes of adverse cardiovascular events. Third, although many possible hypotheses of the mechanisms of GGT have existed, no clear evidence was proven. Even there is no report on the time course of GGT in the setting of AMI like AST, LDH, or CPK. Recently, various subtypes of GGTs with specific functions have been studied.[33,34] These studies might be a key to understand the mechanism of the association between GGT and cardiovascular disease. In addition, a large prospective study with serial GGT values and associated variables will contribute to a detailed understanding of the pathogenesis of cardiovascular disease.
6. Conclusion
In summary, an elevated GGT level is an independent predictor of adverse long-term prognosis and increase of cardiac mortality in patients with AMI. Stronger associations were observed in the young and female patients and in patients who had STEMI and initially preserved LVEF after the indexed PCI.
Author contributions
Conceptualization: Eun Ho Choo, Kiyuk Chang.
Data curation: Eun Ho Choo, Kiyuk Chang, Ki-Bae Seung.
Formal analysis: Jae Gyung Kim, Eun Ho Choo, Kiyuk Chang.
Investigation: Jae Gyung Kim, Kiyuk Chang, Ki-Bae Seung.
Methodology: Jae Gyung Kim, Eun Ho Choo.
Project administration: Jae Gyung Kim, Kiyuk Chang.
Resources: Kiyuk Chang, Ki-Bae Seung.
Software: Jae Gyung Kim.
Supervision: Jong-Min Lee, Kiyuk Chang.
Visualization: Jae Gyung Kim.
Writing – original draft: Jae Gyung Kim.
Writing – review & editing: Jae Gyung Kim, Kiyuk Chang.
Footnotes
Abbreviations: 2xULN = two-times of upper limit of normal, ALP = alkaline phosphatase, ALT = alanine aminotransferase, AMI = acute myocardial infarction, AST = aspartate aminotransferase, AUC = areas under the curve, BMI = body mass index, CAD = coronary artery disease, CI = confidence interval, ECG = electrocardiogram, GGT = gamma-glutamyl transferase, HbAc1 = glycated hemoglobin, HDL-C = High-density lipoprotein cholesterol, HR = hazard ratio, hsCRP = high-sensitivity C-reactive protein, IQR = interquartile range, LDL-C = low-density lipoprotein cholesterol, LVEF = left ventricular ejection fraction, MI = myocardial infarction, NSTEMI = Non ST-segment elevation myocardial infarction, PCI = percutaneous coronary intervention, ROC = receiver-operating characteristic, STEMI = ST-segment elevation myocardial infarction, TIMI = thrombolysis in myocardial infarction.
The authors claim no relationships with industry.
The authors report no conflicts of interest.
References
- [1].Paolicchi A, Minotti G, Tonarelli P, et al. Gamma-glutamyl transpeptidase-dependent iron reduction and LDL oxidation—a potential mechanism in atherosclerosis. J Investig Med 1999;47:151–60. [PubMed] [Google Scholar]
- [2].Emdin M, Pompella A, Paolicchi A. Gamma-glutamyltransferase, atherosclerosis, and cardiovascular disease: triggering oxidative stress within the plaque. Circulation 2005;112:2078–80. [DOI] [PubMed] [Google Scholar]
- [3].Pompella A, Emdin M, Passino C, et al. The significance of serum gamma-glutamyltransferase in cardiovascular diseases. Clin Chem Lab Med 2004;42:1085–91. [DOI] [PubMed] [Google Scholar]
- [4].Liu CF, Zhou WN, Fang NY. Gamma-glutamyltransferase levels and risk of metabolic syndrome: a meta-analysis of prospective cohort studies. Int J Clin Pract 2012;66:692–8. [DOI] [PubMed] [Google Scholar]
- [5].Onat A, Can G, Ornek E, et al. Serum gamma-glutamyltransferase: independent predictor of risk of diabetes, hypertension, metabolic syndrome, and coronary disease. Obesity (Silver Spring) 2012;20:842–8. [DOI] [PubMed] [Google Scholar]
- [6].Lee DS, Evans JC, Robins SJ, et al. Gamma glutamyl transferase and metabolic syndrome, cardiovascular disease, and mortality risk: the Framingham Heart Study. Arterioscler Thromb Vasc Biol 2007;27:127–33. [DOI] [PubMed] [Google Scholar]
- [7].Ruttmann E, Brant LJ, Concin H, et al. Gamma-glutamyltransferase as a risk factor for cardiovascular disease mortality: an epidemiological investigation in a cohort of 163,944 Austrian adults. Circulation 2005;112:2130–7. [DOI] [PubMed] [Google Scholar]
- [8].Lee DH, Silventoinen K, Hu G, et al. Serum gamma-glutamyltransferase predicts non-fatal myocardial infarction and fatal coronary heart disease among 28,838 middle-aged men and women. Eur Heart J 2006;27:2170–6. [DOI] [PubMed] [Google Scholar]
- [9].Ozcan F, Karakas MF, Ozlu MF, et al. Effect of serum gamma-glutamyl transferase levels on myocardial perfusion and long-term prognosis after primary angioplasty in patients with acute ST-elevation myocardial infarction. J Investig Med 2012;60:1186–93. [DOI] [PubMed] [Google Scholar]
- [10].Gul M, Uyarel H, Ergelen M, et al. The relationship between gamma-glutamyl transferase levels and the clinical outcomes in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Coron Artery Dis 2013;24:272–8. [DOI] [PubMed] [Google Scholar]
- [11].Choo EH, Chang K, Ahn Y, et al. Benefit of beta-blocker treatment for patients with acute myocardial infarction and preserved systolic function after percutaneous coronary intervention. Heart 2014;100:492–9. [DOI] [PubMed] [Google Scholar]
- [12].Senior JR. Alanine aminotransferase: a clinical and regulatory tool for detecting liver injury-past, present, and future. Clin Pharmacol Ther 2012;92:332–9. [DOI] [PubMed] [Google Scholar]
- [13].Mannion CM. General Laboratory Manual. Department of Pathology, Hackensack University Medical Centre; 2012:129. [Google Scholar]
- [14].Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115:2344–51. [DOI] [PubMed] [Google Scholar]
- [15].Turgut O, Tandogan I. Gamma-glutamyltransferase to determine cardiovascular risk: shifting the paradigm forward. J Atheroscler Thromb 2011;18:177–81. [DOI] [PubMed] [Google Scholar]
- [16].Akpek M, Elcik D, Kalay N, et al. The prognostic value of serum gamma glutamyl transferase activity on admission in patients with STEMI undergoing primary PCI. Angiology 2012;63:579–85. [DOI] [PubMed] [Google Scholar]
- [17].Reddy VS, Bui QT, Jacobs JR, et al. Investigators of National Registry of Myocardial Infarction b. Relationship between serum low-density lipoprotein cholesterol and in-hospital mortality following acute myocardial infarction (The Lipid Paradox). Am J Cardiol 2015;115:557–62. [DOI] [PubMed] [Google Scholar]
- [18].Fahie-Wilson M, Mills R, Wilson K. HDL cholestrol and the acute phase reaction following myocardial infacrtion and acute pancreatitis. Clin Chim Acta 1987;167:197–209. [DOI] [PubMed] [Google Scholar]
- [19].Emiroglu MY, Esen OB, Bulut M, et al. GGT levels in type II diabetic patients with acute coronary syndrome (does diabetes have any effect on GGT levels in acute coronary syndrome?). Acta Diabetol 2013;50:21–5. [DOI] [PubMed] [Google Scholar]
- [20].Lazzeri C, Valente S, Tarquini R, et al. The prognostic role of gamma-glutamyltransferase activity in non-diabetic ST-elevation myocardial infarction. Intern Emerg Med 2011;6:213–9. [DOI] [PubMed] [Google Scholar]
- [21].Onat A, Can G, Ornek E, et al. Elevated serum uric acid in nondiabetic people mark pro-inflammatory state and HDL dysfunction and independently predicts coronary disease. Clin Rheumatol 2013;32:1767–75. [DOI] [PubMed] [Google Scholar]
- [22].Wang Y, Tuomilehto J, Jousilahti P, et al. Serum gamma-glutamyltransferase and the risk of heart failure in men and women in Finland. Heart 2013;99:163–7. [DOI] [PubMed] [Google Scholar]
- [23].Bucholz EM, Butala NM, Rathore SS, et al. Sex differences in long-term mortality after myocardial infarction: a systematic review. Circulation 2014;130:757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pu J, Shan P, Ding S, et al. Gender differences in epicardial and tissure-level reperfusion in patients undergoing primary angioplasty for acute myocardial infarction. Athrosclerosis 2011;215:203–8. [DOI] [PubMed] [Google Scholar]
- [25].Lee W, Ryoo JH, Suh BS, et al. Association of coronary artery calcification and serum gamma-glutamyl transferase in Korean. Atherosclerosis 2013;226:269–74. [DOI] [PubMed] [Google Scholar]
- [26].Ha KH, Kim HC. Gender differences in the association between serum gamma-glutamyltransferase and blood pressure change: a prospective community-based cohort study. J Korean Med Sci 2014;29:1379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fraser A, Harris R, Sattar N, et al. Gamma-glutamyltransferase is associated with incident vascular events independently of alcohol intake: analysis of the British Women's Heart and Health Study and Meta-Analysis. Arterioscler Thromb Vasc Biol 2007;27:2729–35. [DOI] [PubMed] [Google Scholar]
- [28].Hamm CW, Bassand JP, Agewall S, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2999–3054. [DOI] [PubMed] [Google Scholar]
- [29].Jneid H, Anderson JL, Wright RS, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/Non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2012;126:875–910. [DOI] [PubMed] [Google Scholar]
- [30].O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:529–55. [DOI] [PubMed] [Google Scholar]
- [31].Stone GW, Witzenbichler B, Guagliumi G, et al. Heparin plus a glycoprotein IIb/IIIa inhibitor versus bivalirudin monotherapy and paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction (HORIZONS-AMI): final 3-year results from a multicentre, randomised controlled trial. Lancet 2011;377:2193–204. [DOI] [PubMed] [Google Scholar]
- [32].Leong DP, Smyth A, Teo KK, et al. Patterns of alcohol consumption and myocardial infarction risk: observations from 52 countries in the INTERHEART case-control study. Circulation 2014;130:390–8. [DOI] [PubMed] [Google Scholar]
- [33].Franzini M, Bramanti E, Ottaviano V, et al. A high performance gel filtration chromatography method for gamma-glutamyltransferase fraction analysis. Anal Biochem 2008;374:1–6. [DOI] [PubMed] [Google Scholar]
- [34].Franzini M, Fornaciari I, Rong J, et al. Correlates and reference limits of plasma gamma-glutamyltransferase fractions from the Framingham Heart Study. Clin Chim Acta 2013;417:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]


