Abstract
Background:
Matrix metalloproteinase-2 (MMP-2), a member of the zinc-dependent metalloproteinase gene family, plays a vital role in cancer invasion, metastasis, and progression. This systematic review and meta-analysis aims to explore the clinical significance of MMP-2 expression in endometrial cancer.
Methods:
PubMed, Embase, Cochrane Library, and China National Knowledge Infrastructure databases were systematically searched up to September 30, 2017, supplemented by manual searches of bibliographies. Two reviewers independently identified articles, extracted data, assessed quality, and cross-checked the results. Meta-analysis was conducted to explore the difference in the positive rate of MMP-2 expression between patients with endometrial cancer and those with endometriosis or normal endometrium, and to investigate the associations of MMP-2 expression with clinicopathologic characteristics of patients with endometrial cancer. Weighted mean differences and risk ratios (RRs) with 95% confidence interval (CI) were calculated for continuous and dichotomous variables, respectively.
Results:
Totally 20 studies were selected for this systematic review and meta-analysis. Compared with those with endometriosis or normal endometria, the positive rate of MMP-2 expression is significantly higher in patients with endometrial cancer (RR = 2.31, 95% CI: 1.78–3.00, P < .01). MMP-2 expression was significantly associated with Federation of Gynecology and Obstetrics stage (RR = 1.19, 95% CI: 1.09–1.31, P < .01), histologic grade (RR = 1.10, 95% CI: 1.01–1.19, P = .02), lymph node metastasis (RR = 1.32, 95% CI: 1.15–1.51, P < .01), and myometrial invasion (RR = 1.25, 95% CI: 1.12–1.38, P < .01).
Conclusion:
The results showed that MMP-2 was expressed in high percentage of endometrial cancer and its expression may be associated closely with clinical stage, and tumor invasion and metastasis, indicating that MMP-2 overexpression may serve as a predictive factor for poor prognosis of endometrial cancer.
Keywords: clinicopathologic characteristics, endometrial cancer, meta-analysis, matrix metalloproteinase-2
1. Introduction
Endometrial cancer is a common gynecologic malignancy ranked fourth in developed countries and also a common cause of death from female cancers ranked third.[1] It affected approximately 320,000 patients with the estimated death of 76,000 patients in 2012 worldwide.[2] With the industrialization, urbanization, and westernization of lifestyle, the incidence of endometrial cancer increased significantly, especially in developing countries.[3] Although 5-year survival is estimated to be more than 90% in early stage, those women with advanced stage, high-risk histology, poor differentiation, and metastasis to regional nodes may have poor prognosis, with only 57% in patients with stage III (regional diseases) and 19% in stage IV (distant spread diseases), respectively.[4] Therefore, identification of novel and more reliable markers to accurately predict prognosis of patients with endometrial cancer is urgently needed.
Matrix metalloproteinases (MMPs) represent a family of extracellular zinc-dependent endoproteinases, known for their capacity to degrade extracellular matrix components.[5] They play extremely pivotal roles in tumor invasion and infiltration, as well as in tumor angiogenesis.[6,7] Of the several MMPs analyzed in endometrial tumors, MMP-2 acts as a key enzyme that associated with tumor metastasis and physiologic function.[8] A large amount of studies investigated the expression of MMP-2 in endometrial cancer and its association with clinicopathologic characteristics, but the reported results were inconsistent. For instance, researchers suggested that overexpression of MMP-2 in endometrial cancer was correlated with lymph node metastasis,[9] but others failed to give the same results.[10]
The systematic review and meta-analysis were aimed to explore the difference in the positive rate of MMP-2 between patients with endometrial cancer and the patients with endometriosis or normal endometrium, and to study the associations between MMP-2 expression and clinicopathologic characteristics of patients with endometrial cancer, including clinical stages defined by International Federation of Gynecology and Obstetrics (FIGO) systems,[11] degree of differentiation, depth of myometrial invasion, and metastasis within lymph node.
2. Materials and methods
2.1. Search strategy
The systematic review and meta-analysis were performed in accordance with the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses.[12]
To identify clinical data in published studied for trail, we searched the English database including PubMed, Embase, and Cochrane Library, and the Chinese database, including China National Knowledge Infrastructure (CNKI). In English databases, we combined the search terms “matrix metalloproteinases-2” OR “MMP-2” OR “Gelatinase A” OR “collagenase type IV-A” AND “endometrial cancer” OR “endometrial carcinoma”. The search terms were translated into Chinese when the CNKI was searched. The search was performed at September 30, 2017. In addition, other relevant studies were selected by screened the bibliographies of included articles and reviews. This study was approved by the Ethics Committee of The First Hospital of Lanzhou University, but not involved patient consents that not required.
2.2. Eligibility criteria
Following criteria were used for included studies: patients with endometrial cancer, endometriosis, or normal endometrium; all cases were histologically diagnosed; and the expression of MMP-2 was detected by streptavidin-peroxidase (S-P) immunohistochemistry. We excluded the cell line study, animal study, letter, editorial, and review. If the same study published more than one paper, only the one with abundant information or with largest cases was included.
2.3. Data extraction and quality assessment
An extraction table was developed to assimilate data from included trials, which include general information regarding the identification of the publication, for example, first author's name, title, publication year, median age, affiliations, sample size, and pathologic characteristics such as clinical stages, degree of differentiation, depth of myometrial invasion, and metastasis of lymph node. When the original study not mentioned those information, “not reported (NR)” were present for the corresponding item. The extracted information was checked by 2 reviewers independently. Inspection of article were further done with discussion if the case with conflicting evaluation.
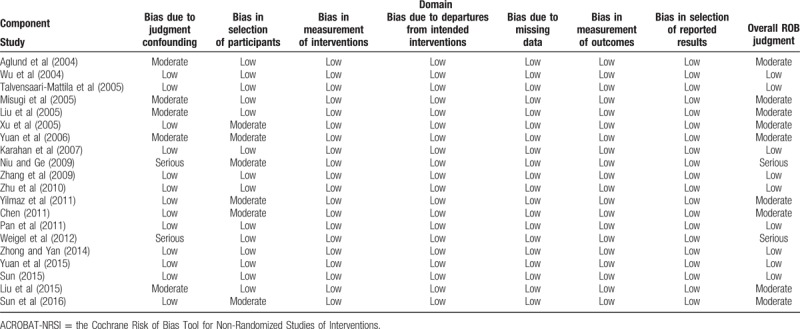
To evaluate the quality of included studies, the Cochrane Risk of Bias (RoB) Tool for Non-Randomized Studies of Interventions (ACROBAT-NRSI) was employed in the meta-analysis. The included studies were assessed based on 7 chronologically arranged bias domains (Table 1). Signaling questions flag potential for bias and help review authors judge RoB. Quality assessment was independently conducted by 2 authors and discussed for resolving disagreement.
Table 1.
Consensus ACROBAT-NRSI judgments between two reviewers by domain of bias–component studies.
2.4. Statistical analysis
Risk ratios (RRs) and weighted mean differences (WMDs) with 95% confidence intervals (CIs) were calculated, respectively, for dichotomous and continuous outcomes. P < .05 was considered statistically significant. Meta-analysis was performed using STATA (version 14; Stata Corp, College Station, TX). Heterogeneity analysis was performed by Cochran Q statistic and I2 statistic. Statistical significance for heterogeneity was considered if P < .05 or I2 > 50%. The fixed-effects model was applied when P > .05 and I2 < 50%, otherwise, the random-effects model was chosen. Additionally, we conducted sensitivity analyses by removing one study each time and recalculating pooled effects. Potential publications bias (considered present if P ≤ .1) was assessed by conducting statistical tests for funnel plot asymmetry as well as Egger test and Begg test.
3. Results
3.1. Characteristics of the included studies
The participant flow diagram for the study inclusion in the meta-analysis is shown in Figure 1. With the initial search strategy mentioned earlier, 318 papers potentially eligible for inclusion were screened. After excluding overlapping studies, irrelevant studies and studies without information of study objectives, 20 articles finally met the inclusion criteria.[9,10,13–30]
Figure 1.
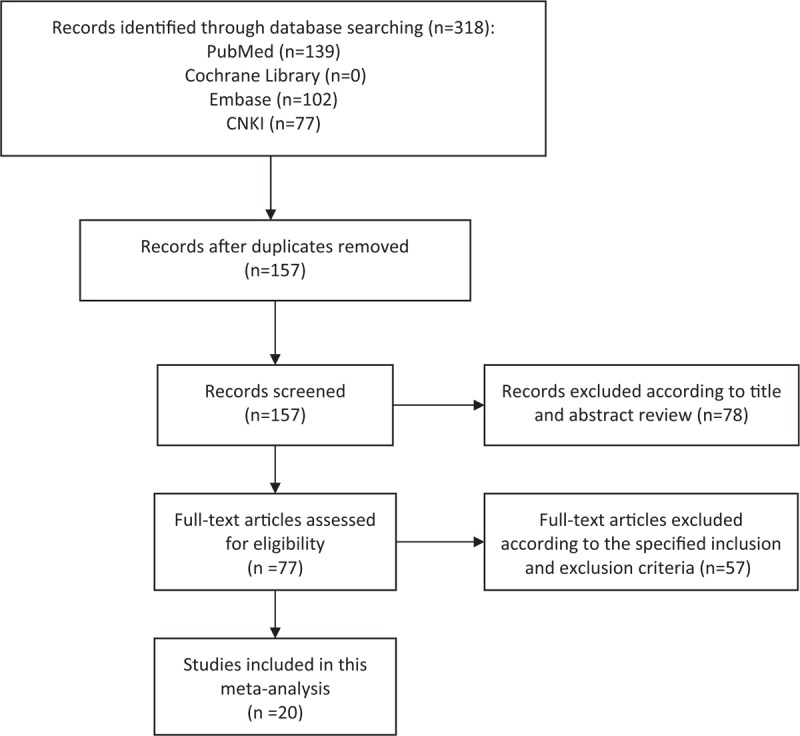
Literature search and selection of articles. CNKI = China National Knowledge Infrastructure.
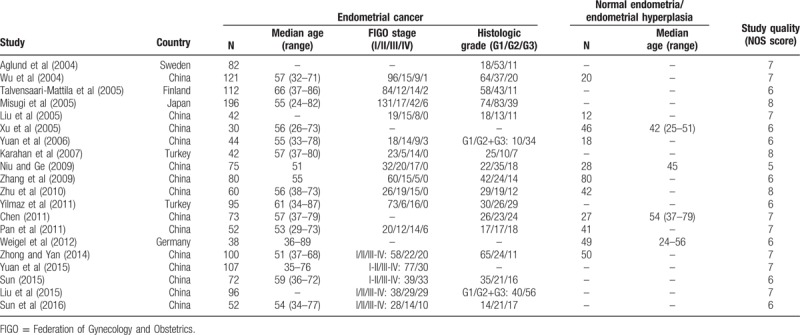
The major characteristics of the included studies were summarized in Table 2. These 20 studies involving 1569 cases with endometrial cancer and 333 cases with normal endometria or endometriosis were published from 2004 to 2016. Most studies (14 studies) evaluated patients from China, 2 from Turkey, and other 4 from Sweden, Finland, Japan, and Germany, respectively. Bias in most studies were low or moderate (Table 1). The bias due to confounding in the studies conducted by Niu and Ge[18] and Weigel et al[22] were serious as they reported neither the baseline distribution between groups nor previous treatment before the surgery. Difference in the positive rate of MMP-2 expression between patients with endometrial cancer and patients with endometriosis or normal endometria.
Table 2.
Basic characteristics and study quality of included studies.
A total of 11 studies compared the positive rate of MMP-2 expression between patients with endometrial cancer and patients with endometriosis or normal endometria. Figure 2 shows that there is substantial between-study heterogeneity in this meta-analysis of 11 studies (I2 = 70%, Cochran Q statistic P < .01), indicating that a random effect model should be employed. Compared with those with endometriosis or normal endometria, the proportion of cases expressing positive MMP-2 is higher in patients with endometrial cancer (RR = 2.31, 95% CI: 1.78–3.00, P < .01).
Figure 2.
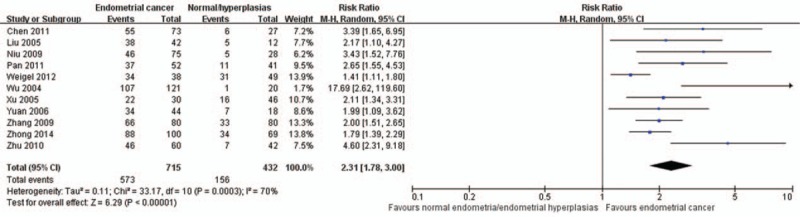
Comparison of positive rate of matrix metalloproteinase-2 expression between patients with endometrial cancer and those with endometriosis or normal endometria. CI = confidence interval.
3.2. Association between MMP-2 expression and clinicopathologic characteristics in patients with endometrial cancer
There are 15, 17, 14, and 18 studies reported the association of MMP-2 expression with FIGO stage (Fig. 3), histologic grade (Fig. 4), lymph node metastasis (Fig. 5), and depth of myometrial invasion (Fig. 6), respectively. Due to the significant heterogeneity among studies, random effect models were applied in all analyses. It is noted that MMP-2 staining was significantly associated with FIGO stage (RR = 1.19, 95% CI: 1.09–1.31, P < .01), histologic grade (RR = 1.10, 95% CI: 1.01–1.19, P = .02), lymph node metastasis (RR = 1.32, 95% CI: 1.15–1.51, P < .01), and myometrial invasion (RR = 1.25, 95% CI: 1.12–1.38, P < .01).
Figure 3.
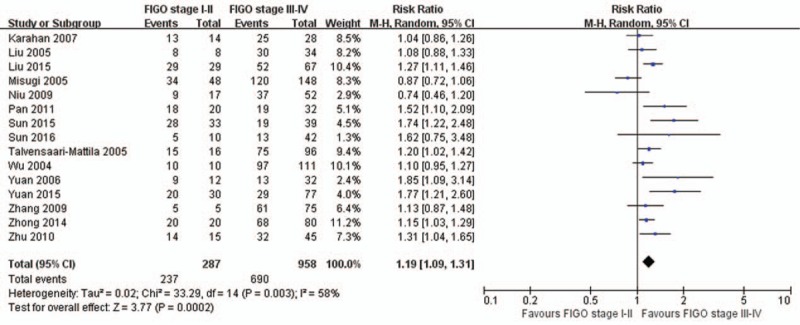
The association of matrix metalloproteinase-2 expression and Federation of Gynecology and Obstetrics (FIGO) stage. CI = confidence interval.
Figure 4.
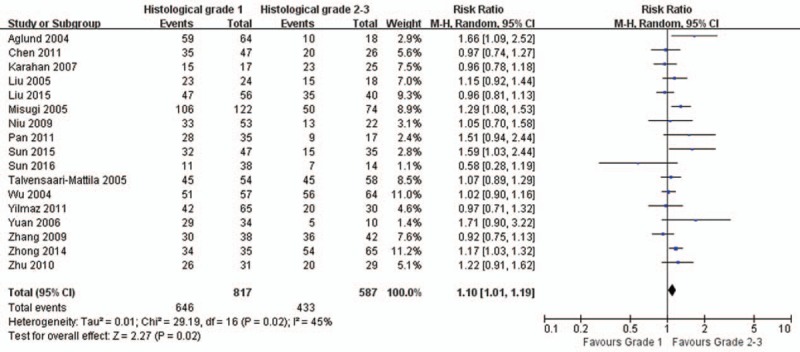
The association of matrix metalloproteinase-2 expression and histologic grade. CI = confidence interval.
Figure 5.
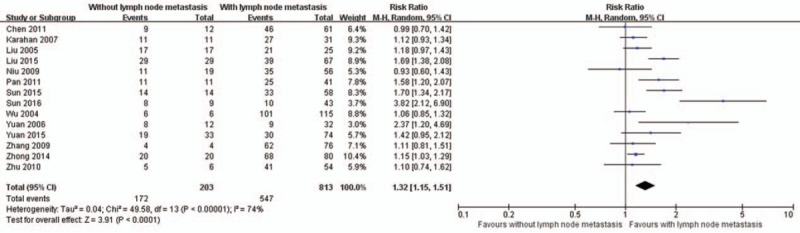
The association of matrix metalloproteinase-2 expression and lymph node metastasis. CI = confidence interval.
Figure 6.
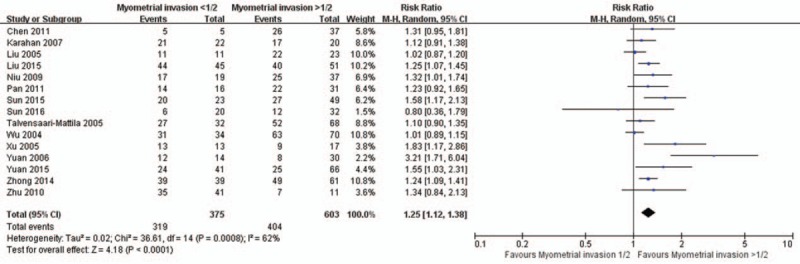
The association of matrix metalloproteinase-2 expression and depth of myometrial invasion. CI = confidence interval.
3.3. Sensitivity analysis
The effect of each study on the overall estimate was verified by calculating the combined results for the remaining studies with omitting the study. Finally, we found that the pooled RR was not significantly affected by individual study. In addition, the removal of 2 studies with serious bias did not significantly affect the outcomes.
3.4. Publication bias
To assess the possibility of publication bias for the association of MMP-2 expression with FIGO stages (Fig. 7A), histologic grades (Fig. 7B), lymph node metastasis (Fig. 7C), and depth of myometrial invasion (Fig. 7D) among the studies, funnel plots were generated. The funnel plot showed no obvious asymmetry, indicating that there was no obvious publication bias in our study, which was supported by Egger test (P = .27, P = .28, P = .73, and P = .27 for FIGO stages, histologic grades, lymph node metastasis, and depth of myometrial invasion, respectively) and Begg test (P = .92, P = .08, P = .58, and P = .32 for FIGO stages, histologic grades, lymph node metastasis, and depth of myometrial invasion, respectively).
Figure 7.
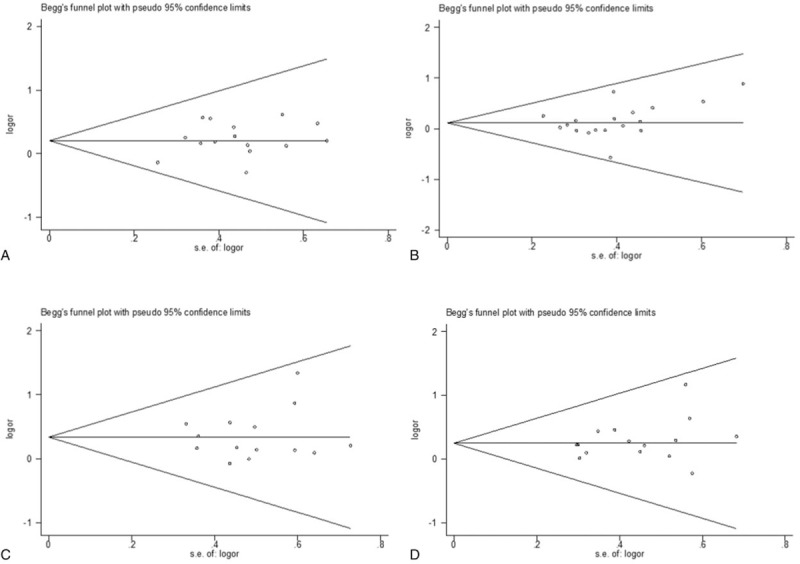
Funnel plots of the meta-analysis assessing the association of matrix metalloproteinase-2 expression with (A) Federation of Gynecology and Obstetrics (FIGO) stage; (B) histologic grade; (C) lymph node metastasis; and (D) depth of myometrial invasion.
4. Discussion
The mechanisms underlying the development and progression of endometrial cancer have not been fully elucidated.[31] Therefore, endometrial cancer is still a serious female health problem in the coming decades, and effective biomarkers with clinical significance are urgently needed.
The extracellular matrix and the basement membrane together constitute the first barrier in the process of tumor metastasis. The components of extracellular matrix are complex. It was reported that type IV collagen is the main component, which could be degrade by MMP-2 after fibrillar collages cleavaged by collagenases. It has been intensively investigated as a potential biomarker and unfavorable factor in a variety of systematic and multi-loci malignant tumors, such as laryngeal, breast, ovarian, and endometrial cancers.[6,32–34] Cymbaluk-Ploska et al[35] indicated that the area under the curve value for identifying patients with endometrial cancer with MMP-2 was 0.79, which was similar to the data for identify the lung cancer (0.75)[36] and bladder cancer (0.83).[37] Though the specificity values were reported in these studies, they cannot be directly compared due to inconsistent cutoff value, which need to be further explored by a large, well-designed clinical study. MMP-2 expression correlates also with tumor progression in neuroblastoma and papillary thyroid carcinoma.[38,39] This systematic review and meta-analysis found that MMP-2 is a potential biomarker for endometrial cancer as the positive rate of MMP-2 expression for patients with endometrial cancer is significantly higher than those with normal endometria or endometriosis.
During cell invasion and migration, extracellular matrix between cell–cell interaction or cell–extracellular matrix attachment were proteolytic modified by MMPs, which facilitated cancer cells metastasis.[40,41] High expression level of MMP-2 has been reported to be associated with in vivo invasion of tumors including oral squamous cell carcinomas (SCCs) and bladder carcinoma.[42,43] The levels of certain MMPs were important predictor of the risk of metastasis in primary tumor. The MMP-2 levels in tumor cells in uveal melanoma and SCC of tongue were associated with increased risk of metastasis.[44,45] Our study illustrated that the MMP-2 level was significantly correlated to myometrial invasion and metastasis within lymph node.
Recent studies have shown that MMPs also contribute to other processes in tumor progression such as cell growth and angiogenesis besides their roles in migration and invasion.[46] Our study demonstrated that the MMP-2 expression was positively associated with the clinical stages. Previous studies have indicated that the upregulation of MMP-2 is associated with the transition from histologic grade 1 to grades 2 and 3,[47,48] which is consistent with our result, that the MMP-2 correlate to the histopathologic grade of the endometrial cancer.
The analysis of the correlation between MMP-2 level and clinicopahologic characteristics revealed no publication bias. The sensitivity analysis showed that the estimation of risk in all the outcomes was not significantly affected by any single study omission. Thus, the results are reliable in the meta-analysis.
Although we have conducted a comprehensive analysis, there are still some limitations that need to be resolved. First, there is no consistent threshold for determining the positive MMP-2 expression in patients with endometrial cancer. The predicted value for MMP-2 should be decided before it used as biomarker. In addition, inaccurate conclusion might obtain due to the potential heterogeneity between studies which was the inherent limitation of meta-analysis.
In summary, the meta-analysis showed that MMP-2 is positively associated with the clinicopathologic characteristics in endometrial cancer. MMP-2 is a potential useful biomarker for predicting the prognosis of patients with endometrial cancer.
Author contributions
Conceptualization: Chang Liu, Ying Li, Shasha Hu, Yongxiu Yang.
Data curation: Chang Liu, Ying Li, Li Gao, Yongxiu Yang.
Formal analysis: Chang Liu, Shasha Hu, Yao Chen, Dajiang Liu, Hongtao Guo, Yongxiu Yang.
Funding acquisition: Yongxiu Yang.
Investigation: Chang Liu.
Methodology: Chang Liu, Yao Chen, Li Gao, Dajiang Liu, Hongtao Guo.
Project administration: Chang Liu.
Resources: Chang Liu, Shasha Hu.
Software: Chang Liu.
Writing – original draft: Chang Liu, Ying Li, Shasha Hu, Yao Chen, Li Gao, Dajiang Liu, Hongtao Guo, Yongxiu Yang.
Writing – review & editing: Chang Liu, Ying Li, Shasha Hu, Yao Chen, Li Gao, Dajiang Liu, Hongtao Guo, Yongxiu Yang.
Footnotes
Abbreviations: ACROBAT-NRSI = the Cochrane Risk of Bias Tool for Non-Randomized Studies of Interventions, CI = confidence interval, CNKI = China National Knowledge Infrastructure, FIGO = Federation of Gynecology and Obstetrics, MMP-2 = matrix metalloproteinase-2, NR = not reported, RoB = Cochrane Risk of Bias, RRs = risk ratios, SCCs = squamous cell carcinomas, S-P = streptavidin-peroxidase, WMDs = weighted mean differences.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Stewart B, Wild C. World Cancer Report. Lyon, France: WHO Press; 2014. [Google Scholar]
- [3].Li X, Zheng S, Chen S, et al. Trends in gynaecological cancers in the largest obstetrics and gynaecology hospital in China from 2003 to 2013. Tumour Biol 2015;36:4961–6. [DOI] [PubMed] [Google Scholar]
- [4].Bradford LS, Rauh-Hain JA, Schorge J, et al. Advances in the management of recurrent endometrial cancer. Am J Clin Oncol 2015;38:206–12. [DOI] [PubMed] [Google Scholar]
- [5].Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002;2:161–74. [DOI] [PubMed] [Google Scholar]
- [6].Rak B, Mehlich D, Garbicz F, et al. Post-transcriptional regulation of MMP16 and TIMP2 expression via miR-382, miR-410 and miR-200b in endometrial cancer. Cancer Genomics Proteomics 2017;14:389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Maquoi E, Munaut C, Colige A, et al. Modulation of adipose tissue expression of murine matrix metalloproteinases and their tissue inhibitors with obesity. Diabetes 2002;51:1093–101. [DOI] [PubMed] [Google Scholar]
- [8].Honkavuori-Toivola M, Santala M, Soini Y, et al. Combination of strong MMP-2 and weak TIMP-2 immunostainings is a significant prognostic factor in endometrial carcinoma. Dis Markers 2013;35:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sun Q, Yin G, Chen M, et al. Expression of vasculogenic mimicry and matrix metalloproteinase-2 in endometrial carcinoma. Chin J Obstet Gynecol Pediatr (Electron Ed) 2016;12:29–34. [Google Scholar]
- [10].Karahan N, Guney M, Baspinar S, et al. Expression of gelatinase (MMP-2 and MMP-9) and cyclooxygenase-2 (COX-2) in endometrial carcinoma. Eur J Gynaecol Oncol 2007;28:184–8. [PubMed] [Google Scholar]
- [11].Benedet JL, Bender H, Jones H, 3rd, et al. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet 2000;70:209–62. [PubMed] [Google Scholar]
- [12].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Aglund K, Rauvala M, Puistola U, et al. Gelatinases A and B (MMP-2 and MMP-9) in endometrial cancer-MMP-9 correlates to the grade and the stage. Gynecol Oncol 2004;94:699–704. [DOI] [PubMed] [Google Scholar]
- [14].Chen X. Expression and clinical significance of CD44 V6 and MMP-2 in endometrial carcinoma. Chin J Fam Plan Gynecotokol 2011;3:60–3. [Google Scholar]
- [15].Liu Y, Gao Y, Chen G, et al. Expression and significance of FHIT and MMP-2 in the endometrial cancer. Chin J Diagn Pathol 2005;12:218–9. [Google Scholar]
- [16].Liu Y, Yang H, Liang L. Expressions of EGFRv III and MMP-2 in endometrial carcinoma and its clinical significance. Acta Med Sin 2015;28:1–4. [Google Scholar]
- [17].Misugi F, Sumi T, Okamoto E, et al. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinase in uterine endometrial carcinoma and a correlation between expression of matrix metalloproteinase-7 and prognosis. Int J Mol Med 2005;16:541–6. [PubMed] [Google Scholar]
- [18].Niu D, Ge X. Expression and significance of claudin-4 and MMP-2 in endometrial carcinoma. Modern Oncology 2009;17:1955–7. [Google Scholar]
- [19].Pan X, Yang Q, Sun Z. Expression of HIF-1, MMP-2 in endometrial carcinoma and its clinical significance. Chin J Pract Gynecol Obstet 2011;27:48–50. [Google Scholar]
- [20].Sun M. Expression and significance of MMP-2, 7, 9 in endometrial cancer. Jilin Med J 2015;36:3256–7. [Google Scholar]
- [21].Talvensaari-Mattila A, Santala M, Soini Y, et al. Prognostic value of matrix metalloproteinase-2 (MMP-2) expression in endometrial endometrioid adenocarcinoma. Anticancer Res 2005;25(6B):4101–5. [PubMed] [Google Scholar]
- [22].Weigel MT, Kramer J, Schem C, et al. Differential expression of MMP-2, MMP-9 and PCNA in endometriosis and endometrial carcinoma. Eur J Obstet Gynecol Reprod Biol 2012;160:74–8. [DOI] [PubMed] [Google Scholar]
- [23].Wu X, Zhang S, Chen A, et al. The expression of matrix metalloproteinase and tissue inhibitor of metalloproteinase in endnometrial child care. J Pract Obstet Gynecol 2004;20:22–4. [Google Scholar]
- [24].Xu L, Han X, Pu P, et al. Expression of E-cad, MMP-2 in human adenomyosis and endometrial carcinoma and correlation with biological behavior. J Pract Obstet Gynecol 2005;21:346–8. [Google Scholar]
- [25].Yilmaz E, Koyuncuoglu M, Gorken IB, et al. Expression of matrix metalloproteinase-2 and survivin in endometrioid and nonendometrioid endometrial cancers and clinicopathologic significance. J Gynecol Oncol 2011;22:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yuan Y, Li F, Zhang L, et al. Expression of matrix metalloproteinase-2 and CD147 in endometrial carcinoma and its clinical meaning. J Harbin Med Univ 2006;40:147–50. [Google Scholar]
- [27].Yuan Y, Shen N, Yang SY, et al. Extracellular matrix metalloproteinase inducer and matrix metalloproteinase-2 overexpression is associated with loss of hormone receptor expression and poor prognosis in endometrial cancer. Oncol Lett 2015;10:342–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhang W, Wei X, Dai S. Expression of MMP-2 and K167 in endometrioid adenocarcinoma and their significance. Med J Qilu 2009;24:489–91. [Google Scholar]
- [29].Zhong X, Yan L. Immunohistochemical studies on the expression of matrix metalloproteinase-2 (MMP-2) and metalloproteinase-9 (MMP-9) in endometrial adenocarcinoma. Med Innov China 2014;11:48–50. [Google Scholar]
- [30].Zhu D, Sheng M, Fu J, et al. Expression of matrix metalloproteinase-2 and matrix metalloproteinase-9 in endometrioid adenocarcinoma tissues and clinical significances. J Jilin Univ (Medicine Edition) 2010;36:965–8. [Google Scholar]
- [31].Xu F, Sun S, Yan S, et al. Elevated expression of RIT1 correlates with poor prognosis in endometrial cancer. Int J Clin Exp Pathol 2015;8:10315–24. [PMC free article] [PubMed] [Google Scholar]
- [32].Liu RR, Li MD, Li T, et al. Matrix metalloproteinase 2 (MMP2) protein expression and laryngeal cancer prognosis: a meta analysis. Int J Clin Exp Med 2015;8:2261–6. [PMC free article] [PubMed] [Google Scholar]
- [33].Chen Y, Wang X, Chen G, et al. The impact of matrix metalloproteinase 2 on prognosis and clinicopathology of breast cancer patients: a systematic meta-analysis. PLoS One 2015;10:e0121404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fu Z, Xu S, Xu Y, et al. The expression of tumor-derived and stromal-derived matrix metalloproteinase 2 predicted prognosis of ovarian cancer. Int J Gynecol Cancer 2015;25:356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cymbaluk-Ploska A, Chudecka-Glaz A, Pius-Sadowska E, et al. Clinical importance of serum HE4 and MMP2 levels in endometrial cancer patients. Onco Targets Ther 2017;10:3169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cao C, Xu N, Zheng X, et al. Elevated expression of MMP-2 and TIMP-2 cooperatively correlates with risk of lung cancer. Oncotarget 2017;8:80560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Eissa S, Ali-Labib R, Swellam M, et al. Noninvasive diagnosis of bladder cancer by detection of matrix metalloproteinases (MMP-2 and MMP-9) and their inhibitor (TIMP-2) in urine. Eur Urol 2007;52:1388–96. [DOI] [PubMed] [Google Scholar]
- [38].Ara T, Fukuzawa M, Kusafuka T, et al. Immunohistochemical expression of MMP-2, MMP-9, and TIMP-2 in neuroblastoma: association with tumor progression and clinical outcome. J Pediatr Surg 1998;33:1272–8. [DOI] [PubMed] [Google Scholar]
- [39].Maeta H, Ohgi S, Terada T. Protein expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinase 1 and 2 in papillary thyroid carcinomas. Virchows Arch 2001;438:121–8. [DOI] [PubMed] [Google Scholar]
- [40].Zeng Y. Endothelial glycocalyx as a critical signalling platform integrating the extracellular haemodynamic forces and chemical signalling. J Cell Mol Med 2017;21:1457–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zeng Y, Yao X, Chen L, et al. Sphingosine-1-phosphate induced epithelial-mesenchymal transition of hepatocellular carcinoma via an MMP-7/syndecan-1/TGF- autocrine loop. Oncotarget 2016;7:63324–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ikebe T, Shinohara M, Takeuchi H, et al. Gelatinolytic activity of matrix metalloproteinase in tumor tissues correlates with the invasiveness of oral cancer. Clin Exp Metastasis 1999;17:315–23. [DOI] [PubMed] [Google Scholar]
- [43].Kanayama H, Yokota K, Kurokawa Y, et al. Prognostic values of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 expression in bladder cancer. Cancer 1998;82:1359–66. [PubMed] [Google Scholar]
- [44].Vaisanen A, Kallioinen M, von Dickhoff K, et al. Matrix metalloproteinase-2 (MMP-2) immunoreactive protein - a new prognostic marker in uveal melanoma? J Pathol 1999;188:56–62. [DOI] [PubMed] [Google Scholar]
- [45].Yoshizaki T, Maruyama Y, Sato H, et al. Expression of tissue inhibitor of matrix metalloproteinase-2 correlates with activation of matrix metalloproteinase-2 and predicts poor prognosis in tongue squamous cell carcinoma. Int J Cancer 2001;95:44–50. [DOI] [PubMed] [Google Scholar]
- [46].Moser PL, Kieback DG, Hefler L, et al. Immunohistochemical detection of matrix metalloproteinases (MMP) 1 and 2, and tissue inhibitor of metalloproteinase 2 (TIMP 2) in stage IB cervical cancer. Anticancer Res 1999;19(5C):4391–3. [PubMed] [Google Scholar]
- [47].Iurlaro M, Loverro G, Vacca A, et al. Angiogenesis extent and expression of matrix metalloproteinase-2 and -9 correlate with upgrading and myometrial invasion in endometrial carcinoma. Eur J Clin Invest 1999;29:793–801. [DOI] [PubMed] [Google Scholar]
- [48].Di Nezza LA, Misajon A, Zhang J, et al. Presence of active gelatinases in endometrial carcinoma and correlation of matrix metalloproteinase expression with increasing tumor grade and invasion. Cancer 2002;94:1466–75. [DOI] [PubMed] [Google Scholar]


