Abstract
Glioblastoma multiforme (GBM) is the most prevalent and aggressive brain tumor. The current standard therapy, which includes radiation and chemotherapy, is frequently ineffective partially because of drug resistance and poor penetration of the blood-brain barrier. Reducing resistance and increasing sensitivity to chemotherapy may improve outcomes. Glioma stem cells (GSCs) are a source of relapse and chemoresistance in GBM; sensitization of GSCs to temozoliomide (TMZ), the primary chemotherapeutic agent used to treat GBM, is therefore integral for therapeutic efficacy. We previously discovered a unique tumor-specific target, cell surface vimentin (CSV), on patient-derived GSCs. In this study, we found that the anti-CSV monoclonal antibody 86C efficiently increased GSC sensitivity to TMZ. The combination TMZ+86C induced significantly greater antitumor effects than TMZ alone in eight of 12 GSC lines. TMZ+86C–sensitive GSCs had higher CSV expression overall and faster CSV resurfacing among CSV− GSCs compared with TMZ+86C–resistant GSCs. Finally, TMZ+86C increased apoptosis of tumor cells and prolonged survival compared with either drug alone in GBM mouse models. The combination of TMZ+86C represents a promising strategy to reverse GSC chemoresistance.
Keywords: Cell surface vimentin, monoclonal antibody 86C, glioma stem cells, temozolomide (TMZ), cell death
1. Introduction
Glioblastoma multiforme (GBM) is the most prevalent and aggressive malignant brain tumor and has a median survival duration of approximately 15 months from diagnosis1. The current standard of care for GBM patients is surgical resection followed by radiotherapy and chemotherapy. This therapy is not effective in most patients because of factors including drug resistance and poor penetration of the blood-brain barrier. The primary chemotherapeutic agent used to treat GBM is temozolomide (TMZ), a methylating agent that does cross the blood-brain barrier2, 3 but whose efficacy is constrained by frequent development of resistance4–6. Median survival duration is increased only 2.5 months by adjuvant combined radiation and TMZ treatment. The efficacy of TMZ is further constrained by toxic effects outside of the central nervous system and by the biologic limits to achieving a sustained tumoricidal concentration in the tissue, as with most other systemic therapies7. Intensification of TMZ, in the form of dose-dense adjuvant TMZ, was associated with significantly greater high-grade toxicity without any survival benefit compared with the standard regimen for GBM8. There is a great need for more efficacious therapeutic strategies to improve clinical management and survival outcomes in GBM patients.
Glioma stem cells (GSCs) exhibit resistance to radiation and to anticancer drugs such as TMZ9–12, and elimination of GSCs is considered key to ensuring the long-term survival of GBM patients13. Since GSCs are also responsible for tumor initiation and recurrence, they are attractive candidate targets for anticancer therapy. Identifying new drugs that can specifically target and kill GSCs is a critical step in improving GBM patient outcomes.
A potential approach to targeting GSCs is through cell surface vimentin (CSV). Unlike intracellular vimentin, which is found in both cancer cells and normal mesenchymal cells, CSV is tumor-specific. It has been found primarily on cancer cells, including circulating tumor cells, GBM cells, and GSCs14–16, and can serve as a therapeutic target for such cells. Our previous study showed that the novel monoclonal antibody 86C, which binds CSV on cancer stem cells and is internalized, induces apoptosis of the target cells, suggesting that targeting GSCs using 86C is a promising approach for the treatment of GBM16. On the basis of these findings, we hypothesized that targeting CSV with 86C would help overcome TMZ resistance in GSCs, increasing cell killing. Our findings show that 86C efficiently targets GSCs expressing CSV and that this intervention increases GSC sensitivity to TMZ. The addition of 86C reduced the dose of TMZ required to eliminate GSCs, which will ultimately reduce its toxic effects. Treatment with 86C combined with TMZ decreased resistance to chemotherapy and resulted in a striking recovery of GSC sensitivity to TMZ.
2. Materials and Methods
2.1 Ethics statement
The mice used in this study were maintained under the guidelines of the National Institutes of Health and euthanized according to procedures approved by the Institutional Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center (MD Anderson). Tumor sample collection from patients with GBM at MD Anderson was conducted under protocol #LAB03-0687, which was approved by the institutional review board, after informed consent was obtained from the patients.
2.2 Cell lines and cell culture
Twelve GSC cell lines (GSC6-27, GSC7-2, GSC8-11, GSC11, GSC17, GSC20, GSC23, GSC28, GSC272, GSC280, GSC295, and GSC300) were provided by Dr. Frederick Lang (MD Anderson) and were cultured in serum-free Dulbecco’s modified Eagle medium supplemented with epidermal growth factor (20 ng/mL), basic fibroblast growth factor (20 ng/mL), and 2% B27 (Life Technologies, Carlsbad, CA). GL261 murine glioma cells were cultured in 10% fetal bovine serum and Dulbecco’s modified Eagle medium. Cells were dissociated using Accutane (Invitrogen, Carlsbad, CA) and then used for experiments. No further authentication of cell lines was conducted in our laboratory.
2.3 Drugs
The stock solution of TMZ (50 mM; Sigma-Aldrich, St. Louis, MO) was prepared by dissolving the drug in dimethyl sulfoxide (DMSO). The final concentration of DMSO was kept to less than 0.5% (v/v), and it did not contribute to toxicity.
2.4 TMZ treatment and analysis of cell viability in vitro
GSCs were plated at a density of 5×103/100 mL of culture medium and treated with TMZ alone or in combination with 86C (TMZ+86C) at various concentrations in triplicate for 3 days. Cell viability was determined by normalizing the absolute values of absorbance for cells that received each treatment with that of controls and is expressed as a percentage.
2.5 Isolation of CSV− and CSV+ cells by flow cytometry
Single-cell suspensions were blocked for 10 minutes at room temperature with FcR blocker (Miltenyi Biotic, Bergisch Gladbach, Germany) in a 1:1000 dilution and then incubated with 2 μg of the CSV-binding antibody 84-1, produced in our laboratory, for 15 minutes at room temperature. After two washings, the cells were incubated with 2 μg of goat anti-mouse Alexa Fluor 405–conjugated secondary antibody in phosphate-buffered saline solution (PBS) plus 2% serum for 15 minutes in the dark at room temperature. CSV− and CSV+ cells were isolated using a FACSAria Fusion cell sorter (BD Biosciences, San Jose, CA). CSV− cells were cultured for 30 minutes, 4 hours, 1 day, or 2 days, and CSV expression was analyzed.
2.6 Detection of CSV expression by flow cytometry
Single-cell suspensions were blocked for 10 minutes at room temperature with FcR blocker in a 1:1000 dilution and then incubated with 0.4 μg of 84-1 for 15 minutes at room temperature. After two washings, cells were incubated with 0.5 μg of goat anti-mouse Alexa Fluor 405–conjugated secondary antibody in PBS plus 2% serum for 15 minutes in the dark at room temperature. Cells were analyzed on an Attune flow cytometer (Life Technologies), and the results were evaluated using FlowJo 10.0 software (Tree Star, Inc., Ashland, OR).
2.7 Western blotting and immunoprecipitation
Vimentin-null T47D cells, LN18-shCtrl cells, and LN18-shVim cells were lysed in RIPA buffer on ice for 30 minutes. Lysates were centrifuged at 15,000 × g for 5 min at 4°C. The supernatant was collected for subsequent procedures. Immunoblotting was performed as previously described17. Briefly, 86C (1:1000), β-actin antibody (1:1000, Biolegend) and secondary antibodies were diluted in 5% nonfat milk/TBST, incubated overnight at 4°C, and blots were developed with Enhanced Chemiluminescence Plus (GE Biosciences). Immunoprecipitation was performed using 2 mg of total protein lysate and 1 μg (30 μl) of agarose beads were added to the mixture of protein lysate and 86C and incubated at 4°C overnight. The lysate mixture was then centrifuged at 3000 rpm for 30 seconds at 4°C. After discarding the supernatant, the pellet was washed 3 times using 1 ml RIPA. Then, 2X SDS loading buffer was added to the beads for western blotting.
2.8 Cell surface vimentin attachment
To detect whether 86C can bind to cell surface vimentin, vimentin-null T47D cells were incubated with 500 ng/mL rhVim (recombinant human vimentin, R&D Systems CF2105-VI-100 with C-terminal 6-His tag) or with serum albumin (AbCAM 94020 with 6-His tag) as developed and described in our previous publication17. For CSV analysis, cells were stained with the 86C monoclonal antibody (1:100) followed by PE-labeled secondary antibody (Invitrogen).
2.9 Tumor models
Logarithmically growing GSC11 cells or GL261 cells that were CVS+ (GL261-CVS+ cells) were collected and washed with PBS. GSC11 cells or GL261-CVS+ cells were pre-incubated with 20 μg of immunoglobulin G (IgG) or 86C, mixed with an equal volume of 3% methylcellulose in PBS. GSC11 cells (5×105 and 20 μg IgG or 86C) or GL261-CVS+ cells (1×104 and 2 μg IgG or 86C) in a total volume of 5 μL were injected intracerebrally into 6-week-old non-obese diabetic/severe combined immunodeficiency gamma (NSG) mice (#005557; The Jackson Laboratory, Bar Harbor, ME) or C57BL/6J mice (#000664, The Jackson Laboratory). Starting on day 3, TMZ (20 mg/kg) was injected intraperitoneally into each mouse each day for 5 days. The mice were observed three times per week, and their survival was monitored.
2.10 Histologic analysis
Twenty days after inoculation of GL261-CVS+ cells, brain tissues were collected, fixed in 10% buffered formalin, and cut at 5 μm. Hematoxylin and eosin staining was performed on formalin-fixed, paraffin-embedded brain tissue sections.
2.11 TUNEL assay for apoptotic cells
For detecting fragmented DNA in situ, formalin-fixed, paraffin-embedded brain tissue sections were subjected to TUNEL (terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling) assay according to the manufacturer’s protocols (Life Technologies, Eugene, USA). Nuclei were counterstained with DAPI.
2.12 Statistical analysis
Results are expressed as mean ± standard deviation. Data were analyzed with GraphPad software (GraphPad Software, Inc., La Jolla, CA) using an unpaired two-tailed Student t-test to determine the significance of differences between groups (except for survival, which was compared between groups using the log-rank test). p<0.05 was considered statistically significant.
3. Results
3.1 GSC sensitivity to TMZ varies widely
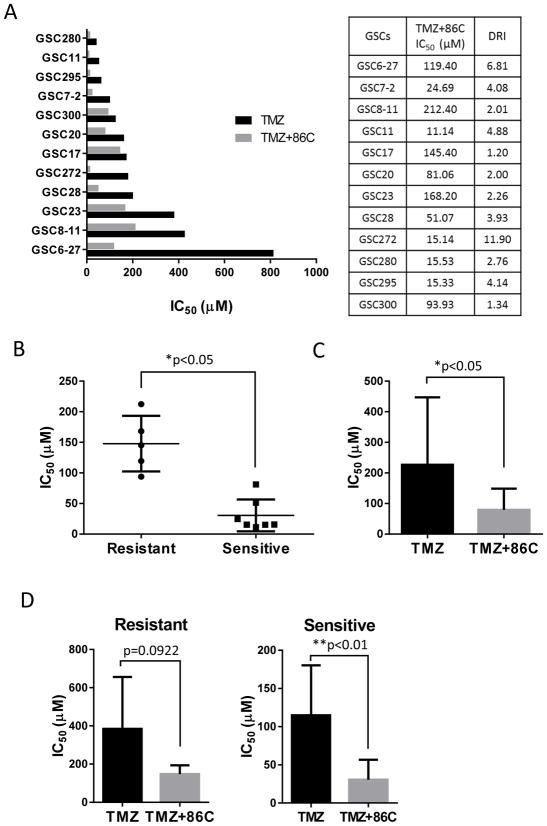
To evaluate GSC resistance to TMZ, we first analyzed the individual median inhibitory concentrations (IC50 values) of TMZ for the 12 GSC cell lines. The 12 GSC cell types were treated with TMZ (0–500 μM) for 72 hours, and the numbers of viable cells were assessed. The cell growth–inhibitory effects of TMZ on all the GSC lines were dose dependent. Most of the GSC lines showed low sensitivity to TMZ, with IC50 values of >100 μM, well above the peak plasma concentration of TMZ reached in cancer patients (20–96 μM at a TMZ dose of 200 mg/m2)18. The GSC280, GSC11, GSC295, and GSC7-2 cells were relatively susceptible to TMZ, with IC50 values between 40 and 100 μM, whereas the GSC300, GSC20, GSC17, GSC272, and GSC28 cells were relatively resistant to TMZ (120–200 μM). The GSC23, GSC8-11, and GSC6-27 cells showed the most resistance to TMZ treatment, with IC50 values of >300 μM (Figure 1A and 1B). In a previous report, GBM cell lines showed a similar range of TMZ IC50 values: A-172, AM-38, U-87MG, and U-251MG cells had IC50 values of <100 μM, while T98G, U-138MG, and YH-13 cells were resistant to TMZ (had IC50 values of >350 μM19. Interestingly, comparison of GSC lines with similar IC50 values revealed significant differences among each the groups (Figure 1B).
Figure 1.
Median inhibitory concentrations (IC50) of temozolomide (TMZ) for 12 GSC cell lines. (A) Effects of TMZ on cell viability were determined. At 72 hours after addition of TMZ (final concentration, 0–500 μM) to the culture medium, the viable cells were counted and the numbers expressed as percentages of the untreated control cells. (B) IC50 represents the TMZ concentration required for a 50% decrease in cell growth compared with the control.
3.2 86C potentiates GSC sensitivity to TMZ
Because the CSV-targeting monoclonal antibody 86C has been shown to induce GSC cell death16, we tested the effects of TMZ+86C on the 12 GSC cell lines. To determine whether 86C can reverse GSC resistance to TMZ, we treated the cells with graded concentrations of TMZ in combination with a fixed dose of 86C; in all 12 cell lines, TMZ+86C showed a greater tumor cell killing effect than TMZ alone did. The combination yielded a consistent, dose-dependent additive effect on the GSCs, reducing cell viability to a significantly greater degree than TMZ alone did (p<0.05; Figure 2). These results validated our hypothesis that 86C worked effectively with TMZ to enhance its cell-killing effects in both sensitive and resistant GSCs. As shown in Figure 2, TMZ+86C inhibited GSC viability at almost all TMZ doses tested (86C dose was fixed), but the extent of the additive effect varied by cell line. 86C had a noticeably greater impact on cell viability in seven of the GSC cell lines (GSC7-2, GSC11, GSC20, GSC28, GSC272, GSC280, and GSC295, sensitive to TMZ) than in the other five GSC cell lines (GSC6-27, GSC8-11, GSC17, GSC23, and GSC300, resistant to TMZ; Figure 2); these two groups of cell lines were hereafter considered TMZ+86C sensitive and TMZ+86C resistant, respectively.
Figure 2.
Reduction of TMZ resistance of GSCs by 86C. Twelve GSC lines were exposed to graded concentrations of TMZ as a single agent (blue) or in combination with a fixed dose of 86C (20 μg/mL) (red) for 72 hours. The graphs represent the means (±standard deviations) from three independent experiments.
Analysis of the dose reduction index (DRI) indicated that the addition of 86C to TMZ decreased TMZ IC50 significantly in all 12 GSC cell lines—to as little as 1/12 of the level without 86C. TMZ+86C–sensitive GSCs showed a significantly lower TMZ IC50 than TMZ+86C–resistant GSCs did (Figure 3B), although the TMZ IC50 was significantly reduced by the addition of 86C in all 12 GSC cell lines (Figure 3C). Interestingly, the difference in TMZ IC50 between TMZ+86C and TMZ alone was significant (p<0.01) in the TMZ+86C–sensitive GSCs but not significant in the TMZ+86C–resistant GSCs (p=0.0922; Figure 3D). In other words, the potentiating effect of 86C on TMZ efficacy was more pronounced in the TMZ+86C–sensitive GSCs.
Figure 3.
Modulation of GSC sensitivity to TMZ by 86C. (A) Bars represent the median inhibitory concentration (IC50) of TMZ, alone (black column) or in combination with 86C (gray column), for 12 GSC cell lines. The dose reduction index (DRI) value refers to the fold-decrease of TMZ IC50 attainable when TMZ was combined with 86C. (B) The IC50 of TMZ+86C–sensitive GSCs was compared with that of TMZ+86C–resistant GSCs. *p<0.05, resistant GSCs (R) versus sensitive GSCs (S) by the Student t-test. (C) The IC50 of TMZ alone was compared to that of TMZ+86C in all 12 GSC cell lines. *p<0.05 for TMZ+86C versus TMZ alone by the Student t-test. (D) The IC50 of TMZ alone was compared with that of TMZ+86C in TMZ+86C–resistant GSCs and TMZ+86C–sensitive GSCs. *p<0.05 for TMZ+86C versus TMZ alone by Student t-test.
3.3 Higher CSV expression and quicker CSV resurfacing in CSV− GSCs is associated with sensitivity to TMZ+86C
To investigate the biologic mechanism underlying the difference in the degree of boosting of TMZ activity by 86C in various GSCs, CSV expression was compared between the TMZ+86C–sensitive and TMZ+86C–resistant groups. The dose reduction index of TMZ+86C was significantly associated with the CSV expression in each GSC cell line. The GSCs relatively resistant to TMZ+86C had lower CSV expression than those that were relatively sensitive. These data suggest that CSV expression is a limiting factor for the antitumor efficacy of TMZ+86C in GSCs. Notably, although about 2%–40% of GSCs expressed CSV, almost 80% of GSCs were eliminated by TMZ+86C (Figures 2 and 4A).
Figure 4.
Higher levels of CSV expression and quicker CSV resurfacing in sorted CSV− TMZ+86C–sensitive GSCs than in sorted CSV− TMZ+86C–resistant GSCs. (A) CSV expression on GSCs was determined. Cells were sorted and CSV expression determined by flow cytometry. CSV expression on TMZ+86C–sensitive GSCs was compared with CSV expression on TMZ+86C–resistant GSCs. *p<0.05, resistant GSCs (R) versus sensitive GSCs (S) by Student t-test. (B) CSV− GSC11 and GSC17 cells were isolated and cultured, and CSV expression was determined at 30 minutes, 4 hours, 1 day, 2 days, and 3 days by flow cytometry. (C) CSV expression (i.e., resurfacing) was analyzed in all 12 GSC lines. CSV resurfacing rates in TMZ+86C–sensitive GSCs and TMZ+86C–resistant GSCs were determined 4 hours and 1 day after sorting and compared at each time point. Resistant GSCs (R) versus sensitive GSCs by Student t-test.
To better understand the mechanism whereby GSCs not expressing CSV (CSV−) were affected by 86C, we hypothesized that CSV resurfaces or is re-expressed by CSV− GSCs. To test this hypothesis, CSV− GSCs were isolated by flow cytometry and analyzed for CSV expression (i.e., resurfacing) at several time points (Figure 4B and Supplemental Figure 1). CSV− GSCs from all 12 GSC lines demonstrated resurfaced CSV expression at day 2 after sorting, showing levels of CSV expression similar to those of untreated parental GSCs. However, the CSV resurfacing rate varied significantly between the GSC lines. Generally, CSV resurfacing in CSV− GSCs was substantially more rapid in GSCs more sensitive to TMZ+86C than in CSV− GSCs less sensitive to TMZ+86C (Figure 4C). The CSV resurfacing rate in CSV− cells on day 1 was significantly different in GSCs sensitive to TMZ+86C than in GSCs resistant to TMZ+86C (p=0.0082), although this difference was not observed 4 hours after sorting (p=0.2291). These results indicate that the CSV resurfacing rate is positively correlated with the enhanced cell killing by TMZ+86C.
Another possibility is that 86C may cross bind other antigens to sensitize TMZ treatment. To determine whether that was the case, the specificity of the 86C mAb was evaluated using both western blot analysis and the pulldown assay. Western blot analysis using the 86C mAb clearly shows the presence of a single band at a MW equivalent to vimentin in the vimentin-positive LN18Ctrl cells and the absence of this band in the vimentin-negative cell T47D, while a very weak band was detected in vimentin knockdown cells LN18shVim (Supplemental Figure 2A). The pulldown assay against these cell lysates, followed by western blot analysis of the pulldown pellets, showed the same detection trend. Importantly, this detection utilized a commercial Rabbit anti-vimentin antibody to independently validate the pulldown protein identity, indicating that the detected band in the western blot is vimentin (Supplemental Figure 2B). Collectively, these data have shown the specificity of this 86C mAb in vimentin binding.
To further validate the specificity of this 86C mAb against vimentin on the cell surface (CSV), we have utilized the exogenous vimentin binding assay. This method was shown in our previous publication, in which exogenous vimentin was added to the tumor cell culture because our previous publication had found that external vimentin is able to bind to the tumor cell surface but not to the normal cell surface17. To ensure that this assay is definitive, a vimentin-null tumor cell line, was used. As expected, 86C only detected CSV from T47D cells post external vimentin spiking (Supplemental Figure 2C), which definitively shows the specificity of this 86C mAb.
3.4 CSV resurfacing correlates with TMZ+86C efficacy in patient-derived tumor cells
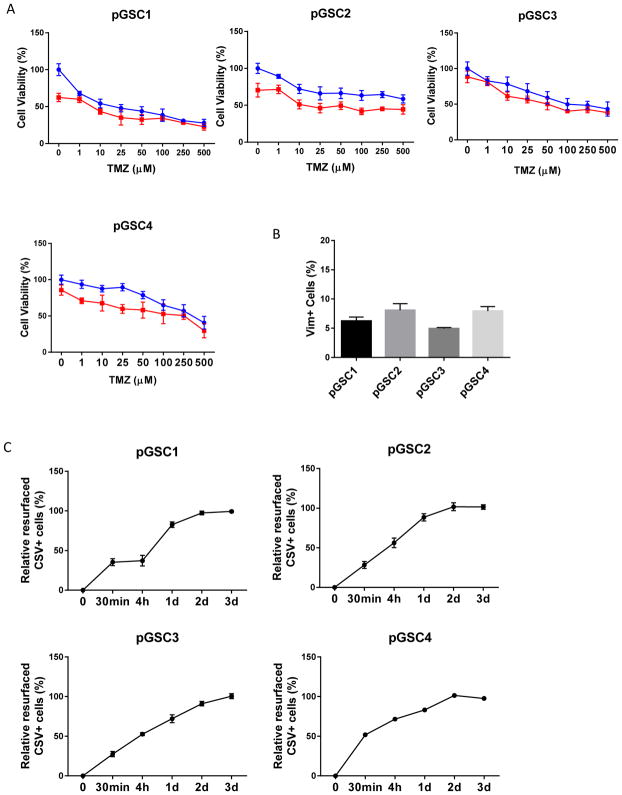
To further validate in clinical specimens our discovery that 86C enhances the effect of TMZ even in CSV− cells, we isolated primary tumor cells from GBMs resected from four patients and treated the cells with TMZ or TMZ+86C ex vivo. The combined treatment synergistically reduced cell viability in all four samples (Figure 5A). CSV was expressed on 6%–7% of tumor cells isolated from these primary tumor tissues (Figure 5B). After these cells were sorted by CSV expression, CSV resurfacing was seen in 80%–90% of CSV− primary GBM tumor cells, a resurfacing rate similar to that of primary GBM tumor cells sensitive to TMZ+86C (Figure 5C).
Figure 5.
CSV resurfacing rate and TMZ+86C efficacy in patient-derived tumor cells. (A) Patient-derived GSC cells pGSC1 and pGSC2 were exposed to graded concentrations of TMZ alone or in combination with a fixed dose of 86C (20 μg/mL) for 72 hours. The graphs represent the mean (±standard deviation) from three independent experiments. (B) CSV expression on pGSC1 and pGSC2 was analyzed by flow cytometry. Data are presented as mean±standard error of three independent experiments. (C) CSV− pGSC1 and pGSC2 cells were sorted by flow cytometry, and the CSV− GSCs were cultured for 30 minutes, 4 hours, 1 day, and 2 days. CSV expression (i.e., resurfacing) in the cells was analyzed by flow cytometry.
3.5 TMZ+86C prolongs survival in a GBM mouse model
To test whether the 86C antibody exerts a proof-of-principle therapeutic effect in combination with TMZ in vivo, we created an orthotopic mouse model of GBM by injecting GSC11 cells with IgG or 86C intracerebrally into immuno-deficient NSG mice. Starting 3 days later, the mice were treated with TMZ daily for 5 days and monitored for survival and weight. The mice treated with TMZ+86C (n=5) survived significantly longer than those treated with TMZ+IgG (p<0.01; n=5), 86C alone (p<0.01; n=5), or the IgG control (p<0.01; n=6) (Figure 6A), and the weights of mice in different treatment groups did not significantly differ.
Figure 6.
TMZ+86C treatment enhanced apoptosis of tumor cells and prolonged survival in an orthotopic GBM mouse model. (A) Tumors were established by injecting GSC11 glioma cells with IgG or 86C intracerebrally into NSG mice. Starting 3 days later, the mice were treated with intraperitoneal injection of TMZ (20 mg/kg). The overall survival of the mice in each group was monitored and compared by the log-rank test. (B) Hematoxylin and eosin staining of sections of mouse GBM tissues was performed on day 20 after intracranial inoculation of GL261-CSV+ cells. (C) Apoptosis was evaluated by TUNEL assay, and the number of TUNEL positive cells was quantified in tumor sections.
To further verify the synergistic anti-tumor effect of TMZ+86C, we performed staining on brain sections 20 days after intracranial inoculation of GL261-CSV+ cells. TMZ+86C treatment strikingly suppressed tumor growth compared with TMZ alone, 86C alone, or the IgG control (Figure 6B). Furthermore, to confirm the previously observed pro-apoptotic effect of TMZ+86C treatment in tumor cells16, we performed assays for TUNEL positive cells in tumor sections from the GL261-CSV+ model. TMZ+86C treatment significantly increased the frequency of TUNEL positive cells in tumor sections compared with 86C alone (Figure 6C). In sum, TMZ+86C enhanced apoptosis of GBM cells and prolonged survival in mice. Thus, the combination of the 86C antibody with TMZ has the potential to treat GBM more effectively than standard TMZ alone.
4. Discussion
In this study, we demonstrate that the anti-CSV monoclonal antibody 86C efficiently increases GSC sensitivity to TMZ. The combination of TMZ+86C induced greater antitumor effects in most GSC lines tested and prolonged survival in a GBM mouse model compared with TMZ alone. TMZ+86C–sensitive GSCs had higher CSV expression overall and faster CSV resurfacing among CSV− GSCs compared with TMZ+86C–resistant GSCs. The combination of TMZ+86C represents a promising strategy to reverse GSC chemoresistance.
Despite the emergence of targeted therapy for cancer over the past decade, no efficient and effective targeted therapies are yet available for GBM. One approach to treating this disease is to overcome its resistance to, and thus improve the efficacy of, TMZ. Some recent reports show the preliminary results of combining TMZ with other drugs active against biologic targets, particularly anti-angiogenic drugs, such as thalidomide and COX-2 inhibitor rofecoxib, as well as other drugs, such as metformin and arsenic trioxide20–22. These combinations yielded acceptable tolerance and favorable survival outcomes in comparison with historical regimens, such as radiotherapy alone or radiotherapy with adjuvant nitrosourea chemotherapy. Several phase I and II trials are exploring possible therapeutic approaches that include combinations of TMZ and new drugs23–25. Many new drugs being developed target specific molecular or genetic lesions, and although targeted therapies alone do not often demonstrate sufficient antitumor effects, it seems likely that combining such agents with standard chemotherapy agents could produce higher response rates and more durable responses26.
The cancer stem cell theory suggests that eradicating cancer stem cells can overturn drug resistance after chemoradiation or targeted therapy. Although 86C was shown in our previous study to have a cell-killing effect on GSCs, the role of 86C as a TMZ chemosensitizer in GSCs has never been investigated. The findings presented here demonstrate for the first time that 86C can efficiently target human GSCs to increase their sensitivity to TMZ. In all GSC lines tested, 86C allowed reduction of TMZ IC50 to as much as 1/3 of levels with 86C.
The association of GSC susceptibility to 86C with CSV expression is illustrated by the higher CSV expression levels in the GSCs with greater sensitivity to TMZ+86C. These data suggest that CSV expression on GSCs is a limiting factor for the antitumor efficacy of TMZ+86C. Another key factor is the rate of CSV resurfacing in CSV− GSCs. The CSV− cells isolated from TMZ+86C–sensitive GSCs showed CSV expression similar to that of unsorted GSCs 1 day after sorting, whereas the CSV− cells isolated from resistant GSCs showed a CSV resurfacing rate equivalent to only 50%–70% of that of unsorted GSCs 1 day after sorting.
In conclusion, combined treatment with TMZ+86C is a promising, novel therapeutic strategy for GBM, especially for counteracting resistance of GSCs to TMZ, which contributes to treatment failure and tumor recurrence in GBM patients. While targeting CSV with 86C may have a anti-tumorigenic effect through inhibition of RP6P phosphorylation16, the exact role of CSV in the therapeutic effect of TMZ+86C requires further study. Further exploration of the mechanisms of CSV translocalization may suggest novel therapeutic targets for GBM.
Supplementary Material
Highlights.
Anti-CSV monoclonal antibody 86C sensitize GSCs to TMZ treatment.
GSCs with higher CSV expression are more sensitive to TMZ+86C.
GSCs with higher CSV resurfacing rate among CSV− cells are more sensitive to TMZ+86C.
TMZ+86C increased apoptosis and prolonged survival in GBM models.
Acknowledgments
We appreciate the Department of Scientific Publications at MD Anderson for helping us edit our manuscript.
Funding: This study was supported by National Institutes of Health grants CA120895, CA208113, P50 CA127001, and P30CA016672.
Abbreviations
- CSV
cell surface vimentin
- DMSO
dimethyl sulfoxide
- GBM
glioblastoma multiforme
- GSC
glioma stem cell
- IC50
median inhibitory concentration
- PBS
phosphate-buffered saline solution
- TMZ
temozolomide
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buckner JC. Factors influencing survival in high-grade gliomas. Seminars in oncology. 2003;30:10–14. doi: 10.1053/j.seminoncol.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The Lancet Oncology. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 4.Friedman HS, McLendon RE, Kerby T, et al. DNA mismatch repair and O6-alkylguanine-DNA alkyltransferase analysis and response to Temodal in newly diagnosed malignant glioma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1998;16:3851–3857. doi: 10.1200/JCO.1998.16.12.3851. [DOI] [PubMed] [Google Scholar]
- 5.Hegi ME, Liu L, Herman JG, et al. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26:4189–4199. doi: 10.1200/JCO.2007.11.5964. [DOI] [PubMed] [Google Scholar]
- 6.Cahill DP, Levine KK, Betensky RA, et al. Loss of the mismatch repair protein MSH6 in human glioblastomas is associated with tumor progression during temozolomide treatment. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13:2038–2045. doi: 10.1158/1078-0432.CCR-06-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newlands ES, Stevens MF, Wedge SR, Wheelhouse RT, Brock C. Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer treatment reviews. 1997;23:35–61. doi: 10.1016/s0305-7372(97)90019-0. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31:4085–4091. doi: 10.1200/JCO.2013.49.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh SK, Clarke ID, Hide T, Dirks PB. Cancer stem cells in nervous system tumors. Oncogene. 2004;23:7267–7273. doi: 10.1038/sj.onc.1207946. [DOI] [PubMed] [Google Scholar]
- 10.Kondo T. Brain cancer stem-like cells. European journal of cancer. 2006;42:1237–1242. doi: 10.1016/j.ejca.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 11.Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nature reviews Cancer. 2006;6:425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 12.Lathia JD, Mack SC, Mulkearns-Hubert EE, Valentim CL, Rich JN. Cancer stem cells in glioblastoma. Genes & development. 2015;29:1203–1217. doi: 10.1101/gad.261982.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer research. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 14.Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cellular and molecular life sciences: CMLS. 2011;68:3033–3046. doi: 10.1007/s00018-011-0735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cutrera J, Dibra D, Xia X, Hasan A, Reed S, Li S. Discovery of a linear peptide for improving tumor targeting of gene products and treatment of distal tumors by IL-12 gene therapy. Molecular therapy: the journal of the American Society of Gene Therapy. 2011;19:1468–1477. doi: 10.1038/mt.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noh H, Yan J, Hong S, et al. Discovery of cell surface vimentin targeting mAb for direct disruption of GBM tumor initiating cells. Oncotarget. 2016;7:72021–72032. doi: 10.18632/oncotarget.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satelli A, Hu J, Xia X, Li S. Potential Function of Exogenous Vimentin on the Activation of Wnt Signaling Pathway in Cancer Cells. J Cancer. 2016;7:1824–1832. doi: 10.7150/jca.15622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panetta JC, Kirstein MN, Gajjar A, et al. Population pharmacokinetics of temozolomide and metabolites in infants and children with primary central nervous system tumors. Cancer chemotherapy and pharmacology. 2003;52:435–441. doi: 10.1007/s00280-003-0670-4. [DOI] [PubMed] [Google Scholar]
- 19.Yoshino A, Ogino A, Yachi K, et al. Gene expression profiling predicts response to temozolomide in malignant gliomas. International journal of oncology. 2010;36:1367–1377. doi: 10.3892/ijo_00000621. [DOI] [PubMed] [Google Scholar]
- 20.Laske DW, Youle RJ, Oldfield EH. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nature medicine. 1997;3:1362–1368. doi: 10.1038/nm1297-1362. [DOI] [PubMed] [Google Scholar]
- 21.Lesser GJ, Grossman S. The chemotherapy of high-grade astrocytomas. Seminars in oncology. 1994;21:220–235. [PubMed] [Google Scholar]
- 22.Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Human gene therapy. 2000;11:2389–2401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 23.Giese A, Westphal M. Glioma invasion in the central nervous system. Neurosurgery. 1996;39:235–250. doi: 10.1097/00006123-199608000-00001. discussion 250–232. [DOI] [PubMed] [Google Scholar]
- 24.Ram Z, Culver KW, Oshiro EM, et al. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nature medicine. 1997;3:1354–1361. doi: 10.1038/nm1297-1354. [DOI] [PubMed] [Google Scholar]
- 25.Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-oncology. 2003;5:79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Augustine CK, Yoo JS, Potti A, et al. Genomic and molecular profiling predicts response to temozolomide in melanoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:502–510. doi: 10.1158/1078-0432.CCR-08-1916. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.