Abstract
Rationale:
Gorham-Stout syndrome in the spine is extremely rare, and there is no standard curative management thus far. The objective of this article is to report a very rare case of Gorham-Stout syndrome of the lumbar and sacral spine with chylothorax and chyloperitoneum successfully treated by combination of vertebroplasty with cement augmentation and medication treatment. We described the clinical characteristics and postoperative therapy of the patient, and reviewed all of the published cases of Gorham-Stout syndrome of the lumbar and sacral spine.
Patient concerns:
A 31-year-old man presented with increasingly serious abdominal distention and back pain. MRI showed massive bony destruction of the spine and pelvis. CT and ultrasonography demonstrated massive ascites and mild hydrothorax.
Diagnoses:
We believe this is the first report of a case of Gorham-Stout syndrome with both chylothorax and chyloperitoneum.
Interventions:
Chest and abdominal cavity puncture was performed for symptomatic relief and the test results confirmed chylothorax and chyloperitoneum. Tissue biopsy and percutaneous vertebroplasty at L5 were performed and the postoperative pathology together with symptoms and examinations were reported to be consistent with Gorham-Stout syndrome. Subsequently, we administered combination medical treatment consisting of interferon-α-2b, zoledronic acid and calcitriol.
Outcomes:
At the 1-year and 2-year follow-up visit, he had nearly full complete remission and reported palliative back pain. Moreover, the amount of pleural and peritoneal fluid was successfully reduced gradually.
Lessons:
Vertebroplasty by cement augmentation may be a treatment option for patients with Gorham-Stout Syndrome in the spine who cannot undergo appropriate surgery or decline open surgery. This represents a safe and minimally invasive approach to sustainably relieve pain and stabilize vertebral bodies with Gorham-Stout syndrome in the spine.
Keywords: chyloperitoneum, chylothorax, Gorham–Stout syndrome, percutaneous vertebroplasty, spine
1. Introduction
Gorham–Stout syndrome (GSS) is an extremely rare disorder that presents with focal or massive bony destruction.[1–3] The characteristics of GSS were first described by Jackson in 1838, and were further clarified by Gorham and Stout in 1955.[2,4] We are presenting a detailed analysis of Gorham–Stout syndrome involving the lumbar and sacral spine with chylothorax and chyloperitoneum in a patient, and no report of GSS presenting in this manner has been documented thus far. Our focus is to emphasize the importance of considering GSS as a diagnosis and guiding the clinical, radiological, and histological features and proper treatment of GSS in the spinal region. Written informed consent was obtained from the patient for publication of this article. A copy of the written consent is available for review by the editors of the Medicine. Because this article does not involve any human or animal trials, there is no need to conduct special ethic review and the ethical approval is not necessary.
2. Case presentation
In 2015, a 31-year-old otherwise healthy man presented to our institution with increasingly serious abdominal distention and back pain. In history of present illness, the patient stated he has been experiencing a progressive back pain, a gradual decrease in muscle strength in his bilateral lower extremities, as well as worsening numbness for approximately 7 years, the pain in his back was alleviated with rest and hot compresses. On physical exam, examination of strength demonstrated a 5-/5 at right leg and 5-/5 at left leg. Sensory examination was significant for decreased sensation in both legs. His reflex was weakened at patella and ankle bilaterally. Ataxia and hyperexplexia were absent. Cranial nerves, mini mental, and the rest of the neurological exam showed no abnormalities. Routine laboratory tests were ordered, including complete blood count, electrolytes, liver and kidney function tests, tumor markers and bone metabolites. Laboratory results revealed a parathyroid hormone (PTH) level of 67.9 pg/mL (normal: 12.0–65.0 pg/mL), a 1, 25-hydroxyvitam D3 level of 6.50 pg/mL (normal: 19.6–54.3 pg/mL), β-crosslaps (β-CTX) level of 1.020 ng/mL (normal: 0.26–0.512 ng/mL), and other results were at the normal range.
Magnetic resonance imaging (MRI) showed massive bony destruction of the lumbar and sacral spine as well as pelvis (Fig. 1). Computed tomography (CT) and ultrasonography demonstrated massive ascites and mild hydrothorax as well as cystic lesions in the spleen (Fig. 2). Chest and abdominal cavity puncture were performed for symptomatic relief and the test results confirmed chylothorax and chyloperitoneum. Furthermore, a positron emission tomography-CT (PET/CT) scan revealed multiple osteolytic lesions of the L4, L5, sacrum, and pelvis girdle (Fig. 2). Based on these findings, GSS with chylothorax and chyloperitoneum was considered.
Figure 1.
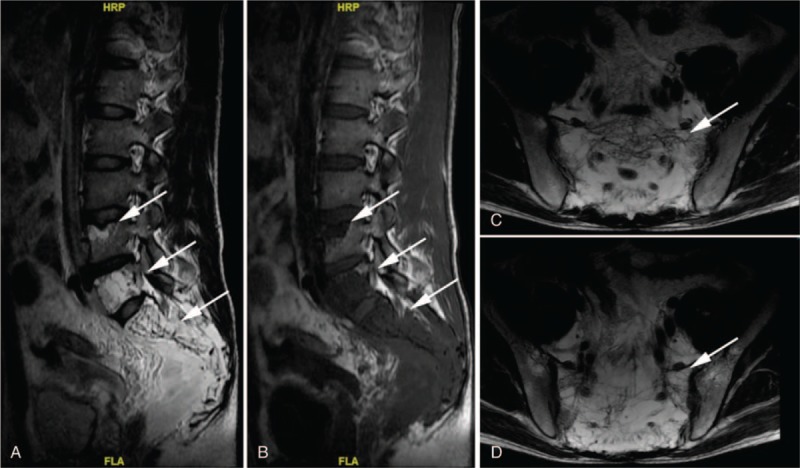
A, B, Sagittal plane of the MRI scan revealed bony destruction of L4, L5 and sacrum appeared high signal intensity on T2 images and low signal intensity on T1 images. C, D Transverse plane of the MRI scan showed bony destruction of L4, L5 and sacrum appeared high signal intensity on T2 images.
Figure 2.
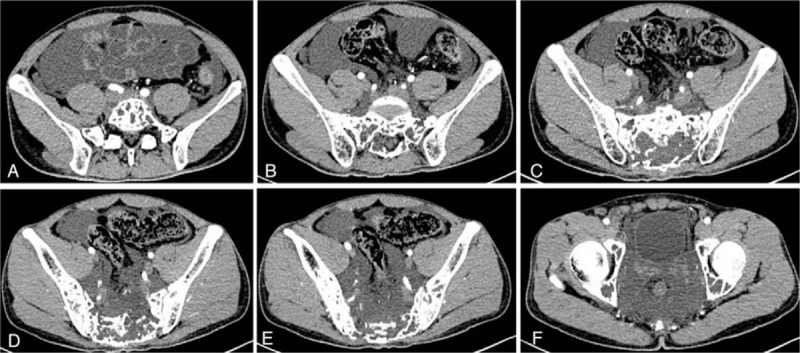
A–F, Transverse plane of the computed tomographic scans revealed massive bony destruction of the lumbar and sacral spine, pelvis.
In brief, percutaneous vertebroplasty at L5 were performed according to the original surgical plan. For the posterior approach, we used C-arm for perspective positioning, L5 vertebral lesion was identified as surgical target, and the L5 pedicle puncture point was located. Then 2% lidocaine was used for local infiltration anesthesia, and the puncture needle was inserted through the cannula. Under the C-arm fluoroscopy, the L5 vertebral lesion was penetrated through the left pedicle of the L5, and pink turbid fluid gushed through the percutaneous puncture cannula during operation (Fig. 3 A, B). Then we send out the pink turbid fluid for pathological examination. Bone cement for vertebroplasty was introduced. Under the perspective, the 10.0 mL cement of L5 was slowly pushed through the putter, and the biopsy passage was closed. Fluoroscopy confirmed the good dispersion of bone cement. The operation was successful and intraoperative bleeding was about 10 mL. The result of the chyle test for the puncture fluid was positive. No malignant cells were found in the puncture fluid. Postoperative posteroanterior and lateral radiographs of the spine showed cement augmentation was satisfactory (Fig. 3 C, D).
Figure 3.
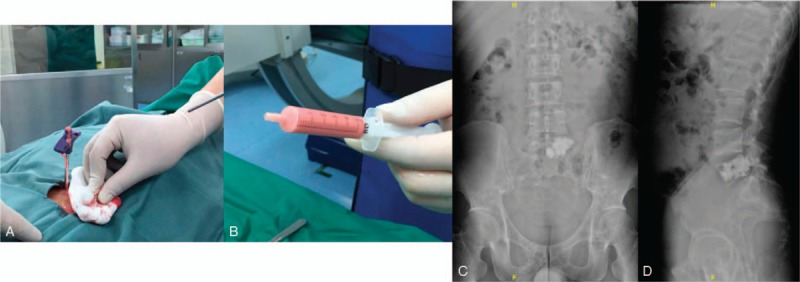
A, B Pink turbid fluid gushed through the percutaneous puncture cannula during operation. C, D, Postoperative posteroanterior and lateral radiographs of the spine.
Histopathologic examination, including immunohistochemical staining, was performed, and the diagnosis of GSS was made according to the Heffez criteria.[3] Microphotograph showed dilated lymphatic channels in hyperplastic fibrous connective tissue with lymphocytic infiltrate among the trabeculae (Fig. 4). Immunohistochemistry of the lesion showed D2–40 strongly positive staining of lymphatic endothelial cells in the dilated lymphatic channels. Pathological result was positive for D2–40, CD3, CD34, and CD20 indicating proliferation of endothelial-lined blood vessels and lymphatic vessels in crushed bone tissue. Biopsy samples were negative for S-100, AE1/AE3, CD4, CD38 and CMV, with 1% Ki-67 positive nuclei. No cellular atypia, inflammatory cell infiltration, or osteoclasts were seen. The postoperative pathology together with symptoms and examinations were reported to be consistent with GSS.
Figure 4.
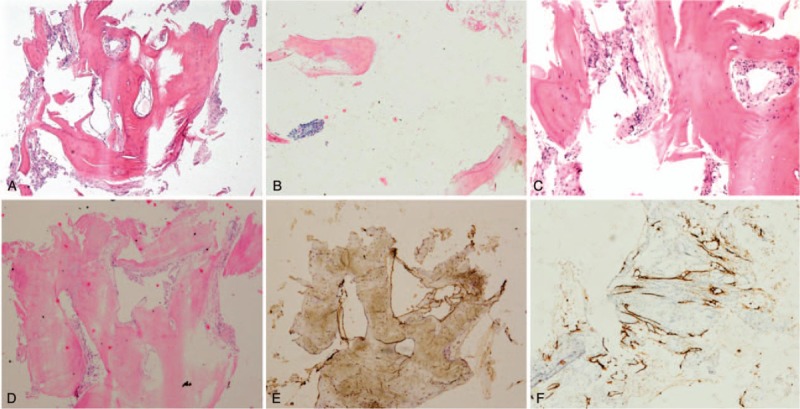
Pathologic histology of spinal specimens. A, Pathological histological examination showed hyperplastic fibrous connective tissue, intercellular substance edema and dilated lymphatic channels with magnification 40 ×. B, Microphotograph showed osseous tissue was broken down with magnification 100 × (C, D). Microphotograph showed dilated lymphatic channels and vessels in hyperplastic fibrous connective tissue (H&E, original magnification 40 × and 100 ×). E, Immunohistochemistry of the lesion showed D2-40 strongly positive staining of lymphatic endothelial cells in the dilated lymphatic channels. F, CD34 immunostaining is positive. (Immunoperoxidases, original magnification 40 × and 100 ×). No cellular atypia, inflammatory cell infiltration or osteoclasts were seen.
One week after the operation, the visual analogue scale score of his back pain improved to 0 to 1 point compared to the preoperative status, 4 to 5 points. On physical exam, the patient showed basically normal sensation to pin-prick and fine-touch of bilateral lower extremities and exhibited a 5/5 strength in the lower extremities. Moreover, his reflex returned to normal at patella and ankle bilaterally. Subsequently, we administered combination medical treatment consisting of interferon-α-2b, zoledronic acid and calcitriol for a year, the amount of pleural and peritoneal fluid was successfully reduced gradually. At the 1-year and 2-year follow-up visit, he had nearly full complete remission and reported palliative back pain. There were no complications during the perioperative period. The patient chose not to undergo another operation of vertebroplasty with cement augmentation at sacrum tentatively because he was nearly asymptomatic at present.
3. Discussion
GSS is an extremely rare disorder characterized by clinical and radiological disappearance of bone by proliferation of non-neoplastic lymphatic, vascular or fibrous tissue.[4,5] No report of GSS of spine with both chylothorax and chyloperitoneum has been documented in literature, and GSS of spine were still short of imaging proof. To identify previously reported cases of GSS in spine, PubMed was searched using the keywords,“Spine,” and, “Gorham-Stout syndrome,” which yielded 9 results of GSS of the lumbar and sacral spine.[6–14] References from these articles were reviewed but yielded no additional results. As we present a rare case of GSS located in the lumbar and sacral spine, an effort was made to search for similar cases published ever (Table 1).[6–14]
Table 1.
Summary of 9 cases of GSS in lumbar and sacral spine reported in literature.
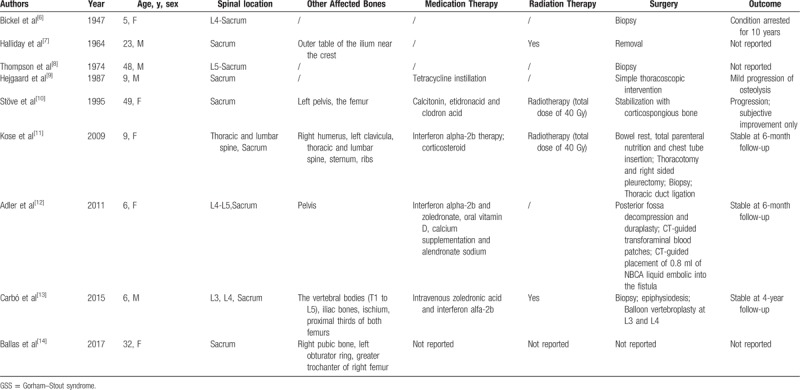
Typical manifestations of a patient suffering from GSS of spine include local pain, swelling of the affected region, low back pain, pathologic fracture, paresthesia, functional impairment, and paralysis.[5,15] Although the clinical course of GSS is reported to be self-limited, the complications of GSS can be potentially fatal.[15] For example, chylothorax that has been reported to be a severe complication of GSS can dramatically influence the respiratory function or even deadly dyspnea.[16] Chyloperitoneum is a rare complication that can cause abdominal distention in certain patients with GSS.[5,15] Chylothorax and chyloperitoneum may occur due to the affected thoracic or lumbar skeleton by the extension of lymphangiectasia into the pleural cavity, intraperitoneal space, or by direct invasion of the thoracic duct.[5,15] However, the specific pathogenesis of chylothorax and chyloperitoneum has not been fully elucidated in literature, to date. Additionally, cystic lesions in visceral organs, pathologic fracture, spinal region involvement, paraplegia due to spinal cord compression, cerebrospinal fluid leakage and meningitis due to the involvement of the skull have also been reported in literature.[5,15,17–19]
To date, laboratory tests may give clinicians additional information for early diagnosis and proper treatment. Several studies have shown that growth factor vascular endothelial growth factor-A (VEGF-A) and cytokine IL-6 can be elevated in the peripheral circulation of patients with GSS and that the level of these biomarkers can significantly decrease following effective treatment.[5,15,20–22] Recently, in a prospective study of 12 GSS patients by Liu et al,[22] erythrocyte sediment rate, serum alkaline phosphatase, and serum β-CTX level were moderately increased or decreased in some patients. Under these circumstances, a combination detection of novel biomarkers to monitor disease activity of GSS may provide more diagnostic, prognostic or predictive information for clinicians to diagnose and choose treatment options. In our patient, laboratory test results showed a high PTH level, a low 1, 25-hydroxyvitam D3 level, and a high β-CTX level. We think the results are closely related to the process of bone resorption and bone remodeling, but the specific mechanism behind the abnormal results is still to be explored. A few cases of GSS in spine have been documented in literature, thus there is still short of imaging proof. On imaging, a diagnostic role could be played by plain radiographs, CT and MRI in previous case reports.[23–25] Based on x-ray, CT, and MRI, bone scintigraphy is frequently used to indicate the skeletal abnormality. The more accurate technique of SPECT/CT scan or 18F-FDG PET/CT scan is currently employed to detect early lesions of GSS.[26] Imaging studies play a crucial role in the management decision making, and can also demonstrate the occurrence of spinal cord compression and pathological vertebral fracture. The “gold-standard” to diagnose GSS relies on pathological findings. Histopathologically, GSS is characterized by an architecture of abnormal proliferation of non-neoplastic lymphatic, vascular or hyperplastic fibrous connective tissue without cellular atypia, inflammatory cell infiltration or osteoclasts.[3,5,22]
Despite the fact that GSS is considered as benign disorder, it may have an unpredictable prognosis and severe complications. Due to its rarity and unclear etiology, its treatment is still an entity in research, although several therapeutic options have been proposed during the last decades. So far, the treatment of GSS includes the following major categories: medication therapy, radiation therapy, surgery and targeted therapies.[6–16,27–30]
Only one case of GSS treated with vertebroplasty by cement augmentation has been reported.[13] In 2015, Carbó described a 10-year-old boy with GSS in spine treated by balloon vertebroplasty with polymethylmethacrylate filler. Balloon vertebroplasty was performed at L3 and L4, and his progress remains satisfactory 4 years after surgery.[13] To most patients in suspicion of GSS, bone tissue biopsy is necessary to obtain bone tissue specimens. In patients with GSS in the spine, surgical treatment are recommended if severe low back pain, pathologic fracture, paresthesia, functional impairment and paralysis occur.[6–14] To date, surgical management of GSS of spine has remained under evaluation, with no standard criteria. We described the case of GSS in lumbar and sacral spine that underwent vertebroplasty with cement augmentation. In our case, we originally planned to perform a 2-stage surgery considering the possible risks such as cement leakage into the canal and subsequent spinal cord compression. After vertebroplasty at L5 was performed, the symptom was successfully controlled with back pain relief and increased strength of bilateral lower limbs. Thus, the patient chose not to undergo another operation of vertebroplasty with cement augmentation at sacrum tentatively because he was nearly asymptomatic. This might be a useful strategy to achieve rapid and sustained neurological improvements for patients with GSS involving the spine and the pelvis. Although there were no complications associated with vertebroplasty in our case, the safety of this approach still needs to be confirmed in further studies with larger sample sizes and longer follow-up periods. One postoperative complication was cement leakage into the canal and subsequent spinal cord compression.[31] Surgical extent, cement volume, and postoperative complications are critical factors that need further investigation. Surgery is also performed to stabilize and reconstruct affected regions of the skeleton once the disease appears to be unstable or neurological deficits occur, especially in the lower spinal region. Vertebroplasty by cement augmentation may be a proper treatment option for patients with GSS who cannot undergo appropriate surgery or decline open surgery.
4. Conclusion
In conclusion, this is the first report of GSS in the spinal region with chylothorax and chyloperitoneum in a patient. Although uncommon, GSS of the spine should be part of the differential when the patient presents with atypical symptoms. Combination of vertebroplasty with cement augmentation and postoperative medical treatment is a definite therapy of choice.
Author contributions
Conceptualization: Shuzhong Liu, Xi Zhou, Xiangyi Kong, Yong Liu.
Investigation: Shuzhong Liu, Xi Zhou, An Song, Yong Liu.
Resources: Shuzhong Liu, Yong Liu.
Supervision: Yipeng Wang, Yong Liu.
Writing – original draft: Shuzhong Liu, Xi Zhou, An Song, Xiangyi Kong, Yong Liu.
Writing – review & editing: Shuzhong Liu, Xiangyi Kong, Yipeng Wang, Yong Liu.
Footnotes
Abbreviations: β-CTX = β-crosslaps, CT = computed tomography, IL-6 = interleukin-6, MRI = magnetic resonance imaging, PET/CT = positron emission tomography-computed tomography, PTH = parathyroid hormone, T1WI = T1-weighted image, T2WI = T2-weighted image, VEGF-A = vascular endothelial growth factor-A.
SL, XZ, and AS contributed equally to this work.
The authors declare no conflicts of interest.
References
- [1].Gorham LW, Wright AW, Shultz HH, et al. Disappearing bones: a rare form of massive osteolysis; report of two cases, one with autopsy findings. Am J Med 1954;17:674–82. [DOI] [PubMed] [Google Scholar]
- [2].Jackson J. A boneless arm. Boston Med Surg J 1838;18:398–9. [Google Scholar]
- [3].Heffez L, Doku HC, Carter BL, et al. Perspectives on massive osteolysis. Report of a case and review of the literature. Oral Surg Oral Med Oral Pathol 1983;55:331–43. [DOI] [PubMed] [Google Scholar]
- [4].Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone); its relation to hemangiomatosis. J Bone Joint Surg Am 1955;37-A:985–1004. [PubMed] [Google Scholar]
- [5].Nikolaou VS, Chytas D, Korres D, et al. Vanishing bone disease (Gorham-Stout syndrome): a review of a rare entity. World J Orthop 2014;5:694–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bickel WH, Brodere AC. Primary lymphangioma of the ilium; report of a case. J Bone Joint Surg Am 1947;29:517–22. [PubMed] [Google Scholar]
- [7].Halliday DR, Dahlin DC, Pugh DG, et al. Massive osteolysis and angiomatosis. Radiology 1964;82:637–44. [DOI] [PubMed] [Google Scholar]
- [8].Thompson JS, Schurman DJ. Massive osteolysis. Case report and review of literature. Clin Orthop Relat Res 1974;206–11. [PubMed] [Google Scholar]
- [9].Hejgaard N, Olsen PR. Massive Gorham osteolysis of the right hemipelvis complicated by chylothorax: report of a case in a 9-year-old boy successfully treated by pleurodesis. J Pediatr Orthop 1987;7:96–9. [DOI] [PubMed] [Google Scholar]
- [10].Stöve J, Reichelt A. Massive osteolysis of the pelvis, femur and sacral bone with a Gorham-Stout syndrome. Arch Orthop Trauma Surg 1995;114:207–10. [DOI] [PubMed] [Google Scholar]
- [11].Kose M, Pekcan S, Dogru D, et al. Gorham-Stout Syndrome with chylothorax: successful remission by interferon alpha-2b. Pediatr Pulmonol 2009;44:613–5. [DOI] [PubMed] [Google Scholar]
- [12].Adler F, Gupta N, Hess CP, et al. Intraosseous CSF fistula in a patient with Gorham disease resulting in intracranial hypotension. AJNR Am J Neuroradiol 2011;32:E198–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Carbó E, Riquelme Ó, García A, et al. Vertebroplasty in a 10-year-old boy with Gorham-Stout syndrome. Eur Spine J 2015;24suppl 4:S590–3. [DOI] [PubMed] [Google Scholar]
- [14].Ballas R, Caduc M, Ovigue J. Fourteen years follow-up of massive pelvic girdle osteolysis caused by lymphatic malformation(Gorham-Stout Disease). Joint Bone Spine 2017;84:625. [DOI] [PubMed] [Google Scholar]
- [15].Dellinger MT, Garg N, Olsen BR. Viewpoints on vessels and vanishing bones in Gorham-Stout disease. Bone 2014;63:47–52. [DOI] [PubMed] [Google Scholar]
- [16].Hu P, Yuan XG, Hu XY, et al. Gorham-Stout syndrome in mainland China: a case series of 67 patients and review of the literature. J Zhejiang Univ Sci B 2013;14:729–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sekharappa V, Arockiaraj J, Amritanand R, et al. Gorham's disease of spine. Asian Spine J 2013;7:242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kakuta Y, Iizuka H, Kobayashi R, et al. Gorham disease of the lumbar spine with an abdominal aortic aneurysm: a case report. Spine J 2014;14:e5–9. [DOI] [PubMed] [Google Scholar]
- [19].Ozbayrak M, Yilmaz MH, Kantarci F, et al. A case of an idiopathic massive osteolysis with skip lesions. Korean J Radiol 2013;14:946–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Torg JS, Steel HH. Sequential roentgenographic changes occurring in massive osteolysis. J Bone Joint Surg Am 1969;51:1649–55. [PubMed] [Google Scholar]
- [21].Motamedi MH, Homauni SM, Behnia H. Massive osteolysis of the mandible: a case report. J Oral Maxillofac Surg 2003;61:957–63. [DOI] [PubMed] [Google Scholar]
- [22].Liu Y, Zhong DR, Zhou PR, et al. Gorham-Stout disease: radiological, histological, and clinical features of 12 cases and review of literature. Clin Rheumatol 2016;35:813–23. [DOI] [PubMed] [Google Scholar]
- [23].Mitchell CS, Parisi MT, Osborn RE. Gorham's disease involving the thoracic skeleton. Plain films and CT in two cases. Pediatr Radiol 1993;23:543–4. [DOI] [PubMed] [Google Scholar]
- [24].Franco-Barrera MJ, Zavala-Cerna MG, Aguilar-Portillo G, et al. Gorham-Stout disease: a clinical case report and immunological mechanisms in bone erosion. Clin Rev Allergy Immunol 2017;52:125–32. [DOI] [PubMed] [Google Scholar]
- [25].Spieth ME, Greenspan A, Forrester DM, et al. Gorham's disease of the radius: radiographic, scintigraphic, and MRI findings with pathologic correlation. A case report and review of the literature. Skeletal Radiol 1997;26:659–63. [DOI] [PubMed] [Google Scholar]
- [26].Marymont JV. Comparative imaging. Massive osteolysis (Gorham's syndrome, disappearing bone disease). Clin Nucl Med 1987;12:153–4. [PubMed] [Google Scholar]
- [27].Brance ML, Castiglioni A, Cóccaro N, et al. Two cases of Gorham-Stout disease with good response to zoledronic acid treatment. Clin Cases Miner Bone Metab 2017;14:250–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Deveci M, Inan N, Corapcioglu F, et al. Gorham-stout syndrome with chylothorax in a six-year-old boy. Indian J Pediatr 2011;78:737–9. [DOI] [PubMed] [Google Scholar]
- [29].Aizawa T, Sato T, Kokubun S. Gorham disease of the spine: a case report and treatment strategies for this enigmatic bone disease. Tohoku J Exp Med 2005;205:187–96. [DOI] [PubMed] [Google Scholar]
- [30].Páez Codeso FM, Morillo Domínguez MC, Dorado Galindo A. A rare case of chylothorax. Gorham-Stout Syndrome. Arch Bronconeumol 2017;53:640. [DOI] [PubMed] [Google Scholar]
- [31].Cai S, Kong X, Yan C, et al. Successful treatment of metastatic pheochromocytoma in the spine with cement augmentation. Medicine (Baltimore) 2017;96:e5892. [DOI] [PMC free article] [PubMed] [Google Scholar]


