Abstract
Background:
High mobility group box 1 (HMGB1) is a kind of proinflammatory mediator to stimulate the innate and adaptive immune system and participates in a number of acute and chronic inflammatory processes after sterile injury or microbial invasion. HMGB1 has been suggested to be involved in the pathogenesis of many autoimmune diseases. However, the results are contradictory or inconclusive among these findings. The aim of this study was to investigate whether serum/plasma HMGB1 levels are associated with autoimmune diseases by comparing the serum/plasma HMGB1 levels in patients with autoimmune disease and healthy controls and to further evaluate whether serum/plasma HMGB1 levels are associated with disease state.
Methods:
PubMed, Medline, and Web of science databases (up to October 1, 2017) were used to obtain all relative published literature. Study quality was assessed by the Newcastle–Ottawa scale (NOS). Pooled standard mean difference (SMD) with 95% confidence interval (CI) was calculated by fixed-effects or random-effect model analysis.
Results:
A total of 23 original articles of autoimmune diseases were finally included in the meta-analysis. Results revealed that the serum/plasma HMGB1 levels were increased in patients with autoimmune disease, compared to healthy controls. Subgroup analysis showed that serum/plasma HMGB1 levels in patients with active disease state were significantly higher than in those with inactive state. In addition, subgroup analysis based on disease type has indicated that the serum/plasma HMGB1 levels in patients with small vessel vasculitis, systemic lupus erythematosus, rheumatoid arthritis, ankylosing spondylitis and sjogren syndrome were significantly higher, compared to healthy controls. Further subgroup analyses by region showed that plasma/serum HMGB1 levels were higher in Asian and European patients with autoimmune diseases.
Conclusions:
Serum/plasma HMGB1 levels in patients with autoimmune diseases are significantly higher than in healthy controls, and may reflect the disease activity.
Keywords: autoimmune diseases, high mobility group box 1, meta-analysis, systemic lupus erythematosus, vasculitis
1. Introduction
Autoimmune diseases are a group of complex chronic inflammatory conditions characterized by immunologic tolerance to self-antigens and immune-mediated tissue destruction, which encompass a variety of autoimmune conditions, involving different organs and showing different clinical manifestations.[1] Autoimmune diseases have been considered rare, whereas studies show that the incidence was 3% to 5% of the population and higher in female than in male population.[2] Thus far, pathogenesis of autoimmune diseases is still unclear. Studies have suggested that immune response abnormalities in autoimmune diseases may result from the genetic and environmental factors.[3–4]
High mobility group box 1 (HMGB1) is a group of non-histone nuclear protein by being actively secreted from activated immune cells or passively released from injured or dying cells and becomes a proinflammatory mediator via binding to various receptors (such as RAGE, TLR-2, TLR-4, and TLR-9) on the surface of responding cells.[5] HMGB1 acts as a damage-associated molecular pattern (DAMP) to stimulate the immune system and play an important role in acute and chronic inflammatory process.[6–7] HMGB1 has been investigated in several systemic disorders including sepsis, cancer, atherosclerosis, and certain chronic inflammatory conditions.[8–10] Recently, a number of studies have focused on the relationship between serum/plasma HMGB1 levels and autoimmune diseases, such as antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV), Behcet disease (BD), systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), ankylosing spondylitis (AS), and Sjogren syndrome (SS) and found that the serum/plasma HMGB1 levels were altered in autoimmune diseases.[11–33] However, some studies showed that patients with autoimmune diseases present similar serum/plasma HMGB1 levels compared to healthy controls. Therefore, whether HMGB1 as a proinflammatory mediator is associated with autoimmune diseases is still controversial. In order to settle these controversial issues, this meta-analysis was performed to evaluate the relationship between serum/plasma HMGB1 levels and autoimmune diseases.
2. Materials and methods
2.1. Search strategy
Our meta-analysis was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Relevant articles and studies up to October 1 2017 were searched via the following electronic databases: PubMed, Medline, and Web of science databases. We searched for articles containing the following terms: “antineutrophil cytoplasmic antibody-associated vasculitis” or “AAV” or “granulomatosis with polyangiitis” or “GPA” or “Kawasaki disease” or “KD” or “Henoch-Schonlein purpura” or “HSP” or “behcet's disease” or “BD” or “Takayasu arteritis” or “TA” or “giant cell arteritis” or “GCA” or “systemic lupus erythematosus” or SLE” or “rheumatoid arthritis” or “RA” or “juvenile idiopathic arthritis” or “JIA” or “ankylosing spondylitis” or “AS” or “Sjogren syndrome” or “SS” combined with “high motility group box 1” or “HMGB1” or “HMGB1 Protein” or “HMG1” or “HMG1 Protein”. We recruited data only from the full-published paper and meeting or conference abstracts were excluded. All resulting articles were then screened for serum/plasma HMGB1 levels measures. Our search was limited to English language publications. In this meta-analysis, ethical approval was not necessary as all the data were based on the previous published studies.
2.2. Inclusion criteria
Relevant articles and studies were included if they satisfied all of the following criteria: correlations of serum/plasma HMGB1; human subjects with AAV or KD or HSP or TA or BD or SLE or RA or AS or SS; case-control, clinical cohort or cross-sectional design; serum/plasma HMGB1 levels measured by enzyme-linked immunosorbent assay (ELISA); articles with more than 10 cases; and detailed data about serum/plasma HMGB1 in both case group and healthy controls was accessible. If the same study was reported in more than 1 publication, we considered largest sample size or the most recent publication were included, but only 1 was included.
2.3. Exclusion criteria
Relevant articles and studies were excluded if any of following criteria were met: conference abstracts, editorials, reviews, meta-analyses, consensus statements, guidelines, case-only reports, and case report; studies were not performed in humans; studies with partially overlapping patient populations; and study with incomplete data.
2.4. Data extraction and quality assessment
Two authors (BZ and QZ) conducted independently extracted the data and quality assessment. The extracted information for each eligible articles included first author's name, publication year, country of origin, study type, study subjects (sample size, age, and sex), diagnostic criteria and assay detection methods of serum/plasma HMGB1 levels. In most studies, the mean and deviation were obtained, but in several studies only the median and quartiles were reported. Therefore, when the original data were median and quartiles, we transformed and calculated the data to gain the appropriate values according to the method recommended by Hozo et al.[34–35] Furthermore, the software of GetData Graph Digitizer 2.22 was applied to digitize and extract the data from the scatter diagrams in some studies. If original important data were unavailable in some articles, we contacted corresponding author by E-mails to obtain further details. The methodologic quality of the studies was evaluated using Newcastle–Ottawa quality assessment scale (NOS).[36] The NOS consists of 3 perspectives with a maximum of 9 stars: a maximum of 4 stars for selection, 2 stars for comparability, and 3 stars for the ascertainment of either the exposure or outcome of interest. A study with >5 stars was included in the meta-analysis.
2.5. Statistical analysis
Stata 12.0 software (StataCorp, College Station, Texas, USA) was used for statistical analyses. The effect size of each study was calculated by the standardized mean difference (SMD) and its 95% confidence intervals (95% CI) were described by a forest plot. The heterogeneity was evaluated by Cochrane Q statistic.[37] We then estimated the effect of heterogeneity in each study by the method (I2 = [(Q - df)/Q] × 100%).[38] If P >.05 for the Q test or effect sizes with I2 value of < 50% were considered to have low heterogeneity, P < .05 for the Q test or I2 value of 50% to 75% were considered moderate and I2 values of > 75% were considered highly heterogeneous.[39] If P > .05 for the Q test or I2 < 50%, there was no significant heterogeneity among these studies, so the fixed-effect model was selected. Otherwise, the random effect model was selected. Sensitivity analyze was used to evaluate the impact of single studies on overall outcomes. Publication bias was visually estimated by a funnel plot, Begg methods, and Egger linear regression test. To detect the sources of heterogeneity, further subgroup analysis was performed according to disease state, disease type, and region.
3. Results
3.1. Characteristics of the included studies
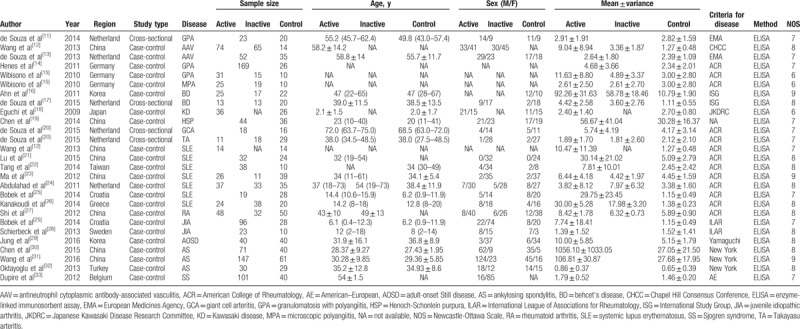
A total of 420 studies were identified, 214 were searched from PubMed databases, 110 were searched from Medline databases, 82 were from Web of science databases, and 14 were from others sources. After removing duplicates and review titles and abstracts, 379 articles were excluded. The remaining 41 articles were further examined and reviewed in full articles, 9 excluded due to methods, 2 insufficient data for detailed analysis, 2 not relevant, 3 reviews, 1 retracted, 1 not in humans. Finally, a subtotal of 23 original articles included in our meta-analysis (Fig. 1). A total of 1528 patients with autoimmune diseases and 680 healthy controls were included in this study. In patients with autoimmune diseases, 585 patients were with small vessel vasculitis (SVV), 36 with medium vessel vasculitis (MVV), 47 with large vessel vasculitis (LVV), 272 with SLE, 80 with RA, 159 with JIA, 248 with AS, and 101 with SS. The baseline characteristics of included studies are shown in Table 1.[11–33] Twenty original articles were case-control studies, 3 were cross-sectional studies. The age and sex were matched between patients with autoimmune diseases and healthy controls in each included study. The ELISA technique was the uniform method for detection of serum/plasma HMGB1 in our meta-analysis. The NOS for case-control studies was used to assess the quality of study and all quality scores of the enrolled original articles were higher than 5 score (Table 1).
Figure 1.
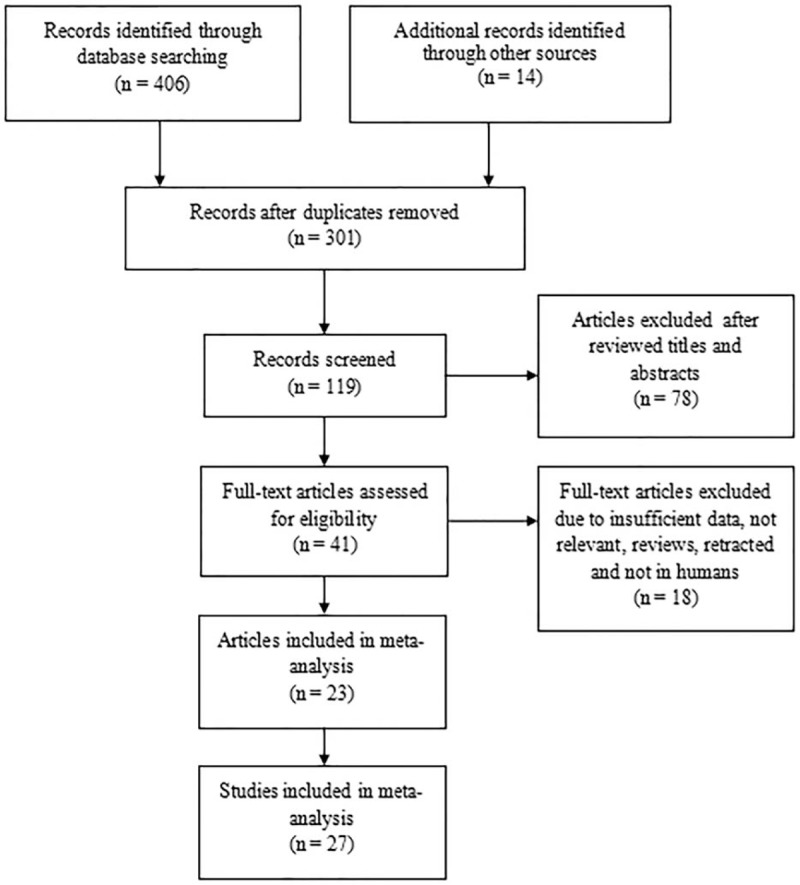
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart.
Table 1.
Characteristics of the included studies.
3.2. Meta-analysis results
Heterogeneity was tested with the heterogeneity statistic Q and quantified using I2. Random-effects model were utilized following the detection of significant heterogeneity across studies (I2 = 92.8%, P < .001). In this study, our meta-analysis results revealed that the serum/plasma HMGB1 levels in patients with autoimmune diseases are higher than in healthy controls (SMD = 1.07, 95% CI: 0.69 −1.45, P < .001) (Fig. 2). To evaluate stability of the results, we performed sensitivity analysis, which showed that the overall statistical significance does not change when any single study was omitted. Therefore, the current meta-analysis data is relatively reliable and credible (Fig. 3). Furthermore, there was no publication bias performing Egger regression test (t = 1.57, P = .129) and Begg methods (Z = 0.96, P = .338) in the 23 original articles (Fig. 4).
Figure 2.
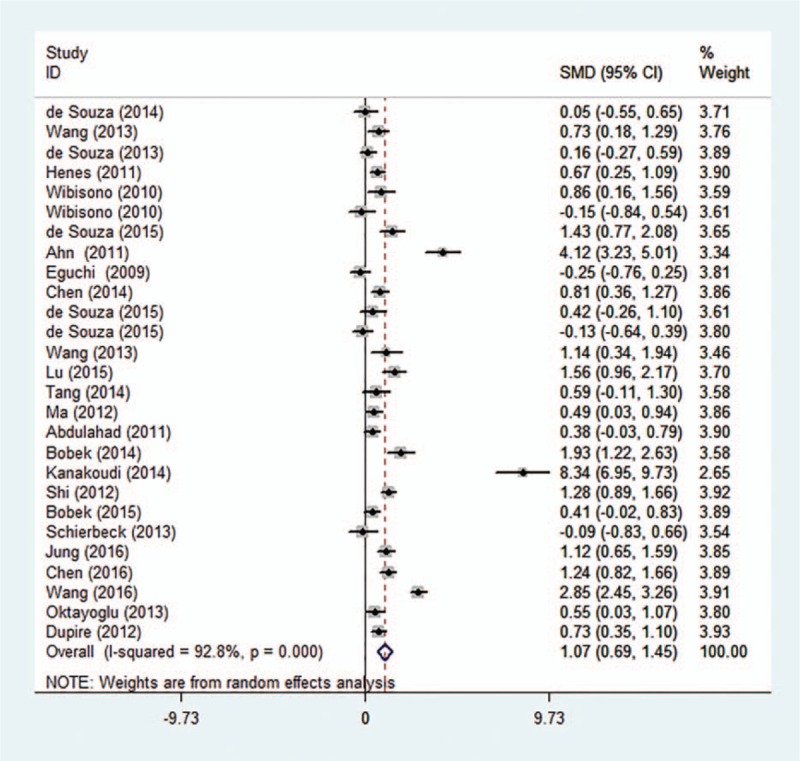
Forest plot of studies in serum/plasma HMGB1 levels for patients with autoimmune diseases compared to healthy controls (23 original articles were included).
Figure 3.
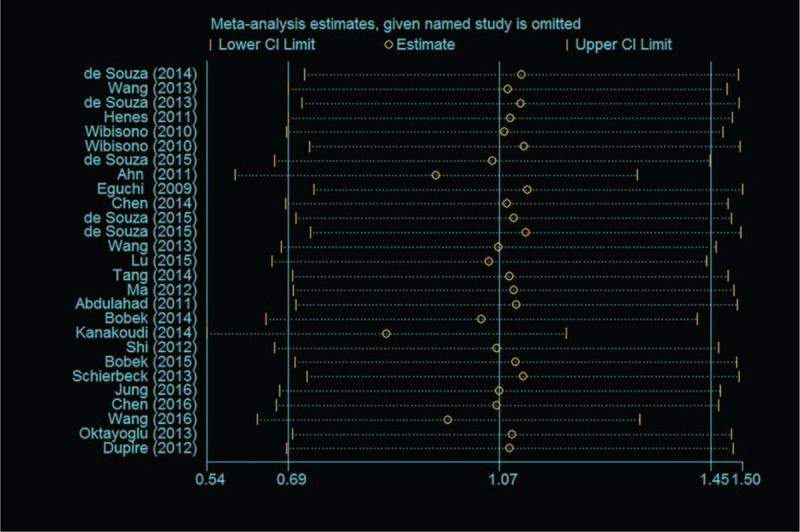
Sensitivity analyses by excluding one study at a time.
Figure 4.
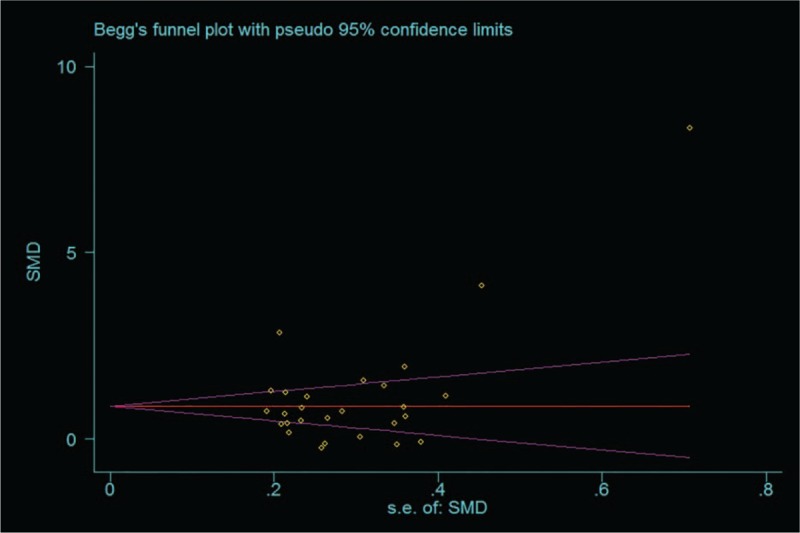
Funnel plots for the difference of serum/plasma HMGB1 levels between autoimmune diseases patients and healthy controls.
3.3. Subgroup analysis
In our study, the correlation of serum/plasma HMGB1 levels between patients with active disease state and healthy controls were discussed in 12 studies. The correlation of serum/plasma HMGB1 levels between patients with inactive disease state and healthy controls, patients with active and inactive diseases were reported in 10 studies. Random-effects model was applied in accordance with the presence of heterogeneity (active vs control: I2 = 93.0%, P < .001; inactive vs control: I2 = 93.1%, P < .001; active vs inactive: I2 = 89.2%, P < .001) (Fig. 5). Our meta-analysis demonstrated that serum/plasma HMGB1 levels of patients with active and inactive disease state was significantly higher than that of healthy controls (active vs control: SMD = 1.34, 95% CI = 0.63–2.05, P < .001; inactive vs control: SMD = 1.37, 95% CI = 0.63–2.18, P = .001) (Fig. 5 A, B). Further comparison revealed that serum/plasma HMGB1 levels of patients with active disease state were higher than in patients with inactive state (active vs inactive: SMD = 0.76, 95% CI = 0.20–1.32, P = .008) (Fig. 5 C). Subgroup analysis based on disease type has indicated that the serum/plasma HMGB1 levels in patients with SVV, SLE, RA, AS, and SS were higher than in healthy controls (SMD = 0.92, 95% CI = 0.33–1.51, P = 0.002, I2 = 89.8%, P < .001; SMD = 1.92, 95% CI = 0.80–3.03, P = .001, I2 = 95.5%, P < .001; SMD = 1.28, 95% CI = 0.89–1.66, P < .001, I2 = not applicable, P = not applicable; SMD = 1.56, 95% CI = 0.22–2.90, P < .023, I2 = 96.3%, P < .001; SMD = 0.73, 95% CI = 0.35–1.10, P < .001, I2 = not applicable, P = not applicable, respectively) (Table 2). Further subgroup analyses by region showed that the plasma/serum HMGB1 levels were higher in Asian and European patients with autoimmune diseases (SMD = 1.23, 95% CI = 0.70–1.75, P < .001, I2 = 92.6%, P < .001; SMD = 0.91, 95% CI = 0.39–1.43, P < .001; I2 = 92.1%, P < .001).
Figure 5.
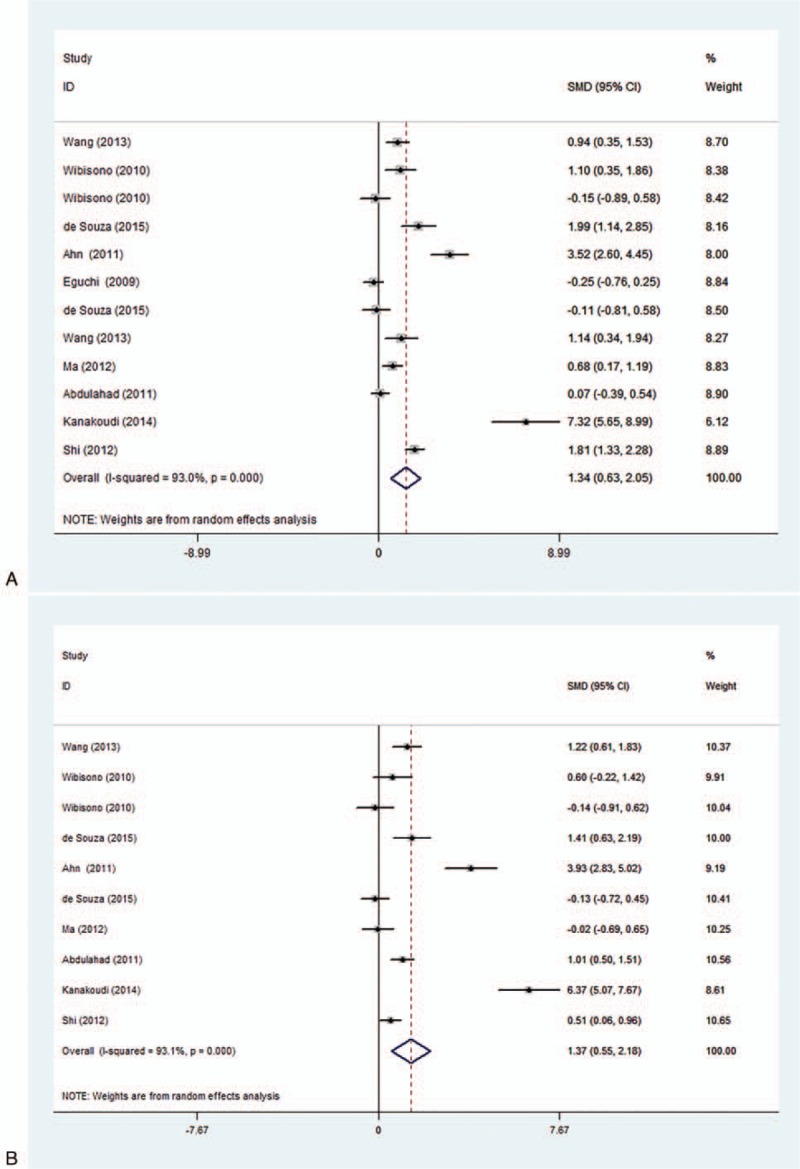
Forest plots for the comparison of serum high mobility group box 1 (HMGB1) among active group, inactive group and healthy controls [(A) active group vs healthy controls; (B) inactive group vs healthy controls; (C) active group vs inactive group].
Table 2.
Subgroup analysis of HMGB1 levels in autoimmune diseases.
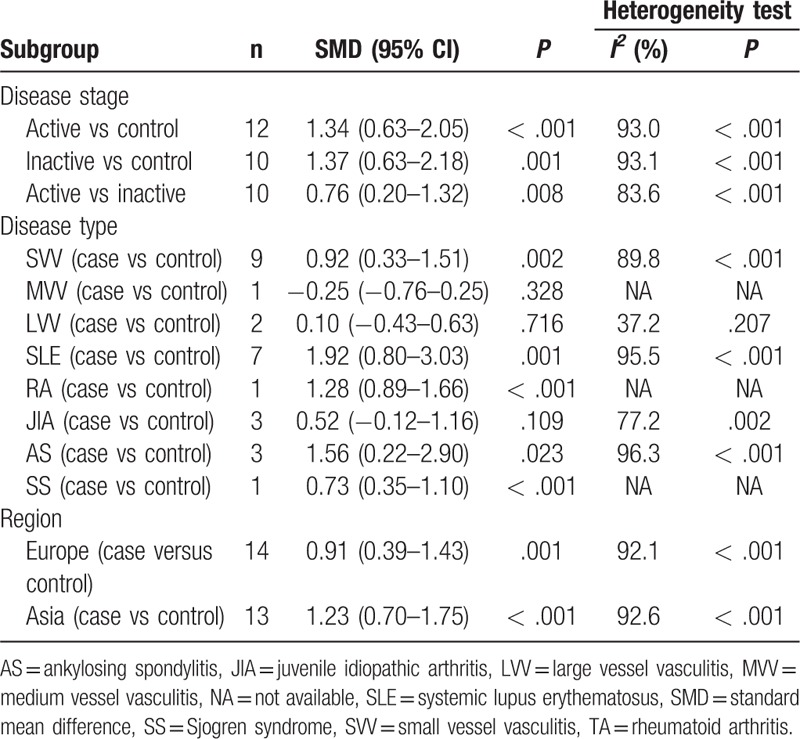
Figure 5 (Continued).
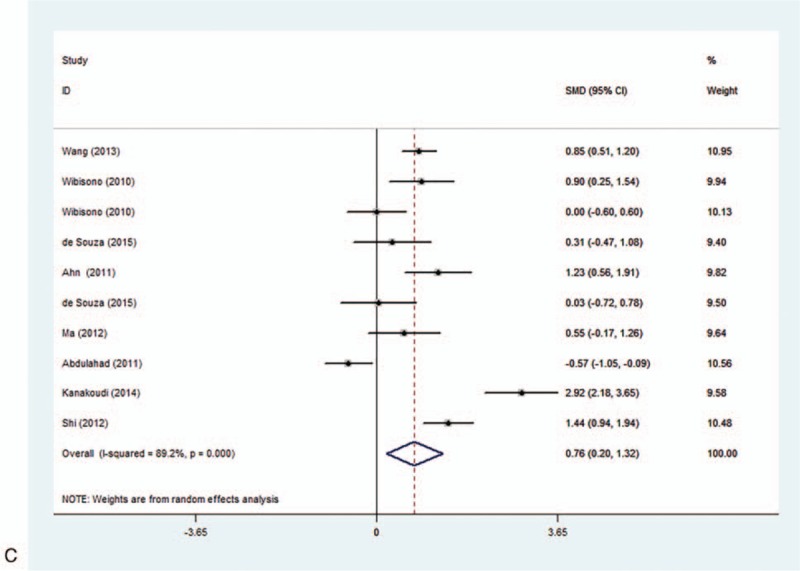
Forest plots for the comparison of serum high mobility group box 1 (HMGB1) among active group, inactive group and healthy controls [(A) active group vs healthy controls; (B) inactive group vs healthy controls; (C) active group vs inactive group].
4. Discussion
In this study, we performed this meta-analysis to observe the alterations of serum/plasma HMGB1 levels in patients with autoimmune diseases, and further to explore the relationship between the serum/plasma HMGB1 levels and disease state. Our study revealed that the serum/plasma HMGB1 levels in patients with autoimmune diseases are significantly higher than in healthy controls. This result showed that HMGB1 might play a crucial role in the pathogenesis of autoimmune diseases. Further analysis showed that the serum/plasma HMGB1 levels in patients with active and inactive disease state were significantly higher than in healthy controls. In addition, compared with patients in inactive disease state, patients in active disease state showed significantly higher serum/plasma HMGB1 levels. These results might indicate that serum/plasma HMGB1 levels could reflect the disease activity of autoimmune diseases.
HMGB1 is an important proinflammatory mediator and binds to cell surface receptors to induce inflammatory responses.[40] Neutrophils, macrophages, monocytes and endothelial cells stimulated by inflammation could release HMGB1, which acts as an inducer of macrophage activation including production of tumor necrosis factor-α, interleukin-1, and other proinflammatory mediators; thus in turn regulates cytokine expression and promotes inflammatory cell recruitment.[41] Furthermore, HMGB1 may be involved in the development and evolution of disease processes through its ability to promote cell proliferation and migration, modulate the adhesive properties, and stimulate neoangiogenesis. In addition, HMGB1 can translocate outside the cell in response to injuries by being actively secreted from activated immune cells or passively released from necrosis cells, extracellular HMGB1 can induce the release of proinflammatory mediators, which can result in highly increased serum levels of proinflammatory cytokines, and then cause the activation of immune system, as well as invasion by inflammatory cells, thereby prolonging and sustaining inflammatory processes.[5,42–43] In this regard, cell injury and necrosis caused by inflammatory response of tissue may be the main cause of elevated serum/plasma HMGB1, and may be closely associated with the disease activity of autoimmune diseases and its development. Wang et al[12] have demonstrated that plasma HMGB1 levels in AAV patients were significantly higher than those in normal controls and might reflect the disease activity. Li et al[44] suggest that increased serum levels of HMGB1 in SLE patients may be associated with lupus disease activity. Chen et al[30] findings show that HMGB1 might be a good laboratory index for the evaluation of disease activities and disease severity in AS patients. These results imply that the elevated serum HMGB1 levels may be a potential predictor in the disease activity of autoimmune diseases patients.
Heterogeneity test results displayed the existence of significant heterogeneity among studies. Strong heterogeneity suggests that the validity of the meta-analysis is impaired, as the included studies reported inconsistent results. We performed a subgroup analysis to investigate the potential influential factors for the results. The subgroup analysis showed that the serum/plasma HMGB1 levels in SVV, SLE, RA, AS, and SS patients were significantly higher than in healthy controls. However, patients with MVV, LVV, and JIA present similar serum/plasma HMGB1 levels compared with healthy controls. This suggests that the disease types may correlate with the serum/plasma HMGB1 levels in autoimmune diseases. Further subgroup analysis based on the region of autoimmune diseases has indicated that the serum/plasma HMGB1 levels were significantly increased in patients with autoimmune diseases in European and Asian populations, implying that region differences may not be the potential heterogeneity source. However, other potential factors such as age, sex ratio, environmental characteristics, and clinical characteristics may also affect the serum/plasma HMGB1 levels. Although many factors have effects on HMGB1 in serum/plasma, increasing studies have investigated the potential mechanisms responsible for the association between HMGB1 levels in serum/plasma and autoimmune diseases. Further studies with an adequate number of subjects according to different factors are needed. In addition, the strong heterogeneity may also be attributed to the two high-weight studies (Ahn et al[16] and Kanakoudi et al[26]), both of which showed significantly higher HMGB1 levels than other studies. Moreover, the serum/plasma HMGB1 detection is based on the ELISA methods in our selected articles, but different ELISA kit manufacturers may also cause strong heterogeneity.
This is the first comprehensive meta-analysis to assess the relationship between serum/plasma HMGB1 levels and autoimmune diseases. However, there exists some limitation in this meta-analysis which should be acknowledged. First, there is the possible existence of biases. Although we performed a methodological assessment of those studies to avoid some selection biases, there was a highly significant heterogeneity among the 23 articles which may be attributed to age, gender, region, disease type and other residual confounding effect. Second, in this study we have not excluded patients with complicated autoimmune diseases such as SVV or SLE with renal involvement due to the limited studies we can include. However, we cannot neglect the effect of other complicated autoimmune diseases on serum/plasma HMGB1 levels. Thirdly, if the original data were median and quartiles in selected articles, and may be cannot to acquire original data from the included studies. We transformed and calculated the data to gain the appropriate values. Thus it may be lead to some errors of in our results. Finally, studies published in languages other than English were not included, which may also indicate a language bias and affect the results in our study. Despite of have some limitations in our study, all the research methods were carried out on strict inclusion and exclusion criteria. Therefore, we obtained information may approximate the actual results.
5. Conclusion
In conclusion, the results from our meta-analysis suggest that serum/plasma HMGB1 levels were significantly higher in patients with autoimmune diseases than in healthy controls, and may reflect disease activity. Further studies focusing on the underlying mechanisms of HMGB1 in autoimmune diseases are also needed.
Author contributions
Conceptualization: Nanfang Li.
Data curation: Bin Zhu, Shasha Liu, Shanshan Liu.
Formal analysis: Bin Zhu, Qing Zhu.
Funding acquisition: Nanfang Li.
Methodology: Qing Zhu, Ting Wu.
Project administration: Nanfang Li.
Writing – original draft: Bin Zhu.
Writing – review & editing: Bin Zhu, Qing Zhu, Ting Wu.
Footnotes
Abbreviations: AAV = antineutrophil cytoplasmic antibody-associated vasculitis, ACR = American College of Rheumatology, AOSD = adult-onset Still disease, AS = ankylosing spondylitis, BD = Behcet disease, CHCC = Chapel Hill Consensus Conference, ELISA = enzyme-linked immunosorbent assay, EMA = European Medicines Agency, GCA = giant cell arteritis, GPA = granulomatosis with polyangiitis, HMGB1 = high mobility group box 1, HSP = Henoch-Schonlein purpura, JIA = juvenile idiopathic arthritis, KD = Kawasaki disease, LVV = large vessel vasculitis, MPA = microscopic polyangiitis, MVV = medium vessel vasculitis, NA = not available, NOS = Newcastle–Ottawa Scale, RA = rheumatoid arthritis, SLE = systemic lupus erythematosus, SMD = standard mean difference, SS = Sjogren syndrome, SVV = small vessel vasculitis, TA = Takayasu arteritis.
BZ and QZ contributed equally to this study and share first authorship.
This work was supported by a grant from the National Natural Science Fund (No. 81460078). The authors declare no conflicts of interest.
References
- [1].Wahren-Herlenius M, Dorner T. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet 2013;382:819–31. [DOI] [PubMed] [Google Scholar]
- [2].Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Intern Med 2015;278:369–95. [DOI] [PubMed] [Google Scholar]
- [3].Cho JH, Gregersen PK. Genomics and the multifactorial nature of human autoimmune disease. N Engl J Med 2011;365:1612e1623. [DOI] [PubMed] [Google Scholar]
- [4].Costenbader KH, Gay S, Alarcon-Riquelme ME, et al. Genes, epigenetic regulation and environmental factors: which is the most relevant in developing autoimmune diseases? Autoimmun Rev 2012;11:604e609. [DOI] [PubMed] [Google Scholar]
- [5].Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol 2012;8:195–202. [DOI] [PubMed] [Google Scholar]
- [6].Hosakote YM, Brasier AR, Casola A, et al. Respiratory syncytial virus infection triggers epithelial HMGB1 release as a damage-associated molecular pattern promoting a monocytic inflammatory response. J Virol 2016;90:9618–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yang H, Antoine DJ, Andersson U, et al. The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol 2013;93:865–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].de Souza AW, Westra J, Limburg PC, et al. HMGB1 in vascular diseases: its role in vascular inflammation and atherosclerosis. Autoimmun Rev 2012;11:909–17. [DOI] [PubMed] [Google Scholar]
- [9].Singh A, Feng Y, Mahato N, et al. Role of high-mobility group box 1 in patients with acute obstructive suppurative cholangitis-induced sepsis. J Inflamm Res 2015;8:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ueda M, Takahashi Y, Shinden Y, et al. Prognostic significance of high mobility group box 1 (HMGB1) expression in patients with colorectal cancer. Anticancer Res 2014;34:5357–62. [PubMed] [Google Scholar]
- [11].Souza AW, de Leeuw K, van Timmeren MM, et al. Impact of serum high mobility group box 1 and soluble receptor for advanced glycation end-products on subclinical atherosclerosis in patients with granulomatosis with polyangiitis. PLoS One 2014;9:e96067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang C, Gou SJ, Chang DY, et al. Association of circulating level of high mobility group box 1 with disease activity in antineutrophil cytoplasmic autoantibody-associated vasculitis. Arthritis Care Res (Hoboken) 2013;65:1828–34. [DOI] [PubMed] [Google Scholar]
- [13].de Souza A, Westra J, Bijzet J, et al. Is serum HMGB1 a biomarker in ANCA-associated vasculitis? Arthritis Res Ther 2013;15:R104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Henes FO, Chen Y, Bley TA, et al. Correlation of serum level of high mobility group box 1 with the burden of granulomatous inflammation in granulomatosis with polyangiitis (Wegener's). Ann Rheum Dis 2011;70:1926–9. [DOI] [PubMed] [Google Scholar]
- [15].Wibisono D, Csernok E, Lamprecht P, et al. Serum HMGB1 levels are increased in active Wegener's granulomatosis and differentiate between active forms of ANCA-associated vasculitis. Ann Rheum Dis 2010;69:1888–9. [DOI] [PubMed] [Google Scholar]
- [16].Ahn JK, Cha HS, Bae EK, et al. Extracellular high-mobility group box 1 is increased in patients with Behcet's disease with intestinal involvement. J Korean Med Sci 2011;26:697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].de Souza AW, Perazzio SF, de França NR, et al. High mobility group box 1 serum levels are increased in Behcet's disease, but not associated with disease activity or disease manifestations. Rheumatology (Oxford) 2015;54:2151–5. [DOI] [PubMed] [Google Scholar]
- [18].Eguchi T, Nomura Y, Hashiguchi T, et al. An elevated value of high mobility group box 1 is a potential marker for poor response to high-dose of intravenous immunoglobulin treatment in patients with Kawasaki syndrome. Pediatr Infect Dis J 2009;28:339–41. [DOI] [PubMed] [Google Scholar]
- [19].Chen T, Guo ZP, Wang WJ, et al. Increased serum HMGB1 levels in patients with Henoch-Schonlein purpura. Exp Dermatol 2014;23:419–23. [DOI] [PubMed] [Google Scholar]
- [20].de Souza AW, van der Geest KS, Brouwer E, et al. High mobility group box 1 levels in large vessel vasculitis are not associated with disease activity but are influenced by age and statins. Arthritis Res Ther 2015;17:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lu M, Yu S, Xu W, et al. HMGB1 promotes systemic lupus erythematosus by enhancing macrophage inflammatory response. J Immunol Res 2015;2015:946748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tang KT, Hsieh TY, Chen YM, et al. Measuring serum levels of high-mobility group box 1 by enzyme-linked immunosorbent assay does not identify bacterial infections in patients with systemic lupus erythematosus. Lupus 2014;23:926–34. [DOI] [PubMed] [Google Scholar]
- [23].Ma CY, Jiao YL, Zhang J, et al. Elevated plasma level of HMGB1 is associated with disease activity and combined alterations with IFN-alpha and TNF-alpha in systemic lupus erythematosus. Rheumatol Int 2012;32:395–402. [DOI] [PubMed] [Google Scholar]
- [24].Abdulahad DA, Westra J, Bijzet J, et al. High mobility group box 1 (HMGB1) and anti-HMGB1 antibodies and their relation to disease characteristics in systemic lupus erythematosus. Arthritis Res Ther 2011;13:R71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bobek D, Grčević D, Kovačić N, et al. The presence of high mobility group box-1 and soluble receptor for advanced glycation end-products in juvenile idiopathic arthritis and juvenile systemic lupus erythematosus. J Immunol Res 2014;12:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kanakoudi-Tsakalidou F, Farmaki E, Tzimouli V, et al. Simultaneous changes in serum HMGB1 and IFN-α levels and in LAIR-1 expression on plasmatoid dendritic cells of patients with juvenile SLE. New therapeutic options? Lupus 2014;23:305–12. [DOI] [PubMed] [Google Scholar]
- [27].Shi Y, Sandoghchian Shotorbani S, Su Z, et al. Enhanced HMGB1 expression may contribute to Th17 cells activation in rheumatoid arthritis. Clin Dev Immunol 2012;2012:295081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schierbeck H, Pullerits R, Pruunsild C, et al. HMGB1 levels are increased in patients with juvenile idiopathic arthritis, correlate with early onset of disease, and are independent of disease duration. J Rheumatol 2013;40:1604–13. [DOI] [PubMed] [Google Scholar]
- [29].Jung JY, Suh CH, Sohn S, et al. Elevated high-mobility group B1 levels in active adult-onset Still's disease associated with systemic score and skin rash. Clin Rheumatol 2016;35:1937–42. [DOI] [PubMed] [Google Scholar]
- [30].Chen Y, Sun W, Li S, et al. Preliminary study of high mobility group box chromosomal protein 1(HMGB1) in ankylosing spondylitis patients. Clin Exp Rheumatol 2015;33:187–94. [PubMed] [Google Scholar]
- [31].Wang C, Miao Y, Wu X, et al. Serum HMGB1 serves as a novel laboratory indicator reflecting disease activity and treatment response in ankylosing spondylitis patients. J Immunol Res 2016;2016:6537248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Oktayoglu P, Em S, Tahtasiz M, et al. Elevated serum levels of high mobility group box protein 1 (HMGB1) in patients with ankylosing spondylitis and its association with disease activity and quality of life. Rheumatol Int 2013;33:1327–31. [DOI] [PubMed] [Google Scholar]
- [33].Dupire G, Nicaise C, Gangji V, et al. Increased serum levels of high-mobility group box 1 (HMGB1) in primary Sjogren's syndrome. Scand J Rheumatol 2012;41:120–3. [DOI] [PubMed] [Google Scholar]
- [34].Whitlock RP, Chan S, Devereaux PJ, et al. Clinical benefit of steroid use in patients undergoing cardiopulmonary bypass: a meta-analysis of randomized trials. Eur Heart J 2008;29:2592e2600. [DOI] [PubMed] [Google Scholar]
- [35].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [37].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [38].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Huang XL, Zhang L, Duan Y, et al. Association of Pentraxin 3 with autoimmune diseases: a systematic review and meta-analysis. Arch Med Res 2016;47:223–31. [DOI] [PubMed] [Google Scholar]
- [40].Yang H, Wang H, Chavan SS, et al. High mobility group box protein 1 (HMGB1): the prototypical endogenous danger molecule. Mol Med 2015;21suppl 1:S6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wang C, de Souza AW, Westra J, et al. Emerging role of high mobility group box 1 in ANCA-associated vasculitis. Autoimmun Rev 2015;14:1057–65. [DOI] [PubMed] [Google Scholar]
- [42].Yanai H, Ban T, Taniguchi T. High-mobility group box family of proteins: ligand and sensor for innate immunity. Trends Immunol 2012;33:633–40. [DOI] [PubMed] [Google Scholar]
- [43].Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol 2011;29:139–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Li J, Xie H, Wen T, et al. Expression of high mobility group box chromosomal protein 1 and its modulating effects on downstream cytokines in systemic lupus erythematosus. J Rheumatol 2010;37:766–75. [DOI] [PubMed] [Google Scholar]


