Abstract
Objectives:
To explore the relationship among the vitamin D receptor (VDR) gene polymorphisms, serum 25-hydroxyvitamin D levels, and vitiligo.
Methods:
Databases including PubMed, Cochrane Library, Ovid, Web of Science, CNKI, SinoMed, and Wanfang Data were systematically searched. The association was assessed using odds ratios (ORs), standard mean difference (SMD), and 95% confidence intervals (CIs). The statistical tests were performed using Review Manager 5.3.3.
Results:
We identified a total of 17 studies. The relationship between VDR gene polymorphisms (BsmI, ApaI, TaqI, and FokI), serum 25 (OH)D levels, and incidence of vitiligo was investigated. The results of this meta-analysis showed that the dominant genetic model (CC+AC vs AA, P = .007, OR = 1.41, 95% CI = 1.10–1.80), recessive genetic model (CC vs AC+AA, P = .01, OR = 4.10, 95% CI = 1.36–12.35), and allelic contrast model (C vs A, P = .005, OR = 1.87, 95% CI = 1.21–2.90) of VDR Apal locus increased the risk of vitiligo, and BsmI, TaqI, and FokI loci and the risk of vitiligo have no obvious correlation. Serum 25 (OH)D deficiency was positively associated with the incidence of vitiligo (P < .0001, SMD = −0.94, 95% CI = −1.39, −0.48).
Conclusion:
This meta-analysis revealed that VDR Apal polymorphism increased the susceptibility risk of vitiligo, and there is a positive correlation between serum 25 (OH)D deficiency and the incidence of vitiligo.
Keywords: meta-analysis, serum 25-(OH)D, vitamin D receptor, polymorphism, vitiligo
1. Introduction
Vitiligo is a polygenic autoimmune disease, which is characterized by acquired localized or generalized depigmentation of the skin. Vitiligo can occur at any part of the body. The exposed regions with the white spots severely reduce patients’ quality of life.[1] Various hypotheses for vitiligo occurrence have been proposed, such as genetic predisposition, neural theory, and autoimmune hypothesis. Among them, the destruction of melanocytes caused by immune function abnormality has become a research hotspot.[2,3]
Vitamin D and its derivatives play a biological role through the vitamin D receptor (VDR).[4] Previous studies have confirmed that VDR is not only involved in the growth of the bone but also related to the regulation of T cell function and the biological function of melanocytes.[5,6] VDR gene is located at chromosome 12q12–q14.[7] The biological activation of vitamin D is the conversion of vitamin D into 25-hydroxyvitamin D [25(OH)D], followed by 1α-hydroxylation to yield 1,25-dihydroxyvitamin D3 [1,25(OH)D].[8] 25(OH)D has a long half-life. It is the most appropriate index to reflect the vitamin D content and can accurately reflect the vitamin D level in the human body.[9]
There have been several meta-analyses reporting the association of VDR gene polymorphisms, serum 25(OH)D levels, and the risk of some diseases. For example, Vaughan–Shaw et al[10] found that a higher 25(OH)D concentration is associated with a better cancer outcome. Randerson-Moor et al[11] conducted a meta-analysis and found that vitamin D and VDR gene may have a potentially important role in melanoma. The association of VDR polymorphisms, serum 25(OH)D levels, and vitiligo caused significant clinical and epidemiological research in recent years, but the reported results have been inconsistent. Thus, we conducted a meta-analysis of the existing published studies on this topic to evaluate the strength of the association among the four main VDR gene polymorphisms (BsmI, ApaI, TaqI, and FokI), serum 25(OH)D levels, and risk of vitiligo.
2. Methods
2.1. Literature search
Databases, including PubMed, Cochrane Library, Ovid, Web of Science, the China National Knowledge Infrastructure (CNKI), the China Biological Medicine Database (SinoMed), and Wanfang Data, were systematically searched. These computer searches were limited to articles published in English and Chinese before December 2016, excluding editorials and reviews. The following keywords were used for the search: Vitiligo AND Vitamin D OR 25-hydroxyvitamin D. Additional published data that met our inclusion criteria were identified by reviewing the bibliographical references listed in the retrieved articles. This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline. The protocol for this meta-analysis is available in PROSPERO (CRD42017076932).
2.2. Inclusion criteria
We included studies that met the following criteria: it is a case-control study of patients with vitiligo and healthy controls; all patients were clinically diagnosed with vitiligo; and studies evaluated the association of VDR gene polymorphisms and/or serum vitamin D levels and susceptibility to vitiligo.
Studies were excluded from the analysis when data cannot be extracted from published results and the reported appropriate outcomes were excluded or if they contained duplicate data.
2.3. Data extraction
Two authors (J-ZZ and MW) independently extracted data from all the included studies. Disagreement was resolved by consensus. If these 2 authors failed to reach a consensus, the results were reviewed by a third author (XJK). The extracted data included the following items: first author's name, publication year, total number of cases and controls, allele frequency (cases), serum 25-hydroxyvitamin D level, methods, population (ethnicity), sex, age (in years), and Newcastle–Ottawa scale (NOS). Articles related to evaluating the association between VDR gene polymorphisms and vitiligo were consistent with the Hardy–Weinberg equilibrium (HWE).
2.4. Quality assessment
The methodological quality of the observational studies was determined using the NOS, and the “star” rating system was used to judge the quality of the study.[12] The NOS ranges from 0 (worst) to 9 (best) stars. Studies with a score equal to or higher than 7 were considered of high quality. The medium-quality studies which with a score equal to or higher than 5 were considered. Two investigators (FG and YD) independently assessed the quality of the included studies, and the results were reviewed by a third investigator (Y-YF). Disagreement was resolved by discussion.
2.5. Statistical analysis
The associations between the BsmI, ApaI, TaqI, and FokI polymorphisms of the VDR gene and vitiligo were compared by using the odds ratio (OR) corresponding to the 95% confidence interval (CI) by using Review Manager 5.33. The standard mean difference (SMD) and corresponding 95% CI were utilized to assess the associations of the serum vitamin D levels and vitiligo. Heterogeneity between the studies was assessed using the I2 statistic; P < .10 and I2 > 50% indicated evidence of obvious heterogeneity.[13,14] If obvious heterogeneity existed between the studies, the random effects model was used (DerSimonian and Laird method).[15] Otherwise, the fixed effects model was adopted (Mantel–Haenszel method).[16] For the VDR gene polymorphisms, we investigated the associations between the genetic variants and vitiligo risk in allelic contrast, recessive, and dominant genetic models. The Z test was used to determine the pooled OR, and significance was set at P < .05. The HWE for each single-nucleotide polymorphism was assessed for the controls in each study using χ2 test at a significant level of P < .05. The potential publication bias was investigated using a funnel plot.
3. Results
3.1. Study characteristics
A total of 147 articles were retrieved. After duplicates were removed, only 97 full-text studies were evaluated. Through further screening, 19 articles met the criteria,[17–35] excluding a repeated study[17] and a study of large heterogeneity,[35] and eventually 17 studies[18–34] were included in the final meta-analysis. These comprised 9[18–23,25–27] studies for serum vitamin D levels and 7[28–34] studies for VDR gene polymorphisms and vitiligo. One study[24] evaluated the correlation of serum 25(OH)D levels, VDR gene polymorphisms, and vitiligo. In the study of VDR gene polymorphisms, the genotype frequencies of the control group were consistent with the HWE. The results of NOS showed that the quality of the methodology was generally good (Fig. 1).
Figure 1.
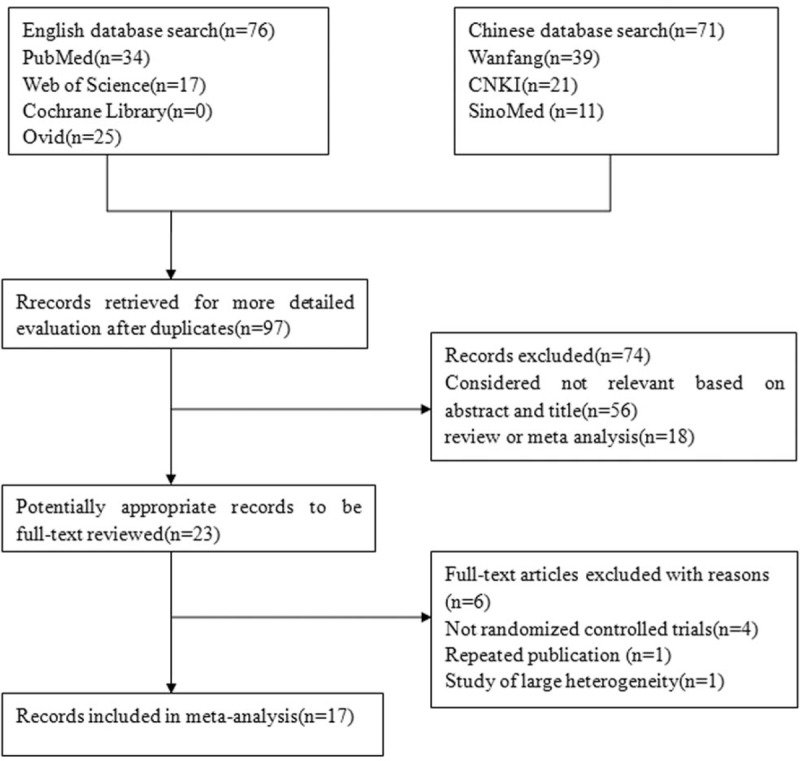
Flow diagram of the study identification.
3.2. Main results, heterogeneity, and sensitivity analysis
3.2.1. VDR gene polymorphism and the risk of vitiligo
For VDR BsmI polymorphism and its association with vitiligo, heterogeneity was found under the dominant genetic model (I2 = 85%, P = .0002) and allelic contrast model (I2 = 82%, P = .0008), so the random effects model was applied. There was no obvious heterogeneity in the recessive genetic model. (I2 = 0%, P = .90). So, the fixed effects model was applied. No statistical association between VDR BsmI polymorphism and vitiligo susceptibility was observed under the dominant genetic model (AA+AG vs GG, P = .79, OR = 1.08, 95% CI = 0.61–1.94), recessive genetic model (AA vs AG+GG, P = .31, OR = 1.21, 95% CI = 0.83–1.77), and allelic contrast model (A vs G, P = .82, OR = 1.05, 95% CI = 0.69–1.61). We considered the forest figure of the dominant genetic model as the representative (Fig. 2). The figures of the recessive gene model and allelic contrast model were not shown.
Figure 2.
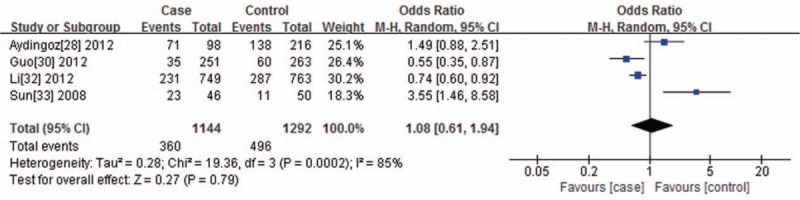
Forest plot of the association between the VDR BsmI polymorphisms and vitiligo under the dominant genetic model (AA+AG vs GG). The horizontal lines correspond to the study-specific OR and 95% CI, respectively. The area of the squares reflects the study-specific weight. The diamond represents the pooled results of the OR and the 95% CI.
For VDR ApaI polymorphism and the risk of vitiligo, 7 studies were included. According to the results of the sensitivity analysis, 6 articles were finally included. No statistically heterogeneity was found under the dominant genetic model (I2 = 0%, P = .69), so the Mantel–Haenszel fixed effects model was used. Heterogeneity was found under the recessive genetic model (I2 = 94%, P < .00001) and allelic contrast model (I2 = 89%, P < .00001), so the random-effects model was applied. A significant statistical association was observed under the dominant genetic model (CC+AC vs AA, P = .007, OR = 1.41, 95% CI = 1.10–1.80) (Fig. 3), recessive genetic model (CC vs AC+AA, P = .01, OR = 4.10, 95% CI = 1.36–12.35), and allelic contrast model (C vs A, P = .005, OR = 1.87, 95% CI = 1.21–2.90).
Figure 3.
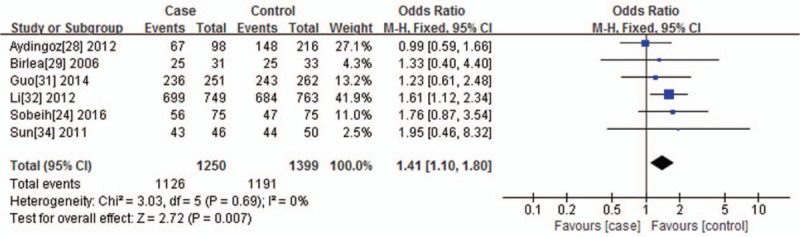
Forest plot of the association between the VDR ApaI polymorphisms and vitiligo under the dominant genetic model (CC+AC vs AA). The horizontal lines correspond to the study-specific OR and 95% CI, respectively. The area of the squares reflects the study-specific weight. The diamond represents the pooled results of the OR and the 95% CI.
As for VDR TaqI polymorphism and its relationship to vitiligo, no significant heterogeneity was found under the dominant genetic model (I2 = 49%, P = .12). For this reason, the Mantel–Haenszel fixed effects model was used. Heterogeneity was found under the recessive genetic model (I2 = 81%, P = .001) and allelic contrast model (I2 = 76%, P = .005); therefore, the random-effects model was applied. Statistical association was observed under the dominant genetic model (CC+CT vs TT, P = .04, OR = 0.81, 95% CI = 0.66–1.00) (Fig. 4). There was no statistical association between VDR TaqI polymorphism and vitiligo susceptibility, which was observed under the recessive genetic model (CC vs CT+TT, P = .94, OR = 1.05, 95% CI = 0.32–3.46) and allelic contrast model (C vs T, P = .68, OR = 0.92, 95% CI = 0.63–1.35).
Figure 4.
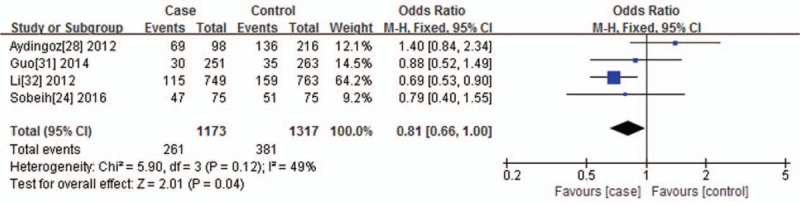
Forest plot of the association between the VDR TaqI polymorphisms and vitiligo under the dominant genetic model (CC+CT vs TT). The horizontal lines correspond to the study-specific OR and 95% CI, respectively. The area of the squares reflects the study-specific weight. The diamond represents the pooled results of the OR and the 95% CI.
For VDR FokI polymorphism and the risk of vitiligo, no significant heterogeneity was found among the dominant genetic model (I2 = 0%, P = .46), recessive genetic model (I2 = 0%, P = .89), and allelic contrast model (I2 = 0%, P = .48); thus, we used the Mantel–Haenszel fixed effects model. There was no statistical association between VDR FokI polymorphism and vitiligo susceptibility, which was observed among the dominant genetic model (TT+TC vs CC, P = .82, OR = 1.02, 95% CI = 0.85–1.23) (Fig. 5), recessive genetic model (TT vs TC+CC, P = .86, OR = 1.02, 95% CI = 0.80–1.31), and allelic contrast model (T vs C, P = .80, OR = 1.02, 95% CI = 0.89–1.16).
Figure 5.
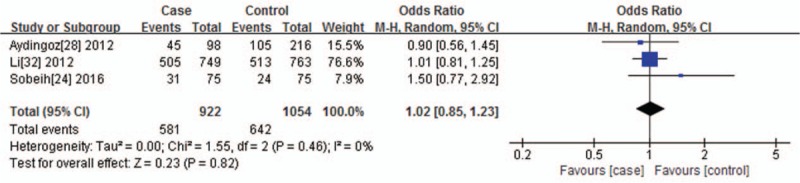
Forest plot of the association between the VDR FokI polymorphisms and vitiligo under the dominant genetic model (TT+TC vs CC). The horizontal lines correspond to the study-specific OR and 95% CI, respectively. The area of the squares reflects the study-specific weight. The diamond represents the pooled results of the OR and the 95% CI.
3.2.2. Association of serum 25(OH)D level with vitiligo
For the serum 25(OH)D levels and its association with vitiligo, I2 test indicated that the heterogeneity was significant (P < .0001, I2 = 92.0%); therefore, the random-effects model was applied in performing the meta-analysis. We found that the serum resisting level in the vitiligo group was higher than that in the normal control group (P < .0001, SMD = −0.94, 95% CI = −1.39, −0.48) (Fig. 6).
Figure 6.
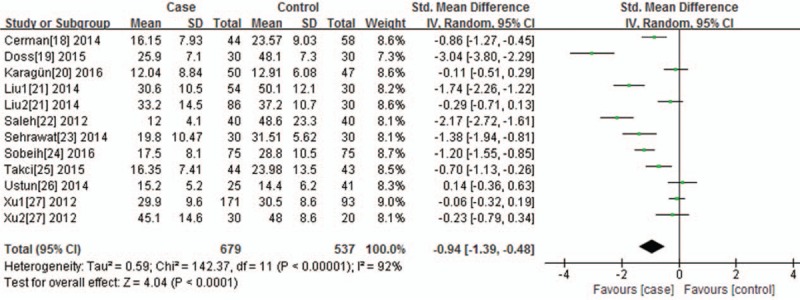
Forest plot of the association between serum 25-(OH) D level and vitiligo, the horizontal lines correspond to the study-specific SMD and 95% CI, respectively. The area of the squares reflects the study-specific weight. The diamond represents the pooled results of SMD and 95%CI.
3.2.3. Sensitivity analysis
The contribution of every included study to the pooled estimate was determined in order to assess the sensitivity analysis. We excluded each study one at a time and recalculated the pooled P or OR estimates for the remaining studies. In order to observe the stability of the results, we also converted to each other the fixed effects model and random effects model. Sobeih[24] had an undue influence on the summary ORs under the dominant genetic model. Birlea[29], Guo[31], Li[32], Sobeih[24], and Sun[34] had an undue influence on the summary ORs under the recessive genetic model. Wei[35] had an undue influence on the summary ORs under the dominant genetic model and allelic contrast model when the fixed effects model and random effects model were converted to each other. After excluding this study, the 2 genetic models became stable. We excluded each study one at a time again and recalculated the pooled P or OR estimates for the remaining studies. Only Li[32] had an undue influence on the summary ORs under the dominant genetic model. According to the results of sensitivity analysis, we removed the study of Wei.[35]
Similarly, Li[32] and Sobeih[24] had an undue influence on the pooled P or OR estimates and their data changed the pooled point estimate when converting the fixed effects model to the random effects model for TaqI polymorphism. The data changed the pooled point estimate when converting the fixed effects model to the random effects model under the dominant genetic model.
For VDR gene polymorphism (BsmI and FokI) and serum 25(OH)D level with vitiligo, their data did not substantially change the estimate of the pooled points when excluding one individual study at a time or converting the random effects model to the fixed effects model. Thus, our results are quite reliable (Tables 1 and 2).
Table 1.
Characteristics of studies reporting the distribution of 4 VDR gene polymorphisms (ApaI, BsmI, FokI, and TaqI) in cases and controls.
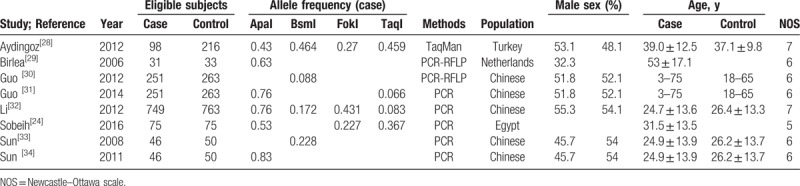
Table 2.
Characteristics of studies reporting the serum 25-(OH)D level in cases and controls.
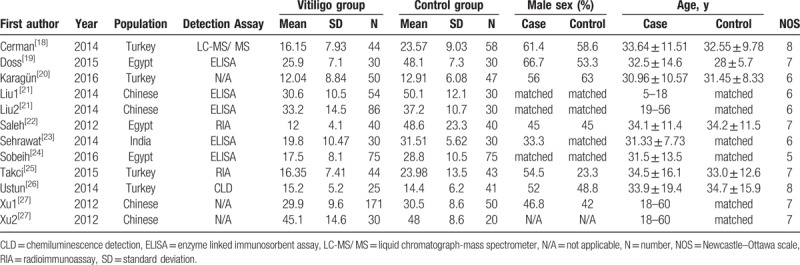
3.3. Publication bias
The publication bias of the individual studies was evaluated using funnel plots. We considered the figure of the dominant genetic model of BsmI, ApaI, TaqI, and FokI polymorphisms as representative. No visual publication bias was found in the funnel plot for the BsmI (Fig. 7A), ApaI (Fig. 7B), TaqI (Fig. 7C), or FokI (Fig. 7D) polymorphisms using allelic contrast. Moreover, no visual publication bias was found under the association of serum 25(OH)D level and vitiligo, as shown in Figure 8.
Figure 7.
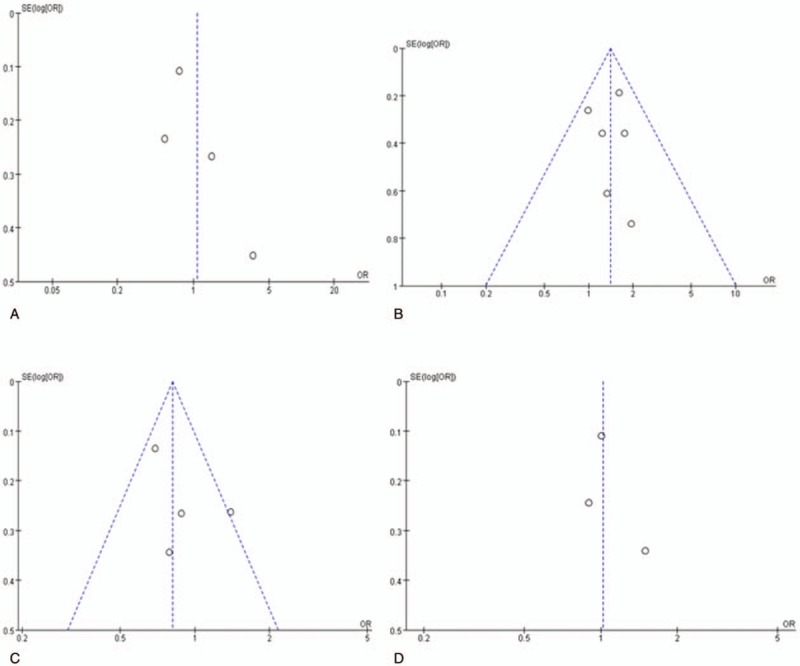
Funnel plot for the publication bias tests. Each point represents a separate study for the indicated association. The horizontal and vertical axis correspond to the OR and confidence limits (OR: odds ratio, SE: standard error). A, VDR BsmI polymorphism and vitiligo under the dominant genetic model (AA+AG vs GG); (B) VDR ApaI polymorphism and vitiligo under the dominant genetic model (CC+AC vs AA); (C) VDR TaqI polymorphism and vitiligo under the dominant genetic model (CC+CT vs TT). (D) VDR FokI polymorphism and vitiligo under the dominant genetic model (TT+TC vs CC).
Figure 8.
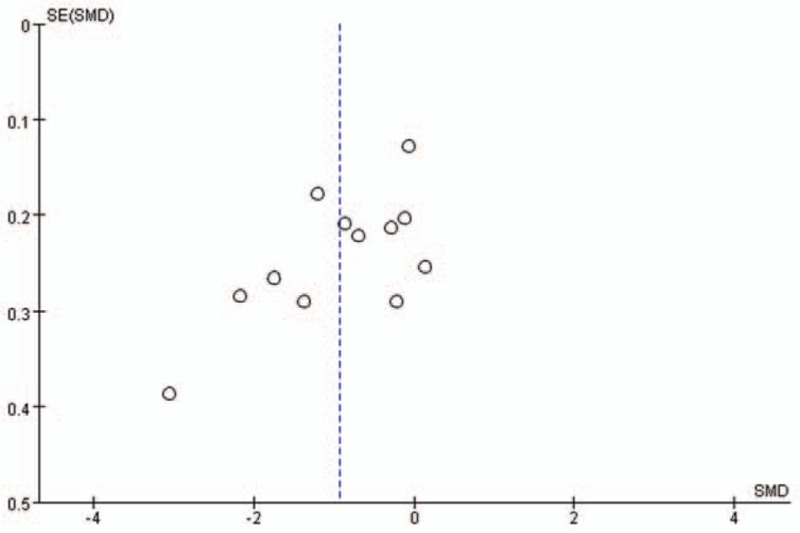
Funnel plot for the publication bias tests of serum 25-(OH) D level and vitiligo. The horizontal and vertical axis correspond to the SMD and confidence limits (SE = standard error, SMD = standard mean difference).
4. Discussion
Vitamin D is a fat-soluble vitamin and 1,25(OH)D is the active form of vitamin D, which mediates the biological effects of VDR on immune diseases.[36] VDR plays an important role in maintaining the dynamic balance of minerals, calcium and phosphate metabolism, bone metabolism, growth and differentiation of a variety of tissue cells, and cardiovascular and cerebrovascular diseases and immune regulation.[37,38] Recent studies have also shown that VDR TaqI and ApaI polymorphisms play a major role in many cancers.[39,40] At present, the etiology and pathogenesis of vitiligo are not entirely clear. The theory of autoimmunity is the focus of the present research. Active vitiligo is characterized by peripheral lymph node invasion, which can invade the basement membrane zone of melanocytes and result in melanocyte decrease or absence, lymphocyte infiltration in the superficial dermis, and visible T lymphocyte, macrophage, and dendritic cell infiltration and aggregation.[41–43] 1,25(0H)D can elevate antigen presenting cells and T cells, produce a large number of regulatory T cells of CD4+ and CD25+, and induce immune tolerance.[12] Therefore, VDR gene polymorphism and serum 25(OH)D may be closely related to the occurrence and development of vitiligo.
Li[44] evaluated the association between VDR gene polymorphisms (ApaI, BsmI) and susceptibility to vitiligo in 2014 and found that the ApaI a allele or BsmI bb genotype are associated with the risk of vitiligo in East Asian populations. The research literature was searched until November 2013. However, the data extraction of a literature was wrong. The numbers of the vitiligo and control groups were 31 and 33, respectively, in the study by Birlea.[29] However, in the meta-analysis of Li et al,[44] the numbers of the vitiligo and control groups were 33 and 31, respectively.
Upala[45] conducted a meta-analysis and identified a significant relationship between 25(OH)D levels and vitiligo, which found that lower 25(OH)D levels were associated with vitiligo. However, the extraction of literature pertaining to the study was also wrong.[27] Additionally, some relevant studies were not included. We therefore conducted this meta-analysis, which included 8 studies on VDR gene polymorphisms. The dominant genetic model, recessive genetic model, and allelic contrast model of ApaI in the VDR gene were shown to be associated with an increased risk of vitiligo; this polymorphism may be a potential biomarker for early detection of vitiligo. Statistical association was also observed under the dominant genetic model of TaqI. However, the data changed the pooled point estimate when converting the fixed effects model to the random effects model. No statistical association was observed under the recessive genetic model and allelic contrast model. It may not be associated with an increased risk of vitiligo. BsmI and FokI loci and the pathogenesis of vitiligo have no obvious correlation. Ten studies evaluated the correlation between serum levels of vitamin D and vitiligo. Our study found that serum 25(OH)D levels in patients with vitiligo were lower than those in normal subjects. Serum 25(OH)D deficiency was positively associated with the incidence of vitiligo. Karagüzel[46] conducted a prospective study and found that oral vitamin D supplementation might be useful for children with vitiligo who also have vitamin D deficiency, which is consistent with our results. Li[32] found that 25(OH)D levels were significantly higher in patients carrying the FokI ff or ApaI AA genotype compared with those carrying the FF or aa genotype. However, Sobeih[24] found that the serum 25(OH)D levels were not significantly different among the various ApaI, TaqI, and FokI genotypes. The association between serum 25(OH)D levels and VDR gene polymorphisms in patients with vitiligo still requires more research.
Heterogeneity is a potential problem that may influence the efficacy of statistical analyses. We attempted to create subgroups by ethnicity, but this cannot explain the heterogeneity observed among the studies. Heterogeneity may be attributed to the potential confounding caused by the diversity of sample sizes, design differences, variations in the ethnicities and regions, subject gender, methods of genotyping, and so on. Other limitations of this meta-analysis should also be acknowledged. First, only full-text articles published in English and Chinese were included. Thus, some eligible studies, which were unpublished or reported in other languages, were missed in this meta-analysis. Second, cultural background factors also affected the publishing decisions, which affect researchers’ likelihood of reporting or editing negative results in some research areas. Third, the studies done on BsmI, TaqI, and FokI are fewer compared to those on ApaI. Is it possible that the available studies are not sufficient to make a conclusive result for these loci compared to ApaI? The statistical ability of the small sample is limited, and the well-designed and large sample studies are necessary to confirm the findings. Furthermore, serum 25(OH)D level can be significantly influenced by the environment surrounding patients such as intensity of sun exposure. The disease activity of vitiligo should also be considered in this meta-analysis. Finally, the segmental and nonsegmental vitiligo is different in many aspects, including genetic susceptibility. The available data do not allow us to analyze each type separately, which requires further research.
Despite these limitations, our meta-analysis also has some advantages. Above all, a meta-analysis can lead to more robust data by increasing the sample size to overcome the small sample size constraints of the studied population. Second, the quality of the case-control studies in our meta-analysis was satisfactory. Third, our research has corrected some mistakes in previous studies and may provide a reference for subsequent vitiligo research.
5. Conclusions
In conclusion, this meta-analysis revealed that VDR Apal locus increased the risk of patients with vitiligo. Serum 25(OH)D deficiency was positively associated with the incidence of vitiligo. However, this result should be interpreted with caution due to the limitations of the present study. Further studies with larger sample sizes that consider gene-gene and gene-environment interactions are thus needed to confirm our findings. Prospective studies are also needed to determine whether vitamin D supplementation in the population can improve the prognosis of vitiligo.
Author contributions
J-ZZ and MW conceived of the study, participated in the design, collected the data, performed statistical analyses, and drafted the article. X-JK, YD, and FG conceived of the study and revised the article. X-JW and Y-YF participated in the study design and helped to draft the article. PW, F-XH, and JX helped to modify the article. All authors read and approved the final article.
Conceptualization: Jing-Zhan Zhang, Xiao-Jing Kang.
Data curation: Jing-Zhan Zhang, Yuan Ding, Feng Gao, Xiao-Jing Kang.
Formal analysis: Jing-Zhan Zhang, Yuan Ding, Feng Gao, Xiao-Jing Kang.
Funding acquisition: Feng Gao, Xiu-Juan Wu, Xiao-Jing Kang.
Investigation: Man Wang, Yuan Ding, Feng Gao, Xiao-Jing Kang.
Methodology: Man Wang, Yuan Ding, Yan-Yan Feng.
Project administration: Yuan Ding, Xiao-Jing Kang.
Software: Man Wang, Feng-Xia Hu.
Supervision: Yuan Ding, Yan-Yan Feng.
Validation: Yuan Ding, Xiu-Juan Wu, Yan-Yan Feng, Xiao-Jing Kang, Feng-Xia Hu, Jun Xian.
Visualization: Yuan Ding, Feng Gao, Xiu-Juan Wu, Yan-Yan Feng, Xiao-Jing Kang, Jun Xian.
Writing – original draft: Jing-Zhan Zhang, Xiao-Jing Kang.
Writing – review & editing: Jing-Zhan Zhang, Man Wang, Xiao-Jing Kang, Buwajieer Yakeya, Peng Wang, Feng-Xia Hu, Jun Xian.
Footnotes
Abbreviations: CI = confidence interval, HWE = Hardy–Weinberg equilibrium, OR = odds ratio, SMD = standard mean difference.
J-ZZ and MW have contributed equally to this work.
The authors declare no conflicts of interest.
References
- [1].Rodrigues M, Ezzedine K, Hamzavi I, et al. Vitiligo Working Group. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol 2017;77:1–3. [DOI] [PubMed] [Google Scholar]
- [2].Ghafourian A, Ghafourian S, Sadeghifard N, et al. Vitiligo: symptoms, pathogenesis, and treatment. Int J Immunopathol Pharmacol 2014;27:485–9. [DOI] [PubMed] [Google Scholar]
- [3].Allam M, Riad H. Concise review of recent studies in vitiligo. Qatar Med J 2013;2013:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Birlea SA, Costin GE, Norris DA. Cellular and molecular mechanisms involved in the action of vitamin D analogs targeting vitiligo depigmentation. Curr Drug Targets 2008;9:345–59. [DOI] [PubMed] [Google Scholar]
- [5].Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J 2001;15:2579–85. [DOI] [PubMed] [Google Scholar]
- [6].Watabe H, Soma Y, Kawa Y, et al. Differentiation of murine melanocyte precursors induced by 1,25-dihydroxyvitamin D3 is associated with the stimulation of endothelin B receptor expression. J Invest Dermatol 2002;119:583–9. [DOI] [PubMed] [Google Scholar]
- [7].Uitterlinden AG, Fang Y, Van Meurs JB, et al. Genetics and biology of vitamin D receptor polymorphisms. Gene 2004;338:143–56. [DOI] [PubMed] [Google Scholar]
- [8].Ersoy-Evans S. Commentary: Vitamin D and autoimmunity: is there an association? J Am Acad Dermatol 2010;62:942–4. [DOI] [PubMed] [Google Scholar]
- [9].Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol 2008;8:685–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vaughan-Shaw PG, O'Sullivan F, Farrington SM, et al. The impact of vitamin D pathway genetic variation and circulating 25-hydroxyvitamin D on cancer outcome: systematic review and meta-analysis. Br J Cancer 2017;116:1092–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Randerson-Moor JA, Taylor JC, Elliott F, et al. Vitamin D receptor gene polymorphisms, serum 25-hydroxyvitamin D levels, and melanoma: UK case-control comparisons and a meta-analysis of published VDR data. Eur J Cancer 2009;45:3271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa Health Research Institute. Available at: www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed on October 20, 2011. [Google Scholar]
- [13].Ioannidis JP, Patsopoulos NA, Evangelou E. Heterogeneity in meta-analyses of genome-wide association investigations. PLoS One 2007;2:e841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Berkey CS, Hoaglin DC, Mosteller F, et al. A random-effects regression model for meta-analysis. Stat Med 1995;14:395–411. [DOI] [PubMed] [Google Scholar]
- [15].DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 2007;28:105–14. [DOI] [PubMed] [Google Scholar]
- [16].Manteln, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. 10. [PubMed] [Google Scholar]
- [17].Li Y, Wang L, Sun LC, et al. Study on the association between VDR polymorphism and vitiligo. Chin J Integr Med 2007;16:302–4. [Google Scholar]
- [18].Aksu Cerman A, Sarikaya Solak S, Kivanc Altunay I. Vitamin D deficiency in alopecia areata. Br J Dermatol 2014;170:1299–304. [DOI] [PubMed] [Google Scholar]
- [19].Doss RW, El-Rifaie AA, Gohary YM, et al. Vitamin D receptor expression in vitiligo. Indian J Dermatol 2015;60:544–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Karagün E, Ergin C, Baysak S, et al. The role of serum vitamin D levels in vitiligo. Postepy Dermatol Alergol 2016;33:300–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liu CG, Li JB, Sun SQ, et al. Detection of serum 25-hydroxy vitamin D3 in patients with vitiligo. J Navy Med 2014;122–4. [Google Scholar]
- [22].Saleh HM, Abdel Fattah NS, Hamza HT. Evaluation of serum 25-hydroxyvitamin D levels in vitiligo patients with and without autoimmune diseases. Photodermatol Photoimmunol Photomed 2013;29:34–40. [DOI] [PubMed] [Google Scholar]
- [23].Sehrawat M, Arora TC, Chauhan A, et al. Correlation of Vitamin D Levels with pigmentation in vitiligo patients treated with NBUVB therapy. ISRN Dermatol 2014;2014:493213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sobeih S, Mashaly HM, Gawdat H, et al. Evaluation of the correlation between serum levels of vitamin D and vitamin D receptor gene polymorphisms in an Egyptian population. Int J Dermatol 2016;55:1329–35. [DOI] [PubMed] [Google Scholar]
- [25].Takci Z, Tekin Ö, Ertuğru DT, et al. A case-control study: evaluation of vitamin D metabolism in patients with vitiligo. Turk J Med Sci 2015;45:837–41. [PubMed] [Google Scholar]
- [26].Ustun I, Seraslan G, Gokce C, et al. Investigation of vitamin D levels in patients with vitiligo vulgaris. Acta Dermatovenerol Croat 2014;22:110–3. [PubMed] [Google Scholar]
- [27].Xu X, Fu WW, Wu WY. Serum 25-hydroxyvitamin D deficiency in Chinese patients with vitiligo: a case-control study. PLoS One 2012;7:e52778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Aydingöz IE, Bingül I, Doğru-Abbasoğlu S, et al. Analysis of vitamin D receptor gene polymorphisms in vitiligo. Dermatology 2012;224:361–8. [DOI] [PubMed] [Google Scholar]
- [29].Birlea S, Birlea M, Cimponeriu D, et al. Autoimmune diseases and vitamin D receptor Apa-I polymorphism are associated with vitiligo in a small inbred Romanian community. Acta Derm Venereol 2006;86:209–14. [DOI] [PubMed] [Google Scholar]
- [30].Guo R, Hao YJ, Wang JX, et al. Investigation on association of VDR gene polymorphism with Hui and Han ethnic vitiligo patients in Ning Xia. Chin J Lab Diag 2012;2223–6. [Google Scholar]
- [31].Guo R, Wang JX, Liu X, et al. Association of vitamin D receptor gene ApaI and TaqI polymorphism with Hui and Han ethnic vitiligo patients in Ning Xia. J Ningxia Med Univ 2014;169–173+177. [Google Scholar]
- [32].Li K, Shi Q, Yang L, et al. The association of vitamin D receptor gene polymorphisms and serum 25-hydroxyvitamin D levels with generalized vitiligo. Br J Dermatol 2012;167:815–21. [DOI] [PubMed] [Google Scholar]
- [33].Sun Y, Han J, Wu RQ, et al. Polymorphism of vitamin D receptor gene BsmI in patients with vitiligo. J Shang Jiaotong Univ (Med Sci) 2008;392–4. [Google Scholar]
- [34].Sun Y, Han J, Wu RQ, et al. Association of vitamin D receptor ApaI gene polymorphism and immunological abnormality in patients with vitiligo. Chin J Dermatovenereol Integ Tradit West Med 2011;148–51. [Google Scholar]
- [35].Wei HP, Zhong YJ, Li XH. Vitamin D receptor ApaI polymorphism in vitiligo and its association with immune abnormalities. China Health Indus 2013;135–6. [Google Scholar]
- [36].Sun J. Vitamin D and mucosal immune function. Curr Opin Gastroenterol 2010;26:591–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gao L, Tao Y, Zhang L, et al. Vitamin D receptor genetic polymorphisms and tuberculosis: updated systematic review and meta-analysis. Int J Tuberc Lung Dis 2010;14:15–23. [PubMed] [Google Scholar]
- [38].Wu-Wong JR. Potential for vitamin D receptor agonists in the treatment of cardiovascular disease. Br J Pharmacol 2009;158:395–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Peterlik M, Grant WB, Cross HS. Calcium, vitamin D and cancer. Anticancer Res 2009;29:3687–98. [PubMed] [Google Scholar]
- [40].Köstner K, Denzer N, Müller CS, et al. The relevance of vitamin D receptor (VDR) gene polymorphisms for cancer: a review of the literature. Anticancer Res 2009;29:3511–36. [PubMed] [Google Scholar]
- [41].Hann SK, Park YK, Lee KG, et al. Epidermal changes in active vitiligo. J Dermatol 1992;19:217–22. [DOI] [PubMed] [Google Scholar]
- [42].Mathieu C, van Etten E, Decallonne B, et al. Vitamin D and 1, 25-dihydroxyvitamin D3 as modulators in the immune system. J Steroid Biochem Mol Biol 2004;89–90:449–52. [DOI] [PubMed] [Google Scholar]
- [43].Cantorna MT, Zhu Y, Froicu M, et al. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr 2004;80(6 Suppl):1717S–20S. [DOI] [PubMed] [Google Scholar]
- [44].Li L, Wu Y, Li L, et al. Association of ApaI and BsmI polymorphisms with vitiligo risk: a meta-analysis. Clin Exp Dermatol 2015;40:794–803. [DOI] [PubMed] [Google Scholar]
- [45].Upala S, Sanguankeo A. Low 25-hydroxyvitamin D levels are associated with vitiligo: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed 2016;32:181–90. [DOI] [PubMed] [Google Scholar]
- [46].Karagüzel G, Sakarya NP, Bahadir S, et al. Vitamin D status and the effects of oral vitamin D treatment in children with vitiligo: a prospective study. Clin Nutr ESPEN 2016;15:28–31. [DOI] [PubMed] [Google Scholar]


