Abstract
Current influenza vaccines do not provide effective protection against heterologous influenza viruses. The ability of the novel M2SR influenza vaccine to protect against drifted influenza viruses was evaluated in naïve ferrets and in ferrets with pre-existing immunity to influenza. In naïve ferrets, M2SR provided similar protection against drifted challenge viruses as the comparator vaccine, FluMist®. However, in ferrets with pre-existing immunity, M2SR provided superior protection than FluMist in two model systems.
In the first model, ferrets were infected with influenza A H1N1pdm and influenza B viruses to mimic the diverse influenza exposure in humans. The pre-infected ferrets, seropositive to H1N1pdm and influenza B but seronegative to H3N2, were then vaccinated with H3N2 M2SR or monovalent H3N2 FluMist virus (A/Brisbane/10/2007, clade 1) and challenged 6 weeks later with a drifted H3N2 virus (clade 3c.2a). Antibody titers to Brisbane/10/2007 were higher in M2SR vaccinated ferrets than in FluMist vaccinated ferrets in the pre-infected ferrets whereas the opposite was observed in naïve ferrets. After challenge with drifted H3N2 virus, M2SR provided superior protection than FluMist monovalent vaccine.
In the second model, the impact of homologous pre-existing immunity upon vaccine-induced protection was evaluated. Ferrets, pre-infected with H1N1pdm virus, were vaccinated 90 days later with H1N1pdm M2SR or FluMist monovalent vaccine and challenged 6 weeks later with a pre-pandemic seasonal H1N1 virus, A/Brisbane/59/2007 (Bris59). While cross-reactive serum IgG antibodies against the Bris59 HA were detected after vaccination, anti-Bris59 hemagglutination inhibition antibodies were only detected post-challenge. M2SR provided better protection against Bris59 challenge than FluMist suggesting that homologous pre-existing immunity affected FluMist virus to a greater degree than M2SR.
These results suggest that the single replication intranasal M2SR vaccine provides effective protection against drifted influenza A viruses not only in naïve ferrets but also in those with pre-existing immunity in contrast to FluMist viruses.
Keywords: M2-deficient, single replication, live influenza, intranasal, heterosubtypic immunity, pre-existing immunity, drifted, hemagglutination inhibition
INTRODUCTION
Currently available inactivated influenza vaccines primarily aim to induce neutralizing antibodies that recognize the hemagglutinin (HA) and depend on a close antigenic match between the vaccine and circulating viruses; mismatching antigens render inactivated vaccines to be less effective against antigenically drifted viruses [1]. Recently the poor efficacy of vaccine used in the 2014–15 influenza season was believed to be due to mismatch between the circulating viruses and the inactivated vaccine, resulting in vaccine effectiveness (VE) of just 6% against the H3N2 subtype [2]. In the 2017 influenza season that just ended in Australia, the inactivated vaccines displayed a preliminary VE estimate of only 10%, again due to mismatch between the vaccine strain and the circulating seasonal H3N2 virus that had accumulated antigenic changes (i.e., “drifted”) [3].
Live influenza virus vaccines are generally believed to offer immunologically superior responses because they are administered via the natural route of infection (intranasally) and induce diverse types of adaptive responses including secretory IgA, serum IgG and cell mediated immune responses [4]. Only one such product, FluMist® (MedImmune, LLC) has been approved in the US, and is presently indicated only for persons 2–49 years of age. Accumulating data with FluMist suggest that pre-existing, cross-reactive immunity present in most adults may limit vaccine virus replication, in turn mitigating a consistently effective immune response [5–8]. In fact, FluMist in Europe (marketed as Fluenz) is not indicated in adults over age 18 due to insufficient efficacy in the adult population [9].
We have previously described a novel M2-deficient single replication (M2SR) influenza vaccine that behaves like wild-type virus during initiation of infection and elicits broad-spectrum immunity similar to that elicited by natural infection; these responses include mucosal and systemic antibodies and cell-mediated immunity [10]. M2SR is intranasally administered and provides heterosubtypic protection in animal models against seasonal and pandemic influenza viruses [10, 11]. These studies were conducted in naïve animals which do not reflect the human condition where pre-existing immunity to influenza exists due to multiple immunizations or natural exposure.
Here we examine the protective efficacy of M2SR against drifted H3N2 and H1N1 viruses in ferrets with preexisting immunity to influenza. We show that M2SR is able to provide effective protection against drifted influenza viruses in naïve ferrets similar to the comparator vaccine FluMist-like monovalent viruses. However, in ferrets with pre-existing immunity, M2SR potency is unaffected whereas FluMist-like virus immunogenicity and efficacy are reduced. These results suggest that the single replication phenotype of M2SR renders the vaccine virus less susceptible to the inhibitory effects of preexisting influenza immunity, in contrast to the replication competent but highly attenuated FluMist component viruses that are dependent upon cell-to-cell spreading for induction of potent immune responses [12].
MATERIALS AND METHODS
Cells and viruses
MDCK (ATCC CCL-34 or Sigma-Aldrich, St. Louis, MO), MDCK-SIAT1 (Sigma-Aldrich) and M2CK [13] and BM2CK [14] cells (MDCK cells that stably express the influenza A M2 or influenza B BM2 protein) were maintained in MEM (Thermo Fisher Scientific, Waltham, MA); 293T HEK (ATCC CRL-3261) cells were maintained in DMEM (Thermo Fisher Scientific, Waltham, MA). Media was supplemented with 10% FCS (Corning, Corning, NY) and cells grown at 37 °C in 5% CO2 as described previously [10, 11].
Live attenuated viruses, Bris10 (H3N2) and CA07 (H1N1pdm) FluMist monovalent vaccines, encoding the HA and NA of influenza A/Brisbane/10/2007 (Bris10)-like influenza A/Uruguay/716/2007 (H3N2) and A/California/07/2009 (A/CA07, H1N1pdm), respectively, were isolated from FluMist® 2009–2010 and 2011–2012 commercial formulations, respectively, by plaque purification followed by amplification and titration in MDCK cells and hereafter referred to as FluMist-like.
M2SR virus (a wild-type like M2-deficient single replication influenza virus) was prepared and titrated in M2CK cells as described previously [10, 15]. Briefly, the vaccine is manufactured in a complementing cell line that stably expresses the influenza A M2 protein resulting in a wild type-like virus that can infect normal cells but not produce a next generation of virus due to the deletion of M2 in the viral genome. The six internal genes of M2SR are derived from A/Puerto Rico/8/1934 (PR8, H1N1) [10]. Bris10 (H3N2) M2SR virus expresses the same HA and NA as Bris10 FluMist component virus (Supplementary Table 1). CA07 (H1N1pdm) M2SR expresses the HA and NA from vaccine virus A/California/07/2009 X-179A (International Reagent Resource, Manassas, VA) that differs from the FluMist-like sequence in 4 positions (Supplementary Table 2).
Wild-type influenza A/California/07/2009 (A/CA07, H1N1pdm), A/Brisbane/59/2007 (A/Bris59, H1N1), A/Brisbane/10/2007 (A/Bris10, H3N2), A/Hong Kong/4801/2014 (A/HK4801, H3N2), A/Switzerland/9715293/2013 (A/Swiss, H3N2), B/Wisconsin/01/2010 (B/WI01, Yamagata Lineage), and B/Brisbane/60/2008 (B/Bris60, Victoria Lineage) were amplified in MDCK cells. Influenza A/Alaska/140/2015 (A/AK140, H3N2), kindly provided by the CDC, was amplified in MDCK-SIAT1cells.
BM2SR viruses (BM2-deficient recombinant influenza B viruses) encoding the HA and NA from Victoria-lineage (B/Brisbane/60/2008, Bris60 BM2SR) or from Yamagata-lineage (B/Wisconsin/01/2010, WI01 BM2SR) were generated using a plasmid rescue system described previously [14]; resulting BM2SR viruses were amplified and titrated in BM2CK cells. All viruses were stored at <−65°C until use.
Animals
Ferrets that were seronegative to influenza viruses were purchased from Triple F Farms, Inc. (Sayre, PA,) or from Marshall BioResources (North Rose, NY). All animal study protocols were approved by IIT Research Institute Institutional Animal Care and Use Committee and all experiments were performed in accordance with the National Institute of Health guidelines for the care and use of laboratory animals.
Model 1: H3N2 vaccination and drifted H3N2 virus challenge in ferrets with pre-existing immunity to H1 and B viruses
Three- to five-month-old male ferrets were intranasally (IN) infected twice with 107 TCID50 of WI01 BM2SR or Bris60 BM2SR 28 days apart followed by infection with wild-type influenza A/CA07 (H1N1pdm) on day 70. The influenza exposed ferrets were confirmed to be seropositive for A/CA07, B/WI01 and B/Bris60 by ELISA as previously described [10].
After 42 days rest, the flu-exposed ferrets were randomized into three groups and vaccinated intranasally with Bris10 M2SR or FluMist-like (N=6, 107 TCID50) virus or PBS (N=4). Age-matched naïve ferrets (N=6/group) were used as controls and administered IN Bris10 M2SR or FluMist-like (107 TCID50) virus or PBS. Ferrets were challenged IN 42 days later with A/AK140 (106 PFU). Ferret body weight, body temperature and clinical symptoms were monitored for 12 days after challenge. Nasal washes were collected from all ferrets on days 1, 3, 5 and 7 after challenge and virus load determined by TCID50 assay in MDCK cells as previously described [16].
Serum samples were collected on days -5 (pre-Bris10 immunization), 21, 35 (post-Bris10 immunization), and 56 (post-challenge). Serum anti-HA IgG ELISA and hemagglutination inhibition (HAI) antibody titers were determined as previously described [10].
Model 2: H1N1pdm vaccination and variant H1N1 challenge in ferrets with pre-existing H1N1pdm immunity
Three-to-five months old male seronegative ferrets were infected IN with wild-type A/CA07 (H1N1pdm). Seroconversion against H1N1pdm was confirmed by ELISA as previously described [10].
After 90 days rest, the ferrets were randomized into three groups and vaccinated IN with CA07 M2SR or FluMist-like vaccine viruses (N=6, 107 TCID50) or PBS (N=4). Similarly, comparison groups of age-matched H1N1 naïve ferrets (N=6/group) were vaccinated IN with CA07 M2SR or FluMist like viruses (107 TCID50) or PBS. Ferrets were challenged IN with A/Bris59 (H1N1, 106 PFU) 42 days post-vaccination. Ferret body weight, temperature and clinical symptoms were monitored for 12 days after challenge. Nasal washes were collected from all ferrets on days 1, 3, 5 and 7 after challenge and viral load was determined by TCID50 assay in MDCK cells.
Serum samples were collected on days -3, 21, 35, and 56 (14 days post-challenge). Serum anti-HA IgG ELISA and hemagglutination inhibition (HAI) antibody titers were determined as previously described [10].
RESULTS
H3N2 Bris10 M2SR antibody responses in ferrets with pre-existing immunity to H1 and B viruses
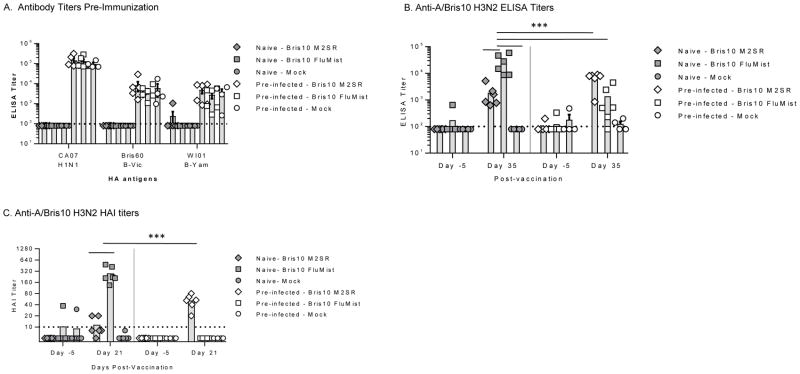
H1N1 and influenza B antibody was generated prior to H3N2 immunization by intranasal infection with wild-type influenza A H1N1pdm (A/CA07) and influenza B BM2SR viruses (B/Bris60 BM2SR and B/WI01 BM2SR) to mimic the complex immunity present in partially immune humans as shown in Table 1. Age-matched naïve ferrets were used as controls. Sera from all ferrets were tested by ELISA for HA antibodies against H1 A/CA07, H3 A/Bris10, B/WI01, B/Bris60. The naïve ferrets were seronegative to all antigens whereas the pre-infected ferrets were seropositive for H1N1pdm and influenza B HA antigens but were seronegative for H3N2 as expected (Fig 1A).
Table 1.
Study design to evaluate M2SR vaccine vs FluMist-like vaccine against drifted H3N2 challenge
| Group | N | Infection with Influenza B Vaccine | Infection with H1N1pdm Virus | Intranasal Vaccine1 | N | Wildtype Challenge2 |
|---|---|---|---|---|---|---|
| Day 0 and Day 28 | Day 70 | Day 112 | Day 154 | |||
| Naive | 18 | None | None | M2SR (H3N2, clade 1) | 6 | A/Alaska/140/2015 (H3N2, clade 3C.2a) |
| FluMist (H3N2, clade 1) | 6 | |||||
| Mock | 6 | |||||
| Pre-infected | 16 | WI01 BM2SR or Bris60 BM2SR3 | A/California/07/2009 (H1N1pdm) | M2SR (H3N2, clade 1) | 6 | |
| FluMist (H3N2, clade 1) | 6 | |||||
| Mock | 4 |
Vaccines contained HA and NA from A/Brisbane/10/2007 (H3N2).
Intranasal challenge at day 42 post-vaccination.
Modified M2-deficient influenza B vaccine viruses of influenza B Victoria and Yamagata lineages.
Figure 1. Serum antibody titers in naïve and pre-infected ferrets.
Ferrets were infected with influenza A H1N1 and influenza B viruses to generate pre-existing immunity (pre-infected). Forty-five days later age-matched naive ferrets (filled symbols) and pre-infected ferrets (open symbols) were intranasally inoculated with H3N2 vaccine viruses Bris10 M2SR or FluMist-like (107 TCID50) or mock inoculated with PBS. Serum samples were collected before immunization (day -5) and after immunization (day 21 or day 35). A. Individual pre-vaccination serum IgG titers against recombinant HA of A/California/07/2009 (H1N1pdm), B/Brisbane/60/2007 (Victoria lineage), and B/Wisconsin/01/2010 (Yamagata lineage) were determined by ELISA (day -5). B. Individual serum IgG titers against recombinant HA of A/Brisbane/10/2007 (A/Bris10, H3N2) were measured by ELISA for pre-vaccination (day -5) and post-vaccination (day 35) time-points. Limit of detection is indicated by horizontal dashed line. C. Hemagglutination inhibition (HAI) titers against vaccine strain (A/Bris10, H3N2) were determined using 0.5% turkey RBC. Horizontal dash line is limit of detection (HAI = 10). In all panels, symbols indicate individual animals; bars indicate group mean with standard error. Vertical dashed line separates naïve and pre-infected groups. *** p<0.0001, Two-way ANOVA.
The pre-infected and naïve ferrets were vaccinated with H3N2 Bris10 M2SR or FluMist-like or mock-immunized with PBS 45 days after influenza infection. Sera after immunization was analyzed for presence of anti-A/Bris10 H3 HA IgG antibody by ELISA (Figure 1B). H3N2 Bris10 M2SR elicited higher antibody titers in the pre-infected ferrets than it did in the naïve ferrets whereas H3N2 FluMist-like antibody titers in the pre-infected ferrets were significantly reduced relative to titers generated in the naïve animals (Figure 1B). Antibody titers in the mock-immunized ferrets, pre-infected or naïve, remained at background levels.
Similar to the ELISA results, H3N2 Bris10 M2SR elicited higher HAI titers in pre-infected ferrets than in naïve ferrets, whereas H3N2 Bris10 FluMist-like vaccine elicited high HAI titers in the naïve ferrets but not in the pre-infected ferrets (Figure 1C). These results suggest that the wild-type like H3N2 Bris10 M2SR was more effective at inducing immunity to a novel HA in the face of pre-existing heterotypic and heterosubtypic immunity whereas FluMist-like vaccine virus was inhibited by the pre-existing immunity to H1 and/or B viruses.
H3N2 Bris10 M2SR protects against drifted H3N2 virus challenge in naïve and pre-infected ferrets
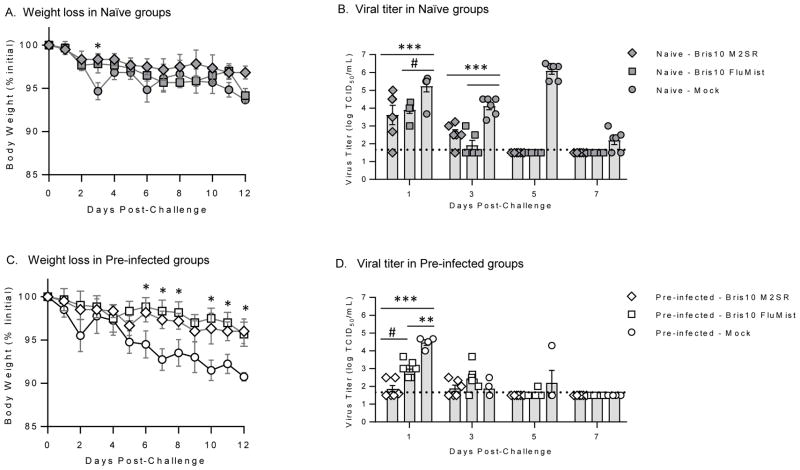
To assess the ability of the H3N2 Bris10 vaccines to cross-protect against significantly drifted H3N2 challenge, the vaccinated ferrets were challenged with A/Alaska/140/2015 (H3N2; clade 3c.2a) 42 days after vaccination. The phylogenetic relationship between the vaccine and challenge viruses are shown in Supplementary Figure 1. Weight loss and body temperatures were monitored for 12 days following challenge (Figure 2A, C and Supplementary Figure 2). In the naïve ferrets, both H3N2 Bris10 M2SR and FluMist-like vaccine viruses performed similarly with respect to weight loss and nasal wash titers (Figure 2A and 2B), despite higher serum antibody titers in the naïve H3N2 Bris10 FluMist-like vaccine group. Both vaccine groups had similar levels of H3N2 A/AK shedding on day 1 post-challenge whereas on day 3, the Bris10 FluMist-like group had a lower incidence of shedding (2/6) compared to the M2SR Bris10 group (5/6) (Figure 2B). By day 5 post-challenge, virus was not detected in the nasal washes of the vaccinated ferrets, whereas ferrets in naïve-mock group still shed high titer of virus. These results suggest that the replication-restricted M2SR virus provides effective protection in naïve ferrets similar to the replication-competent FluMist virus.
Figure 2. M2SR vaccine controls drifted H3N2 challenge virus in naïve and pre-infected ferrets.
Bris10 (clade 1; H3N2) or mock vaccinated ferrets (in naïve and pre-infected groups) were challenged with drifted H3N2 strain, A/AK140 (clade 3c.2a) six weeks after vaccination. Weight loss was monitored for 12 days post-challenge. Nasal wash samples were collected on days 1, 3, 5, and 7 post-challenge. Virus loads in the samples were determination by 50% tissue culture infection (TCID50) method in MDCK cells. A. Weight loss in naïve-vaccine groups after challenge. B. Virus titers in nasal wash from naïve-vaccine animals after challenge. C. Weight loss in pre-infected vaccine ferrets after challenge. D. Virus titers in nasal wash in pre-infected ferrets after challenge. The detection limit of the TCID50 assay (horizontal dashed line) was 1.5 log10 TCID50/mL. Symbols are individual ferret and bars are mean titers with standard error for group at indicated time-point. *** p<0.0001, ** p=0.0001, # p<0.0008, * p<0.05; Two-way ANOVA.
In the pre-infected ferrets, both H3N2Bris10 M2SR and FluMist-like H3 vaccinated groups displayed ~4% weight loss whereas the pre-infected mock vaccinated group lost ~9% (Figure 2C,) of their starting weight after challenge. H3N2Bris10 M2SR controlled the replication of the challenge virus in nasal wash better than H3N2Bris10 FluMist-like (Figure 2D). Only 2/6 H3N2Bris10 M2SR vaccinated ferrets shed virus on day 1 post-challenge whereas all animals in the H3N2Bris10 FluMist-like and mock vaccinated groups shed virus in nasal wash at the same time-point.
Previous infection with influenza B and H1N1pdm provided a level of protection against H3N2 challenge as shown by the nasal wash virus titer differences between the two mock groups (Figure 2B and 2D). The virus titers after H3N2 challenge were higher in the naïve mock than the pre-infected mock group suggesting that cross-reactive cellular immune responses may have been contributing to virus control after wild type challenge among ferrets with previous infections with influenza A H1 or B viruses.
Post-challenge serum was evaluated for HAI antibodies against the vaccine virus strain, A/Bris10 (H3N2, clade 1), and the recent 2017–18 WHO recommended H3N2 strain, A/HK4801 (H3N2, clade 3C2.a), to further assess the ability of the vaccines to prime for cross-protective responses. HAI antibody titers to H3N2 Bris10 (vaccine HA) on day 56, two weeks after A/AK140 challenge, were higher than the mock vaccinated and challenged animals in both the naïve and pre-infected group against both A/Bris10 and A/HK4801 (Supplementary Figure 3A and B). In contrast, FluMist-like vaccine demonstrated high HAI titers only in the naïve ferrets; HAI titers in the pre-infected ferrets were similar to the mock group for both viruses (Supplementary Figure 3A and B). These results show that the two intranasal live vaccines have the ability to prime for cross-reactive antibody responses in naïve ferrets but that only M2SR can maintain this ability in the face of pre-existing immunity. As expected, the H1N1pdm HAI titers did not increase after H3N2 vaccination or challenge (data not shown).
H1N1pdm CA07 M2SR cross-reactive antibody responses to pre-pandemic seasonal H1N1 in ferrets with pre-existing H1N1pdm immunity
In the second model of pre-existing immunity, the performance of M2SR was evaluated in the presence of homologous immunity; that is, H1N1pdm CA07 M2SR vaccine was administered to ferrets that were previously infected with H1N1pdm virus as shown in Table 2. Age-matched naïve ferrets were used as controls.
Table 2.
Study design to evaluate M2SR vaccine vs FluMist-like vaccine against drifted H1N1 challenge
| Group | N | Previous Infection With H1N1pdm Virus | Intranasal Vaccine1 | N | Wildtype Challenge2 |
|---|---|---|---|---|---|
| Day 0 | Day 90 | Day 132 | |||
| Naive | 18 | None | M2SR (H1N1pdm) | 6 | A/Brisbane/59/2007 (H1N1 seasonal) |
| FluMist (H1N1pdm) | 6 | ||||
| Mock | 6 | ||||
| Pre-infected | 16 | A/California/07/2009 (H1N1pdm) | M2SR (H1N1pdm) | 6 | |
| FluMist (H1N1pdm) | 6 | ||||
| Mock | 4 |
Vaccines contained HA and NA from A/California/07/2009 (H1N1pdm).
Intranasal challenge at day 42 post-vaccination.
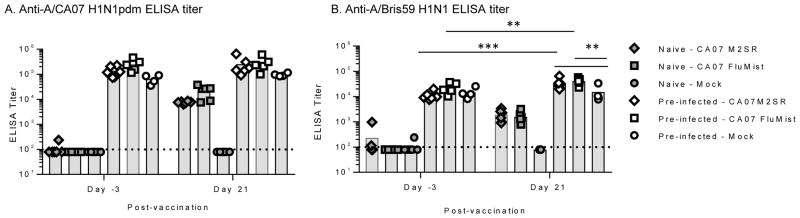
The pre-infected and naïve ferrets were vaccinated with H1N1pdm CA07 M2SR or FluMist-like or mock-immunized with PBS 90 days after CA07 pre-infection. Sera after immunization (day 21) was analyzed by ELISA for vaccine-induced anti-HA antibody titers. The H1N1pdm CA07 M2SR and FluMist-like vaccines elicited CA07 HA specific antibodies to similar levels in naïve animals whereas the mock-immunized did not elicit any detectable antibody (Figure 3A). Vaccine-induced CA07 HA-specific responses were not discernable in the CA07 pre-infected ferrets due to high baseline titers even after 90 days of rest post-infection (Figure 3A). Therefore, to demonstrate vaccine take, ELISA titers were measured against the pre-pandemic seasonal H1N1 A/Brisbane/59/2007 (A/Bris59) reasoning that baseline antibody titers for the cross-reactive H1N1 A/Bris59 HA would be lower than H1N1pdm CA07. Indeed, day 21 ELISA titers against H1N1 Bris59 HA were higher than the baseline titers and higher than the mock group in the pre-infected ferrets suggesting that there was a de novo response to each of the vaccines (Figure 3B). As expected, the vaccine induced H1N1pdm CA07 titers in the naïve animals were cross-reactive with H1N1 Bris59 (Figure 3B).
Figure 3. Cross-reactive serum antibody responses in naïve and pre-infected ferrets.
Ferrets were infected with A/California/07/2009 (H1N1pdm) virus to generate homologous pre-existing immunity in ferrets (pre-infected). Age-matched naïve ferrets (filled symbols) and A/CA07 (H1N1pdm) pre-infected ferrets (open symbols) were intranasally inoculated with CA07 M2SR or FluMist-like (107 TCID50) or mock immunized with PBS. Serum samples were collected before immunization (day -3) and after vaccine inoculations (day 21). Serum IgG titer against recombinant A/CA07 HA (A) and recombinant A/Bris59 HA (H1N1) (B) were measured by ELISA. Limit of detection is indicated by horizontal dashed line. Symbols indicate individual ferret titers; bars indicate group mean. *** p<0.0006, ** p<0.009; Two-way ANOVA.
H1N1pdm CA07 M2SR protects against pre-pandemic seasonal H1N1 virus challenge in naïve and seropositive ferrets
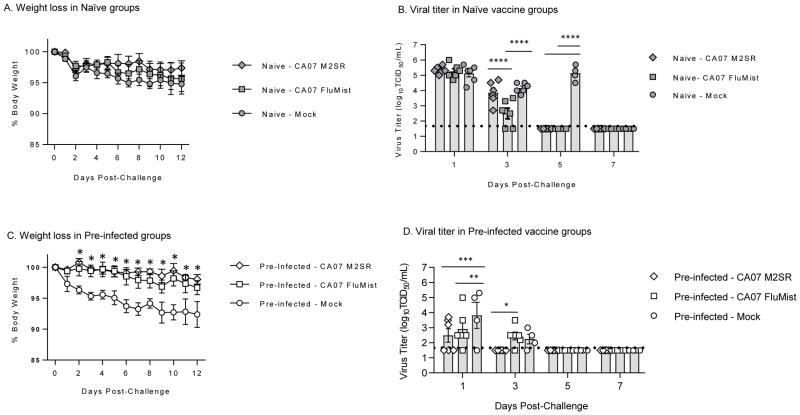
Ferrets were intranasally challenged with pre-pandemic seasonal H1N1 virus A/Bris59 42 days after vaccination. The phylogenetic relationship between the vaccine and challenge viruses are shown in Supplementary Figure 1. All groups in the naïve animals lost weight after challenge with the mock group demonstrating the most weight loss (Figure 4A). The mock group also demonstrated a spike in body temperature on day 2 post-challenge (Supplementary Figure 4). Nasal wash collected post-challenge was evaluated for the presence of challenge virus in all ferrets. Both H1N1pdm CA07 M2SR and FluMist-like controlled the virus to similar levels in contrast to the mock group that had detectable virus for 5 days (Figure 4B).
Figure 4. M2SR vaccine controls drifted H1N1 virus challenge in naïve and pre-infected ferrets.
Six weeks after vaccination, ferrets were challenged with drifted H1N1 strain, A/Brisbane/59/2007 (H1N1). Weight loss was monitored for 12 days post-challenge. Nasal wash samples were collected on days 1, 3, 5, and 7 post-challenge. Viral load was determined by 50% tissue culture infection (TCID50) in MDCK cells. A. Weight loss in naïve-vaccine groups after challenge. B. Virus titers in nasal wash from naïve-vaccine animals after challenge. C. Weight loss in pre-infected vaccine ferrets after challenge. D. Virus titers in nasal wash in pre-infected ferrets after challenge. The detection limit of the TCID50 assay (horizontal dashed line) was 1.5 log10 TCID50/mL. Symbols are individual ferrets and bars are group mean titers with standard error at indicated time-point. **** p<0.0001, ** p<0.005, * p<0.05; Two-way ANOVA.
In the pre-infected ferrets, both H1N1pdm M2SR and FluMist-like groups lost less weight than the mock group (Figure 4C). M2SR demonstrated better protection than FluMist-like with a lower incidence of shedding on day 1 (3/6 vs 5/6, respectively). M2SR cleared challenge the virus faster than both the FluMist-like and mock groups (Figure 4D).
The pre-infection with H1N1pdm did provide some protection against the pre-pandemic H1N1 A/Bris59 virus as shown by the difference in peak viral load between the mock groups for the naïve and pre-infected ferrets (Figure 4B and D). However, despite better control of challenge virus and reduced body temperature (Supplementary Figure 4), this partial protection did not prevent weight loss in pre-infected mock-vaccinated ferrets and may have enhanced morbidity (also observed in Figure 2C).
Post-challenge serum was evaluated for functional HAI titers against the H1N1 Bris59 challenge virus. No Bris59 HAI was present in any group before challenge (day 21, Supplementary Figure 5). After challenge, the vaccinated and mock groups in the naïve ferrets showed high Bris59 HAI titers whereas in the pre-infected ferrets the HAI titers appear to parallel the viral load in the nasal wash; that is, the H1N1pdm M2SR group that controlled the challenge virus the best had the lowest HAI whereas the mock group with the highest viral load had the highest HAI against the challenge virus (Supplementary Figure 5). Interestingly, although clinical disease after challenge was relatively mild in all groups, the M2SR group demonstrated fewer clinical symptoms after challenge than FluMist-like in both the naïve and pre-infected ferrets (Supplementary Table 3). These results further support that M2SR and FluMist-like have the ability to prime for cross-reactive antibody responses in naïve ferrets but that only M2SR boosts the cross-reactive capacity of pre-existing immunity.
DISCUSSION
We show that the novel intranasal influenza vaccine, M2SR, provides effective protection against heterologous influenza viruses (H3N2 and H1N1) in naïve ferrets and in ferrets with pre-existing immunity; thus addressing two fundamental issues that impact the effectiveness of licensed influenza vaccines: drifted influenza viruses and pre-existing immunity. Vaccine efficacy can be as low as 6% in flu seasons in which the vaccine is a poor match to the circulating influenza virus [1, 2, 17]. In addition, recent data has suggested that pre-existing immunity in human subjects, whether due to annual vaccination and/or natural infection, may result in reduced vaccine responses and vaccine induced protection [18–20]. For the only licensed intranasal live flu vaccine, FluMist, vaccine effectiveness appears to diminish in adults relative to children [17].
We show that M2SR virus is superior to the FluMist-like virus in providing protection against drifted challenge viruses (H3N2 and H1N1) in ferrets with pre-existing immunity. M2SR immunogenicity was not reduced in ferrets with pre-existing immunity when compared to naïve ferrets whereas FluMist-like virus immune responses were significantly reduced in the pre-infected ferrets relative to the naïve ferrets. These results suggest that the single-cycle replication M2SR virus is not susceptible to pre-existing immunity to influenza, whereas the replicating FluMist-like virus may be prevented from undergoing the multi-cycle replication needed to elicit protective responses. The cold-adaptation process that generated the FluMist virus has resulted in a highly attenuated virus with multiple mutations that affect the viral replication complex [21, 22].
The inverse correlation between the overall immunological experience of the host and FluMist replication in the respiratory tract has been recognized since the early development of the vaccine [23]. Indeed, post-licensure efficacy studies have further corroborated that FluMist is not as efficacious in adults due to pre-existing immunity preventing the virus from undergoing multiple replication cycles [5–8, 17]. The ferret data obtained in this study are consistent with results in humans that demonstrate that FluMist is inhibited by the presence of prior immunity to influenza. We show that in the same ferret model, the single-replication M2SR vaccine is much less susceptible to inhibition by pre-existing immunity.
Two models of pre-existing immunity were used. In the first case, heterosubtypic immunity was generated by pre-infection of the ferrets with H1N1pdm and influenza B virus infection. Ferrets were not pre-infected with H3N2 virus. M2SR induced serum H3N2 antibody responses in these pre-infected animals were similar to those induced in naïve ferrets. However, serum H3N2 antibody responses induced by FluMist-like were higher in naïve animals and significantly reduced in the ferrets with pre-existing immunity suggesting that cross-reactive immune responses such as secretory IgA antibodies and/or T cells (and not homologous antibody directed to the HA) may have prevented the sustained replication of FluMist necessary for effective responses. Similar observations have been observed in human subjects, in which higher baseline levels of influenza immunity have affected the immune responses to the FluMist vaccine [24–27].
We also evaluated whether pre-existing homologous antibodies (systemic and/or mucosal) would inhibit the ability of the live vaccines to protect against challenge with a drifted influenza virus. H1N1pdm M2SR and FluMist-like vaccines were administered to ferrets that had previously been infected with the wildtype H1N1pdm virus. We show that M2SR provided superior protection against challenge with pre-pandemic seasonal H1N1 A/Brisbane/59/2007 than did FluMist-like in the ferrets pre-infected with H1N1pdm.
These results suggest that despite the presence of homologous serum antibodies, M2SR was able to infect for a single-cycle and provide effective protection. In contrast, FluMist-like was not able to protect as well in the pre-infected ferrets presumably due to the immunity preventing spread of the virus.
Interestingly, in naïve animals, a single-dose of M2SR provided comparable protection to FluMist-like against both of the heterologous challenges (H3N2 and H1N1) even though FluMist-like induced higher serum antibody titers. These results suggest that the two intranasal live vaccines may differ in their mechanisms of action in providing effective protection. We have previously shown that M2SR elicits broad-spectrum immune responses including systemic and mucosal antibody responses in addition to cell-mediated responses that provide heterosubtypic protection in animal models [10, 11]. Future studies will evaluate the multi-factorial nature of protection provided by M2SR relative to FluMist to understand the differences between the two vaccines.
In summary, we show that the novel intranasal single-replication M2SR vaccine provides effective protection against heterologous H3N2 and H1N1 viruses in both naïve ferrets and ferrets with pre-existing immunity. These results suggest that M2SR has the desired attributes of a broadly protective vaccine for all age groups, a need unmet by current inactivated and live vaccines.
Supplementary Material
Highlights.
Pre-existing immunity to influenza affects the response to influenza vaccinations
M2SR protects against drifted H3N2 virus in ferrets with pre-existing heterosubtypic immunity.
M2SR protects against drifted H1N1 virus in ferrets with pre-existing homologous immunity.
Acknowledgments
We thank Dr. Robert Belshe for suggestions on the manuscript. This work was supported, in part, by National Institutes of Health grants AI 111451 and AI 109925 to S.S. and P.B.
ABBREVIATIONS
- CDC
Centers for Disease Control and Prevention
- ELISA
enzyme-linked immunosorbent assay
- FCS
fetal calf serum
- HA
hemagglutinin
- HAI
hemagglutination inhibition
- HEK
human embryonic kidney
- M2SR
M2-deficient single replication vaccine virus
- M2CK
Madin-Darby canine kidney cells expressing M2 protein
- MDCK
Madin-Darby canine kidney
- MEM
minimal essential medium
- NA
neuraminidase
- OD
optical density
- PBS
phosphate buffered saline
- pdm
pandemic
- PFU
plaque-forming unit
- RBC
red blood cell
- RDE
receptor destroying enzyme
- sIgA
secretory IgA
- TCID50
50% tissue culture infectious dose
- TMB
tetramethylbenzidine
- VE
vaccine effectiveness
Footnotes
Conflict of interest statements: S.S. and D.B. have no conflicts of interest. G.N. and Y.K. are founders of FluGen. Y.H. and P.B. are employees of FluGen.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Belongia EA, et al. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004–2005 season to the 2006–2007 season. J Infect Dis. 2009;199(2):159–67. doi: 10.1086/595861. [DOI] [PubMed] [Google Scholar]
- 2.Zimmerman RK, et al. 2014–2015 Influenza Vaccine Effectiveness in the United States by Vaccine Type. Clin Infect Dis. 2016;63(12):1564–1573. doi: 10.1093/cid/ciw635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paules CI, et al. Chasing Seasonal Influenza - The Need for a Universal Influenza Vaccine. N Engl J Med. 2018;378(1):7–9. doi: 10.1056/NEJMp1714916. [DOI] [PubMed] [Google Scholar]
- 4.Sridhar S, Brokstad KA, Cox RJ. Influenza Vaccination Strategies: Comparing Inactivated and Live Attenuated Influenza Vaccines. Vaccines (Basel) 2015;3(2):373–89. doi: 10.3390/vaccines3020373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eick AA, et al. Comparison of the trivalent live attenuated vs. inactivated influenza vaccines among U.S. military service members. Vaccine. 2009;27(27):3568–75. doi: 10.1016/j.vaccine.2009.03.088. [DOI] [PubMed] [Google Scholar]
- 6.Ohmit SE, et al. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N Engl J Med. 2006;355(24):2513–22. doi: 10.1056/NEJMoa061850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z, et al. Live attenuated or inactivated influenza vaccines and medical encounters for respiratory illnesses among US military personnel. JAMA. 2009;301(9):945–53. doi: 10.1001/jama.2009.265. [DOI] [PubMed] [Google Scholar]
- 8.Monto AS, et al. Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med. 2009;361(13):1260–7. doi: 10.1056/NEJMoa0808652. [DOI] [PubMed] [Google Scholar]
- 9.Agency EM. Fluenz Assessment Report. London: 2011. pp. 1–85. [Google Scholar]
- 10.Sarawar S, et al. M2SR, a novel live single replication influenza virus vaccine, provides effective heterosubtypic protection in mice. Vaccine. 2016;34(42):5090–5098. doi: 10.1016/j.vaccine.2016.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatta Y, et al. M2SR, a novel live influenza vaccine, protects mice and ferrets against highly pathogenic avian influenza. Vaccine. 2017;35(33):4177–4183. doi: 10.1016/j.vaccine.2017.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter NJ, Curran MP. Live attenuated influenza vaccine (FluMist(R); Fluenz): a review of its use in the prevention of seasonal influenza in children and adults. Drugs. 2011;71(12):1591–622. doi: 10.2165/11206860-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe S, Watanabe T, Kawaoka Y. Influenza A virus lacking M2 protein as a live attenuated vaccine. J Virol. 2009;83(11):5947–50. doi: 10.1128/JVI.00450-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatta M, Goto H, Kawaoka Y. Influenza B virus requires BM2 protein for replication. J Virol. 2004;78(11):5576–83. doi: 10.1128/JVI.78.11.5576-5583.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neumann G, et al. Generation of influenza A viruses entirely from cloned cDNAs. Proc Natl Acad Sci U S A. 1999;96(16):9345–50. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe T, et al. Novel approach to the development of effective H5N1 influenza A virus vaccines: use of M2 cytoplasmic tail mutants. J Virol. 2008;82(5):2486–92. doi: 10.1128/JVI.01899-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osterholm MT, et al. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(1):36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 18.Skowronski DM, et al. Beyond Antigenic Match: Possible Agent-Host and Immuno-epidemiological Influences on Influenza Vaccine Effectiveness During the 2015–2016 Season in Canada. J Infect Dis. 2017;216(12):1487–1500. doi: 10.1093/infdis/jix526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLean HQ, et al. Impact of repeated vaccination on vaccine effectiveness against influenza A(H3N2) and B during 8 seasons. Clin Infect Dis. 2014;59(10):1375–85. doi: 10.1093/cid/ciu680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohmit SE, et al. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis. 2014;58(3):319–27. doi: 10.1093/cid/cit736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin H, et al. Multiple amino acid residues confer temperature sensitivity to human influenza virus vaccine strains (FluMist) derived from cold-adapted A/Ann Arbor/6/60. Virology. 2003;306(1):18–24. doi: 10.1016/s0042-6822(02)00035-1. [DOI] [PubMed] [Google Scholar]
- 22.Tannock GA, Paul JA, Barry RD. Relative immunogenicity of the cold-adapted influenza virus A/Ann Arbor/6/60 (A/AA/6/60-ca), recombinants of A/AA/6/60-ca, and parental strains with similar surface antigens. Infect Immun. 1984;43(2):457–62. doi: 10.1128/iai.43.2.457-462.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy BR, Coelingh K. Principles underlying the development and use of live attenuated cold-adapted influenza A and B virus vaccines. Viral Immunol. 2002;15(2):295–323. doi: 10.1089/08828240260066242. [DOI] [PubMed] [Google Scholar]
- 24.He XS, et al. Baseline levels of influenza-specific CD4 memory T-cells affect T-cell responses to influenza vaccines. PLoS One. 2008;3(7):e2574. doi: 10.1371/journal.pone.0002574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasaki S, et al. Comparison of the influenza virus-specific effector and memory B-cell responses to immunization of children and adults with live attenuated or inactivated influenza. virus vaccines. J Virol. 2007;81(1):215–28. doi: 10.1128/JVI.01957-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoft DF, et al. Comparisons of the Humoral and Cellular Immune Responses Induced by Live Attenuated Influenza Vaccine and Inactivated Influenza Vaccine in Adults. Clin Vaccine Immunol. 2017;24(1) doi: 10.1128/CVI.00414-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barria MI, et al. Localized mucosal response to intranasal live attenuated influenza vaccine in adults. J Infect Dis. 2013;207(1):115–24. doi: 10.1093/infdis/jis641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.