Abstract
Background:
This study sought to identify factors that impact the total health care costs associated with hospitalization of young Japanese children with respiratory syncytial virus (RSV).
Methods:
Children admitted between April 2014 and March 2015 with at least a confirmed diagnosis of RSV and 2 days of hospital stay were considered for inclusion. Data analyses of hospital claims were performed using a structural equation modeling approach.
Results:
A total of 6811 Japanese inpatients (<5 years old) diagnosed with RSV were included. The average length of stay was 7.5 days with a mean total health care cost of US Dollars (USD) $3344 per hospitalization. Intensive care unit hospitalizations were associated with greater costs (USD +$4951) compared to routine hospitalizations. The highest procedure-related cost drivers were blood transfusions (USD +$6402) and tube feedings (USD +$3512).
Conclusion:
The economic burden of RSV-related infection hospitalizations in Japan is considerable. Efforts should be toward immunization and therapeutic treatment strategies that reduce severity, prevent, or reduce the duration of hospitalization.
Keywords: economic burden, health care costs, hospitalization costs, Japan, length of stay of Japanese children, prevention, respiratory syncytial virus, respiratory syncytial virus treatment, structural equation modeling, vaccine
1. Introduction
Respiratory syncytial virus (RSV) is a highly prevalent and contagious virus that often results in lower respiratory tract infections which may cause other serious complications. RSV is the primary cause of hospitalization among infants with almost all experiencing their first RSV infection by the age of 2.[1–3] In developed countries 1% to 3% of all children with RSV infection are hospitalized.[4] According to the latest epidemiologic study, the mortality from complications due to this disease stands at close to 118,200 cases annually (uncertainty range 94,600–149,400).[5] Moreover, the study reported a significant burden of RSV infection in neonates with an annual occurrence of nearly 40 episodes per 1000 neonates. Collectively, these data together with the significance of the disease in the <6 months age group indicate development of protective measures such as immunization or therapeutics against the RSV infection at birth or treatments to reduce severity of RSV infection would be highly desirable to control the disease.[5]
There are 2 subtypes of RSV namely type A and type B, with differences in the envelope proteins on the viral shell. While both subtypes of the virus are equally infectious, emerging evidence indicates that type A may result in more severe disease.[6] Other associated risk factors include young age, premature birth, passive smoke exposure, lack of breastfeeding, chronic lung disease, and congenital heart disease.[7–10] Children <2 years of age often present with initial clinical symptoms of cough, mild fever, and rhinorrhoea which develop into wheezing, tachypnea, crackles, as well as increased work of breathing. Also, the infant may exhibit characteristics of poor feeding, vomiting, or irritability. Despite significant improvement in most of the affected children within 3 to 5 days, those with underlying comorbidities such as immune or chronic lung disease may progressively become worse.[11] The infection caused by RSV is a seasonal disease; in temperate climates, the RSV activity typically peaks during the winter months,[12] while in tropical climates, RSV infection tends to peak during the rainy season and the hottest months.[13]
There are considerable costs associated with RSV-related infections. In 2000, close to 98% of all hospitalizations related to RSV infections in the United States were reported in children <5 years of age. In addition, hospitalizations due to RSV-related infections (US Dollars [USD] $394 million) together with all other medical encounters (USD $258 million) for these children were an estimated USD $652 million of the total annual medical costs.[14] The average cost of RSV-related hospitalization was an estimated USD $14,832 annually for all infants. Furthermore, the health care costs of RSV-related hospitalization in the United States for high-risk infants up to 1 year of age are reportedly from USD $20,160 to $39,399 annually.[15] At the same time, the average costs of intensive care unit (ICU) admissions were an estimated USD $35,000 to $89,000 for RSV-related hospitalizations with higher costs for younger infants (<90 days old) compared to the older infants.[16] While the cost of RSV-related hospitalization for infants <6 months in Canada was CAN $23,030,[17] in China this health care cost was USD $571.8 (US$ 909.6 for ICU admission).[18] In addition, results of multivariable logistic regression demonstrated that children > 6 months of age and with comorbidities such as chronic lung disease had higher hospitalization costs when compared to those ≤6 months of age.[18]
While a previous study in Japan reported hospitalization of a significant number of children <3 years of age (31.4%) with RSV-related infections, to date there are no published studies on the economic burden of RSV-related health care utilization in Japan. Therefore, given that in recent years the number of new RSV cases reported is at all-time high,[19] the purpose of this study is to determine the cost of health care associated with RSV-related hospitalization using structural equation modeling (SEM) approach.
2. Methods
2.1. Patient selection
We utilized a commercially available hospital claims data bank from Medical Data Vision Co Ltd (MDV). This is a national administrative database of approximately 4,400,000 patients that represent approximately 3% of the total Japanese population.[20] Previously, the MDV database has been used to examine a wide range of medical conditions in Japan such as rheumatoid arthritis,[21–23] schizophrenia,[24] infectious diseases,[25] multiple sclerosis,[26] hypertension,[27] or prostate cancer.[28,29] We considered the inpatient claims from patients with hospital admission between April 1, 2014 and March 31, 2015 and at least 1 confirmed RSV-related diagnosis (International Classification of Diseases 10th Revision codes: J12.1, J20.5, J21.0, and B34.8) as well as a minimum hospital stay of 2 days (confirmed by at least 1-night stay in the hospital). We limited our analysis to patients of up to 5 years of age. We performed subgroup analysis for the following age groups: <1, 1, 2, and 3 to 5 years.
2.2. Calculations of hospitalization associated costs
Total health care cost (total hospitalization cost) comprised all costs of health care services incurred during each hospitalization period. These included basic management fees, examination, diagnostic and medical procedures, and medications. In addition, Diagnosis Procedure Combination (DPC) cost (which is a case-mix reimbursement cost) was defined as the insurance reimbursed costs. All costs were converted from Japanese yen (JPY) to USD according to the average exchange rate during the April 2014 to March 2015 period (Financial Market Department, Bank of Japan; 1 USD = 109.33 JPY).[30]
2.3. Statistical analysis
Descriptive analyses were performed on baseline characteristics as well as resource use, length of stay (LOS) and total health care cost. Since LOS is usually an important driver of the total hospitalization costs,[31,32] we considered a SEM approach to assess the relationship between the characteristics of patients, hospital procedures as well as LOS, and hospitalization costs by considering LOS as an intermediate effect. SEM is a flexible multivariate statistical framework with a wide range of applications that can be used to model complex relationships between variables.[33] The SEM framework allows assessment of relationships among variables by integrating the strengths of factor analysis and multiple regression in a single model that can be tested statistically.[34] More specifically, in this study, we performed a path analysis which is a special case of the SEM framework that allows an exploration of the causal links (direct and indirect effects) between exogenous variables and one or more endogenous variables. In this framework, the total effects of a covariate on the main dependent variable can be divided into 2 categories of effects: the indirect effects, consisting of the effect of the covariate on one or more intermediary endogenous variables, which in turn translates into an effect on the main variable; and the direct effect, which is the remaining effect of the covariate on the main variable while controlling for their indirect effects.[35] In our analysis, the main endogenous variable of interest was the total hospitalization cost expressed in Japanese yen. The independent variables were assumed to have both a direct effect on total hospitalization costs and indirect effects through the LOS. The relationship between each variable is depicted as a flow diagram in Figure 1. Statistical analyses were performed using Stata 15.0 (StataCorp LLC, College Park, Taxas).[36]
Figure 1.
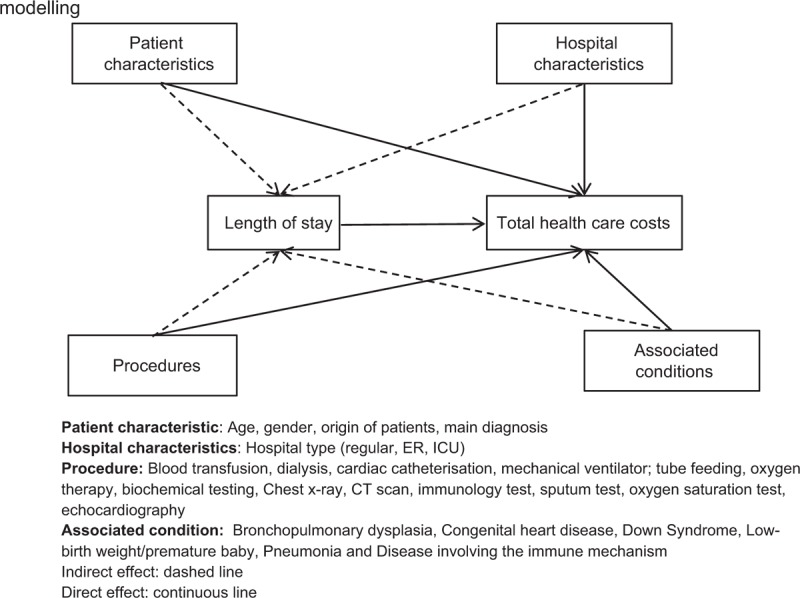
Path showing the relationship between each variable using structural equation modeling.
3. Results
The final analysis included a total of 6811 Japanese children <5 years old hospitalized with RSV-related infections. Patients >5 years (n = 25) were excluded from the analysis. We also excluded 42 rehospitalized admissions due to the limited number of patients (Fig. 2).
Figure 2.
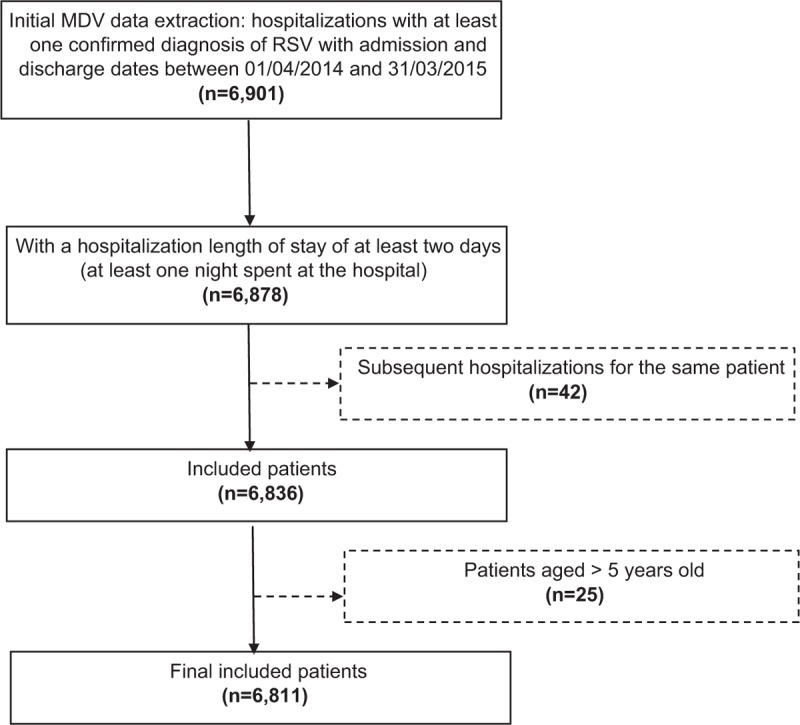
Patient flowchart.
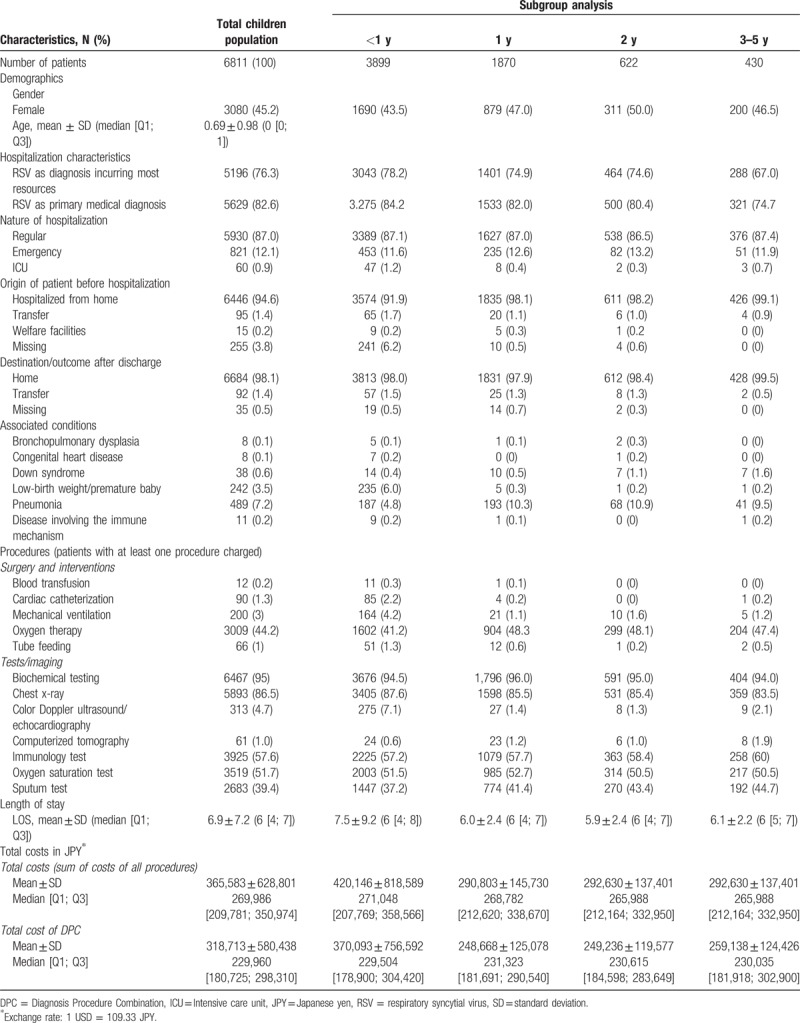
The baseline characteristics for all patients and each subgroup are presented in Table 1. Majority of patients were <1 year old (74.1%), with 12.1% admitted to hospital emergency room (ER), while 0.9% were admitted to the ICU. Among the children, pneumonia (7.2%) was the most common comorbidity followed by low-birth weight/premature baby (3.5%). Common procedures were oxygen therapy (44.2%) and mechanical ventilation (3%). Most patients (95%) had laboratory testing and chest x-ray (86.5%). Overall, the average length of hospital stay was 6.9 days with only minor variations across the age groups. The mean total health care cost was JPY 365,583 (USD $3344). Hospitalization of infants <1 year of age incurred higher costs of JPY 420,146 (USD $3843) with DPC costs of JPY 318,713 (USD $2915).
Table 1.
Characteristics of included patients with RSV.
The results of SEM analysis are summarized in Table 2 and the results revealed that infants <1 year of age had higher direct costs but lower indirect costs through lengths of stay resulting in insignificant total effect. The cost of hospital stay was USD $316 per day. As expected, ICU stays were significantly higher in cost (USD +$4951) than routine hospital stays. The ER hospitalizations had a direct positive impact on costs (USD +$458 per day) but apparently shortened LOS and therefore the indirect effect was USD −$242 per day. The small number of infants admitted from welfare institutions resulted in a lower cost (USD −$377 per day).
Table 2.
Results of the standard linear structural equation modeling for total costs of hospitalizations: direct, indirect, and total effects.
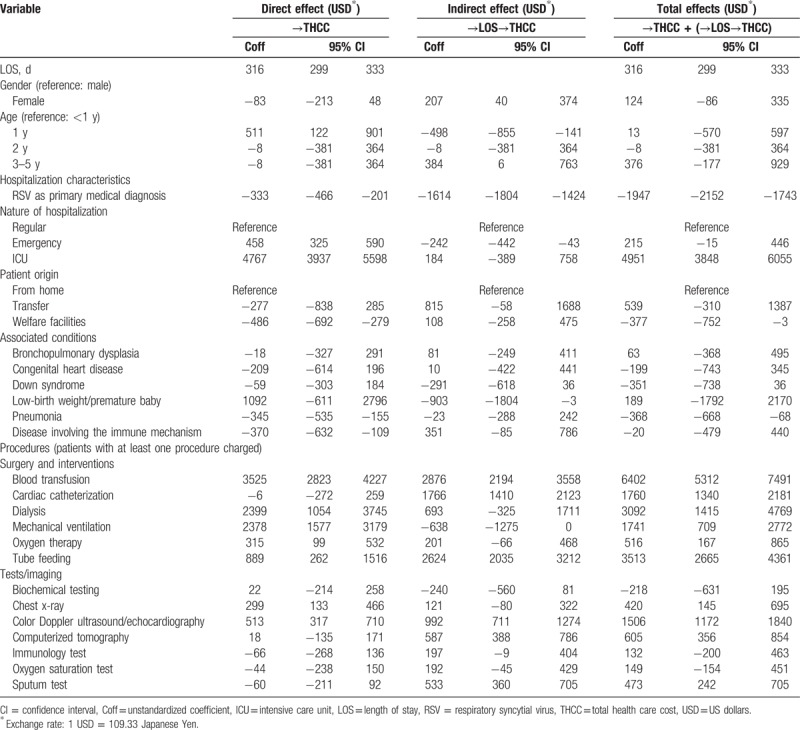
In general, RSV infection as the primary diagnosis reduced the total cost by USD −$2152 mainly through a decrease in LOS. Among comorbidities, low-birth weight/premature infant had a very high albeit insignificant effect on costs. A significantly negative overall relation was found with pneumonia (USD −$368 per day).
The greatest cost-drivers in terms of medical procedures were: blood transfusion (USD +$6402), tube feeding (USD +$3513), dialysis (USD +$3092), cardiac catheterization (USD +$1760), and mechanical ventilation (USD +$1741). The costs associated with the diagnostic imaging procedures of echocardiography (USD +$1506) increased overall health care costs significantly.
4. Discussion
This retrospective cross-sectional study utilized a national administrative database to measure the direct health care costs associated with RSV-related hospitalization. We have demonstrated that in Japan 74% of RSV-related hospitalization occur in children who are <1 year old. We further found that costs are lower when RSV is the primary diagnosis. Clearly, physicians code serious manifestations of the RSV infection as the primary diagnosis. The average LOS was 6.9 days in the overall sample with 7.5 days within the age group <1 year of age. The hospital stay cost on an average USD $316 per day. LOS in Japan appears to be longer compared to that reported for other countries. In Malaysia for instance, 4 days were reported as the average LOS.[37] Similarly, in the United States, the average LOS in hospital is 3.5 days for infants with bronchiolitis, a common clinical presentation of RSV infection,[38] while it was 5.7 days for Canada.[39] Interestingly, in Japan long hospital stays have been reported for other medical conditions in particular mental disorders.[40] Nevertheless, the total health care costs in Japan are significantly lower than in the United States or Canada; the costs for RSV-related hospitalization were JPY 365,583 (USD $3344) in our study which is nearly 78% lower than that reported for the US hospital cost (USD $14,832).[15]
Lower health care costs in Japan compared to the United States for other conditions such as leukemia[41] have also been reported or antimicrobial-resistant infections.[42] The main reason is due to differences in health care systems between the 2 countries. For instance, the health care market in the United States is largely unregulated in terms of price setting with much higher prices for both health care and therapeutic services.[43,44]
We found the most common procedure, oxygen therapy, administered to 44.2% of the patients increased the total costs by USD $516 per hospitalization. Although only performed for severe infection cases, blood transfusions and tube feedings were identified as the most expensive medical procedures in the hospital.
Our findings of a high cost burden associated with ICU or ER admissions when compared with routine hospitalizations are consistent with previous studies and underscore the importance of preventing ICU admissions whenever possible.
Compared to other pediatric diseases, RSV seems to incur higher costs as well. A recent study analyzed the disease burden of measles in Japan and reported total costs for inpatients of USD $2525[45] which is significantly lower than the results we found in this study.
4.1. Implications
We have identified considerable economic burden of RSV infection in Japan which warrants implementation of strategies to manage the impact of the disease. However, since most children affected with RSV infection are usually healthy prior to hospitalization, control strategies targeting only high-risk children will have a limited effect on the overall disease burden of RSV infections.[2] Therefore, the development of a vaccine to immunize infants against RSV infection should be a high public health priority.[46] At present, several live-attenuated and chimeric virus vaccine approaches are being developed. Despite the challenges faced with stabilizing the genetics as well as striking a balance between attenuation (safety) and immunogenicity, much progress has been achieved in developing a recombinant RSV vaccine.[47]
Another potential option available for patients at high risk of infection includes pharmacotherapy with drugs such as palivizumab that is used to prevent the infection. In a recent study, treatment of infants at 32 to 34 weeks gestational age with palivizumab was found to be a more economical strategy and improved quality adjusted life years compared to no prophylactic treatment.[15] In other subpopulations, however, the calculated incremental cost-effectiveness ratio ranged from USD $44,774 to $464,476 which is not considered to be cost-effective in various health care systems. The availability of more effective treatments than pavilizumab that reduce severity and shorten the duration of infection could avert the need for hospitalization or reduce the level and duration of hospital care required.
4.2. Limitations
There are several limitations to our study. Firstly, this analysis is based on a 1-year database. Consequently, we were unable to capture possible changes due to prescribing behavior changes and any treatment guideline modifications implemented over time. Secondly, due to the inherent limitations of the database, potentially useful information that might explain associated costs was lacking. For instance, we could not retrieve hospital ID numbers, which may have assisted in identification of heterogeneity between hospitals as well as patient characteristics such as region, social status, professional position, clinical severity of their disease, etc. Nevertheless, we examined all available patient characteristics (such as age, gender, and relevant comorbidities) that could be retrieved from the database. Finally, bias may have been introduced from the current DPC system that allows hospitals to select for the diagnosis that is incurring the most medical resource utilization as the principle diagnosis. In general, patients with comorbidities will receive a higher reimbursement if hospitals choose comorbidities as the primary diagnosis.
Acknowledgment
The authors are grateful to Dr Negar Jamshidi for editing and proofreading the manuscript.
4.3. Declarations and ethical approval
This study was conducted in line with the guidelines provided by Johnson & Johnson and approved by the Janssen Approval Committee. This was a retrospective study carried out using hospital claims data from the Medical Data Vision database; the authors were not involved in the collection of this data. Patients were informed that their data would be used for research purposes (opt-out system) and their data were de-identified before addition to the research database. Retrieval of the data from this database occurred in an unlinked fashion. As the data had been anonymized, the Ethical Guidelines for Epidemiological Research (Ministry of Education, Culture, Sports, Science and Technology, and Ministry of Health, Labour and Welfare of Japan), which require ethics approval and informed consent, are not applicable to this study. Based on the Ethical Guidelines on Biomedical Research Involving Human Subjects (Ministry of Education, Culture, Sports, Science and Technology, and Ministry of Health, Labour and Welfare of Japan), pharmacoepidemiologic studies conducted on medical databases constitute research carried out on pre-existing material and information, that did not require any interventions or interactions with patients. For such studies, including this study, obtaining written informed consent from patients is not compulsory.
Author contributions
Conceptualization: Jörg Mahlich.
Formal analysis: Rosarin Sruamsiri.
Validation: Hiroshi Kubo.
Writing – original draft: Jörg Mahlich.
Footnotes
Abbreviations: DPC = Diagnosis Procedure Combination, ER = emergency room, ICU = intensive care unit, JPY = Japanese yen, LOS = length of stay, MDV = Medical Data Vision Co Ltd, RSV = respiratory syncytial virus, SEM = structural equation modeling, THCC = total health care cost, USD = US dollars.
This work was supported and funded by Janssen Pharmaceutical KK Company which at no time made any decisions or had a role in the study design, data collection and analysis, data interpretation, or writing up of the report. Data were acquired by Janssen Pharmaceutical KK. The funding body provided support in the form of salaries for authors (JM, HK, RS).
JM, HK and RS were affiliated with Janssen Pharmaceutical KK at the time the study was conducted.
RS, HK, and JM were employed at Janssen KK at the time the study was conducted.
References
- [1].Hall CB, Weinberg GA, Blumkin AK, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics 2013;132:e341–8. [DOI] [PubMed] [Google Scholar]
- [2].Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009;360:588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr 2003;143:S127–32. [DOI] [PubMed] [Google Scholar]
- [4].Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010;375:1545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017;390:946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gilca R, De Serres G, Tremblay M, et al. Distribution and clinical impact of human respiratory syncytial virus genotypes in hospitalized children over 2 winter seasons. J Infect Dis 2006;193:54–8. [DOI] [PubMed] [Google Scholar]
- [7].Alvarez A, Marson FA, Bertuzzo CS, et al. Epidemiological and genetic characteristics associated with the severity of acute viral bronchiolitis by respiratory syncytial virus. J Pediatr 2013;89:531–43. [DOI] [PubMed] [Google Scholar]
- [8].Gouyon JB, Rozé JC, Guillermet-Fromentin C, et al. Hospitalizations for respiratory syncytial virus bronchiolitis in preterm infants at <33 weeks gestation without bronchopulmonary dysplasia: the CASTOR study. Epidemiol Infect 2013;141:816–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Semple MG, Taylor-Robinson DC, Lane S, et al. Household tobacco smoke and admission weight predict severe bronchiolitis in infants independent of deprivation: prospective cohort study. PLoS One 2011;6:e22425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Koehoorn M, Karr CJ, Demers PA, et al. Descriptive epidemiological features of bronchiolitis in a population-based cohort. Pediatrics 2008;122:1196–203. [DOI] [PubMed] [Google Scholar]
- [11].Ramagopal G, Brow E, Mannu A, et al. Clinical and hematological profile of children with bronchiolitis: a comparative study between respiratory synctial virus [RSV] and [Non RSV] groups. J Clin Diagn Res 2016;10:SC05–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Carbonell-Estrany X, Quero J. The IRIS Study Group. RSV hospitalization rates in premature infants born over two consecutive seasons. Pediatr Infect Dis J 2001;20:874–9. [DOI] [PubMed] [Google Scholar]
- [13].Cherian T, Simoes EA, Steinhoff MC, et al. Bronchiolitis in tropical south India. Am J Dis Child 1990;144:1026–30. [DOI] [PubMed] [Google Scholar]
- [14].Paramore LC, Ciuryla V, Ciesla G, et al. Economic impact of respiratory syncytial virus-related illness in the US: an analysis of national databases. Pharmacoeconomics 2004;22:275–84. [DOI] [PubMed] [Google Scholar]
- [15].Mahadevia PJ, Masaquel AS, Polak MJ, et al. Cost utility of palivizumab prophylaxis among pre-term infants in the United States: a national policy perspective. J Med Econ 2012;15:987–96. [DOI] [PubMed] [Google Scholar]
- [16].McLaurin KK, Farr AM, Wade SW, et al. Respiratory syncytial virus hospitalization outcomes and costs of full-term and preterm infants. J Perinatol 2016;36:990–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Banerji A, Lanctôt KL, Paes BA, et al. Comparison of the cost of hospitalization for respiratory syncytial virus disease versus palivizumab prophylaxis in Canadian Inuit infants. Pediatr Infect Dis J 2009;28:702–6. [DOI] [PubMed] [Google Scholar]
- [18].Zhang T, Zhu Q, Zhang X, et al. Clinical characteristics and direct medical cost of respiratory syncytial virus infection in children hospitalized in Suzhou, China. Pediatr Infect Dis J 2014;33:337–41. [DOI] [PubMed] [Google Scholar]
- [19].Japan Times. RSV infections in Japan surge to highest levels in last decade 2016. Available at: https://www.japantimes.co.jp/news/2016/10/09/national/science-health/rsv-infections-japan-surge-highest-levels-last-decade/#.WaoKVLIjHIU Accessed August 30, 2017. [Google Scholar]
- [20].Saokaew S, Sugimoto T, Kamae I, et al. Healthcare Databases in Thailand and Japan: potential sources for health technology assessment research. PLoS One 2015;10:e0141993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mahlich J, Sruamsiri R. Treatment patterns of rheumatoid arthritis in Japanese hospitals and predictors of the initiation of biologic agents. Curr Med Res Opin 2017;33:101–7. [DOI] [PubMed] [Google Scholar]
- [22].Mahlich J, Sruamsiri R. Persistence with biologic agents for the treatment of rheumatoid arthritis in Japan. Patient Prefer Adherence 2016;10:1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Guelfucci F, Kaneko Y, Mahlich J, et al. Cost of depression in Japanese patients with rheumatoid arthritis: evidence from administrative data. Rheumatol Ther 2018;5:171–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cheung S, Hamuro Y, Mahlich J, et al. Drug utilization of japanese patients diagnosed with schizophrenia: an administrative database analysis. Clin Drug Investig 2017;37:559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Udagawa Y, Ohno S, Nakagawa S, et al. Using clinical databases to verify the impact of regulatory agency alerts in Japan: hepatitis B testing behavior after an alert regarding risk of viral reactivation. Drugs Real World Outcomes 2015;2:227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ogino M, Kawachi I, Otake K, et al. Current treatment status and medical cost for multiple sclerosis based on analysis of a Japanese claims database. Clin Exp Neuroimmunol 2016;7:158–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hashikata H, Harada KH, Kagimura T, et al. Usefulness of a large automated health records database in pharmacoepidemiology. Environ Health Prev Med 2011;16:313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mahlich J, Tsubota A, Imanaka K, et al. Burden of illness of chemotherapy in castration-resistant prostate cancer patients in Japan: a retrospective database analysis. Current Medical Research and Opinion 2018;1–6. [DOI] [PubMed] [Google Scholar]
- [29].Cheung S, Hamuro Y, Mahlich J, et al. Treatment pathways of Japanese prostate cancer patients - a retrospective transition analysis with administrative data. PLoS One 2018;13:e0195789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Japan Bo. Exchange rate 2016. Available at: http://www.boj.or.jp/en/statistics/outline/notice_2015/not151120a.htm/ Accessed March 9, 2017. [Google Scholar]
- [31].Cupurdija V, Lazic Z, Petrovic M, et al. Community-acquired pneumonia: economics of inpatient medical care vis-à-vis clinical severity. J bras pneumol 2015;41:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yang J, Jit M, Leung KS, et al. The economic burden of influenza-associated outpatient visits and hospitalizations in China: a retrospective survey. Infect Dis Poverty 2015;4:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Stein CM, Morris NJ, Hall NB, Nock NL. Structural equation modeling. Methods Mol Biol 2017;1666:557–80. [DOI] [PubMed] [Google Scholar]
- [34].Hays RD, Revicki D, Coyne KS. Application of structural equation modeling to health outcomes research. Eval Health Prof 2005;28:295–309. [DOI] [PubMed] [Google Scholar]
- [35].Cella D, Nichol MB, Eton D, et al. Estimating clinically meaningful changes for the Functional Assessment of Cancer Therapy - Prostate: results from a clinical trial of patients with metastatic hormone-refractory prostate cancer. Value Health 2009;12:124–9. [DOI] [PubMed] [Google Scholar]
- [36].Chan CM, Ahmad WA. Differences in physician attitudes towards patient-centredness: across four medical specialties. Int J Clin Pract 2012;66:16–20. [DOI] [PubMed] [Google Scholar]
- [37].Chan PW, Abdel-Latif ME. Cost of hospitalization for respiratory syncytial virus chest infection and implications for passive immunization strategies in a developing nation. Acta Paediatr 2003;92:481–5. [DOI] [PubMed] [Google Scholar]
- [38].Schroeder AR, Marmor AK, Pantell RH, et al. Impact of pulse oximetry and oxygen therapy on length of stay in bronchiolitis hospitalizations. Arch Pediatr Adolesc Med 2004;158:527–30. [DOI] [PubMed] [Google Scholar]
- [39].Mitchell I, Defoy I, Grubb E. Burden of respiratory syncytial virus hospitalizations in Canada. Can Respir J 2017;2017:4521302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mahlich J, Nishi M, Saito Y. Modelling the budget impact of long-acting injectable paliperidone palmitate in the treatment of schizophrenia in Japan. Clinicoecon Outcomes Res 2015;7:267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mahlich J, Okamoto S, Tsubota A. Cost of illness of Japanese patients with chronic lymphocytic leukemia (CLL) and budget impact of the market introduction of ibrutinib. Pharmacoecon Open 2017;1:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Uematsu H, Yamashita K, Kunisawa S, et al. Estimating the disease burden of methicillin-resistant Staphylococcus aureus in Japan: retrospective database study of Japanese hospitals. PLoS One 2017;12:e0179767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Laugesen M, Glied S. Higher fees paid to US physicians drive higher spending for physician services compared to other countries. Health Aff (Millwood) 2011;30:1647–56. [DOI] [PubMed] [Google Scholar]
- [44].Danzon PM, Chao L-W. Cross-national price differences for pharmaceuticals: how large, and why? J Health Econ 2000;19:159–95. [DOI] [PubMed] [Google Scholar]
- [45].Takahashi K, Ohkusa Y, Kim J-Y. The economic disease burden of measles in Japan and a benefit cost analysis of vaccination, a retrospective study. BMC Health Serv Res 2011;11:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Neuzil KM. Progress toward a respiratory syncytial virus vaccine. Clin Vaccine Immunol 2016;23:186–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Karron RA, Luongo C, Thumar B, et al. A gene deletion that up-regulates viral gene expression yields an attenuated RSV vaccine with improved antibody responses in children. Sci Transl Med 2015;7:312ra175. [DOI] [PMC free article] [PubMed] [Google Scholar]


