Abstract
To identify whether marital status is associated with survival in patients with bladder urothelial carcinoma (UC). Using Surveillance, Epidemiology, and End Results population-based data, 133,846 patients diagnosed with bladder UC between 1988 and 2009 were identified. Kaplan–Meier methods and multivariable Cox regression models were used for survival analyses and evaluation of the association between marital status and survival, after controlling for gender, age, race, primary site, tumor (topography), lymph node, metastasis stage, pathological grading, and surgery. Patients in the married group had a higher proportion of men within group comparisons, more often white, older, earlier clinical stage at diagnosis, surgical treatment, all of which were statistically significant (P < .001). Widowed patients had the worst bladder UC cause-specific survival (CSS) compared with married, never married, and so on groups classified by stage and grade. The 5-year CSS of widowed patients compared with that of married patients was, respectively, all (P < .001), 89.8% versus 95.8% at noninvasive papillary carcinoma stage, 84.1% versus 91.6% at occur in situ stage, 74.3% versus 86.1% at I stage, 41.2% versus 61.6% at II stage, 39.2 versus 52.5% at III stage, and 8.8% versus 17.0% at IV stage. Widowed patients tend to have a significantly higher risk of bladder-cancer-specific mortality. Marital status was relevant to improved CSS in patients with bladder UC.
Keywords: bladder urothelial carcinoma; grade; marital status; Surveillance, Epidemiology, and End Results (SEER); TNM stage
1. Introduction
Bladder cancer is the fourth most common cancer in the United States and the ninth most common malignancy worldwide, urothelial carcinoma (UC) that originates from the bladder is the most common subtype.[1,2] Marital status has been confirmed to affect the survival rates in many tumors. Cancer patients being married have better survival, with colorectal, gallbladder, prostate, and breast carcinoma.[3–6] Similarly, Klaassen et al[7] and Gore et al[8] reported that patients with bladder cancer who were unmarried had higher mortality than those who had been married. There are many different mechanisms to explain the association between cancer survival and marital status. Marital status is commonly used as a marker of social support. Those patients who are married may enjoy increased financial resources, can experience social support,[9] may have high quality of life, tend to have a healthier lifestyle,[10] will receive better treatment[11] than those who are unmarried.
In addition, Li et al[12] have demonstrated that despite favorable clinic-pathological characteristics, widowed patients in colorectal cancer were at highest risk of death compared with other groups in a larger population-based study on data from the Surveillance, Epidemiology, and End Results (SEER) database. However, there are few studies explored the effect of marital status on the survival of bladder UC according to stage and grade at diagnosis. Therefore, we used the data from the SEER cancer registries diagnosed between 1988 and 2009 to explore the relationship between marital status and the survival of bladder UC.
2. Methods
2.1. Patient selection in the SEER database
The SEER Cancer Statistics Review is an authoritative source of information on cancer incidence and survival in the United States. The current SEER database consists of 17 population-based cancer registries that represent approximately 28% of the population in the United States. The SEER data contains cancer-based demographics, the tumor primary site and stage at diagnosis, surgical treatment, the follow-up of survival, and so on.
Using the National Cancer Institute's SEER∗Stat software (Version 8.3.4; www.seer.cancer.gov/seerstat), we identified bladder UC patients diagnosed between 1988 and 2009 with a known marital status. Primary site codes C67.0 to C67.9 and histological type codes were UC (8120/3, 8122/3, 8130/3, 8131/3, 8082/3, 8020/3, 8031/3). Patients with nonprimary bladder UC were excluded. The cause of death and survival of all patients were clearly known, as well as, ethnic information and tumor (topography), lymph node, metastasis (TNM) stage.
2.2. Ethical approval
The current research does not contain any studies with human participants or animals performed by any of the authors.
2.3. Description of covariates
Gender, age, race, primary site, pathology grade, survival, cause-specific survival (CSS), and reason no cancer-directed surgery were recruited from the SEER database. The TNM stage group derived by the American Joint Committee on Cancer, Cancer Staging Manual (7th edition, 2010). We divided patients into 4 groups “‘married,” “widowed,” “single (never married),” and “divorced/separated.” Patients with unknown marital status and TNM stage were excluded. Pathology grade was divided into 3 groups, low grade (well differentiated; grade I/moderately differentiated; grade II), high grade (poorly differentiated; grade III /undifferentiated; anaplastic; grade IV), and unknown group. Primary sites include wall of bladder (C67.2, C67.3, C67.4), trigone of bladder (C67.0, 67.5, C67.6), dome of bladder (C67.1, C67.7), bladder (C67.8, C67.9). Other covariates involved, race recode (white, black, other), age group (<60 or ≥60), and surgery performed (yes or no).
2.4. Statistical analysis
Descriptive statistics for the patient baseline characteristics were analyzed using the chi-square test. Using the Kaplan–Meier method compared the death rate of bladder UC between groups and generated the survival curves. Multivariate Cox regression models were built to analyze the risk factors on survival outcomes. The primary observation point of present study was the CSS of bladder UC, which referred to the time between the date of diagnosis and cancer-specific death. Deaths attributed to bladder UC were treated as events and deaths from other causes were treated as censored observations. All statistical analyses were performed using the statistical software package SPSS (SPSS Inc, Chicago, IL) 22.0. All tests were 2-sided, and statistical significance was defined as P < .05.
3. Results
3.1. Patient baseline characteristics
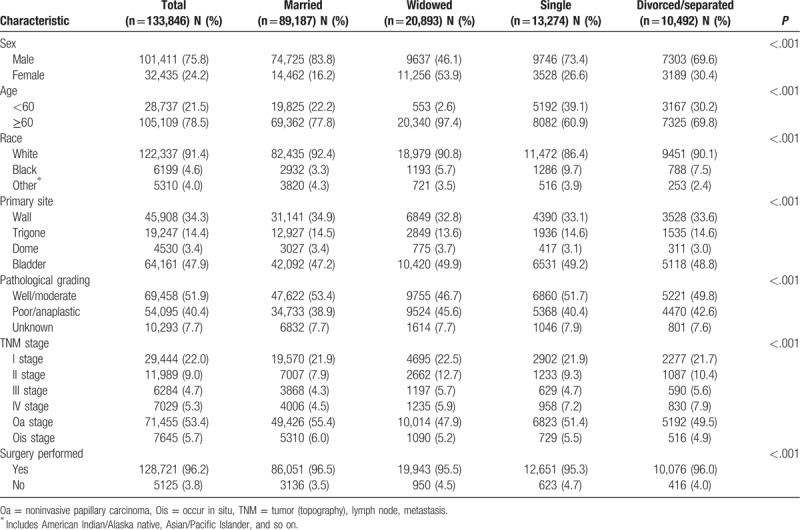
A total of 133,846 eligible patients were identified from SEER database during the 21-year study period (between 1988 and 2009), including 101,411 male and 32,435 female patients. Bladder UC is 3 times more common in men than in women in the United States. Among these patients, 89,187 (66.6%) married, 20,893 (15.6%) widowed, 13,274 (9.9%) single (never married), and 10,492 (7.8%) divorced/separated. Within group comparisons, the widowed group had the higher proportion of women (53.9%), white race (90.8%), older (≥60 years) patients (97.4%), bladder (primary site) (49.9%), low grade (46.7%), noninvasive papillary carcinoma (Oa) stage (47.9%), and surgery performed (95.5%), all of which were statistically significant (P < .001). In our view, it is interesting about gender (man vs women) in different marital status, married group (83.8% vs 16.2%), widowed group (46.1% vs 53.9%), single group (73.4% vs 26.6%), and divorced /separated group (69.6% vs 30.4%). It seemed that only widowhood had higher effect on women than man. Patient demographics and clinical characteristics are summarized in Table 1.
Table 1.
Baseline demographic and tumor characteristics of bladder urothelial carcinoma patients in the SEER database.
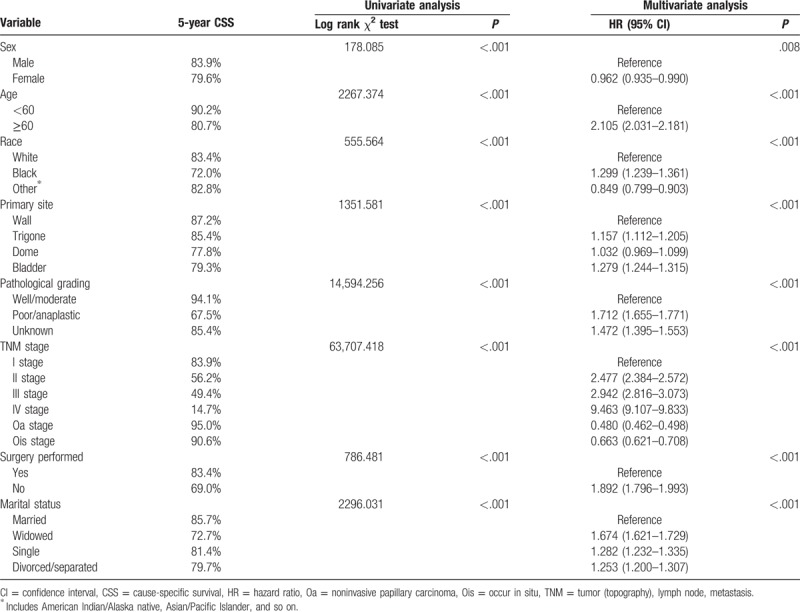
3.2. Effect of marital status on CSS in the SEER database
The 5-year CSS of married group (85.7%) was highest than other groups. The widowed group had the lowest 5-year CSS (72.7%) of bladder UC. All the differences were significant according to the univariate log-rank test (P < .001) (Table 2). Female sex (P < .001), black race (P < .001), older patients (P < .001), dome of bladder (P < .001), high grade (P < .001), IV stage (P < .001), no surgery performed (P < .001), and the widowed group (P < .001) had been confirmed as significant risk predictors for poor survival on univariate analysis (Table 2). Multivariate modeling analysis with Cox regression revealed that all the aforementioned variables were validated as independent risk predictors associated with poor survival (Table 2). These consisted of sex (female, hazard ratio [HR] 0.962, 95% confidence interval [CI] [0.935–0.990]), age (≥60 years, HR 2.105, 95% CI [2.031–2.181]), race (black, HR 1.299, 95% CI [1.239–1.361]; other, HR 0.849, 95% CI [0.799–0.903]), primary site (trigone, HR 1.157, 95% CI [1.112–1.205]; dome, HR 1.032, 95% CI [0.969–1.099]; bladder, HR 1.279, 95% CI [1.244–1.315]), high grade (poor/anaplastic, HR 1.712, 95% CI [1.655–1.771]; unknown, HR 1.472, 95% CI [1.395–1.553]), TNM stage (II stage, HR 2.477, 95% CI [2.384–2.572]; III stage, HR 2.942, 95% CI [2.816–3.073]; IV stage, HR 9.463, 95% CI [9.107–9.833]; Oa stage, HR 0.480, 95% CI [0.462–0.498]; occur in situ (Ois) stage, HR 0.663, 95% CI [0.621–0.708]), marital status (widowed, HR 1.674, 95% CI [1.621–1.729]; single, HR 1.282, 95% CI [1.232–1.335]; divorced/separated, HR 1.253, 95% CI [1.200–1.307]), surgery performed (no, HR 1.892, 95% CI [1.796–1.993]).
Table 2.
Univariate and multivariate survival analysis for evaluating the influence of marital status on bladder urothelial carcinoma cause-specific survival in SEER database.
3.3. Subgroup analysis for evaluating the effect of marital status according to TNM stage
We analyzed the effects of marital status on survival in bladder UC of different clinical stage. First, marital status was an independent factor for CSS in each TNM stage, both in univariate and multivariate analysis (P < .001). Second, the widowed group patients always had the lowest 5-year survival rate compared with other groups. The married group patients almost had the highest 5-year survival rate compared with other groups, except Ois stage. Married patients 5-year CSS compared with widowed patients at Oa stage 95.8% versus 89.8% (P < .001), Ois stage 91.6% versus 84.1% (P < .001), I stage 86.1% versus 74.3% (P < .001), II stage 61.6% versus 41.2% (P < .001), III stage 52.5% versus 39.2% (P < .001), IV stage 17.0% versus 8.8% (P < .001) (Table 3). Interestingly, the 5-year CSS of divorced/separated group compared with married group at Ois stage was 92.5% versus 91.6% (P = .160) (Table 3). This phenomenon may be related to the good prognosis of bladder carcinoma in situ. Third, the single (never married) group and the divorced/separated group had no significant difference. They have an approximate 5-year CSS and a similar survival curve (Fig. 1).
Table 3.
Univariate and multivariate analysis of marital status on bladder urothelial carcinoma cause-specific survival based on different TNM stage.
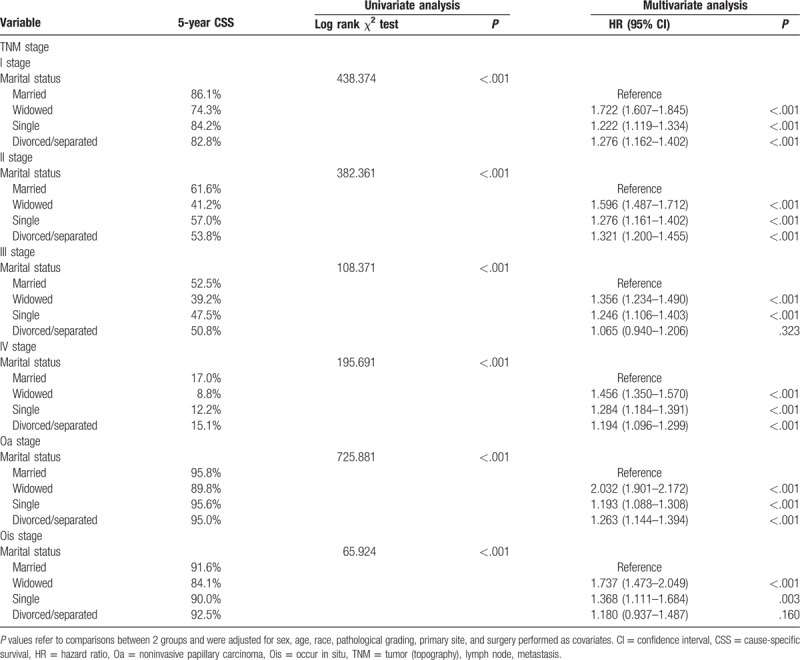
Figure 1.
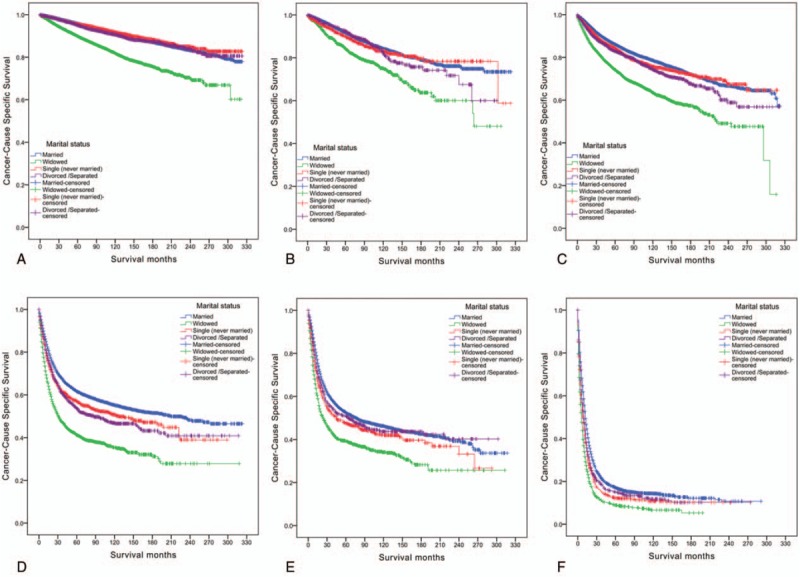
Survival curves in tumor (topography), lymph node, metastasis stage of bladder urothelial carcinoma patients according to marital status. (A) Noninvasive papillary carcinoma stage: χ2 = 725.881, P < .001; (B) occur in situ stage: χ2 = 65.924, P < .001; (C) I stage: χ2 = 438.374, P < .001; (D) II stage: χ2 = 382.361, P < .001; (E) III stage: χ2 = 108.371, P < .001; (F) IV stage: χ2 = 195.691, P < .001.
3.4. Subgroup analysis for evaluating the effect of marital status according to pathology grade
We further analyzed the effects of marital status on survival in bladder UC of different pathology grades. We observed same interesting findings in the subgroup of pathological grading among the different marital status groups (Table 4). First, pathology grade was an independent factor for CSS, both in the univariate and multivariate analysis (P < .001). Second, widowed patients had the lowest survival rate in comparisons at all grades: For low-grade (well differentiated/moderately differentiated) carcinoma, 5-year CSS of widowed patients had 6.6%, 5.6%, and 5.5% reductions compared with that of married patients, single (never married) patients, and divorced/separated patients, respectively (all P < .001). For high-grade (poorly differentiated/undifferentiated; anaplastic) carcinoma, 5-year CSS of widowed patients had 16%, 8.2%, and 6.6% reductions compared with that of married patients, single (never married) patients, and divorced/separated patients, respectively (all P < .001). Even for unknown pathological grading carcinoma, widowed patients had a 13.9% reduction in 5-year CSS compared with married patients (P < .001), a 9.7% reduction in 5-year CSS compared with single patients (P = .001), and a 5.9% reduction in 5-year CSS compared with divorced/separated patients (P < .001) (Table 4 and Fig. 2).
Table 4.
Univariate and multivariate analysis of marital status on bladder urothelial carcinoma cause-specific survival based on different pathological grading.
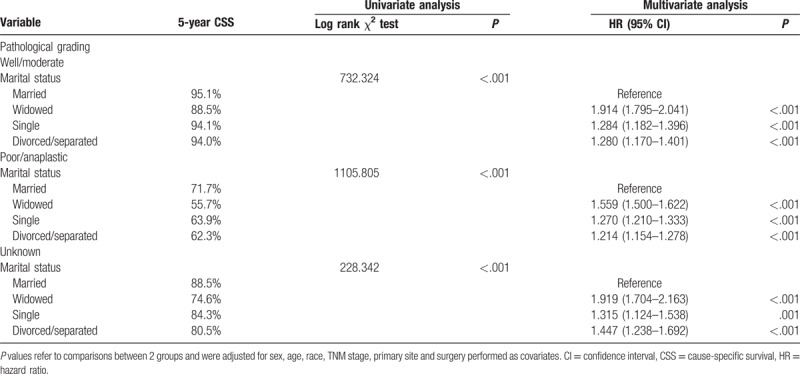
Figure 2.
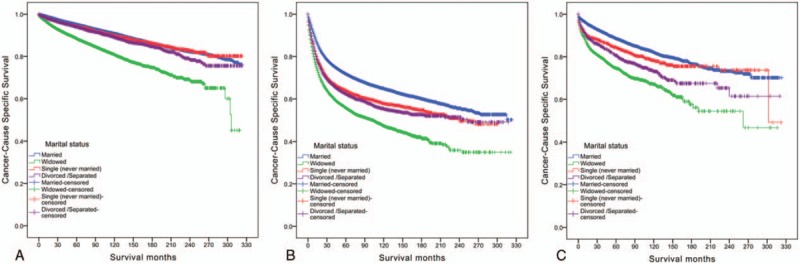
Survival curves in pathology grade of bladder urothelial carcinoma patients according to marital status. (A) Well/moderate: χ2 = 732.324, P < .001; (B) poor/anaplastic: χ2 = 1105.805, P < .001; (C) unknown: χ2 = 228.342, P < .001.
4. Discussion
In present study, female gender, black race, older (≥60), dome of bladder, high grade, IV stage, no surgery performed, and widowed patients had worst CSS (Table 2). Approximately 70% of all suicides in patients aged >60 years are attributed to physical illness, with higher rates noted in patients with cancer.[13,14] Schiffmann et al[15] pointed that presence of lymph node invasion at radical cystectomy regardless of T2 or T3/4a stage was the most important variable that increased the use of adjuvant chemotherapy. However, older individuals were less likely to receive adjuvant chemotherapy. Because age influenced the choice of treatment[16] and age was related to the decline of immunity in the elderly.[17] These factors may explain why older patients had lower survival.
Although men are diagnosed with bladder cancer with a rate 3 times higher than women, women experience poorer survival.[18] Meanwhile, there are many studies reported that women diagnosed with bladder cancer commonly had lower survival.[19–22] On the other hand, Soave et al[23] made a study on 398 (77%) male patients and 119 (23%) female patients. At a median follow-up of 44 months, there was no statistical difference in disease recurrence, cancer-specific mortality, and overall survival between both genders. However, multivariate Cox regression analyses in our study show there is a significantly statistical difference of bladder UC cancer-specific survival between male and female (P = .008). It was reported that sex steroid hormones and their receptors play an active role in bladder cancer development and progression.[24]
White patients with urinary tumors consistently have a survival advantage over black patients despite similar patient and treatment characteristics.[25,26] Previous reports also indicated that blacks presented with higher stage disease and had worse disease-specific survival compared to whites.[7,27,28] In our study, black patients tend to have significantly lower 5-year CSS compared to whites (72.0% vs 83.4%), which may be associated with black race having worse social status, economic income, medical insurance, and other unknown factors.[29]
In the present study, we found that approximately 66.6% of patients with bladder UC were married. The effect of marital status on cancer-specific survival of many cancers had been reported by using SEER database; married group always had higher CSS and lower mortality than other groups.[3,6,30,31] Marital status also had been implicated as a prognostic factor in bladder cancer survival.[8,20] We identified marital status was an independent prognostic factor in each pathological grading and each TNM stage in patients with bladder UC. Meanwhile, we found the widowed group had lowest 5-year CSS in each classification compared with other groups (Figs. 1 and 2).
Psychosocial factors and social support may play an important role in the relationship between marital status and survival. Epidemiological studies indicate that stress, chronic depression, and lack of social support might serve as risk factors for cancer development and progression.[32] Married people have better health, because they have more material resources, less stress, indulge in less risky health behavior, and have more social support.[33] Unmarried and especially widowed patients may suffer from a lack of emotional support and social attention.[34] One meta-analysis presented reasonable evidence that depression predicts mortality in cancer patients and depression may play a causal role.[35] Meanwhile, another meta-analysis presented that depression diagnosis and higher levels of depressive symptoms predicted elevated mortality.[36] Higher levels of social wellbeing were correlated with lower vascular endothelial growth factor (VEGF) levels in presurgical patients with ovarian carcinoma suggest that poor social support may be associated with disease progression.[37]
There are many different mechanisms explain that the level of physiological stress and depression may affect cancer outcomes. Fang et al[38] suggest that poorer psychosocial functioning before surgery is associated with greater VEGF expression in tumor, which is a clinically relevant biomarker among head and neck squamous cell carcinoma patients. Greater VEGF expression in tumor is predictive of poorer overall and disease-free survival. There was evidence that chronic stress impaired the immune system's response to anti-inflammatory signals: The capacity of a synthetic glucocorticoid hormone to suppress in vitro production of the proinflammatory cytokine interleukin-6 was diminished among parents of cancer patients.[39] Chronic stress results in the activation of specific signaling pathways in cancer cells and the tumor microenvironment, leading to tumor growth and progression.[40] In addition, negative emotions also contribute to prolonged infection and delayed wound healing, processes that fuel sustained proinflammatory cytokine production.[41]
In the present study, we divide the patients into low-grade, high-grade, and unknown groups according to the tumor differentiation. In high-grade group, widowed patients had worst 5-year CSS (55.7%) compared with married (71.7%), single (never married) (63.9%), and divorced/separated (62.3%) patients (all P < .001). Similarly, the patients with low grade, the widowed group had worst 5-year CSS (88.5%) compared with married (95.1.0%), never married (94.1%), and divorced/separated (94.0%) patients (all P < .01). However, 1 study reported that after controlling for stage and grade, no survival difference could be detected between micropapillary urothelial bladder carcinoma (MPBC) and UC. Low-grade nonmuscle invasive MPBC behaved similarly to both high-grade MPBC and high-grade UC.[42] We can see the higher stage have lower bladder UC CSS from present study. In the IV stage, widowed patients had the worst 5-year CSS (8.8%) compared with married (17.0%), single (never married) (12.2%), and divorced/separated (15.1%) patients (all P < .001).
We identified the relationship between marital status and the CSS of bladder UC by using the SEER database. However, there are some potential limitations in our study. First, some data of patient were not complete. Health behaviors including past/present smoking and alcohol use were factors linked to survival among patients with cancer.[9,10] Smoking is a risk factor for bladder cancer diagnosis and recurrence.[43] But the SEER database lacks the information about smoking. Second, the SEER database only provides marital status at the time of tumor diagnosis. We could not determine whether the marital status whether had a change or not after the diagnosis of bladder cancer. Moreover, the quality of marriage is not clear. It is reported that marital distress had a variety of negative effect on health and immunity.[44] Furthermore, the SEER database lacks enough information on therapy options, subsequent therapy, comorbidities, and recurrence. Finally, our study is a retrospective research, which may weaken our conclusion.
Despite there are some potential limitations, the results in present study confirmed that unmarried patients had greater risk of cancer-specific mortality. Furthermore, our study showed the widowed patients were at the highest risk of bladder-cancer-specific mortality than those in other groups. Social and psychosocial factors may be some of main reasons for poor survival outcomes in unmarried patients. Therefore, to improve postoperative survival, close social and family care may improve the survival outcomes for unmarried patients, especially for those who were widowed.
Acknowledgments
The authors acknowledge the efforts of the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER database. The authors gratefully thank their colleagues from the Subei People's Hospital of Jiangsu Province (Clinical Medical Academy of Yangzhou University) and Qindao University Medical College Affiliated Yantai Yuhuangding Hospital for their kind help.
Author contributions
Conceptualization: Guangchen Zhou.
Data curation: Quan Niu, Yinxia Wu.
Formal analysis: Quan Niu, Shigao Xu, Guangchen Zhou.
Funding acquisition: Yinxia Wu, Junjie Yu.
Investigation: Youyi Lu, Shigao Xu, Qun Shi.
Methodology: Quan Niu, Tianbao Huang.
Project administration: Yinxia Wu, Guangchen Zhou, Xiao Gu, Junjie Yu.
Resources: Youyi Lu, Xiao Gu, Junjie Yu.
Software: Youyi Lu, Qun Shi, Tianbao Huang.
Writing – original draft: Quan Niu.
Writing – review & editing: Xiao Gu, Junjie Yu.
Footnotes
Abbreviations: CI = confidence interval, CSS = cause-specific survival, HR = hazard ratio, MPBC = micropapillary urothelial bladder carcinoma, Oa = noninvasive papillary carcinoma, Ois = occur in situ, SEER = Surveillance, Epidemiology, and End Results, TNM = tumor (topography), lymph node, metastasis, UC = urothelial carcinoma, VEGF = vascular endothelial growth factor.
QN, YL, and YW have contributed equally to the work.
Funding/support: This study was supported by grants from the National Natural Science Foundation of China (nos. 81402101 and 81572702) and grants from the Postdoctoral Science Foundation of China (no. 2017M611755).
The authors have no conflicts of interest to disclose.
References
- [1].Antoni S, Ferlay J, Soerjomataram I, et al. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol 2017;71:96–108. [DOI] [PubMed] [Google Scholar]
- [2].Clark PE, Agarwal N, Biagioli MC, et al. Bladder cancer. J Natl Compr Canc Netw 2013;11:446–75. [DOI] [PubMed] [Google Scholar]
- [3].Wang L, Wilson SE, Stewart DB, et al. Marital status and colon cancer outcomes in US Surveillance, Epidemiology and End Results registries: does marriage affect cancer survival by gender and stage? Cancer Epidemiol 2011;35:417–22. [DOI] [PubMed] [Google Scholar]
- [4].Bai DS, Chen P, Qian JJ, et al. Effect of marital status on the survival of patients with gallbladder cancer treated with surgical resection: a population-based study. Oncotarget 2017;8:26404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tyson MD, Andrews PE, Etzioni DA, et al. Marital status and prostate cancer outcomes. Can J Urol 2013;20:6702–6. [PubMed] [Google Scholar]
- [6].Croft L, Sorkin J, Gallicchio L. Marital status and optimism score among breast cancer survivors. Support Care Cancer 2014;22:3027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Klaassen Z, DiBianco JM, Jen RP, et al. Female, black, and unmarried patients are more likely to present with metastatic bladder urothelial carcinoma. Clin Genitourin Cancer 2016;14:e489–92. [DOI] [PubMed] [Google Scholar]
- [8].Gore JL, Kwan L, Saigal CS, et al. Marriage and mortality in bladder carcinoma. Cancer 2005;104:1188–94. [DOI] [PubMed] [Google Scholar]
- [9].Lindstrom M. Social capital, economic conditions, marital status and daily smoking: a population-based study. Public Health 2010;124:71–7. [DOI] [PubMed] [Google Scholar]
- [10].Gritz ER, Demark-Wahnefried W. Health behaviors influence cancer survival. J Clin Oncol 2009;27:1930–2. [DOI] [PubMed] [Google Scholar]
- [11].Merrill RM, Johnson E. Benefits of marriage on relative and conditional relative cancer survival differ between males and females in the USA. J Cancer Surviv 2017;11:578–89. [DOI] [PubMed] [Google Scholar]
- [12].Li Q, Gan L, Liang L, et al. The influence of marital status on stage at diagnosis and survival of patients with colorectal cancer. Oncotarget 2015;6:7339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Klaassen Z, Jen RP, DiBianco JM, et al. Factors associated with suicide in patients with genitourinary malignancies. Cancer 2015;121:1864–72. [DOI] [PubMed] [Google Scholar]
- [14].Chamie K, Litwin MS, Bassett JC, et al. Recurrence of high-risk bladder cancer: a population-based analysis. Cancer 2013;119:3219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schiffmann J, Sun M, Gandaglia G, et al. Use of adjuvant chemotherapy in radical cystectomy patients aged >65 years: a population-based study from the surveillance epidemiology and end results (SEER)-Medicare database. Minerva Urol Nefrol 2017;69:173–80. [DOI] [PubMed] [Google Scholar]
- [16].Serra-Rexach JA, Jimenez AB, Garcia-Alhambra MA, et al. Differences in the therapeutic approach to colorectal cancer in young and elderly patients. Oncologist 2012;17:1277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].van der Geest KS, Abdulahad WH, Tete SM, et al. Aging disturbs the balance between effector and regulatory CD4+ T cells. Exp Gerontol 2014;60:190–6. [DOI] [PubMed] [Google Scholar]
- [18].Patel MI, Bang A, Gillett D, et al. Poor survival of females with bladder cancer is limited to those aged 70 years or over: a population-wide linkage study, New South Wales, Australia. Cancer Med 2015;4:1145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dobruch J, Daneshmand S, Fisch M, et al. Gender and bladder cancer: a collaborative review of etiology, biology, and outcomes. Eur Urol 2016;69:300–10. [DOI] [PubMed] [Google Scholar]
- [20].Datta GD, Neville BA, Kawachi I, et al. Marital status and survival following bladder cancer. J Epidemiol Community Health 2009;63:807–13. [DOI] [PubMed] [Google Scholar]
- [21].Tracey E, Watt H, Currow D, et al. Investigation of poorer bladder cancer survival in women in NSW, Australia: a data linkage study. BJU Int 2014;113:437–48. [DOI] [PubMed] [Google Scholar]
- [22].Puente D, Malats N, Cecchini L, et al. Gender-related differences in clinical and pathological characteristics and therapy of bladder cancer. Eur Urol 2003;43:53–62. [DOI] [PubMed] [Google Scholar]
- [23].Soave A, Dahlem R, Hansen J, et al. Gender-specific outcomes of bladder cancer patients: a stage-specific analysis in a contemporary, homogenous radical cystectomy cohort. Eur J Surg Oncol 2015;41:368–77. [DOI] [PubMed] [Google Scholar]
- [24].Lucca I, Fajkovic H, Klatte T. Sex steroids and gender differences in nonmuscle invasive bladder cancer. Curr Opin Urol 2014;24:500–5. [DOI] [PubMed] [Google Scholar]
- [25].Chow W-H, Shuch B, Linehan WM, et al. Racial disparity in renal cell carcinoma patient survival according to demographic and clinical characteristics. Cancer 2013;119:388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Erickson BK, Doo DW, Zhang B, et al. Black race independently predicts worse survival in uterine carcinosarcoma. Gynecol Oncol 2014;133:238–41. [DOI] [PubMed] [Google Scholar]
- [27].Kaye DR, Canner JK, Kates M, et al. Do African American patients treated with radical cystectomy for bladder cancer have worse overall survival? Accounting for pathologic staging and patient demographics beyond race makes a difference. Bladder Cancer 2016;2:225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gaines AR, Turner EL, Moorman PG, et al. The association between race and prostate cancer risk on initial biopsy in an equal access, multiethnic cohort. Cancer Causes Control 2014;25:1029–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sun M, Abdollah F, Liberman D, et al. Racial disparities and socioeconomic status in men diagnosed with testicular germ cell tumors: a survival analysis. Cancer 2011;117:4277–85. [DOI] [PubMed] [Google Scholar]
- [30].Ikeda A, Iso H, Toyoshima H, et al. Marital status and mortality among Japanese men and women: the Japan Collaborative Cohort Study. BMC Public Health 2007;7:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Aizer AA, Chen MH, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol 2013;31:3869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer 2006;6:240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wyke S, Ford G. Competing explanations for associations between marital status and health. Soc Sci Med 1992;34:523–32. [DOI] [PubMed] [Google Scholar]
- [34].Goldzweig G, Andritsch E, Hubert A, et al. Psychological distress among male patients and male spouses: what do oncologists need to know? Ann Oncol 2010;21:877–83. [DOI] [PubMed] [Google Scholar]
- [35].Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer 2009;115:5349–61. [DOI] [PubMed] [Google Scholar]
- [36].Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med 2010;40:1797–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lutgendorf SK, Johnsen EL, Cooper B, et al. Vascular endothelial growth factor and social support in patients with ovarian carcinoma. Cancer 2002;95:808–15. [DOI] [PubMed] [Google Scholar]
- [38].Fang CY, Egleston BL, Ridge JA, et al. Psychosocial functioning and vascular endothelial growth factor in patients with head and neck cancer. Head Neck 2014;36:1113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol 2002;21:531–41. [DOI] [PubMed] [Google Scholar]
- [40].Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol 2010;6:1863–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kiecolt-Glaser JK, McGuire L, Robles TF, et al. Emotions, morbidity, and mortality: new perspectives from psychoneuroimmunology. Annu Rev Psychol 2002;53:83–107. [DOI] [PubMed] [Google Scholar]
- [42].Vourganti S, Harbin A, Singer EA, et al. Low grade micropapillary urothelial carcinoma, does it exist?—Analysis of management and outcomes from the Surveillance, Epidemiology and End Results (SEER) Database. J Cancer 2013;4:336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Berg CJ, Thomas AN, Mertens AC, et al. Correlates of continued smoking versus cessation among survivors of smoking-related cancers. Psychooncology 2013;22:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jaremka LM, Glaser R, Malarkey WB, et al. Marital distress prospectively predicts poorer cellular immune function. Psychoneuroendocrinology 2013;38:2713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


