Abstract
Purpose of review
We summarize what is known about neutrophils in HIV infection, focusing on their potential roles in HIV protection, acquisition, and pathogenesis.
Recent findings
Recent studies have demonstrated that neutrophil-associated proteins and cytokines in genital tissue pre-infection associate with HIV acquisition. However, recent in vivo assessment of highly exposed seronegative individuals and in vitro studies of anti-HIV functions of neutrophils add to older literature evidence that neutrophils may be important in a protective response to HIV infection.
Summary
Neutrophils are important for containment of pathogens, but can also contribute to tissue damage due to their release of reactive oxygen species, proteases, and other potentially harmful effector molecules. Overall, there is clear evidence for both helpful and harmful roles of neutrophils in HIV acquisition and pathogenesis. Further study, particularly of tissue neutrophils, is needed to elucidate the kinetics, phenotype, and functionality of neutrophils in HIV infection to better understand this dichotomy.
Keywords: neutrophils, HIV mucosal dysfunction, HIV infection, tissue damage, mucosal immunology, HIV protection
Introduction
Neutrophils, the most abundant immune cell, are the first responders to infection and are crucial in the immune response to pathogens[1]. However they can also contribute to tissue damage through their release of reactive oxygen species and other potentially harmful effector molecules. This dichotomy could have important implications for HIV as a tissue-resident pathogen that is transmitted across mucosal tissue surfaces. Therefore, the balance of neutrophil antimicrobial function and tissue damage caused by neutrophils could greatly impact both HIV transmission and pathogenesis in several capacities as depicted in Figure 1. Indeed, studies linking tissue neutrophils to HIV transmission as well as studies suggesting that neutrophils have a role in HIV protection have both been published[2–5]. Neutrophils, especially tissue neutrophils, have been understudied in HIV infection due to the cells’ susceptibility to freezing injury and reduced viability after cryopreservation, which require that they be assessed fresh after isolation from blood or tissue[6]. For example, while it is known that peripheral neutrophils have reduced antimicrobial function in HIV infection and this is related to increased risk of secondary infection[7],[8],[9], the functionality of intestinal and reproductive tract neutrophils in HIV infection remains unexamined. Further, distinct neutrophil phenotypes have been described in various states of health and disease, yet limited work has been done to assess these phenotypes in relevant mucosal tissues in HIV infection. Here, we summarize what is known about neutrophils in HIV infection, including studies assessing peripheral neutrophil phenotype and function, the potential for tissue neutrophils to contribute to HIV pathogenesis, and the evidence that neutrophils may contribute to both HIV acquisition and the antiviral immune response to HIV.
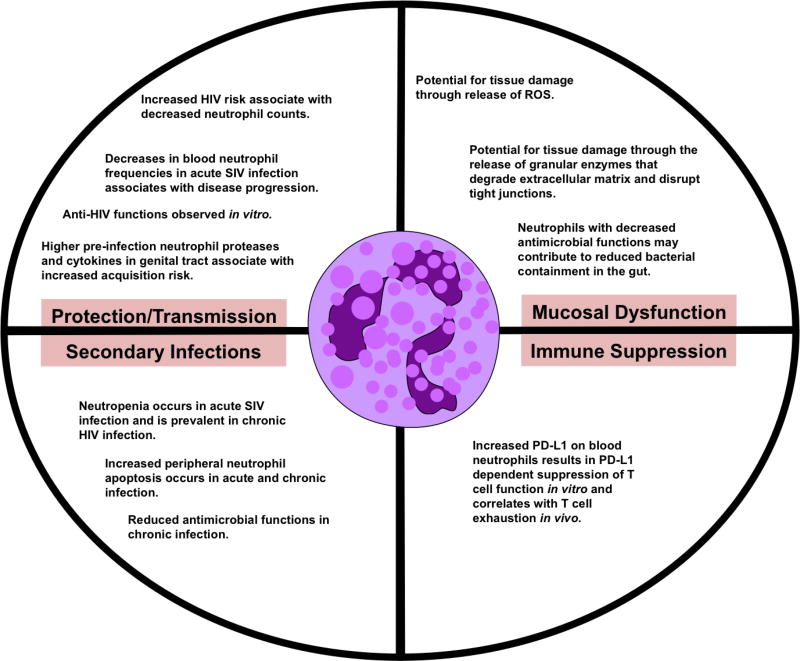
Figure 1. Neutrophils in HIV/SIV infection.
Neutrophils could have a diverse impact on HIV infection depending on their location, kinetics, and functionality. Most of what is known about neutrophils in acute infection is based on non-human primate SIV infection studies. Protection/transmission: In vitro studies demonstrate anti-HIV functions of neutrophils[10–13], [14], [15, 16], [17], [18], and reports correlate reduced peripheral blood neutrophil frequencies with increased HIV acquisition risk and SIV disease progression in vivo[4, 5, 19]. There have been no studies assessing mucosal neutrophil frequencies and acquisition risk in SIV or HIV infection. Contrarily, higher levels of neutrophil factors in the genital tract pre-infection associate with HIV acquisition risk in vivo[3, 20, 21]. Secondary infections: Neutropenia and neutrophil dysfunction are associated with increased risk of secondary infections in HIV-infected individuals[22–24]. Mucosal dysfunction: A direct link between neutrophils and mucosal dysfunction has yet to be assessed but it is proposed that neutrophils could contribute to tissue damage and that neutrophil dysfunction may allow microbial translocation[25, 26]. Immune suppression: Neutrophils can act as granulocytic myeloid derived suppressor cells that suppress T cell function and increased suppressor activity of peripheral neutrophils has been observed in HIV infection[27]. PD-L1 expression on neutrophils correlates with PD-1 expression on CD4+ T cells, which is a marker of exhaustion and marks cells enriched for integrated HIV DNA during suppressive ART[28].
Neutrophil Recruitment, Antimicrobial Functions, and Tissue Damage
Neutrophils are actively recruited to sites of infection by chemotactic factors as they roll along the walls of post-capillary venules searching for signs of distress[29]. Specifically, chemoattractants act on endothelial cells to upregulate selectin molecules involved in neutrophil tethering and integrins involved in neutrophil adhesion. The most potent of the host-derived chemoattractants is the chemokine interleukin-8 (IL-8), which is released by monocytes, macrophages, epithelial cells, mast cells, keratinocytes, fibroblasts, endothelial cells, and neutrophils during inflammation[30]. Importantly, circulating IL-8 levels are increased in HIV-infected persons on ART[31]. High levels of IL-8 cause increased endothelial expression of adhesion molecules and increased leukocyte transmigration, which have been proposed to contribute to the increased risk of comorbidities in treated HIV infection[32].
Once in the tissue, neutrophils employ several potent antimicrobial mechanisms to fight invading pathogens. Central to all of these mechanisms are cytotoxic granules within neutrophils that contain different types of antimicrobial molecules including 1) cationic peptides such as defensins; 2) proteases such as cathepsins, lysozyme, gelatinase, and elastase; and 3) reactive oxygen and reactive nitrogen species[33]. In degranulation, granular contents are exocytosed to kill pathogens or promote transmigration[34, 35]. Additionally, through phagocytosis, neutrophils internalize microorganisms and sequester them in a phagosome, which then merges with granules to kill the pathogen. Finally, neutrophils can release neutrophil extracellular traps (NETs) consisting of DNA to trap microbes and kill them using granule components[36]. These antimicrobial functions of neutrophils are mediated through recognition of pathogen-associated molecular patterns (PAMPs). PAMPs interact with pattern-recognition receptors (PRRs) on the neutrophil surface, including toll-like receptors (TLRs), peptidoglycan-recognition protein (PGRP), and collectins[37, 38]. Neutrophils express TLRs 1, 2 and 4–10, and TLR ligation mediates cell survival, cytokine release, superoxide generation, degranulation, and phagocytosis[39]. Additionally, neutrophils recognize complement-opsonized pathogens by surface receptors such as CD11b/CD18 and CD11c/CD18 and antibody-opsonized pathogens through Fc receptors[39].
The antimicrobial functions of neutrophils make them a necessary component of the immune system’s ability to fight pathogens. However, since their discovery they have also been viewed as inflammatory cells that cause destruction in their wake, and collateral tissue damage is often observed as a result of neutrophils’ antimicrobial activities[40]. Indeed, excessive host tissue damage can be caused by unregulated control of granule proteases, and the three most commonly associated with damage are the serine proteases elastase, proteinase-3 and cathepsin G[41]. Additionally, NETs can trigger antibody-mediated autoimmune responses and organ dysfunction, and the release of toxic reactive oxygen species can cause extracellular matrix damage and tissue necrosis[42, 43].
Neutropenia, neutrophil dysfunction and secondary infections
HIV-infected individuals often experience decreased peripheral blood neutrophil counts compared to uninfected individuals, and the degree and nature of neutropenia in HIV infection has been extensively reviewed[44]. One of the largest and most recent cohort studies found that at baseline 44% of HIV-infected women had neutrophil counts less than 2000 cells/µl and during a 7.5 year follow-up period, and 79% of the HIV-infected women presented with neutrophil counts less than 2000 cells/µl on at least one occasion[45]. Importantly, this study also demonstrated that decreased neutrophil counts associate with more advanced disease progression including lower CD4+ T cell counts and higher HIV-1 RNA levels, and ART treatment protected against the development of neutropenia.
Several factors have been suggested to contribute to neutropenia in HIV infection. These have been reviewed extensively elsewhere[44], but the main contributing immunological factors will be briefly summarized here. Given the relationship between neutropenia and HIV-1 RNA levels, it has been proposed that HIV-induced cytotoxicity contributes to neutropenia. Although there are no studies demonstrating that HIV directly infects and kills mature neutrophils, HIV has been shown to destroy multipotent hematopoietic stem cells (HSC) through direct infection and Fas-dependent apoptosis, and HIV proteins suppress proliferation of granulomonocytic progenitor cells[46–49]. Additionally, HIV infection of stromal cells can disrupt the bone marrow microenvironment, thus reducing support for progenitor development and decreasing factors important for granulocyte development such as G-CSF[50],[51]. HIV also reduces the production of the neutrophil supporting cytokine GM-CSF by T cells and other mononuclear cells, and reduced GM-CSF levels correlated with fewer granulomonocytic progenitor cells in one study[52, 53]. Taken together, these studies provide evidence that HIV cytoxicity of progenitor cells and other leukocytes could contribute to reduced neutrophil production in the bone marrow. In addition to hematopoietic defects, antineutrophil antibodies produced by polyclonal B cell activation in HIV infection have been reported and associated with neutropenia[54, 55]. Finally, neutrophils from people with AIDS exhibited accelerated apoptosis compared to healthy individuals, and neutrophils in SIV-infected rhesus macaques similarly exhibited increased apoptosis, suggesting that reduced survival of peripheral neutrophils may also contribute to neutropenia[56, 57]. However, a recent study reported decreased peripheral neutrophil apoptosis and increased neutrophil necrosis in ART-treated HIV infection, suggesting an inflammatory switch in cell death mechanism rather than overall increased neutrophil cell death may be occurring in individuals on ART[58]. In addition, there is also the possibility that increased homing to effector sites such as the mucosa or marginal pools such as those in the lung may contribute to peripheral neutropenia; however tissue neutrophils have been vastly understudied in the context of HIV infection.
Beyond neutropenia, peripheral neutrophils in HIV-infected individuals have reductions in several functions, including chemotaxis, phagocytosis, bactericidal activity, and oxidative burst abilities, which have been observed in both untreated individuals and those on ART and worsen during the course of infection[7],[8],[9],[59],[60],[61]. Importantly, the antimicrobial function of neutrophils in the tissues has not been assessed, and it is therefore unclear how neutrophil dysfunction may contribute to the lack of HIV containment in initial infection, HIV pathogenesis, or HIV reservoir. However, both neutropenia and decreased peripheral blood neutrophil functionality are linked to an increased risk of secondary infections in people with HIV, such as bacteremia, pneumonia, and aspergillosis[22–24]. Furthermore, several studies indicate that recombinant G-CSF therapy can prevent neutropenia, improve neutrophil function, and increase survival through the prevention of serious bacterial and fungal infections in people with advanced HIV disease [62–64]. Importantly, while multiple studies by the same group have revealed that neutrophil fungicidal activity is not returned to normal in patients on ART treatment despite viral suppression and CD4 reconstitution[65, 66], a comprehensive evaluation of other antimicrobial neutrophil functions following treatment has yet to be performed. This is further complicated by the fact that different ART drugs may directly impact neutrophil functionality by vastly different mechanisms, with one previous study demonstrating that dideoxynucleosides enhanced neutrophil antimicrobial functions while another study found that protease inhibitors inhibited neutrophil functions[67, 68]. These data suggest that different ART regimens likely differentially contribute to neutrophil dysfunction in treated HIV infection. Overall, the level of residual neutrophil dysfunction in treated HIV infection and the contribution of different ART regimens should be further evaluated in order to understand the extent to which neutrophil dysfunction may contribute to HIV pathogenesis in the era of ART.
The potential role of neutrophils in mucosal dysfunction and HIV pathogenesis
GI mucosal dysfunction is a defining feature of HIV infection and is characterized by structural damage to the epithelial barrier [69, 70]. This damages manifests as structural abnormalities including atrophy and blunting of enterocyte villi, crypt hyperplasia, and breaches in tight junctions that lead to increased intestinal permeability[71]. The causes and consequences of mucosal dysfunction are multifaceted and complex, and are still not completely understood[70, 72, 73]. However, putative mechanisms have been shown to contribute to reduced GI barrier integrity in HIV infection. These include: 1) death, dysfunction, and abnormal proliferation of enterocytes caused by HIV proteins[74, 75, 25, 76, 77]; 2) enterocyte apoptosis and tight junction downregulation caused by inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and IL-1β[75, 78]; and 3) massive CD4+ T cell depletion in the GI tract in acute infection[79], including the loss of IL-17- and IL-22-producing T cells known to homeostatically maintain the epithelial barrier[80]. This mucosal dysfunction results in focal breaches in the GI epithelial barrier and allows microbial products to translocate across the GI barrier and circulate in the blood, known as microbial translocation[81, 72, 82, 83]. Ongoing microbial translocation is a central factor in persistent systemic immune activation and inflammation that occur despite ART and is associated with increased morbidities and mortality in people with treated HIV infection[84–87].
During chronic HIV infection, neutrophils infiltrate the GI tract at high levels, yet their contribution to the pathology of mucosal dysfunction in GI tissue is unknown[25]. As previously mentioned, while neutrophils are critical in protection from infections, aberrant neutrophil responses can also be harmful. Models of inflammatory bowel disease (IBD) suggest that neutrophils in the GI tract may contribute to disease, and neutrophil infiltration correlates with disease severity in patients with ulcerative colitis[88, 89]. However, some IBD models also demonstrate that depletion of neutrophils can exacerbate disease, illustrating a controversy over the role of these cells[90–92]. In favor of neutrophils contributing to mucosal damage in settings other than HIV are several studies indicating that transepithelial migration of neutrophils creates gaps between epithelial cells, alters levels of tight junction proteins, and increases epithelial permeability[93],[94],[95]. The potential role of neutrophil recruitment in inducing mucosal damage could have a critical impact on microbial translocation, systemic immune activation, and the resulting comorbidities in HIV infection.
In the SIV model of infection, increased neutrophil infiltration in the GI tract has been observed in association with increased disease progression and damage to the epithelial barrier[26], but it is unclear if this infiltration contributes to the barrier damage or is a response to contain microbial products that have translocated to the lamina propria. One study reported increased peripheral neutrophil death in acute SIV infection associated with more rapid progression to AIDS in rhesus macaques, suggesting that neutrophils may be important for controlling factors such as microbial translocation that are associated with HIV pathogenesis[19]. In rats, increased microbial translocation after neutrophil depletion has been reported, suggesting neutrophils may be critical in clearing translocated bacteria and preventing access of these bacteria and bacterial products to the periphery[90]. Importantly, the inability of neutrophils to contain microbial translocation in the context of SIV and HIV infection may be due to decreased antimicrobial function of the recruited neutrophils. Indeed, evidence that ART may improve chemotaxis but not the crucial microbe-killing functions of neutrophils further supports the hypothesis that decreased neutrophil function may contribute to microbial translocation in the setting of ART-treated, chronic HIV infection[65]. It will be critical to evaluate the antimicrobial functions of GI neutrophils during HIV infection to further assess their potential contribution to the containment of microbial translocation.
Neutrophils, inflammation, and immune dysfunction in HIV
Importantly, most of what is known about neutrophils in HIV infection pertains only to peripheral blood neutrophils and there have been no studies directly assessing the role of neutrophils in gastrointestinal immunity or dysfunction in HIV or SIV infection. Peripheral blood neutrophils of ART-treated, HIV-infected individuals have recently been described as hyperactivated, with reduced L-selectin (CD62L) and FcgRIIIb (CD16) and increased integrin (CD11b) expression, and this hyperactivation is more pronounced in individuals with inflammatory comorbidities[58]. However, it remains unclear if neutrophils directly contribute to inflammation or if microbial translocation drives both inflammation and neutrophil activation in the periphery.
Neutrophils in HIV infection may also be interfacing with the adaptive immune system and driving dysfunction. Neutrophils that act as granulocytic myeloid-derived suppressor cells (G-MDSCs) have been identified as a specific phenotype of neutrophils with the ability to suppress the adaptive immune response, particularly through suppression of T cell cytokine production and proliferation[96]. A recent study elucidated a role for suppressive neutrophils in T cell exhaustion and immune suppression in HIV infection[28]. Specifically, the authors found that blood neutrophils in HIV-1 infected individuals have increased PD-L1 expression and suppress T cell function via a mechanism involving reactive oxygen species and PD-L1 interaction with the T cell surface molecule PD-1. Another recent study demonstrated that low density granulocytes, including G-MDSCs with increased arginase release, inversely correlated with CD4+ T cell count and positively correlated with plasma HIV RNA[27]. Importantly, the activation and functionality of gastrointestinal neutrophils in HIV infection remains unknown, and the expression of functional markers such and PD-L1 has yet to be assessed on GI neutrophils in HIV infection, making this an important area for future study to better elucidate a role for these cells in GI immune dysfunction.
Neutrophils and HIV protection
Two studies have demonstrated associations between increased risk of HIV acquisition and fewer peripheral blood neutrophils, implicating neutrophils in a protective role against HIV in vivo[4, 5]. In one such study of South African high-risk female sex workers, HIV-uninfected women with a genetic basis for ethnic neutropenia and circulating neutrophil counts <2500 cells/µl had a ~3-fold increased risk of acquiring HIV infection compared to those with higher neutrophil counts[4]. Additionally, in another cohort of South African women, higher neutrophil counts in the mother and infant were each associated with lower risk of perinatal HIV infection, with each 1000 cells/µl increase in the infant's neutrophil count at birth associated with an 11% reduction in the risk of perinatal HIV acquisition[5].
A pivotal role for neutrophils in viral immunity has been more recently established and neutrophils could contribute to an anti-HIV response in several ways. First, α-defensins, also known as human neutrophil peptides (HNP), are produced mainly by neutrophils and have potent antiviral properties[12]. HNP1, HNP2, and HNP3 inhibit HIV infection in vitro by directly inactivating the virus or by blocking viral replication by altering target cell signaling pathways[10–13]. Importantly, highly exposed seronegative (HESN) men in Uganda had elevated α-defensins in the foreskin, suggesting they may contribute to a protective mucosal environment[97]. The release of myeloperoxidase (MPO) and reactive oxygen species by neutrophils to form hypochlorous acid has also been demonstrated to be viricidal to HIV-1 in vitro[14]. The anti-HIV properties of defensins and MPO can be potentially concentrated and directed by capturing the virus in extracellular traps, as was recently demonstrated by neutrophils in vitro[17]. Neutrophils participate in antibody-dependent cell-mediated cytotoxic killing of HIV-infected cells, but less effectively than monocytes and NK cells[15, 16]. Lastly, neutrophils can perform antibody-dependent cellular phagocytosis of infected cells and immune complexes, a function that constitutes part of the polyfunctional HIV-specific antibody response described in elite controllers[18]. In a recent in vitro study comparing phagocytic ability of tissue resident cells, neutrophils exhibited more robust phagocytosis of gp120-coated fluorescent beads compared to macrophages from colon and similar phagocytic ability compared to cervical macrophages, further highlighting a role for neutrophil phagocytosis in the anti-HIV immune response[98].
Neutrophils and HIV acquisition
Despite studies indicating a potential role for neutrophils in the anti-HIV response and protection against HIV, several other studies have linked neutrophils or neutrophil-associated factors to increased HIV acquisition. In one study, neutrophils isolated from the blood of HESN individuals expressed lower levels of PRR and cytokine mRNAs ex vivo and demonstrated reduced cytokine production in response to TLR and HIV-1 stimulation when compared to neutrophils from infected individuals[2]. These data suggest reduced neutrophil responses may be associated with protection from HIV infection. Although the HESN individuals also expressed lower levels of some of the PRR and cytokines examined when compared to uninfected controls, it remains unclear if neutrophils in HIV-infected individuals are more responsive overall compared to uninfected individuals and how that may confound comparisons between HESN and infected individuals. Additionally, a study of Kenyan female sex workers demonstrated that high levels of HNPs 1–3 and the cathelicidin LL-37 in cervicovaginal secretions were associated with subsequent HIV acquisition despite their contribution to the ability of the genital secretions to neutralize HIV in vitro[20]. Additionally, the potent neutrophil chemokine IL-8 was among the pro-inflammatory cytokines increased in cervicovaginal lavages (CVL) from South African women who acquired HIV infection when compared to women who remained uninfected in the microbicide trial CAPRISA 004[21].
As mentioned previously, the unregulated release of proteases and other factors into the extracellular space can paradoxically damage host tissues by degrading structural proteins of mucosal surfaces[99, 34]. As proteases help neutrophil migration and penetration through tissue via tissue remodeling and extracellular matrix degradation, proteases therefore facilitate barrier disruption. Indeed, further study of CVL from the CAPRISA 004 trial associated increased neutrophil proteases with increased inflammatory cytokines, an altered cytoskeleton, and increased endocervical CD4+ T cells[100]. The authors also demonstrated that neutrophil proteases correlated positively with IL-17 expression. This is not surprising given that neutrophils and Th17 cells participate in reciprocal recruitment through the production of chemokines, and the Th17/neutrophil axis has been well studied in several bacterial and viral infections[101–103]. Importantly, this axis could represent a role for neutrophils in increasing the number of target cells for HIV acquisition and replication as Th17 cells have been demonstrated to be preferentially infected in the female genital tract[104, 105]. Finally, this link between neutrophils and HIV target cells has also been observed in men. Specifically, one study associated increased odds of seroconversion with penile coronal sulcus IL-8 levels, and the authors further demonstrated that high IL-8 levels associated with increased neutrophils and increased HIV target cell density, including Th17 and Th1 cells, in the foreskin[3]. Thus, overall, there is clear evidence for both helpful and harmful roles of neutrophils in the context of HIV infection. It is possible that this dichotomy is due to a lack of studies assessing tissue neutrophil functionality in vivo or ex vivo in high risk groups, as the protective evidence is based mainly on peripheral blood neutrophil frequencies or the in vitro anti-HIV function of neutrophils while the acquisition evidence is based mainly on neutrophil supporting cytokines or neutrophil factors measured in tissues ex vivo. Studies assessing the anti-HIV functions of tissue neutrophils and/or their contribution to tissue damage in relation to HIV acquisition would be useful in understanding the conflicting results of the studies performed thus far and better elucidate their potential role in protection and acquisition.
Conclusion
There are many unanswered questions regarding neutrophil kinetics and functionality that could greatly impact the role of neutrophils in HIV pathogenesis and acquisition. HIV infection results in neutrophil dysfunction in chronic infection, yet it is unclear when neutrophil dysfunction occurs and to what extent neutrophil dysfunction alters the ability of neutrophils to participate in an antiviral response or contribute to HIV mucosal dysfunction and pathogenesis. With regards to tissue neutrophils in the genital tract, further studies are necessary to fully elucidate the dichotomy of ongoing neutrophil accumulation pre-infection and the association with HIV risk and a transient neutrophil recruitment to the genital mucosa that may contribute to a protective antiviral response. However, reducing neutrophil accumulation and unresolved neutrophil-driven inflammation in high-risk individuals should be investigated as a potential HIV prevention strategy. Additionally, the capacity in which neutrophils contribute to the antiviral response to HIV and SIV remains unclear. While studies have linked low peripheral neutrophil frequencies to increased HIV acquisition risk and SIV disease progression, no studies have fully assessed the necessity of neutrophils in a protective response, particularly at mucosal sites. Finally, while the propensity for neutrophils to damage mucosal tissue is apparent, the extent to which they contribute to mucosal dysfunction in HIV remains unknown. The field would benefit greatly from in vivo studies in HIV-infected individuals, as well as in the nonhuman primate or humanized mouse models, that target neutrophil frequency or functionality to better elucidate the role of neutrophils in protection and/or acquisition and their contribution to mucosal damage in HIV pathogenesis.
Acknowledgments
Conflict of Interest
Tiffany Hensley-McBain and Nichole R. Klatt declare grants from National Institutes of Health.
Footnotes
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
* Of Importance
- 1.Nathan C. Neutrophils and immunity: challenges and opportunities. Nature reviews Immunology. 2006;6(3):173–82. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez JC, Giraldo DM, Paul S, Urcuqui-Inchima S. Involvement of neutrophil hyporesponse and the role of toll-like receptors in human immunodeficiency virus 1 protection. PLoS One. 2015;10(3):e0119844. doi: 10.1371/journal.pone.0119844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3*.Prodger JL, Gray RH, Shannon B, Shahabi K, Kong X, Grabowski K, et al. Chemokine Levels in the Penile Coronal Sulcus Correlate with HIV-1 Acquisition and Are Reduced by Male Circumcision in Rakai, Uganda. PLoS pathogens. 2016;12(11):e1006025. doi: 10.1371/journal.ppat.1006025. A randomized longitudinal trial of uncircumcised men found that HIV acquisition was associated with detectable IL-8 levels in the penile coronal sulcus at the visit prior to seroconversion. Authors also demononstrated that IL-8 levels associated with neutrophils and HIV target cells in the foreskin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramsuran V, Kulkarni H, He W, Mlisana K, Wright EJ, Werner L, et al. Duffy-null-associated low neutrophil counts influence HIV-1 susceptibility in high-risk South African black women. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;52(10):1248–56. doi: 10.1093/cid/cir119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kourtis AP, Hudgens MG, Kayira D Team BANS. Neutrophil count in African mothers and newborns and HIV transmission risk. The New England journal of medicine. 2012;367(23):2260–2. doi: 10.1056/NEJMc1202292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham-Pole J, Davie M, Willoughby ML. Cryopreservation of human granulocytes in liquid nitrogen. Journal of clinical pathology. 1977;30(8):758–62. doi: 10.1136/jcp.30.8.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flo RW, Naess A, Nilsen A, Harthug S, Solberg CO. A longitudinal study of phagocyte function in HIV-infected patients. Aids. 1994;8(6):771–7. doi: 10.1097/00002030-199406000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Elbim C, Prevot MH, Bouscarat F, Franzini E, Chollet-Martin S, Hakim J, et al. Polymorphonuclear neutrophils from human immunodeficiency virus-infected patients show enhanced activation, diminished fMLP-induced L-selectin shedding, and an impaired oxidative burst after cytokine priming. Blood. 1994;84(8):2759–66. [PubMed] [Google Scholar]
- 9.Lazzarin A, Uberti Foppa C, Galli M, Mantovani A, Poli G, Franzetti F, et al. Impairment of polymorphonuclear leucocyte function in patients with acquired immunodeficiency syndrome and with lymphadenopathy syndrome. Clinical and experimental immunology. 1986;65(1):105–11. [PMC free article] [PubMed] [Google Scholar]
- 10.Chang TL, Vargas J, Jr, DelPortillo A, Klotman ME. Dual role of alpha-defensin-1 in anti-HIV-1 innate immunity. The Journal of clinical investigation. 2005;115(3):765–73. doi: 10.1172/JCI21948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Z, Cocchi F, Gentles D, Ericksen B, Lubkowski J, Devico A, et al. Human neutrophil alpha-defensin 4 inhibits HIV-1 infection in vitro. FEBS letters. 2005;579(1):162–6. doi: 10.1016/j.febslet.2004.11.062. [DOI] [PubMed] [Google Scholar]
- 12.Klotman ME, Chang TL. Defensins in innate antiviral immunity. Nature reviews Immunology. 2006;6(6):447–56. doi: 10.1038/nri1860. [DOI] [PubMed] [Google Scholar]
- 13.Mackewicz CE, Yuan J, Tran P, Diaz L, Mack E, Selsted ME, et al. alpha-Defensins can have anti-HIV activity but are not CD8 cell anti-HIV factors. Aids. 2003;17(14):F23–32. doi: 10.1097/01.aids.0000088209.77946.21. [DOI] [PubMed] [Google Scholar]
- 14.Klebanoff SJ, Coombs RW. Viricidal effect of polymorphonuclear leukocytes on human immunodeficiency virus-1. Role of the myeloperoxidase system. The Journal of clinical investigation. 1992;89(6):2014–7. doi: 10.1172/JCI115810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baldwin GC, Fuller ND, Roberts RL, Ho DD, Golde DW. Granulocyte- and granulocyte-macrophage colony-stimulating factors enhance neutrophil cytotoxicity toward HIV-infected cells. Blood. 1989;74(5):1673–7. [PubMed] [Google Scholar]
- 16.Smalls-Mantey A, Connors M, Sattentau QJ. Comparative efficiency of HIV-1-infected T cell killing by NK cells, monocytes and neutrophils. PloS one. 2013;8(9):e74858. doi: 10.1371/journal.pone.0074858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saitoh T, Komano J, Saitoh Y, Misawa T, Takahama M, Kozaki T, et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell host & microbe. 2012;12(1):109–16. doi: 10.1016/j.chom.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Ackerman ME, Mikhailova A, Brown EP, Dowell KG, Walker BD, Bailey-Kellogg C, et al. Polyfunctional HIV-Specific Antibody Responses Are Associated with Spontaneous HIV Control. PLoS pathogens. 2016;12(1):e1005315. doi: 10.1371/journal.ppat.1005315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elbim C, Monceaux V, Mueller YM, Lewis MG, Francois S, Diop O, et al. Early divergence in neutrophil apoptosis between pathogenic and nonpathogenic simian immunodeficiency virus infections of nonhuman primates. Journal of immunology. 2008;181(12):8613–23. doi: 10.4049/jimmunol.181.12.8613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levinson P, Kaul R, Kimani J, Ngugi E, Moses S, MacDonald KS, et al. Levels of innate immune factors in genital fluids: association of alpha defensins and LL-37 with genital infections and increased HIV acquisition. Aids. 2009;23(3):309–17. doi: 10.1097/QAD.0b013e328321809c. [DOI] [PubMed] [Google Scholar]
- 21*.Masson L, Passmore JA, Liebenberg LJ, Werner L, Baxter C, Arnold KB, et al. Genital inflammation and the risk of HIV acquisition in women. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;61(2):260–9. doi: 10.1093/cid/civ298. A study assessing cytokines in CVL of women enrolled in the CAPRISA 004 microbicide trial found that IL-8 was among inflammatory cytokines increased in women who subsequently acquired HIV infection compared to women who remained uninfected. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore RD, Keruly JC, Chaisson RE. Neutropenia and bacterial infection in acquired immunodeficiency syndrome. Archives of internal medicine. 1995;155(18):1965–70. [PubMed] [Google Scholar]
- 23.Keiser P, Higgs E, Smith J. Neutropenia is associated with bacteremia in patients infected with the human immunodeficiency virus. The American journal of the medical sciences. 1996;312(3):118–22. doi: 10.1097/00000441-199609000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Roilides E, Holmes A, Blake C, Pizzo PA, Walsh TJ. Impairment of neutrophil antifungal activity against hyphae of Aspergillus fumigatus in children infected with human immunodeficiency virus. The Journal of infectious diseases. 1993;167(4):905–11. doi: 10.1093/infdis/167.4.905. [DOI] [PubMed] [Google Scholar]
- 25.Somsouk M, Estes JD, Deleage C, Dunham RM, Albright R, Inadomi JM, et al. Gut epithelial barrier and systemic inflammation during chronic HIV infection. Aids. 2015;29(1):43–51. doi: 10.1097/QAD.0000000000000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Estes JD, Harris LD, Klatt NR, Tabb B, Pittaluga S, Paiardini M, et al. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS pathogens. 2010;6(8):e1001052. doi: 10.1371/journal.ppat.1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cloke T, Munder M, Taylor G, Muller I, Kropf P. Characterization of a novel population of low-density granulocytes associated with disease severity in HIV-1 infection. PloS one. 2012;7(11):e48939. doi: 10.1371/journal.pone.0048939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowers NL, Helton ES, Huijbregts RP, Goepfert PA, Heath SL, Hel Z. Immune suppression by neutrophils in HIV-1 infection: role of PD-L1/PD-1 pathway. PLoS pathogens. 2014;10(3):e1003993. doi: 10.1371/journal.ppat.1003993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65(5):859–73. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi SD, Voyich JM, Burlak C, DeLeo FR. Neutrophils in the innate immune response. Archivum immunologiae et therapiae experimentalis. 2005;53(6):505–17. [PubMed] [Google Scholar]
- 31.Ronsholt FF, Ullum H, Katzenstein TL, Gerstoft J, Ostrowski SR. Persistent inflammation and endothelial activation in HIV-1 infected patients after 12 years of antiretroviral therapy. PloS one. 2013;8(6):e65182. doi: 10.1371/journal.pone.0065182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Gaetano Donati K, Rabagliati R, Iacoviello L, Cauda R. HIV infection, HAART, and endothelial adhesion molecules: current perspectives. The Lancet Infectious diseases. 2004;4(4):213–22. doi: 10.1016/S1473-3099(04)00971-5. [DOI] [PubMed] [Google Scholar]
- 33.Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89(10):3503–21. [PubMed] [Google Scholar]
- 34.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–75. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 35.Hager M, Cowland JB, Borregaard N. Neutrophil granules in health and disease. Journal of internal medicine. 2010;268(1):25–34. doi: 10.1111/j.1365-2796.2010.02237.x. [DOI] [PubMed] [Google Scholar]
- 36.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 37.Kang D, Liu G, Lundstrom A, Gelius E, Steiner H. A peptidoglycan recognition protein in innate immunity conserved from insects to humans. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(17):10078–82. doi: 10.1073/pnas.95.17.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu J, Teh C, Kishore U, Reid KB. Collectins and ficolins: sugar pattern recognition molecules of the mammalian innate immune system. Biochimica et biophysica acta. 2002;1572(2–3):387–400. doi: 10.1016/s0304-4165(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 39.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102(7):2660–9. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 40.Kumar V, Sharma A. Neutrophils: Cinderella of innate immune system. International immunopharmacology. 2010;10(11):1325–34. doi: 10.1016/j.intimp.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Korkmaz B, Horwitz MS, Jenne DE, Gauthier F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacological reviews. 2010;62(4):726–59. doi: 10.1124/pr.110.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nature reviews Immunology. 2010;10(12):826–37. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barnado A, Crofford LJ, Oates JC. At the Bedside: Neutrophil extracellular traps (NETs) as targets for biomarkers and therapies in autoimmune diseases. Journal of leukocyte biology. 2016;99(2):265–78. doi: 10.1189/jlb.5BT0615-234R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi X, Sims MD, Hanna MM, Xie M, Gulick PG, Zheng YH, et al. Neutropenia during HIV infection: adverse consequences and remedies. International reviews of immunology. 2014;33(6):511–36. doi: 10.3109/08830185.2014.893301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levine AM, Karim R, Mack W, Gravink DJ, Anastos K, Young M, et al. Neutropenia in human immunodeficiency virus infection: data from the women's interagency HIV study. Archives of internal medicine. 2006;166(4):405–10. doi: 10.1001/archinte.166.4.405. [DOI] [PubMed] [Google Scholar]
- 46.Carter CC, Onafuwa-Nuga A, McNamara LA, Riddell Jt, Bixby D, Savona MR, et al. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nature medicine. 2010;16(4):446–51. doi: 10.1038/nm.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Banda NK, Tomczak JA, Shpall EJ, Sipple J, Akkina RK, Steimer KS, et al. HIV-gp120 induced cell death in hematopoietic progenitor CD34+ cells. Apoptosis : an international journal on programmed cell death. 1997;2(1):61–8. doi: 10.1023/a:1026439726053. [DOI] [PubMed] [Google Scholar]
- 48.Calenda V, Graber P, Delamarter JF, Chermann JC. Involvement of HIV nef protein in abnormal hematopoiesis in AIDS: in vitro study on bone marrow progenitor cells. European journal of haematology. 1994;52(2):103–7. doi: 10.1111/j.1600-0609.1994.tb01294.x. [DOI] [PubMed] [Google Scholar]
- 49.Rameshwar P, Denny TN, Gascon P. Enhanced HIV-1 activity in bone marrow can lead to myelopoietic suppression partially contributed by gag p24. Journal of immunology. 1996;157(9):4244–50. [PubMed] [Google Scholar]
- 50.Bahner I, Kearns K, Coutinho S, Leonard EH, Kohn DB. Infection of human marrow stroma by human immunodeficiency virus-1 (HIV-1) is both required and sufficient for HIV-1-induced hematopoietic suppression in vitro: demonstration by gene modification of primary human stroma. Blood. 1997;90(5):1787–98. [PubMed] [Google Scholar]
- 51.Moses AV, Williams S, Heneveld ML, Strussenberg J, Rarick M, Loveless M, et al. Human immunodeficiency virus infection of bone marrow endothelium reduces induction of stromal hematopoietic growth factors. Blood. 1996;87(3):919–25. [PubMed] [Google Scholar]
- 52.Re MC, Zauli G, Furlini G, Giovannini M, Ranieri S, Ramazzotti E, et al. GM-CSF production by CD4+ T-lymphocytes is selectively impaired during the course of HIV-1 infection. A possible indication of a preferential lesion of a specific subset of peripheral blood CD4+ T-lymphocytes. Microbiologica. 1992;15(3):265–70. [PubMed] [Google Scholar]
- 53.Bagnara GP, Zauli G, Re MC, Furlini G, Giovannini M, Ranieri S, et al. Impaired GM-CSF production by cultured light density mononuclear cells and T lymphocytes correlates with the number of circulating CFU-gm in HIV-1 seropositive subjects. International journal of cell cloning. 1991;9(3):239–50. doi: 10.1002/stem.5530090308. [DOI] [PubMed] [Google Scholar]
- 54.Rubinstein DB, Farrington GK, O'Donnell C, Hartman KR, Wright DG. Autoantibodies to leukocyte alphaMbeta2 integrin glycoproteins in HIV infection. Clinical immunology. 1999;90(3):352–9. doi: 10.1006/clim.1998.4668. [DOI] [PubMed] [Google Scholar]
- 55.Ribera E, Ocana I, Almirante B, Gomez J, Monreal P, Martinez Vazquez JM. Autoimmune neutropenia and thrombocytopenia associated with development of antibodies to human immunodeficiency virus. The Journal of infection. 1989;18(2):167–70. doi: 10.1016/s0163-4453(89)91206-1. [DOI] [PubMed] [Google Scholar]
- 56.Pitrak DL, Tsai HC, Mullane KM, Sutton SH, Stevens P. Accelerated neutrophil apoptosis in the acquired immunodeficiency syndrome. The Journal of clinical investigation. 1996;98(12):2714–9. doi: 10.1172/JCI119096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elbim C, Monceaux V, Francois S, Hurtrel B, Gougerot-Pocidalo MA, Estaquier J. Increased neutrophil apoptosis in chronically SIV-infected macaques. Retrovirology. 2009;6:29. doi: 10.1186/1742-4690-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Campillo-Gimenez L, Casulli S, Dudoit Y, Seang S, Carcelain G, Lambert-Niclot S, et al. Neutrophils in antiretroviral therapy-controlled HIV demonstrate hyperactivation associated with a specific IL-17/IL-22 environment. The Journal of allergy and clinical immunology. 2014;134(5):1142–52. e5. doi: 10.1016/j.jaci.2014.05.040. [DOI] [PubMed] [Google Scholar]
- 59.Roilides E, Mertins S, Eddy J, Walsh TJ, Pizzo PA, Rubin M. Impairment of neutrophil chemotactic and bactericidal function in children infected with human immunodeficiency virus type 1 and partial reversal after in vitro exposure to granulocyte-macrophage colony-stimulating factor. The Journal of pediatrics. 1990;117(4):531–40. doi: 10.1016/s0022-3476(05)80684-5. [DOI] [PubMed] [Google Scholar]
- 60.Ellis M, Gupta S, Galant S, Hakim S, VandeVen C, Toy C, et al. Impaired neutrophil function in patients with AIDS or AIDS-related complex: a comprehensive evaluation. The Journal of infectious diseases. 1988;158(6):1268–76. doi: 10.1093/infdis/158.6.1268. [DOI] [PubMed] [Google Scholar]
- 61.Dobmeyer TS, Raffel B, Dobmeyer JM, Findhammer S, Klein SA, Kabelitz D, et al. Decreased function of monocytes and granulocytes during HIV-1 infection correlates with CD4 cell counts. European journal of medical research. 1995;1(1):9–15. [PubMed] [Google Scholar]
- 62.Kuritzkes DR, Parenti D, Ward DJ, Rachlis A, Wong RJ, Mallon KP, et al. Filgrastim prevents severe neutropenia and reduces infective morbidity in patients with advanced HIV infection: results of a randomized, multicenter, controlled trial. G-CSF 930101 Study Group. Aids. 1998;12(1):65–74. doi: 10.1097/00002030-199801000-00008. [DOI] [PubMed] [Google Scholar]
- 63.Roilides E, Walsh TJ, Pizzo PA, Rubin M. Granulocyte colony-stimulating factor enhances the phagocytic and bactericidal activity of normal and defective human neutrophils. The Journal of infectious diseases. 1991;163(3):579–83. doi: 10.1093/infdis/163.3.579. [DOI] [PubMed] [Google Scholar]
- 64.Coffey MJ, Phare SM, George S, Peters-Golden M, Kazanjian PH. Granulocyte colony-stimulating factor administration to HIV-infected subjects augments reduced leukotriene synthesis and anticryptococcal activity in neutrophils. The Journal of clinical investigation. 1998;102(4):663–70. doi: 10.1172/JCI2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mastroianni CM, Lichtner M, Mengoni F, D'Agostino C, Forcina G, d'Ettorre G, et al. Improvement in neutrophil and monocyte function during highly active antiretroviral treatment of HIV-1-infected patients. Aids. 1999;13(8):883–90. doi: 10.1097/00002030-199905280-00003. [DOI] [PubMed] [Google Scholar]
- 66.Mastroianni CM, d'Ettorre G, Forcina G, Lichtner M, Mengoni F, D'Agostino C, et al. Interleukin-15 enhances neutrophil functional activity in patients with human immunodeficiency virus infection. Blood. 2000;96(5):1979–84. [PubMed] [Google Scholar]
- 67.Roilides E, Venzon D, Pizzo PA, Rubin M. Effects of antiretroviral dideoxynucleosides on polymorphonuclear leukocyte function. Antimicrobial agents and chemotherapy. 1990;34(9):1672–7. doi: 10.1128/aac.34.9.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hadad N, Levy R, Schlaeffer F, Riesenberg K. Direct effect of human immunodeficiency virus protease inhibitors on neutrophil function and apoptosis via calpain inhibition. Clinical and vaccine immunology : CVI. 2007;14(11):1515–21. doi: 10.1128/CVI.00130-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382(9903):1525–33. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burgener A, McGowan I, Klatt NR. HIV and mucosal barrier interactions: consequences for transmission and pathogenesis. Current opinion in immunology. 2015;36:22–30. doi: 10.1016/j.coi.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 71.Batman PA, Miller AR, Forster SM, Harris JR, Pinching AJ, Griffin GE. Jejunal enteropathy associated with human immunodeficiency virus infection: quantitative histology. Journal of clinical pathology. 1989;42(3):275–81. doi: 10.1136/jcp.42.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brenchley JM, Douek DC. HIV infection and the gastrointestinal immune system. Mucosal immunology. 2008;1(1):23–30. doi: 10.1038/mi.2007.1. doi:mi20071 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nature reviews Microbiology. 2012;10(9):655–66. doi: 10.1038/nrmicro2848. [DOI] [PubMed] [Google Scholar]
- 74.Sankaran S, George MD, Reay E, Guadalupe M, Flamm J, Prindiville T, et al. Rapid onset of intestinal epithelial barrier dysfunction in primary human immunodeficiency virus infection is driven by an imbalance between immune response and mucosal repair and regeneration. Journal of virology. 2008;82(1):538–45. doi: 10.1128/JVI.01449-07. doi:JVI.01449-07 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS pathogens. 2010;6(4):e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buccigrossi V, Laudiero G, Nicastro E, Miele E, Esposito F, Guarino A. The HIV-1 transactivator factor (Tat) induces enterocyte apoptosis through a redox-mediated mechanism. PloS one. 2011;6(12):e29436. doi: 10.1371/journal.pone.0029436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Canani RB, Cirillo P, Mallardo G, Buccigrossi V, Secondo A, Annunziato L, et al. Effects of HIV-1 Tat protein on ion secretion and on cell proliferation in human intestinal epithelial cells. Gastroenterology. 2003;124(2):368–76. doi: 10.1053/gast.2003.50056. [DOI] [PubMed] [Google Scholar]
- 78.Schmitz H, Rokos K, Florian P, Gitter AH, Fromm M, Scholz P, et al. Supernatants of HIV-infected immune cells affect the barrier function of human HT-29/B6 intestinal epithelial cells. Aids. 2002;16(7):983–91. doi: 10.1097/00002030-200205030-00004. [DOI] [PubMed] [Google Scholar]
- 79.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280(5362):427–31. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 80.Klatt NR, Estes JD, Sun X, Ortiz AM, Barber JS, Harris LD, et al. Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection. Mucosal immunology. 2012;5(6):646–57. doi: 10.1038/mi.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends in microbiology. 2013;21(1):6–13. doi: 10.1016/j.tim.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Klatt NR, Chomont N, Douek DC, Deeks SG. Immune activation and HIV persistence: implications for curative approaches to HIV infection. Immunol Rev. 2013;254(1):326–42. doi: 10.1111/imr.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature medicine. 2006;12(12):1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 84.Lichtfuss GF, Hoy J, Rajasuriar R, Kramski M, Crowe SM, Lewin SR. Biomarkers of immune dysfunction following combination antiretroviral therapy for HIV infection. Biomark Med. 2011;5(2):171–86. doi: 10.2217/bmm.11.15. [DOI] [PubMed] [Google Scholar]
- 85.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. The Journal of infectious diseases. 2011;203(6):780–90. doi: 10.1093/infdis/jiq118. doi:jiq118 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rodger AJ, Fox Z, Lundgren JD, Kuller LH, Boesecke C, Gey D, et al. Activation and coagulation biomarkers are independent predictors of the development of opportunistic disease in patients with HIV infection. The Journal of infectious diseases. 2009;200(6):973–83. doi: 10.1086/605447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. doi:08-PLME-RA-0628 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Natsui M, Kawasaki K, Takizawa H, Hayashi SI, Matsuda Y, Sugimura K, et al. Selective depletion of neutrophils by a monoclonal antibody, RP-3, suppresses dextran sulphate sodium-induced colitis in rats. Journal of gastroenterology and hepatology. 1997;12(12):801–8. doi: 10.1111/j.1440-1746.1997.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 89.Bressenot A, Salleron J, Bastien C, Danese S, Boulagnon-Rombi C, Peyrin-Biroulet L. Comparing histological activity indexes in UC. Gut. 2015;64(9):1412–8. doi: 10.1136/gutjnl-2014-307477. [DOI] [PubMed] [Google Scholar]
- 90.Kuhl AA, Kakirman H, Janotta M, Dreher S, Cremer P, Pawlowski NN, et al. Aggravation of different types of experimental colitis by depletion or adhesion blockade of neutrophils. Gastroenterology. 2007;133(6):1882–92. doi: 10.1053/j.gastro.2007.08.073. [DOI] [PubMed] [Google Scholar]
- 91.Nemoto Y, Kanai T, Tohda S, Totsuka T, Okamoto R, Tsuchiya K, et al. Negative feedback regulation of colitogenic CD4+ T cells by increased granulopoiesis. Inflammatory bowel diseases. 2008;14(11):1491–503. doi: 10.1002/ibd.20531. [DOI] [PubMed] [Google Scholar]
- 92.Zhang R, Ito S, Nishio N, Cheng Z, Suzuki H, Isobe K. Up-regulation of Gr1+CD11b+ population in spleen of dextran sulfate sodium administered mice works to repair colitis. Inflammation & allergy drug targets. 2011;10(1):39–46. doi: 10.2174/187152811794352114. [DOI] [PubMed] [Google Scholar]
- 93.Nash S, Stafford J, Madara JL. Effects of polymorphonuclear leukocyte transmigration on the barrier function of cultured intestinal epithelial monolayers. The Journal of clinical investigation. 1987;80(4):1104–13. doi: 10.1172/JCI113167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nusrat A, Parkos CA, Liang TW, Carnes DK, Madara JL. Neutrophil migration across model intestinal epithelia: monolayer disruption and subsequent events in epithelial repair. Gastroenterology. 1997;113(5):1489–500. doi: 10.1053/gast.1997.v113.pm9352851. [DOI] [PubMed] [Google Scholar]
- 95.Kucharzik T, Walsh SV, Chen J, Parkos CA, Nusrat A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. The American journal of pathology. 2001;159(6):2001–9. doi: 10.1016/S0002-9440(10)63051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pillay J, Tak T, Kamp VM, Koenderman L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cellular and molecular life sciences : CMLS. 2013;70(20):3813–27. doi: 10.1007/s00018-013-1286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Prodger JL, Hirbod T, Kigozi G, Nalugoda F, Reynolds SJ, Galiwango R, et al. Immune correlates of HIV exposure without infection in foreskins of men from Rakai, Uganda. Mucosal immunology. 2014;7(3):634–44. doi: 10.1038/mi.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sips M, Krykbaeva M, Diefenbach TJ, Ghebremichael M, Bowman BA, Dugast AS, et al. Fc receptor-mediated phagocytosis in tissues as a potent mechanism for preventive and therapeutic HIV vaccine strategies. Mucosal immunology. 2016;9(6):1584–95. doi: 10.1038/mi.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gursoy UK, Kononen E, Luukkonen N, Uitto VJ. Human neutrophil defensins and their effect on epithelial cells. Journal of periodontology. 2013;84(1):126–33. doi: 10.1902/jop.2012.120017. [DOI] [PubMed] [Google Scholar]
- 100.Arnold KB, Burgener A, Birse K, Romas L, Dunphy LJ, Shahabi K, et al. Increased levels of inflammatory cytokines in the female reproductive tract are associated with altered expression of proteases, mucosal barrier proteins, and an influx of HIV-susceptible target cells. Mucosal immunology. 2016;9(1):194–205. doi: 10.1038/mi.2015.51. [DOI] [PubMed] [Google Scholar]
- 101.Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2010;115(2):335–43. doi: 10.1182/blood-2009-04-216085. [DOI] [PubMed] [Google Scholar]
- 102.Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, et al. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS pathogens. 2009;5(12):e1000703. doi: 10.1371/journal.ppat.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal immunology. 2009;2(5):403–11. doi: 10.1038/mi.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rodriguez-Garcia M, Barr FD, Crist SG, Fahey JV, Wira CR. Phenotype and susceptibility to HIV infection of CD4+ Th17 cells in the human female reproductive tract. Mucosal immunology. 2014;7(6):1375–85. doi: 10.1038/mi.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105*.Stieh DJ, Matias E, Xu H, Fought AJ, Blanchard JL, Marx PA, et al. Th17 Cells Are Preferentially Infected Very Early after Vaginal Transmission of SIV in Macaques. Cell host & microbe. 2016;19(4):529–40. doi: 10.1016/j.chom.2016.03.005. A study assessing the phenotype of cells found in distinct foci of infection early after vaginal inoculation of rhesus macaques with SIV found that CCR6+ TH17 cells are primary targets during vaginal transmission. [DOI] [PMC free article] [PubMed] [Google Scholar]