Abstract
Rationale:
Tuberculous meningitis is a highly morbid, often fatal disease.
Patient concern:
We describe a case of an Italian child.
Diagnoses:
we diagnosed early a Tuberculous meningitis complicated by the occurrence of hydrocephalus, stroke, and paradoxical reaction with brain pseudo-abscesses.
Interventions:
The child started readily a specific therapy associated with steroids and thalidomide was introduced few month later.
Outcomes:
the patient had a favorable outcome without neurologic sequelae.
Lessons:
Despite the prompt specific anti-tubercular and adjuvant corticosteroid therapies, only the addition of thalidomide to the treatment allow to a favorable clinical outcome.
Keywords: cerebral vasculitis, children, hydrocephalus, thalidomide, tuberculous meningitis
1. Introduction
According to the World Health Organization, in 2015 approximately 10.5 million cases of tuberculosis (TB) occurred globally, 10% being among children, mainly in resource-limited countries.[1] Tuberculous meningitis (TBM) represents roughly 1% of all TB cases and affects primarily 2 to 4 years old children.[2,3] The diagnosis of TBM could be difficult, mainly because of paucibacillary nature of the infection of the central nervous system (CNS). Early diagnosis and prompt treatment are the main determinants of a good outcome in people with TBM which, despite an appropriate therapy, accounts for high mortality and neurologic sequelae.[4] The most serious complications of TBM are hydrocephalus, stroke, and tuberculoma formation occurring up to 80% of pediatric patients.[5,6] Tuberculous vasculitis and stroke are more frequent in children, and seem related to the basal cisternal meningeal reaction, with hemiplegia being the most frequent clinical-related manifestation.[4] Enlargement of the existing cerebral tuberculoma or appearance of a new tuberculoma is due to a paradoxical reaction observed in patients with TBM, mainly within 3 months after the onset of anti-TB therapy.[7] This reaction is not related to the efficacy of the ongoing anti-TB therapy, but it is possibly due to an exaggerate immune response to Mycobacterium tuberculosis antigens,[8,9] similarly to what happens in immune reconstitution inflammatory syndrome among HIV-positive patients.[10,11] Currently, data on the paradoxical reaction among HIV-negative individuals with TBM are poor.[7] There is no standard treatment for the paradoxical reactions in patients with TBM, but in some severe cases, an immune-modulatory drug, thalidomide, has been used because of its inhibitory action on tumor necrosis factor-alfa (TNF-α).[12–15]
We present the case of a child affected by TBM in whom the early diagnosis and adjuvant treatment with thalidomide allowed a favorable outcome despite the occurrence of a hydrocephalus, stroke, and TB pseudo-abscesses that did not respond to standard therapy.
2. Case description
A 9-year-old Italian girl was admitted to Policlinico Umberto I of Rome (Italy) because of 1-month history of headache, fatigue, slight fever, reported weight loss (6 kg), and sudden late appearance of diplopia, vomit, dizziness, and slight sleepiness. One week before the admission to our hospital, the patient attended the emergency department of another hospital where a head computed tomography scan without contrast was reported as normal and the patient discharged. At the admission to our hospital, general laboratory examinations (including inflammatory values) were normal, and a fundoscopy examination and a brain magnetic resonance imaging (MRI) were negative (Fig. 1A), and an electroencephalogram showed slow focal abnormalities. The day after the sleepiness increased and slight central facial nerve palsy appeared and the patient was transferred to the infectious disease pediatric division. A lumbar puncture (LP) was performed, showing a clear cerebrospinal fluid (CSF) with pleocytosis (90% lymphocytes), white blood cell count of 372 cells/μL with lymphocytes predominant, high protein (1317 mg/dL), and low glucose (13 mg/dL). The microscopic examination of CSF, microbiologic culture test for common bacteria, antigens test for Streptococcus pneumoniae and Neisseria meningitidis, and polymerase chain reaction (PCR) for common viruses (herpes simplex virus [HSV]-1, HSV-2, human herpesvirus-6, cytomegalovirus, Epstein–Barr virus, and Varicella-Zoster Virus) were negative. Table 1 summarizes CSF analysis results during the follow-up. The patient had no TB contact history and chest x-ray was negative. Even though a TB screening was performed: tuberculosis skin test was negative and QuantiferonTB-gold in peripheral whole blood (QuantiFERON-TB© Gold In Tube [QFT-IT]; Cellestis Limited Chadstone, Vic, Australia) was indeterminate. A PCR for M tuberculosis (GeneXpert MTB/RIF, Cepheid) on CSF was also negative. On day 2, due to the persistence of clinical symptoms and the appearance of patient uncertainty at the clinical coordination tests, another brain MRI was performed (Fig. 1B,C) showing hypertensive hydrocephalus and a diffuse leptomeningeal enhancement of the basal cistern, in particular of the interpeduncular cistern. An external ventricular drain (EVD) was placed by neurosurgeon and a sample of ventricular CSF was analyzed in comparison to the CSF obtained through a second LP: a dissociation between samples of cellular and biochemical parameters was evident (Table 1). The microscopic examination and the GeneXpert MTB/RIF assay of CSF obtained through LP were still negative, whereas a nested-PCR amplifying a 123-bp fragment of the M tuberculosis DNA was positive (Table 1). On the same CSF sample, a lymphocyte T-CD4 polyfunctional response pattern was observed, and an adenosine deaminase activity (ADA) test was positive (18 U/L). Thus, a TBM was suspected and a 4-drug anti-tubercular therapy (rifampicin [R] = 10 mg/kg/d, isoniazid [H] = 8 mg/kg/d, ethambutol [E] = 25 mg/kg/d, pyrazinamide [Z] = 25 mg/kg/d), plus and intravenous steroids (dexamethasone 4 mg every 8 hours) was started. A screening of familial contact was performed and the father was found having a latent TB infection. After few days of HERZ regimen, the sleepiness improved, while the diplopia was stable. On day 27, the patient showed an acute onset of clumsy speech and a right hemiplegia. A control brain MRI (Fig. 1D) showed “2 focal areas of signal restriction in correspondence of the left caudate nucleus and of the posterior arm of the left internal capsule, compatible with tuberculous cerebral vasculitis (TVC).” Acetylsalicylic acid and enoxaparin were added to the ongoing treatment. A neurologic rehabilitation was then started and the clinical conditions of the patients progressively improved. On day 33, the EVD was internalized. On day 74, considering the disappearance of neurologic symptoms/signs, the patient was discharged with the recommendation to continue treatment with HERZ regimen plus betamethasone (1 mg/d) and acetylsalicylic acid, and a follow-up schedule was established. After 3 months of anti-TB regimen, the patient was readmitted to our division because of reappearance of slight facial nerve palsy. General laboratory examinations were normal, whereas a brain MRI revealed a radiologic deterioration in comparison to previous MRIs with the appearance of new tuberculomas with perilesional edema within the middle cerebellar peduncle (Fig. 2A). An LP was performed and CSF analysis revealed a reduction of leukocytes and proteins, with a slight increase of glucose in comparison with previous CSF analysis, whereas microbiologic examinations were still negative (Table 1). The HERZ was continued and the corticosteroid (CCS) dosage increased (dexamethasone 4 mg every 8 hours) with progressive improvement of clinical condition. Two months later (5th month of anti-TB therapy), ethambutol was stopped and the patient was discharged with the indication to continue HRZ regimen and prednisone (12.5 mg/d). Two months later (7th month of anti-TB therapy), the patient began to complain of a worsening low back pain. A column MRI revealed recent D9 and L1 somatic fractures; a bone densitometry examination showed only osteopenia (DEXA: lumbar z-score -1). According to these features, a cholecalciferol (vitamin D3), therapy was prescribed as well as the use of an orthopedic bust. The vertebral fractures, the excess of weight gain and the appearance of hirsutism showed by the patient were all related to the long CCS ongoing therapy. After 8-months of anti-TB therapy, the patient was readmitted to our division because of the appearance of sleepiness and vomit. General laboratory examinations were always normal and a brain MRI revealed a worsening of radiologic features (“increased size and perilesional edema of the pseudo-abscess involving the cerebellar peduncle”) (Fig. 2B). Then, CCS was administered at higher dose (dexamethasone 16 mg/d) in association with anti-edema therapy (Mannitol 100 cc × 4/d). Moreover, to strengthen anti-TB therapy, isoniazid daily dose was increased (up to 10 mg/kg/d) for improving CNS penetration; levofloxacin (18 mg/kg/d) and linezolid (20 mg/kg/d) were also added because of their anti-tubercular activity as well as their good CNS penetration. Finally, to gradually reduce CCS therapy, after obtaining parental written informed consent, thalidomide (1.2 mg/kg/d) administration was started. Two weeks later the patient was discharged because of clinical improvement: CCS dosage was progressively reduced and then stopped, while thalidomide and HRZ were continued without evidence of significant clinical or laboratory toxicity. After 5 months after the introduction of thalidomide therapy (13th month of anti-TB therapy), a control brain MRI was improving (“reduction of the lesion's size, hyper-intensity signal, and surrounding vasogenic edema”) (Fig. 2C), and the drug was administered every other day for additional 2 months. An additional control brain MRI performed showed a further improvement (Fig. 2D). After 1 month, pyrazinamide was stopped, while HR regimen was continued until completing 24 months of therapy.
Figure 1.

Brain magnetic resonance imaging. At the onset of the symptoms, normal imaging was found (A), after 2 days leptomeningeal enhancement over the basilar cistern and hydrocephalus were relieved (B, C). Focal areas of signal restriction in correspondence of the left caudate nucleus and of the posterior arm of the left internal capsule (D) were found, suggesting a tuberculous cerebral vasculitis as a complication.
Table 1.
Serial CSF data from lumbar puncture and ventricular drain.
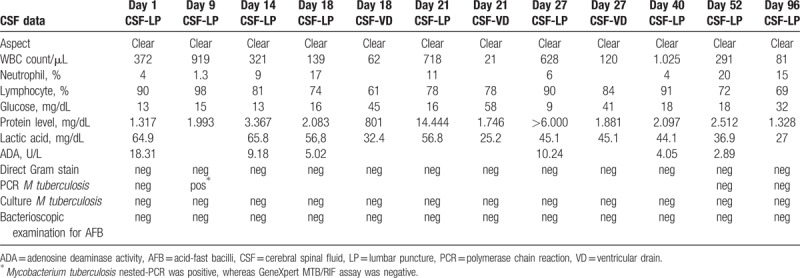
Figure 2.
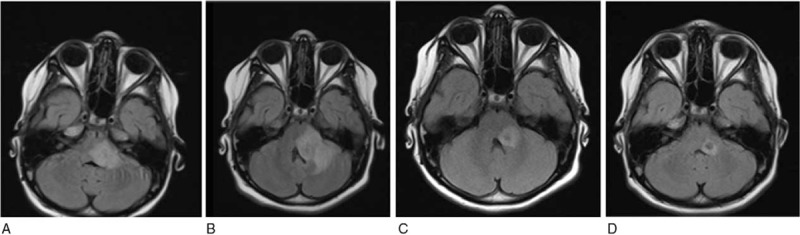
Brain magnetic resonance imaging. Abscess lesions (0.6–1.3 cm) in correspondence of brain stem at the base of the left cerebellar peduncle with surrounding edema reaction and an increase of diffuse leptomeningeal enhancement of the base and small millimeter abscess collections (A). Volumetric increment of the back cranial portion of the abscess lesion (B). Five months after the introduction of thalidomide, follow-up (C, D).
3. Discussion and conclusion
The TBM is the most severe extrapulmonary complication of TB, associated to high mortality rates and neurologic sequelae.[3,4] It results from hematogenous spread of primary or postprimary pulmonary disease, or from the rupture of a sub-ependymal tubercle into the sub-arachnoid space. The clinical presentation of TBM may be heterogenous, from pauci symptomatic to severely symptomatic cases, largely depending on the CNS localization of tuberculoma or tuberculous pseudo-abscess. Often, TBM-affected patients attend a doctor because of headache, seizures, diplopia, and/or other clinical signs related to increased intracranial pressure.
Based on clinical signs, the Medical Research Council described 3 stages of TBM (Medical Research Council), which has been shown in numerous series to have considerable prognostic value.[3,11] stage I (mild cases) is characterized by fully consciousness and no focal deficits; stage II (moderately advanced cases) by consciousness but with inattention, confusion, lethargy, and focal neurologic signs such as cranial nerve palsies; and stage III by stupor or comatose, multiple cranial nerve palsies, or complete hemiparesis or paralysis. Clinical manifestations of tuberculoma or tuberculous brain abscess depend largely on their location, and patients often present with headache, seizures, papilledema, or other signs of increased intracranial pressure.
We described a case of TBM in stage III with hydrocephalus, stroke, and paradoxical reaction with tubercular abscesses where the prompt diagnosis and the addition of thalidomide to the anti-TB and steroid therapy allowed a favorable outcome.
The TBM diagnosis is generally difficult, mainly because of the pauci-bacillary nature of the infection leading to a low sensitivity of traditional microbiologic methods (microscopic direct examination and mycobacterial culture).[4]
In the present case, diagnostic suspicion was based on clinical presentation and basic characteristics of CSF analysis, but not supported by CNS neuro-radiology at the begin. The diagnosis was then confirmed by a M tuberculosis nested-PCR supported by immunologic tools based on intracellular staining of mononuclear cells from blood and CSF,[16,17] ADA test, and neuro-radiologic features observed during the follow-up.
Treating TBM is a clinical challenge, because it responds poorly to conventional TB treatment; moreover, it needs 2 kinds of treatment: the microbiologic and the inflammatory ones.
The choice of anti-TB combination drugs in TBM management should consider the bactericidal activity of each drug, as well as its capacity to penetrate into the CNS, but the optimal drug regimen and duration of each phase are not clearly established.[3] Moreover, the individual immunity response may play a major role in defining the patient outcome.[9] According to a review on CSF concentration of anti-tuberculosis agents,[18] isoniazid well penetrates the CSF in a concentration-dependent manner and has the highest early bactericidal activity; pyrazinamide also achieves acceptable CSF concentration at ordinary drug dosage, whereas rifampicin shows relatively poor CSF concentration in a dose-dependent manner, and ethambutol penetrates poorly into CSF. Although these evidences related to the CSF concentration, the WHO recommends to treat pediatric TBM using a 4-drug (HERZ) regimen for 2 months, followed by a 2-drug (HR) regimen for 10 months.[19] In our patient, we administered a 4-drug (HERZ) regimen and, to allow a better CSF concentration of the drugs, we increased the dosage during the treatment. Moreover, we added levofloxacin to the anti-TB therapy because of its potent bactericidal activity and good penetration into CSF and its relatively safe administration in children affected by TB.[18] Furthermore, considering the worsening clinical evolution observed at some points during the follow-up, although there were no data on mycobacterial susceptibility, we added linezolid to the treatment because of its good CNS penetration and its effectiveness in drug-resistant TB cases, including children.[20]
Anti-TB therapy alone does not improve outcome significantly despite reductions in bacillary load. Microglial cells are the principal target of M tuberculosis in CNS and TNF-α released by microglial infected cells play a crucial role in the pathogenesis of the infection.[21] In fact, low TNF-α levels result in mycobacterial overgrowth, whereas high levels may lead to exaggerated immune reaction and tissue destruction possibly related to mycobacterial antigen release.[7,21] The addition of CCS to the anti-TB therapy reduces TBM-related mortality, at least in the short term,[22] possibly because of its anti-inflammatory effect that may lead also to reduce intracranial pressure. But long duration of CCS administration is associated to significant toxicity, as shown in the present case in which the children had vertebral fractures, significant weight gain, and hirsutism. At the same time, a worsening situation was documented by brain MRI. This was compatible with a paradoxical reaction, which is defined as the worsening of pre-existing tuberculous lesions and/or the appearance of new tuberculous lesions in patients whose clinical symptoms initially improved with anti-TB therapy.[7] Considering that the paradoxical reaction is related to TNF-α over-release in the CNS, and to reduce CCS-related toxicity, thalidomide was added to the therapy leading to a clinical and radiologic response, as previously reported.[14] Thalidomide has a strong anti-TNF activity[23,24] and stimulates CD8-T lymphocytes that play a protective role during M tuberculosis infection.[25] Thus, although further studies are needing, thalidomide, although is off-label, could be considered a safe adjuvant drug for the management of TBM, at least in clinical situations in which paradoxical reactions are observed.
In conclusion, we present the case of a 9-year-old Italian children affected by TBM, complicated by hydrocephalus, stroke, and tubercular pseudo-abscesses, in which the association of an immune-modulatory therapy to the anti-TB treatment conducted to a favorable outcome without neurologic sequelae.
Acknowledgment
The authors thank Ronald Van Toorn, Johan F. Schoeman, Claudio Di Biasi, Laura Lancella, and Piero Valentini for their useful clinical advices.
Author contributions
Conceptualization: Serena Vita.
Data curation: Gianluca Russo.
Funding acquisition: Vincenzo Vullo.
Investigation: Emanuela Caraffa, Anna Paola Massetti.
Methodology: Miriam Lichtner, Claudio Maria Mastroianni.
Project administration: Maria Rosa Ciardi, Camilla Ajassa.
Supervision: Miriam Lichtner, Claudio Maria Mastroianni, Maria Rosa Ciardi, Camilla Ajassa.
Writing – original draft: Emanuela Caraffa, Gianluca Russo, Serena Vita, Anna Paola Massetti.
Writing – review & editing: Gianluca Russo, Serena Vita, Miriam Lichtner, Claudio Maria Mastroianni, Vincenzo Vullo, Maria Rosa Ciardi, Camilla Ajassa.
Footnotes
Abbreviations: ADA = adenosine deaminase activity, CCS = corticosteroid, CNS = central nervous system, CSF = cerebrospinal fluid, DEXA = dual energy x-ray absorptiometry, EVD = external ventricular drain, HERZ = isoniazid, ethambutol, rifampin, pyrazinamide daily, HHV-6 = human herpesvirus-6, HR = isoniazid, rifampin daily, HRZ = isoniazid, rifampin, pyrazinamide daily, HSV = herpes simplex virus, LP = lumbar puncture, MRI = magnetic resonance imaging, MTB/RIF = Mycobacterium tuberculosis/rifampicin, PCR = polymerase chain reaction, TB = tuberculosis, TBM = tuberculous meningitis, TNF-α = tumor necrosis factor-alfa, TVC = tuberculous cerebral vasculitis.
Maria Rosa Ciardi and Camilla Ajassa contributed equally to this study.
Written informed consent for publication of patient's clinical details and clinical images was obtained from the parent of the patient. A copy of the consent form is available for review with the editor of this journal. Ethical approval to report this case was not required from our institution.
The authors have no funding and conflicts of interest to disclose.
References
- [1].World Health Organization. WHO Global Tuberculosis Report 2016. Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- [2].van Well GT, Paes BF, Terwee CB, et al. Twenty years of pediatric tuberculous meningitis: a retrospective cohort study in the western cape of South Africa. Pediatrics 2009;123:e1–8. [DOI] [PubMed] [Google Scholar]
- [3].Thwaites G, Fisher M, Hemingway C, et al. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect 2009;59:167–87. [DOI] [PubMed] [Google Scholar]
- [4].Thwaites GE, van Toorn R, Schoeman J. Tuberculous meningitis: more questions, still too few answers. Lancet Neurol 2013;12:999–1010. [DOI] [PubMed] [Google Scholar]
- [5].Schoeman JF, Van Zyl LE, Laubscher JA, et al. Serial CT scanning in childhood tuberculous meningitis: prognostic features in 198 cases. J Child Neurol 1995;10:320–9. [DOI] [PubMed] [Google Scholar]
- [6].Yaramiş A, Gurkan F, Elevli M, et al. Central nervous system tuberculosis in children: a review of 214 cases. Pediatrics 1998;102:E49. [DOI] [PubMed] [Google Scholar]
- [7].Garg RK, Malhotra HS, Kumar N. Paradoxical reaction in HIV negative tuberculous meningitis. J Neurol Sci 2014;340:26–36. [DOI] [PubMed] [Google Scholar]
- [8].Hawkey CR, Yap T, Pereira J, et al. Characterization and management of paradoxical upgrading reactions in HIV-uninfected patients with lymph node tuberculosis. Clin Infect Dis 2005;40:1368–71. [DOI] [PubMed] [Google Scholar]
- [9].Sáenz B, Hernandez-Pando R, Fragoso G, et al. The dual face of central nervous system tuberculosis: a new Janus Bifrons? Tuberculosis (Edinb) 2013;93:130–5. [DOI] [PubMed] [Google Scholar]
- [10].Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis 2008;8:516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Marais S, Meintjes G, Pepper DJ, et al. Frequency, severity, and prediction of tuberculous meningitis immune reconstitution inflammatory syndrome. Clin Infect Dis 2013;56:450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tavares JL, Wangoo A, Dilworth P, et al. Thalidomide reduces tumour necrosis factor-alpha production by human alveolar macrophages. Respir Med 1997;91:31–9. [DOI] [PubMed] [Google Scholar]
- [13].de la Riva P, Urtasun M, Castillo-Trivino T, et al. Clinical response to thalidomide in the treatment of intracranial tuberculomas: case report. Clin Neuropharm 2013;36:70–2. [DOI] [PubMed] [Google Scholar]
- [14].van Toorn R, du Plessis AM, Schaaf HS, et al. Clinicoradiologic response of neurologic tuberculous mass lesions in children treated with thalidomide. Pediatr Infect Dis J 2015;34:214–8. [DOI] [PubMed] [Google Scholar]
- [15].Viel-Thériault I, Thibeault R, Boucher FD, et al. Thalidomide in refractory tuberculomas and pseudoabscesses. Pediatr Infect Dis J 2016;35:1262–4. [DOI] [PubMed] [Google Scholar]
- [16].Vita S, Ajassa C, Caraffa E, et al. Immunological diagnosis as an adjunctive tool for an early diagnosis of tuberculous meningitis of an immune competent child in a low tuberculosis endemic country: a case report. BMC Res Notes 2017;10:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lichtner M, Mascia C, Sauzullo I, et al. Multifunctional analysis of CD4+ T-cell response as immune-based model for tuberculosis detection. J Immunol Res 2015;2015:217287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Donald PR. Cerebrospinal fluid concentrations of antituberculosis agents in adults and children. Tuberculosis (Edinb) 2010;90:279–92. [DOI] [PubMed] [Google Scholar]
- [19].World Health Organization. Rapid Advice: Treatment of Tuberculosis in Children 2010. Available at: http://whqlibdoc.who.int/publications/2010/9789241500449_eng.pdf Accessed April 18, 2012. [PubMed] [Google Scholar]
- [20].Garcia-Prats AJ, Rose PC, Hesseling AC, et al. Linezolid for the treatment of drug-resistant tuberculosis in children: a review and recommendations. Tuberculosis (Edinb) 2014;94:93–104. [DOI] [PubMed] [Google Scholar]
- [21].Rock RB, Gekker G, Hu S, et al. Role of microglia in central nervous system infections. Clin Microbiol Rev 2004;17:942–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Prasad K, Singh MB, Ryan H. Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst Rev 2016;4: CD002244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schoeman JF, Springer P, van Rensburg AJ, et al. Adjunctive thalidomide therapy for childhood tuberculous meningitis: results of a randomized study. J Child Neurol 2004;19:250–7. [DOI] [PubMed] [Google Scholar]
- [24].Schoeman JF, Fieggen G, Seller N, et al. Intractable intracranial tuberculous infection responsive to thalidomide: report of four cases. J Child Neurol 2006;21:301–8. [DOI] [PubMed] [Google Scholar]
- [25].Haslett PA, Corral LG, Albert M, et al. Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8+ subset. J Exp Med 1998;187:1885–92. [DOI] [PMC free article] [PubMed] [Google Scholar]


