Abstract
This study investigated the efficacy of components of licorice root to alter performance on two different recognition tasks, a hippocampus-sensitive metric change in object location (MCOL) task and a striatum-sensitive double object recognition (DOR) task. Isoliquiritigenin (ISL), licorice root extract (LRE), and whole licorice root powder (LRP) were assessed. Young adult female rats were ovariectomized (OVX) and exposed to ISL, LRE or LRP at 0.075%, 0.5% or 5% respectively in the diet. An estradiol group was included as a positive control based on our prior findings. Rats were allowed to explore two objects for three 5-min study trials (separated by 3-min intervals) before a fourth 5-min test trial where the objects were moved closer together (MCOL task) or replaced with two new objects (DOR task). Rats typically habituate to the objects across the three study trials. An increase in object exploration time in the test trial suggests the rat detected the change. Estradiol improved MCOL performance and impaired DOR performance, similar to previously shown effects of estradiol and other estrogens, which tend to improve learning and memory on hippocampus-sensitive tasks and impair striatum-sensitive cognition. LRP had no effect on recognition while exposure to ISL and LRE improved MCOL performance. Exposure to ISL, LRE and LRP failed to attenuate DOR, contrary to effects of estradiol shown here and to previous reports in young-adult OVX rats. These findings suggest components of licorice root may prove to be effective therapies targeting memory enhancement without unintended deleterious cognitive effects.
A. Introduction
Botanical estrogens are non-steroidal plant compounds that can mimic estrogens in the body (Glazier and Bowman, 2001). These compounds are widely sold as dietary supplements despite a dearth of research on their health effects. Many over the counter botanical supplements contain licorice root powder (LRP) or licorice root extracts (LREs), shown to exert estrogenic effects both in vitro in MCF7 breast cancer cells and in vivo in various tissues including the heart and the pituitary (Maggiolini et al., 2002, Tamir et al., 2001). Isoliquiritigenin (ISL) and liquiritigenin (LIQ) are two of the primary bioactive compounds in licorice root (Mersereau et al., 2008, Miksicek et al., 1993). ISL and LIQ convert readily back and forth in the body (Simmler et al., 2013). Both compounds are lipophilic and have relatively low molecular weight and thus likely cross into the brain through the blood brain barrier (Srihari et al., 2012).
Estrogens have broad ranging actions believed to modulate cognition (for reviews see Galea et al., 2017; Korol and Pisani, 2015; Korol and Wang, 2018; Luine and Frankfurt, 2015). However, the relationship between estrogens and cognition is a complicated one. The effects of estrogens on cognition vary widely depending on a variety of factors including the cognitive task and the brain areas engaged by the task. Much of the existing literature suggests that estrogen supplementation to ovariectomized (OVX) rodents improves performance on hippocampus-sensitive tasks, but impairs performance on striatum-sensitive tasks. (e.g Davis et al., 2005; Korol and Kolo, 2002; Pisani et al., 2012). Studies using the same basic training paradigm to assess different cognitive attributes are especially useful in comparing the effects of estrogens on different brain systems, given that several factors like the dose of estradiol used, the pattern of administration as well as aspects of the task environment are held constant. For example, compared to OVX vehicle-treated controls, administration of estradiol to OVX young adult female rats improves performance on an allocentric, place learning version of a 4-arm radial maze task, known to rely on intact functioning of the hippocampus (Chang and Gold, 2003), but impairs performance on an egocentric, response learning version of the same maze task that engages the striatum during learning (Davis et al., 2005; Korol & Kolo, 2002; Korol & Pisani, 2012; Zurkovsky et al., 2006). Moreover, two days of oral dosing with the estrogen receptor (ER) ERβ-selective botanical estrogen genistein produced a similar shift in learning strategy by enhancing place learning while impairing response learning. These bidirectional effects are not limited to ERβ activation, as several estrogen receptor agonists have also been investigated using this place and response learning paradigm (Korol & Pisani, 2015). The ERα-selective compound propyl pyrazole triol (PPT) and the ERβ-selective compounds diarylpropionitrile (DPN) and Br-ERb-041 were all found to improve place learning and impair response learning, albeit at different doses (Pisani et al., 2015). These findings suggest that estrogen supplementation to OVX young adult rodents facilitates learning and memory that depends on the hippocampus and impairs cognition that depends on the striatum. It should be noted that these bidirectional effects depend on dosing, timing of exposure, and other aspects of the animal history and training environment such as stress history, parity, and age (Korol & Pisani, 2015).
Estrogens have also been shown to affect performance on object recognition and placement memory in paradigms developed to match task attributes much like the place and response learning paradigm described above. Administration of estrogens to OVX adult rodents enhance performance on hippocampus-sensitive object location tasks (Luine et al., 2003; Tunur et al., 2012). The effects of estrogens on object recognition are mixed with some evidence that estrogens enhance performance on these tasks (Gresack and Frick, 2006) and some evidence that they impair performance (Tunur and Korol, 2015).
Diet has also been shown to affect cognition, with several studies showing that a high fat diet can impair various aspects of cognition including spatial learning, working memory, object recognition, and fear conditioning (for a review see Freeman et al., 2014). Impairments have been found on a hippocampus-sensitive radial arm maze task (Granholm et al., 2008; Winocur and Greenwood, 2005), a prefrontal cortex-dependent operant delayed spatial alternation task (Winocur and Greenwood, 2005), an operant-based delayed matching to position task (McNeilly et al., 2011), and an object recognition task (Camer et al., 2015; Carey et al., 2014; Jurdak and Kanarek, 2009; Kaczmarczyk et al., 2013). However, it is worth noting that nearly all of the studies investigating the effects of a high fat diet on cognition in a rodent model, including all of the studies mentioned above, used only male rodents. In the few studies investigating both males and females, some have found sex differences in response to a high fat diet in peripheral metabolism, performance on a contextual fear conditioning task, as well as the magnitude of long-term potentiation (Hwang et al., 2010; Underwood and Thompson, 2016), highlighting the need to investigate the effects of a high fat diet in females as well as males.
In the present studies, we investigated the effects of ISL, LRE and LRP on a hippocampus-sensitive metric change in object location (MCOL) task and a striatum-sensitive double object recognition (DOR) task (Goodrich-Hunsaker et al., 2008, Korol and Pisani, 2015). Importantly, the tasks differ only in the final trial and are otherwise identical. Thus, the roles of ISL, LRE and LRP on two distinct cognitive tasks engaging distinct brain areas/memory systems can be compared using these tasks. We chose to investigate LRP and LRE because those are the forms of licorice root typically found in dietary supplements. We included the pure compound, ISL because it is a component of licorice root that has demonstrated estrogenic properties both in vitro and in vivo (Maggiolini et al, 2002; Miksicek et al, 1993; Tamir et al 2001).
We used an OVX rat model to investigate the ability of ISL, LRE and LRP to impact cognition in the absence of endogenous estrogens that would compete for ERs. Additionally, we wished to address the issue that the standard rodent diet is much lower in fat than the typical western diet, and previous rodent studies have reported cognitive deficits in rats fed high fat diets relative to those fed standard laboratory chow. Thus, the inclusion of high fat diet groups allowed us to evaluate a diet that more closely models the typical western diet consumed by humans and to investigate whether these botanicals interact with this high fat diet to produce a pattern of effects different from that seen in rats consuming a low fat diet.
B. Materials and Methods
Animals and treatment
Due to the large number of rats required in these studies, they were each conducted in a series of cohorts or replicates, as described below. The effects of ISL, LRE and LRP on the MCOL task were investigated in three separate studies consisting of three cohorts each. The effects of ISL, LRE and LRP on the DOR task were investigated in one study consisting of three cohorts. Estradiol groups were also included in all of the studies because previous work has shown that estradiol improves performance on the MCOL task and impairs performance on the DOR task in OVX young adult rats (Korol and Pisani, 2015; Tunur et al., 2012; Tunur and Korol, 2015). For each MCOL study, 72 young adult virgin female Long-Evans rats (53 days old) were obtained from Harlan (Indianapolis, IN) in 3 cohorts of 24 rats each, spaced 1 week apart. In each of the three cohorts 4 rats were assigned to each of 6 treatment groups (high fat control, low fat control, high fat estradiol, low fat estradiol, high fat botanical, low fat botanical). With all cohorts included, there was a total of 12 rats per treatment group in each of the three studies (Table 1A). For the DOR study, a total of 90 female Long-Evans rats was obtained from Harlan (Indianapolis, IN) in cohorts of 30 rats each, spaced 1 week apart. In each cohort 4 rats were assigned to each of five treatment groups (control, estradiol, ISL, LRE and LRP). With all cohorts included, there was a total of 12 rats per treatment group (Table 1B).
Table 1A.
MCOL was assessed in three separate studies that tested the effects of ISL, LRE or LRP, respectively. Each study consisted of three cohorts, each with the study design shown above.
| Control | Estradiol | Botanical (ISL, LRE or LRP) | |
|---|---|---|---|
|
| |||
| High fat | Cohort 1: n=4 | Cohort 1: n=4 | Cohort 1: n=4 |
| Cohort 2: n=4 | Cohort 2: n=4 | Cohort 2: n=4 | |
| Cohort 3: n=4 | Cohort 3: n=4 | Cohort 3: n=4 | |
| Total: n=12 | Total: n=12 | Total: n=12 | |
|
| |||
| Low fat | Cohort 1: n=4 | Cohort 1: n=4 | Cohort 1: n=4 |
| Cohort 2: n=4 | Cohort 2: n=4 | Cohort 2: n=4 | |
| Cohort 3: n=4 | Cohort 3: n=4 | Cohort 3: n=4 | |
| Total: n=12 | Total: n=12 | Total: n=12 | |
Table 1B.
DOR was assessed in one study that tested the effects of ISL, LRE or LRP, respectively. The study consisted of three cohorts with the study design shown above.
| Control | Estradiol | ISL | LRE | LRP | |
|---|---|---|---|---|---|
|
| |||||
| Low fat | Cohort 1: n=4 | Cohort 1: n=4 | Cohort 1: n=4 | Cohort 1: n=4 | Cohort 1: n=4 |
| Cohort 2: n=4 | Cohort 2: n=4 | Cohort 2: n=4 | Cohort 2: n=4 | Cohort 2: n=4 | |
| Cohort 3: n=4 | Cohort 3: n=4 | Cohort 3: n=4 | Cohort 3: n=4 | Cohort 3: n=4 | |
| Total: n=12 | Total: n=12 | Total: n=12 | Total: n=12 | Total: n=12 | |
Rats were housed in a temperature and humidity controlled room (22 °C, 40–55% humidity) on a 12-h light–dark cycle (lights on at 7:00 am). Rats were pair-housed in standard plastic cages (17.7× 9.4 × 7.9 inches) with Beta Chip® bedding, and food and water were available ad libitum. The housing facility was fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Illinois at Urbana-Champaign and were in accordance with the guidelines of the Public Health Service Policy on Humane Care and Use of Laboratory Animals and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (NIH, 1986; Van Sluyters and Obernier, 2004).
In the MCOL studies the diet was switched from standard rodent chow (Harlan 8604) to either a high fat (TD.06415) or a low fat (TD.94045) diet (Harlan, Madison, WI) on the third day after arrival to the vivarium. Both diets were phytoestrogen free to ensure no additional exposure to estrogenic compounds in the food. The high fat diet included 44.8% kcal from fat with a fatty acid profile as follows: 36% saturated, 47% mono unsaturated, 17% polyunsaturated. The low fat diet included 17.2% kcal from fat with a fatty acid profile as follows: 16% saturated fat, 23% mono unsaturated, 58% polyunsaturated (See table 2). Vitamins and minerals were balanced in the two diets. In the DOR study, the diet was switched from standard rodent chow (Harlan 8604) to the same low fat diet (TD.94045) used in the MCOL studies on the third day after arrival to the vivarium. Given the lack of diet effects in all three of the MCOL studies and limited resources, we did not include high fat groups for the DOR study. Food and water were available to all rats ad libitum. Both rats and their remaining food were weighed every three days so that food intake and the amount of the botanical consumed per μg body weight could be estimated. Rats with same treatments were pair housed, so intake for each rat was estimated to be half of the total intake for that cage.
Table 2.
fatty acid profiles of the high fat and the low fat diets used in the MCOL study
| High fat diet (44.8% kcal from fat) | Low fat diet (17.2% kcal from fat) |
|---|---|
| 36% saturated fat | 16% saturated fat |
| 47% mono unsaturated fat | 23% mono unsaturated fat |
| 17% polyunsaturated fat | 58% polyunsaturated fat |
In both the MCOL and the DOR studies, all rats were OVX 9 days after arrival. Rats were allowed to recover from OVX for one week before experimental diets were started. ISL, LRE or LRP was incorporated into the diet of a subset of the animals at a concentration of .075%, 5% or 0.5% of the diet respectively for three weeks prior to testing. ISL, LRE and LRP were obtained from the Botanical Center at the University of Mississippi. ISL and LRE were purified through methanol extraction. LRP was made by powdering the whole root. The identity and purity of all botanical components was verified by chromatography and spectroscopy. The ISL was over 95% pure. The concentrations were selected for relevance to predicted human consumption in dietary supplements as detailed in Madak-Erdogan et al. (2016).
Four weeks after OVX and two days before testing, rats in the estradiol groups received a single daily injection of estradiol in sesame oil (s.c. 45μg/kg), 48 and 24 hours prior to testing. This dose was selected based on previous work showing estradiol-induced improvements in hippocampus-sensitive tasks, and impairments in striatum-sensitive tasks in OVX young adult rats (Korol and Pisani, 2015; Pisani et al., 2012; Tunur et al., 2012; Tunur and Korol, 2015). All rats in the non-estradiol groups in all studies received vehicle injections of sesame oil (s.c 0.45 mL/kg.of oil). Each rat was tested one time during the light portion of the light/dark cycle. Testing occurred between 8am and 12pm Monday-Saturday (See Figure 1A for timeline).
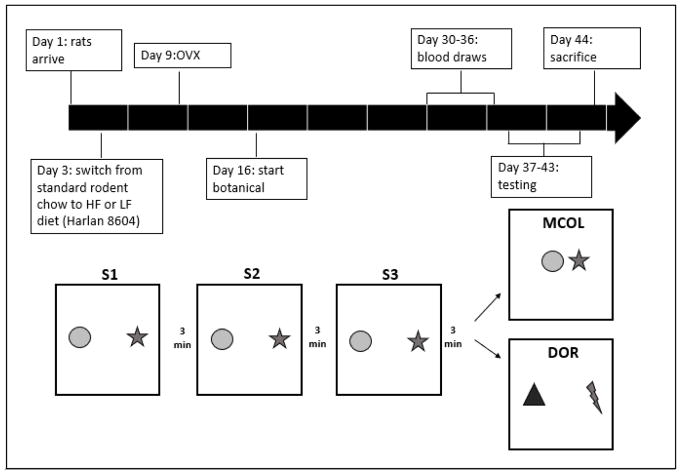
Figure 1.
Figure 1A. (Top) Timeline of the MCOL and DOR studies. Rats were approximately 53 days old upon arrival and approximately 90 days old (89–96 days) when tested. Figure 1B. (Bottom) In the MCOL procedure, two objects are placed 16 inches apart for three study sessions. After the final study session (S3) the objects are moved symmetrically closer together for the test session (T). In the DOR procedure, two objects are placed 16 inches apart for three study sessions. After the final study session (S3) the objects are replaced with two new objects for the test session (T). (Figure adapted from Korol and Pisani, 2015.)
Behavioral testing
Apparatus
The testing apparatus was an opaque, black Plexiglas chamber measuring 28×28 inches, at the base and 21inches tall and surrounded by a black curtain to block peripheral light. The height of the walls of the chamber reduced the possibility of the rat using visual cues outside of the chamber. In both MCOL and DOR studies, two ceramic stimulus objects, 7–8 inches tall were used. Both objects were cookie jars of similar dimensions and were filled with glass marbles to prevent movement of the objects during testing. The objects were chosen to have patterned exterior surfaces that are easy to clean to remove olfactory cues. For the test session in DOR, two novel objects with similar characteristics were substituted. The novel objects were similar in material, texture, and height to the previous objects, but differed in shape and color. A camera was mounted above the chamber to record behavioral testing sessions.
MCOL procedure
Our MCOL task represents a novelty detection paradigm (as described in Goodrich-Hunsaker et al, 2008). The behavioral testing was conducted in one session with four 5-minute trials consisting of three study sessions (S1, S2, S3) followed by one test session (T; Figure 1B). A 3-minute intertrial interval was imposed between each trial, during which rats were returned to their holding cage. Rats were videotaped during the task with a webcam for later scoring.
At the start of each trial, rats were placed in the same location in the chamber. During S1–S3, the two objects were placed 16 inches apart in the chamber. For T, the objects were moved symmetrically closer together so that they were 12 inches apart (Figure 1B). Between each trial and between rats, the objects and the chamber (sides and base) were cleaned with a 10% ethanol solution to minimize odor cues. The positions of the individual objects (left or right), the time of day within the testing window, and the tester were counterbalanced across groups. The two testers were blind to the treatment groups of the rats.
DOR procedure
The DOR task differed from the MCOL task only during T. Instead of changing the distance between objects as in MCOL, the objects were replaced with two new objects that differed in size and shape from the original two objects and from each other (Figure 1B). All other aspects of DOR were as described above for MCOL.
Behavior analysis
In all studies herein, the videos were scored by two people who were blind to the group assignment of the rat. Reliability between scorers was determined with a test set of videos. A correlation coefficient of greater than 95% between coders was required before coding began. Measures of exploration were positively correlated between testers for both the MCOL and DOR studies (r(47)=0.996, p=<0.001) and r(47)=0.990, p<0.001) respectively). Object exploration time was recorded for each trial using a stopwatch. Exploration was defined as activity directed at the object: including sniffing, whisking, or looking at the object with the nose pointed towards the object. Sitting or climbing on top of the object was not considered object exploration.
ISL and LIQ concentrations
To determine circulating amounts of ISL and LIQ in the blood, tail vein blood samples were collected from each rat two weeks after starting exposure to the botanical diet and one week prior to behavioral testing, as well as at the time of sacrifice. Blood collection was timed so that it fell within the testing window, which was between 8:00 am and 12:00 pm. Rats were euthanized up to one week after the conclusion of behavioral testing, during which trunk blood was collected, again at a time that fell within the behavioral testing window. Rats in the botanical groups received the compounds in their feed until euthanasia. The concentrations of ISL, LRE, and LRP in the diets were chosen to produce similar blood concentrations of ISL/LIQ in all three exposure groups. Blood was centrifuged and serum collected and frozen at −20°C for later analysis at the National Center for Toxicological Research (NCTR). Levels of total ISL and LIQ were determined using LC/MS/MS following quantitative hydrolysis of conjugates by β-glucuronidese/arylsulfatase (H. pomatia; Sigma H-1, 2 units/assay) using procedures previously described (Madak-Erdogan et al., 2016). Levels of aglycone ISL and LIQ were determined without the enzymatic hydrolysis step.
Organ weights
At the time of sacrifice, liver and uterine horn weights were taken. The liver weight was divided by the body weight of the animal to derive a liver/body weight ratio. A higher liver/body weight ratio implies increased induction of liver enzymes, which is suggestive of possible hepatotoxicity (Maronpot et al., 2010). As a bioassay for estrogen activity at ERα (Matthiessen, 2013) the uterine horn was removed and trimmed of proximal fat and vasculature to yield accurate weight (g)/length (cm) of the horn sample (described in Pisani et al., 2015).
Statistical analysis
For all studies, the primary dependent variable was the total number of seconds spent exploring both objects during a trial. The derived score for statistical analysis (pattern separation index) was calculated as follows (as described in Goodrich-Hunsaker et al, 2008). The number of seconds spent exploring the objects in the test trial was divided by the sum of the number of seconds spent exploring the objects in the third study trial and the test trial. This measure has been used in previous published literature (e.g Goodrich-Hunsaker et al., 2008). The pattern separation index scores and the organ weights from the MCOL studies were analyzed via two-way ANOVA with treatment and diet as between-subjects factors and with one-way ANOVAs using treatment as the between subjects variable. The pattern separation index scores and organ weights from the DOR study were analyzed via one-way ANOVA with treatment as a between subjects factor. The object exploration times for the MCOL and DOR studies were analyzed via a repeated measures ANOVA with trial as a repeated measures factor and treatment as a between subjects factor. When appropriate, Tukey post hoc tests for pairwise comparisons were done. All statistical analyses were done with Systat for Windows Version 13.1 (systatsoftware.com/products/systat/). Significance was set at p <.05.
C. Results
Behavioral analysis
MCOL studies
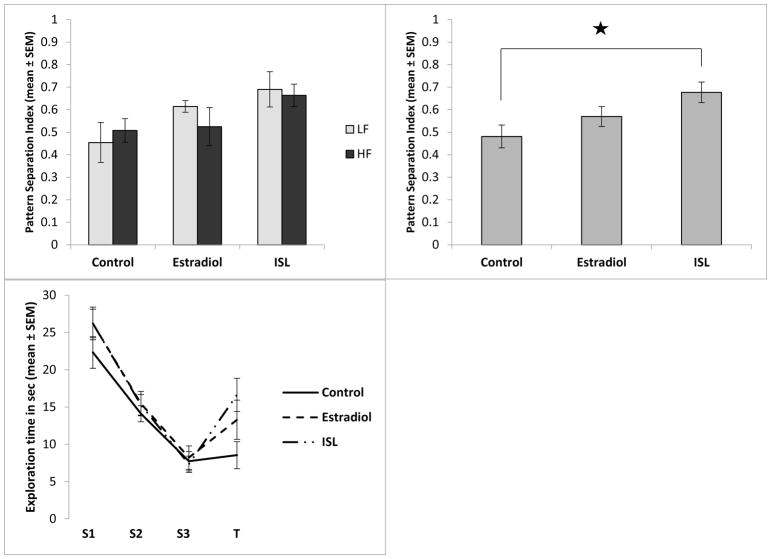
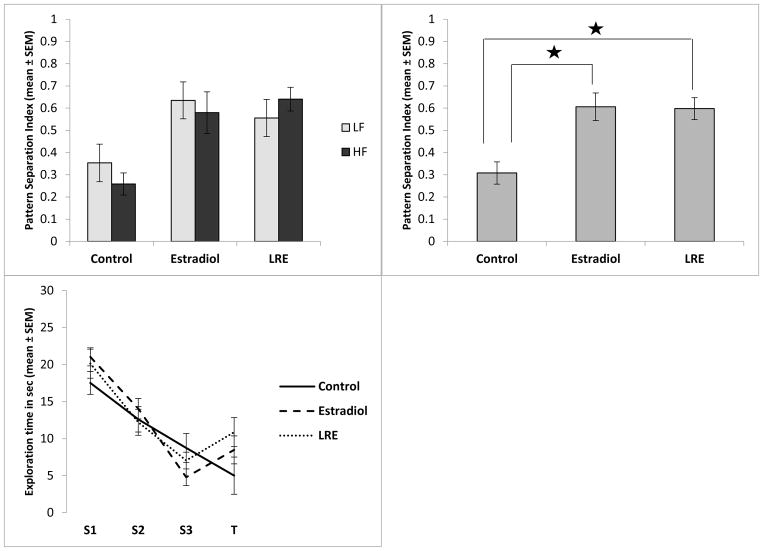
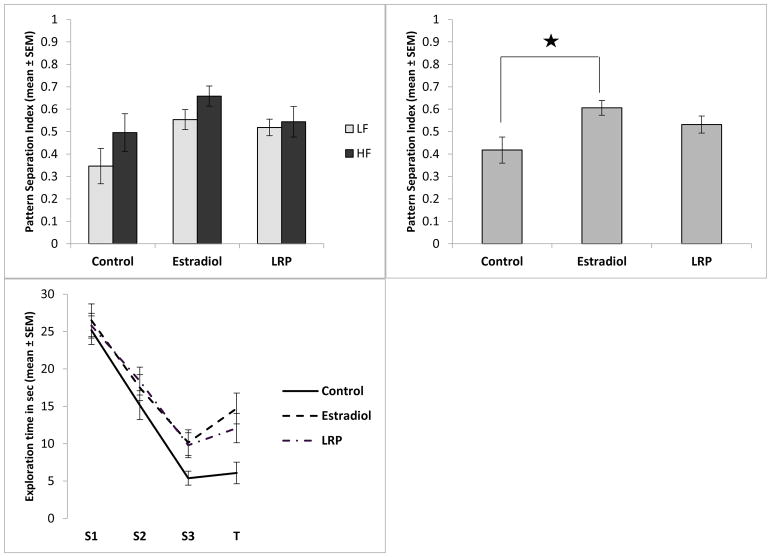
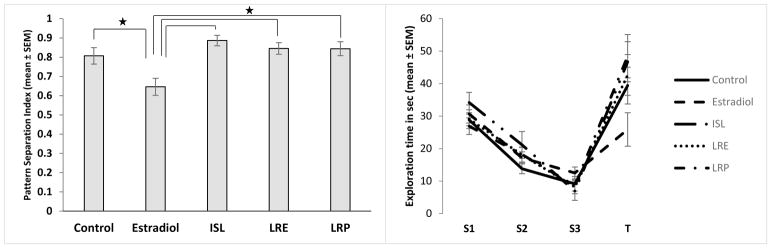
In the ISL MCOL study, the ANOVA revealed a significant main effect of exposure on the pattern separation index (F[2,64]=4.27, p=0.018,η2=0.12There was no significant effect of dietary fat and no interaction between dietary fat and exposure (Figure 2A). A Tukey post-hoc analysis revealed that the rats exposed to ISL had a significantly higher pattern separation index score than the control group (p=0.014, d=0.83, Figure 2B), indicating that ISL rats performed better on the task than did control rats. The estradiol group showed a similar pattern, but there was not a statistically significant difference between the control group and the estradiol group. The exploration times across trials reflects this, showing habituation in all rats from S1 to S3 as expected (F[2,134]=147.05, p<0.001, ηp2=0.69), and an increase in exploration in T in ISL and estradiol treated rats (Figure 2C). In the LRE MCOL study, the ANOVA revealed a main effect of exposure on the pattern separation index (F[2,64]=9.76, p<0.000, η2=0.23). Similar to the ISL study, there was no significant effect of dietary fat and no interaction between exposure and dietary fat on the pattern separation index (Figure 3A). A Tukey post-hoc analysis revealed that both the estradiol group and the LRE group had significantly higher pattern separation index scores than the control group (p=0.001, d=1.1 and p=0.001, d=1.21 respectively, Figure 3B), indicating that both the estradiol and LRE rats performed better on the task than did control rats. As with the ISL study, the exploration times across trials reflects this, showing habituation in all rats from S1 to S3 as expected (F[2,134]=114.10, p<0.001, ηp2=0.63), and an increase in exploration during T in LRE and estradiol treated rats but not in control rats (Figure 3C). In the LRP MCOL study, the ANOVA revealed a main effect of exposure on the pattern separation index (F[2,65]=4.51, p=0.015, η2=0.12). There was no significant effect of dietary fat and no interaction between dietary fat and exposure on the pattern separation index (Figure 4A). A Tukey post-hoc analysis revealed that the rats exposed to estradiol had a significantly higher pattern separation index score than did the control group (p=0.011, d=0.83, Figure 4B), indicating that estradiol rats performed better on the task than control rats. However, there was no significant difference between the LRP group and the control group. Again, the exploration times across trials reflects this, showing habituation in all rats from S1 to S3 as expected (F[2,136]=112.41, p<0.001, ηp2=0.62), and a larger increase in exploration during T in estradiol treated rats compared to both other groups (Figure 4C).
Figure 2.
A). (Top left panel) In the ISL MCOL study, there was a main effect of exposure on the pattern separation index, but no effect of dietary fat and no interaction between dietary fat and exposure. Low fat and high fat groups are represented with light or dark bars respectively. B). (Top right panel) In panel B low fat and high fat groups are combined showing the main effect of exposure. Animals exposed to ISL had a significantly higher pattern separation index score than the control group (p=0.012). C). (Bottom left panel) Raw exploration time of both objects in each trial, with low fat and high fat groups combined.
Figure 3.
A). (Top left panel) In the LRE MCOL study, there was a main effect of exposure on the pattern separation index, but no effect of dietary fat and no interaction between dietary fat and exposure. Low fat and high fat groups are represented with light or dark bars respectively. B). (Top right panel) In panel B low fat and high fat groups are combined showing the main effect of exposure. Both the estradiol group and the LRE group had significantly higher pattern separation index scores than the control group (Control vs Estradiol, p=0.001, Control vs LRE, p=0.001). C). (Bottom left panel) Raw exploration time of both objects in each trial, with low fat and high fat groups combined.
Figure 4.
A). (Top left panel) In the LRP MCOL study, there was a main effect of exposure on the pattern separation index, but no effect of dietary fat and no interaction between dietary fat and exposure. Low fat and high fat groups are represented with light or dark bars respectively. B). (Top right panel) In panel B low fat and high fat groups are combined showing the main effect of exposure. Animals exposed to estradiol had a significantly higher pattern separation index score than the control group. The LRP group did not differ significantly from the control group (p=0.01). C). (Bottom left panel) Raw exploration time of both objects in each trial, with low fat and high fat groups combined.
DOR study
The ANOVA revealed a significant main effect of exposure (F[4,54]= 6.59, p<0.001, η2=0.33). A Tukey post-hoc analysis revealed the rats exposed to estradiol had significantly lower pattern separation index scores than all other groups (Figure 5A). There was no significant difference between the control group and any of the botanical groups. The exploration times across trials reflects this, showing habituation in all rats from S1 to S3 as expected (F[2,106]=157.63, p<0.001, ηp2=0.75), and the smallest increase in exploration during T in estradiol treated rats (Figure 5B).
Figure 5.
A). (Left panel) In the DOR study, the ANOVA revealed a main effect of exposure on the pattern separation index. The animals exposed to estradiol had significantly lower pattern separation index scores than all other groups (p<0.05 for all). There was no significant difference between the control group and any of the botanical groups. B). (Right panel) Raw exploration time of both objects in each trial.
Serum ISL and LIQ levels
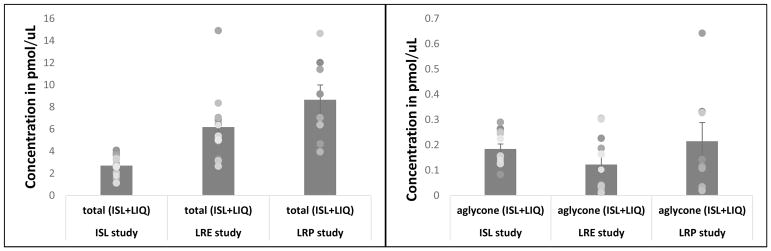
For the purposes of this analysis, levels of isoliquiritigenin and its active metabolite liquiritigenin were combined because ISL and LIQ readily convert back and forth in the body and both compounds have actions at estrogen receptors (Maggiolini et al., 2002; Mersereau et al., 2008; Miksicek et al., 1993; Simmler et al., 2013; Tamir et al., 2001). Analysis of blood samples from the MCOL studies showed that rats treated with ISL, LRE or LRP had significantly elevated levels of ISL+LIQ, with approximately 7%, 2% and 2%, respectively of that being aglycone, which is the active form. The mean serum levels of ISL+LIQ for the ISL, LRE and LRP groups were 2.71, 6.20 and 8.66 pmol/μl respectively (Figure 6A). The mean serum levels of aglycone ISL+LIQ for the ISL, LRE and LRP groups were 0.18, 0.12 and 0.21 pmol/μl respectively (Figure 6B). The levels of ISL+LIQ in control rats was lower than the level of detection.
Figure 6.
A). (Left panel) The mean serum levels of isoliquiritigenin plus liquiritigenin for the ISL, LRE and LRP MCOL studies. B). (Right panel) The mean serum levels of aglycone isoliquiritigenin plus liquiritigenin for the ISL, LRE and LRP MCOL studies.
Organ weights
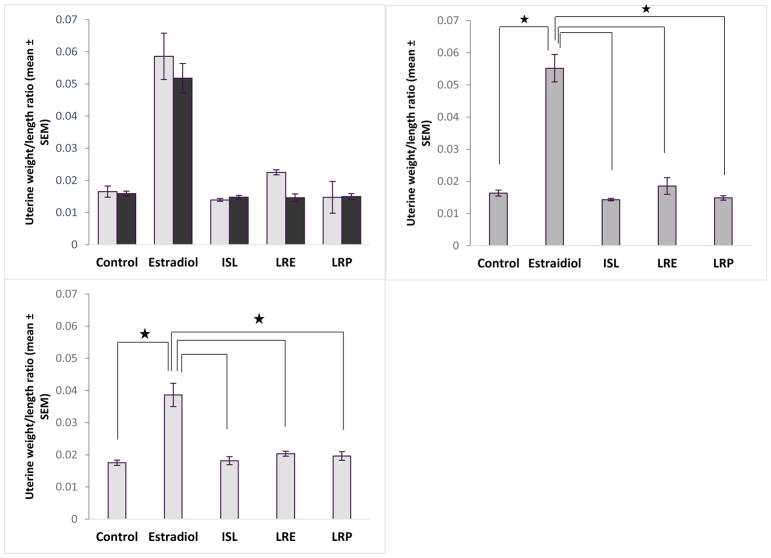
The analysis of uterine horn size in all three of the MCOL studies showed that only estradiol treated rats differed significantly from controls. Because of this, the data were combined across the three MCOL studies for uterine horn size. In the MCOL (combined) and DOR studies, an ANOVA revealed a significant main effect of exposure on uterine horn size (F[4,192]=41.87, p<0.0001, η2=0.46 and F[4,84]=22.21, p<0.0001, η2=0.55 respectively; Figure 7). A Tukey post hoc analysis revealed that rats exposed to estradiol had a significantly larger uterine horn than did rats in all other groups (p<0.0001 for all, Figure 7). Rats in the botanical groups did not differ significantly from rats in the control group. In the MCOL studies, there was no effect of dietary fat on uterine weight and no interaction between exposure and dietary fat (Figure 7A).
Figure 7.
Data across the three MCOL studies were combined in panels A). (top left panel) and B). (top right panel) to illustrate that only the estradiol exposed groups showed increases in uterine horn weight (p<0.05 for all). Low fat and high fat groups are represented with light or dark bars respectively in panel A). In panel B), low fat and high fat groups are combined to show the main effect of exposure. There was no effect of dietary fat and no interaction between exposure and dietary fat on uterine horn weight in the MCOL studies. Panel C) (bottom left panel) show that was the same was true in the DOR study (p<0.05 for all).
In both the ISL and LRE MCOL studies, an ANOVA revealed significant effects of exposure (F[2, 64]=3.21, p=0.047, ηp2=0.09 and F[2,64]=5.98, p=0.004, ηp2=0.14 respectively) as well as dietary fat (F[1,64]=13.86, p<0.001, ηp2=0.18 and F[1,64]=20.99, p<0.001, ηp2=0.21 respectively) on the liver to body weight ratio (data not shown). However, the exposure effect was driven by estradiol-treated rats having slightly smaller livers than those in both other groups. ISL and LRE treated rats did not differ from control rats in liver weight. High fat rats had slightly smaller liver to body weight ratios across all groups. In the LRP MCOL study, an ANOVA revealed no significant effect of exposure and no effect of dietary fat on the liver to body weight ratio. In the DOR study, the ANOVA revealed no main effect of exposure on the size of the liver relative to the body weight.
D. Discussion
The current study investigated the effects of ISL, LRE or LRP in young adult, OVX Long-Evans rats on performance on the MCOL task, which assesses hippocampal function, or the DOR task, which assesses striatal function. Treatment with ISL or LRE significantly improved performance on the MCOL task compared to OVX control rats. Treatment with estradiol also improved performance relative to control rats with the effect reaching statistical significance in the LRP MCOL and LRE MCOL studies, but not in the ISL MCOL study. In the DOR study, the estradiol group was impaired on the DOR task compared to the control group and to all three botanical groups. For DOR, none of the botanical groups differed significantly from the control group. However, it should be noted that in all of the studies reported here, the control group was OVX rats without hormone or botanical supplementation; intact (non-OVX) rats were not included. Thus, it is difficult to know the effects of these compounds in the presence of naturally occurring ovarian steroids.
MCOL task
In the MCOL studies, we found that treatment with ISL or LRE significantly improved the performance of OVX rats on this hippocampus-sensitive task, whereas treatment with LRP did not affect performance on the task. Interestingly in the ISL study, the effect of ISL on task performance was greater than the effect of estradiol. This was an unexpected finding given the relatively low affinity of ISL for the estrogen receptor compared to estradiol. Both estradiol- and ISL-treated rats performed better than control rats did, but the effect was only significant for ISL treated rats. This fits well with our preliminary findings showing that although estradiol supplementation did improve performance on this task, the administration of DPN, an ERβ agonist, led to a larger improvement in task performance than administration of PPT, an ERα agonist. PPT led to an improvement similar in magnitude to that of estradiol (Tunur et al., 2012). These findings suggest that ERβ-selective compounds may be more effective than ER-α selective compounds or estradiol in improving performance on this task.
Our hypothesis that treatment with estradiol would significantly improve performance on the MCOL task was partially supported. In all of the MCOL studies, the estradiol group did, as expected, have a higher average pattern separation index than the control group. While this effect was significant in the LRP and LRE studies, it was not significant in the ISL study, although the effect was in the same direction. The results from the MCOL studies are consistent with those from several other studies showing that treating OVX rats with estrogens improves performance on hippocampus-sensitive tasks (e.g. Galea et al., 2017; Gibbs, 2000; Korol and Kolo, 2002; Korol and Pisani, 2015; Korol and Wang, 2018; Luine et al, 1998). Specifically, the results from the MCOL studies are consistent with the finding that treating OVX rats with the ER agonists PPT or DPN—agonists selective for ERα or ERβ respectively—or estradiol improves performance on the MCOL task (Tunur et al., 2012). However, some investigators have found that the elevated ovarian hormones in cycling rats can impair acquisition of a spatial version of the Morris water maze (Frye, 1995; Warren and Juraska, 1997) Although, others found that cycling females show place learning biases at high hormone stages but response learning biases at low hormone stages in a dual-solution T-maze (Hussain et al., 2014; Korol et al., 2004; McElroy and Korol, 2005). Thus, there are some mixed results in investigations of ovarian hormone levels on spatial learning and memory in naturally cycling rats, likely a result of task variables such as stress (e.g Perrot-Sinal et al., 2000; Shansky et al., 2006).
A high fat and a low fat subgroup were included for each exposure group in the MCOL studies. However, there was no significant effect of diet and no interaction between botanical treatment and diet. This is in contrast to findings that high fat diets cause impairments on a variety of cognitive tasks, including hippocampus-sensitive tasks, prefrontal cortex-sensitive tasks, and object recognition tasks (Camer et al., 2015; Carey et al., 2014; Freeman et al, 2014; Jurdak and Kanarek, 2009; Kaczmarczyk et al., 2013; Underwood and Thompson, 2016; Winocur and Greenwood, 2005).
The lack of a high fat diet-induced impairment in the MCOL studies could be due to a number of factors. First, most studies examining the effects of a high fat diet on cognition utilized only males, and we studied OVX females (e.g Freeman et al, 2014; Winocur and Greenwood, 2005). Additionally, many studies investigating the effects of a high fat diet on cognition in rodents used levels of fat that were higher than the ones used in the MCOL studies. Several studies have used diets containing as much as 60% kcal from fat, which is well above the upper limit of human consumption (Buettner et al, 2007; Winocur and Greenwood, 2005). There is some evidence that a Western diet (41% kcal from fat) and a very high fat diet (60% kcal from fat) can affect the brain differently. When administered to mice, both diets led to an increase in body weight, only the very high fat diet impaired performance on a T-maze, increased brain inflammation and reduced brain-derived neurotrophic factor (BDNF) levels (Pistell et al., 2010), shown to play a crucial role in hippocampal learning and long-term potentiation (Korol et al., 2013; Yamada and Nabeshima, 2003). Thus, it is possible that the cognitive effects of a high fat diet can differ depending on how much fat the diet contains.
Given the lack of diet effects in all three of the MCOL studies and limited resources, we did not include high fat groups for the DOR study. While high fat diets can affect aspects of cognition differentially, previous research has shown that hippocampal function is especially vulnerable to the deleterious effects of a high fat diet (Kanoski et al., 2010). Thus, it is perhaps unlikely that our high fat diet paradigm would have spared performance on the hippocampus-sensitive MCOL task, but impaired performance on the non-hippocampal DOR task.
We also measured serum levels of ISL and its active metabolite LIQ. For the purposes of the analysis, the levels of ISL and LIQ were combined, because regardless of which compound is fed the two readily convert back and forth in the body. We found that while levels of total ISL+LIQ differed between the ISL, LRE and LRP groups, with rats in the LRP group showing the highest level of total ISL+LIQ, the total levels of aglycone in the three groups were quite similar. Aglycone levels are more biologically relevant because in this free form, the compounds are able to bind to receptors and cause biological activity such as activating transcriptional changes or second messenger cascades. In the MCOL studies, a significant behavioral effect was seen in rats treated with ISL or LRE, but not with LRP, despite the fact that aglycone levels were similar between the three studies. Licorice root powder is a complex mixture containing hundreds of different compounds, many of which are not found in LRE or in the pure compound ISL. It is possible that LRP contained compounds that counteracted the behavioral actions of ISL, LIQ or both leading to null effects.
DOR task
In the DOR study we found that treatment with ISL, LRE or LRP had no significant effects on recognition in this striatum-sensitive task in OVX rats, contrary to predictions based on previous results that estrogens, including estradiol, would impair performance on this task (Tunur and Korol, 2015). We did indeed find that estradiol treated rats were impaired on the DOR task compared to all other groups.
While most estrogenic compounds tested to date, including estradiol, PPT, DPN, and genistein, impair performance on striatum-sensitive tasks, ISL, LRE and LRP did not. It is possible that higher doses than the ones used in this study are needed to see a striatum-sensitive learning impairment. It is also possible that these compounds, unlike other estrogens or selective ER modulators, improve hippocampal function without impairing striatal function. We previously found in a dose-effect study that none of the doses of ISL used had an effect on a prefrontally mediated DSA working memory task, (Kundu et al., 2018), similar to our lack of effects on DOR. However, estradiol as well as the ERβ-selective agonist DPN, were previously found to impair performance on this DSA task (Neese et al., 2010; Wang et al., 2009). In a pilot study conducted prior to our DSA study we found that aglycone levels of ISL+LIQ in the ISL-treated rats were likely within the range of those found in rats in the DOR study, although the method of dosing did differ between the two studies. In our DSA study, rats were dosed with ISL 60 minutes prior to testing each day. In the DOR study, the botanical compounds were mixed into the diet, which was available ad libitum. Thus, while most studies to date have found that administering estrogens to OVX rodents results in impairment on striatum-sensitive tasks, the compounds tested in the present study did not, suggesting these botanical compounds in particular might avoid one potential adverse side effect of estrogen supplementation on cognition.
Estrogen effects on object location and object recognition tasks
Our results from the MCOL studies are supported by previous literature on object location tasks showing that administration of estrogens to OVX adult rodents enhances performance on these tasks (Luine et al., 2003; Tunur et al., 2012). However, our results from the DOR study are contrary to findings that estrogens can enhance performance on other types of object recognition tasks (Gresack and Frick, 2006; Luine et al., 2003). This could be because the MCOL and DOR tasks used here differ from most novel object tasks in the literature in several important ways. Previous novel object studies have been conducted by exposing rodents to 2 objects for one training session and then either replacing or moving one of the objects in the test session. Exploration time of the novel vs the familiar object is then used as the measure of learning. The MCOL and DOR tasks differ from those tasks because there are three training sessions instead of one, allowing examination of habituation over study sessions. Additionally, both of the objects are replaced or moved in the final session, perhaps changing the cognitive dimensions of the task that require associations between stimuli. Administration of estradiol, DPN, and PPT, improve performance on the MCOL task while estradiol impairs performance on the DOR task (Tunur et al., 2012; Tunur and Korol, 2015). Previous work has shown that with this paradigm, there is a double dissociation between task and brain area, meaning that hippocampal activation does not seem to be necessary for performing the DOR task and striatum activation does not seem to be necessary to perform the MCOL task (Tunur and Korol, 2015; Korol and Pisani, 2015). In contrast, there is some evidence that the traditional novel object paradigm (e.g Gresack and Frick, 2006; Luine et al., 2003) is hippocampus-sensitive. It has been found that an intrahippocampal infusion of 17β-estradiol enhances performance on this version of the object recognition task via dorsal hippocampal ERK activation (Fernandez et al., 2008). This could be due, at least in part, to the longer delay (between 4 and 48 hours) between presentation of the familiar and the novel objects used in previous studies. It is also possible that the timing of treatment is partially responsible for these disparate results. In most studies investigating the effects of estrogens on object recognition tasks, estrogens were administered either a few minutes prior to the training session or immediately after it (e.g Gresack and Frick, 2006; Luine et al., 2003; Walf et al., 2008). Some studies have administered estrogens up to 4 hours before testing on an object recognition task (e.g Luine et al., 2003; Scharfman et al., 2007). However, in the DOR study, estrogens were administered 48 and 24 hours before training began. Thus, it is possible that estrogens can enhance performance on this task if given close to the time of training, perhaps because of their functions on memory consolidation, but not if given 24 hours prior to training. It is also possible that estrogens can enhance performance on this task through the rapid actions of membrane-associated receptors, but that those actions were not seen in the DOR study due to the timing of administration. These factors could also account for the different findings of the DOR study in the current experiment and other published reports on object recognition
Organ Weights
The uterine proliferation we detected is suggestive of estrogenic activity, especially activity at ERα (Matthiessen et al., 2013). As expected, rats in the ISL, LRE and LRP groups in all studies had uterine horns that did not differ significantly in size from those of control rats. ISL, LRE and LRP can act through estrogen signaling pathways, however, the estrogen-mediated enlargement of the uterus is largely an ERα effect, and these botanicals are ERβ-selective. This is consistent with the lack of a uterotrophic effect from ISL treatment of adult OVX C57BL6 mice in a previous study (Malak-Erdogan et al., 2016). Additionally, while ISL and LIQ have some potential to affect gene expression through the ERα receptor, in addition to the lower binding affinity for the ERα receptor, these compounds fail to form the stable ER-coregulator complexes that are required for stimulation of reproductive tissues (Malak-Erdogan et al., 2016).
Liver toxicity most likely did not account for the collective results following exposures to ISL, LRP or LRE. Even though licorice root contains glycyrrhizin, which has been implicated for its hepatotoxic effects at high doses (Isbrucker and Burdock, 2006), ISL, LRE and LRP did not significantly increase the size of the liver relative to body size. In two of the three MCOL studies, estradiol treated rats had a smaller liver to body weight ratio than control rats as well as botanical treated rats. It is not clear why this was the case, as this effect has not been previously reported in the literature to our knowledge. Additionally, in two of the three MCOL studies, rats fed a high fat diet had a smaller liver to body weight ratio than rats fed a low fat diet. This effect was partially, but not wholly driven by a higher body weight in rats fed a high fat diet. Thus, there were some inconsistent effects of estradiol supplementation as well as dietary fat on the liver to body weight ratio, but no effect of the botanical compounds ISL, LRE or LRP.
E. Summary and Conclusions
In the current studies, treatment with ISL or LRE improved performance of OVX rats on the hippocampus-sensitive MCOL task. Treatment with LRP did not affect performance on the MCOL task. Treatment with ISL, LRE or LRP did not affect performance on the striatum-sensitive DOR task. This is in contrast to most estrogenic compounds tested to date, which typically improve hippocampally-based cognitive functions but impair striatally-based learning and memory. Taken together, these results suggest that ISL and LRE could potentially be promising selective estrogen receptor modulators (SERMs) for improving some aspects of cognitive function in a low estrogen state. Importantly, in all of the studies referenced above, the botanical compounds were administered orally, making the results particularly applicable to human health. Dietary supplements containing licorice root and licorice root components are currently taken as oral supplements and thus go through first pass metabolism, as they did in the present set of studies. However, more studies are needed to show how the doses used in the present studies compare to human exposure from supplements and also to ensure that these or higher doses do not have negative effects such as hepatotoxicity. Additionally, the current studies were done in a surgical menopause model, using OVX young adult rats. Since phytoestrogenic dietary supplements are primarily marketed to post-menopausal women, it will be important to perform similar studies with older OVX rats to determine whether the same pattern of improvement on hippocampus-sensitive tasks and no effect on prefrontal cortex-sensitive and striatum-sensitive tasks are observed across aging.
Highlights.
Rats were OVX and treated with licorice root components, estradiol, or vehicle control
They were then tested on an object location or an object recognition task
Estradiol improved performance on the object location task but impaired performance on the object recognition task
The pure compound isoliquiritigenin and a methanol extract of licorice root both mimicked estradiol and improved performance on the object location task
Unlike estradiol, none of the licorice root components affected performance on the object recognition task
Acknowledgments
Funding: This work was supported by NIH grant 5P50AT006268-05.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. European Journal of Neuroscience. 2006;24(1):229–242. doi: 10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]
- Buettner R, Schölmerich J, Bollheimer LC. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity. 2007;15(4):798–808. doi: 10.1038/oby.2007.608. [DOI] [PubMed] [Google Scholar]
- Burger HG, Dudley EC, Robertson DM, Dennerstein L. Hormonal changes in the menopause transition. Recent progress in hormone research. 2002;57:257–276. doi: 10.1210/rp.57.1.257. [DOI] [PubMed] [Google Scholar]
- Camer D, Yu Y, Szabo A, Fernandez F, Dinh CH, Huang XF. Bardoxolone methyl prevents high-fat diet-induced alterations in prefrontal cortex signalling molecules involved in recognition memory. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2015;59:68–75. doi: 10.1016/j.pnpbp.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Carey AN, Gomes SM, Shukitt-Hale B. Blueberry supplementation improves memory in middle-aged mice fed a high-fat diet. Journal of agricultural and food chemistry. 2014;62(18):3972–3978. doi: 10.1021/jf404565s. [DOI] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Intra-hippocampal lidocaine injections impair acquisition of a place task and facilitate acquisition of a response task in rats. Behavioural brain research. 2003;144(1–2):19–24. doi: 10.1016/S0166-4328(03)00063-9. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Roberts SL, Dohanich GP. Effects of ovarian hormones and environment on radial maze and water maze performance of female rats. Physiology & Behavior. 1999;66(1):11–20. doi: 10.1016/S0031-9384(98)00272-8. [DOI] [PubMed] [Google Scholar]
- Davis DM, Jacobson TK, Aliakbari S, Mizumori SJY. Differential effects of estrogen on hippocampal-and striatal-dependent learning. Neurobiology of learning and memory. 2005;84(2):132–137. doi: 10.1016/j.nlm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Dohanich G, Korol D, Shors T. Hormones, Brain and Behavior Online. Elsevier Inc; 2010. Steroids, learning and memory. [DOI] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, … Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. Journal of Neuroscience. 2008;28(35):8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman LR, Haley-Zitlin V, Rosenberger DS, Granholm AC. Damaging effects of a high-fat diet to the brain and cognition: a review of proposed mechanisms. Nutritional neuroscience. 2014;17(6):241–251. doi: 10.1179/1476830513Y.0000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA. Estrus-associated decrements in a water maze task are limited to acquisition. Physiology & behavior. 1995;57(1):5–14. doi: 10.1016/0031-9384(94)00197-D. [DOI] [PubMed] [Google Scholar]
- Galea LA, Wide JK, Paine TA, Holmes MM, Ormerod BK, Floresco SB. High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects on working memory. Behavioural brain research. 2001;126(1–2):115–126. doi: 10.1016/S0166-4328(01)00255-8. [DOI] [PubMed] [Google Scholar]
- Galea LA, Frick KM, Hampson E, Sohrabji F, Choleris E. Why estrogens matter for behavior and brain health. Neuroscience & Biobehavioral Reviews. 2017;76:363–379. doi: 10.1016/j.neubiorev.2016.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats☆. Neurobiology of aging. 2000;21(1):107–116. doi: 10.1016/S0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Glazier MG, Bowman MA. A review of the evidence for the use of phytoestrogens as a replacement for traditional estrogen replacement therapy. Archives of internal medicine. 2001;161(9):1161–1172. doi: 10.1001/archinte.161.9.1161. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. The interactions and dissociations of the dorsal hippocampus subregions: how the dentate gyrus, CA3, and CA1 process spatial information. Behavioral neuroscience. 2008;122(1):16. doi: 10.1037/0735-7044.122.1.16. [DOI] [PubMed] [Google Scholar]
- Granholm AC, Bimonte-Nelson HA, Moore AB, Nelson ME, Freeman LR, Sambamurti K. Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. Journal of Alzheimer’s Disease. 2008;14(2):133–145. doi: 10.3233/JAD-2008-14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacology Biochemistry and Behavior. 2006;84(1):112–119. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Hussain D, Shams W, Brake W. Estrogen and memory system bias in females across the lifespan. Translational Neuroscience. 2014;5(1):35–50. [Google Scholar]
- Huang HH, Steger RW, Bruni JF, Meites J. Patterns of sex steroid and gonadotropin secretion in aging female rats. Endocrinology. 1978;103(5):1855–1859. doi: 10.1210/endo-103-5-1855. [DOI] [PubMed] [Google Scholar]
- Hwang LL, Wang CH, Li TL, Chang SD, Lin LC, Chen CP, … Chiou LC. Sex differences in high-fat diet-induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice. Obesity. 2010;18(3):463–469. doi: 10.1038/oby.2009.273. [DOI] [PubMed] [Google Scholar]
- Isbrucker RA, Burdock GA. Risk and safety assessment on the consumption of Licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regulatory Toxicology and Pharmacology. 2006;46(3):167–192. doi: 10.1016/j.yrtph.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Jang EY, Choe ES, Hwang M, Kim SC, Lee JR, Kim SG, … Yang CH. Isoliquiritigenin suppresses cocaine-induced extracellular dopamine release in rat brain through GABAB receptor. European journal of pharmacology. 2008;587(1–3):124–128. doi: 10.1016/j.ejphar.2008.03.054. [DOI] [PubMed] [Google Scholar]
- Jurdak N, Kanarek RB. Sucrose-induced obesity impairs novel object recognition learning in young rats. Physiology & behavior. 2009;96(1):1–5. doi: 10.1016/j.physbeh.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Kaczmarczyk MM, Machaj AS, Chiu GS, Lawson MA, Gainey SJ, York JM, … Woods JA. Methylphenidate prevents high-fat diet (HFD)-induced learning/memory impairment in juvenile mice. Psychoneuroendocrinology. 2013;38(9):1553–1564. doi: 10.1016/j.psyneuen.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Zhang Y, Zheng W, Davidson TL. The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat. Journal of Alzheimer’s Disease. 2010;21(1):207–219. doi: 10.3233/JAD-2010-091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behavioral neuroscience. 2002;116(3):411. doi: 10.1037/0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. Shifts in preferred learning strategy across the estrous cycle in female rats. Hormones and behavior. 2004;45(5):330–338. doi: 10.1016/j.yhbeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Korol DL, Pisani SL. Estrogens and cognition: Friends or foes?: An evaluation of the opposing effects of estrogens on learning and memory. Hormones and behavior. 2015;74:105–115. doi: 10.1016/j.yhbeh.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol DL, Wang W. Using a memory systems lens to view the effects of estrogens on cognition: Implications for human health. Physiology & behavior. 2018 doi: 10.1016/j.physbeh.2017.11.022. [DOI] [PMC free article] [PubMed]
- Kundu P, Neese SL, Bandara S, Monaikul S, Helferich WG, Doerge DR, … Schantz SL. The effects of the botanical estrogen, isoliquiritigenin on delayed spatial alternation. Neurotoxicology and teratology. 2018;66:55–62. doi: 10.1016/j.ntt.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre J, Mcclintock MK. Reproductive senescence in female rats: a longitudinal study of individual differences in estrous cycles and behavior. Biology of Reproduction. 1988;38(4):780–789. doi: 10.1095/biolreprod38.4.780. [DOI] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Hormones and behavior. 1998;34(2):149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, MacLusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144(7):2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Luine V, Frankfurt M. Introduction to the Special Issue Estradiol and Cognition: Molecules to Mind. Hormones and behavior. 2015;74:1. doi: 10.1016/j.yhbeh.2015.08.011. [DOI] [PubMed] [Google Scholar]
- Madak-Erdogan Z, Gong P, Zhao YC, Xu L, Wrobel KU, Hartman JA, … Twaddle NC. Dietary licorice root supplementation reduces diet-induced weight gain, lipid deposition, and hepatic steatosis in ovariectomized mice without stimulating reproductive tissues and mammary gland. Molecular nutrition & food research. 2016;60(2):369–380. doi: 10.1002/mnfr.201500445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggiolini M, Statti G, Vivacqua A, Gabriele S, Rago V, Loizzo M, … Amdò S. Estrogenic and antiproliferative activities of isoliquiritigenin in MCF7 breast cancer cells. The Journal of steroid biochemistry and molecular biology. 2002;82(4–5):315–322. doi: 10.1016/S0960-0760(02)00230-3. [DOI] [PubMed] [Google Scholar]
- Maronpot RR, Yoshizawa K, Nyska A, Harada T, Flake G, Mueller G, … Ward JM. Hepatic enzyme induction: histopathology. Toxicologic pathology. 2010;38(5):776–795. doi: 10.1177/0192623310373778. [DOI] [PubMed] [Google Scholar]
- Martin AL, Brown RE. The lonely mouse: verification of a separation-induced model of depression in female mice. Behavioural brain research. 2010;207(1):196–207. doi: 10.1016/j.bbr.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Maruki K, Izaki Y, Hori K, Nomura M, Yamauchi T. Effects of rat ventral and dorsal hippocampus temporal inactivation on delayed alternation task. Brain research. 2001;895(1–2):273–276. doi: 10.1016/S0006-8993(01)02084-4. [DOI] [PubMed] [Google Scholar]
- Matthiessen P. Endocrine Disrupters: Hazard Testing and Assessment Methods. John Wiley & Sons; 2013. [Google Scholar]
- McElroy MW, Korol DL. Intrahippocampal muscimol shifts learning strategy in gonadally intact young adult female rats. Learning & Memory. 2005;12(2):150–158. doi: 10.1101/lm.86205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Invited review: estrogens effects on the brain: multiple sites and molecular mechanisms. Journal of applied physiology. 2001;91(6):2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- McNeilly AD, Williamson R, Sutherland C, Balfour DJ, Stewart CA. High fat feeding promotes simultaneous decline in insulin sensitivity and cognitive performance in a delayed matching and non-matching to position task. Behavioural brain research. 2011;217(1):134–141. doi: 10.1016/j.bbr.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Mersereau JE, Levy N, Staub RE, Baggett S, Zogric T, Chow S, … Leitman DC. Liquiritigenin is a plant-derived highly selective estrogen receptor β agonist. Molecular and cellular endocrinology. 2008;283(1–2):49–57. doi: 10.1016/j.mce.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miksicek RJ. Commonly occurring plant flavonoids have estrogenic activity. Molecular Pharmacology. 1993;44(1):37–43. [PubMed] [Google Scholar]
- National Institutes of Health (US). Office for Protection from Research Risks. Public Health Service policy on humane care and use of laboratory animals. Office for Protection from Research Risks, National Institutes of Health; 1986. [Google Scholar]
- Neese SL, Korol DL, Katzenellenbogen JA, Schantz SL. Impact of estrogen receptor alpha and beta agonists on delayed alternation in middle-aged rats. Hormones and behavior. 2010;58(5):878–890. doi: 10.1016/j.yhbeh.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LF, Radow BL. Isolation stress and volitional ethanol consumption in the rat. Physiology & behavior. 1974;12(1):1–3. doi: 10.1016/0031-9384(74)90060-2. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal T, Ossenkopp KP, Kavaliers M. Influence of a natural stressor (predator odor) on locomotor activity in the meadow vole (Microtus pennsylvanicus): modulation by sex, reproductive condition and gonadal hormones. Psychoneuroendocrinology. 2000;25(3):259–276. doi: 10.1016/S0306-4530(99)00054-2. [DOI] [PubMed] [Google Scholar]
- Pisani SL, Neese SL, Doerge DR, Helferich WG, Schantz SL, Korol DL. Acute genistein treatment mimics the effects of estradiol by enhancing place learning and impairing response learning in young adult female rats. Hormones and behavior. 2012;62(4):491–499. doi: 10.1016/j.yhbeh.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani SL, Neese SL, Katzenellenbogen JA, Schantz SL, Korol DL. Estrogen receptor-selective agonists modulate learning in female rats in a dose-and task-specific manner. Endocrinology. 2016;157(1):292–303. doi: 10.1210/en.2015-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, Bruce-Keller AJ. Cognitive impairment following high fat diet consumption is associated with brain inflammation. Journal of neuroimmunology. 2010;219(1):25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Hintz TM, Gomez J, Stormes KA, Barouk S, Malthankar-Phatak GH, … MacLusky NJ. Changes in hippocampal function of ovariectomized rats after sequential low doses of estradiol to simulate the preovulatory estrogen surge. European journal of neuroscience. 2007;26(9):2595–2612. doi: 10.1111/j.1460-9568.2007.05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Rubinow K, Brennan A, Arnsten AF. The effects of sex and hormonal status on restraint-stress-induced working memory impairment. Behavioral and Brain Functions. 2006;2(1):8. doi: 10.1186/1744-9081-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler C, Hajirahimkhan A, Lankin DC, Bolton JL, Jones T, Soejarto DD, … Pauli GF. Dynamic residual complexity of the isoliquiritigenin–liquiritigenin interconversion during bioassay. Journal of agricultural and food chemistry. 2013;61(9):2146–2157. doi: 10.1021/jf304445p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srihari S, Shanthibhai PK, Rajagopala S. Efficacy of Glycyrrhiza Glabra Linn (Yastimadhu) in Learning, Memory and Cognitive Activity- Current Findings and Future Avenues. Global Journal of Research on Medicinal Plants & Indigenous Medicine. 2012;1(6):247. [Google Scholar]
- Tamir S, Eizenberg M, Somjen D, Izrael S, Vaya J. Estrogen-like activity of glabrene and other constituents isolated from licorice root. The Journal of steroid biochemistry and molecular biology. 2001;78(3):291–298. doi: 10.1016/S0960-0760(01)00093-0. [DOI] [PubMed] [Google Scholar]
- Tunur T, Zendeli L, Korol DL. Nuances of pattern separation determine modulation by estradiol. 42nd Annual meeting for the Society for Neuroscience, Society for Neuroscience.2012. [Google Scholar]
- Tunur T, Zendeli L, Korol DL. Opposing Effects of Estrogens on Two Different Pattern Separation Tasks. 45th Annual meeting for the Society for Neuroscience, Society for Neuroscience.2015. [Google Scholar]
- Underwood EL, Thompson LT. A high-fat diet causes impairment in hippocampal memory and sex-dependent alterations in peripheral metabolism. Neural plasticity. 2016;2016 doi: 10.1155/2016/7385314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guidelines for the care and use of mammals in neuroscience and behavioral research. National Academies Press; 2003. [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor beta knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiology of learning and memory. 2008;89(4):513–521. doi: 10.1016/j.nlm.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang VC, Sable HJ, Ju YH, Allred CD, Helferich WG, Korol DL, Schantz SL. Effects of chronic estradiol treatment on delayed spatial alternation and differential reinforcement of low rates of responding. Behavioral neuroscience. 2008;122(4):794. doi: 10.1037/a0012513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang VC, Neese SL, Korol DL, Schantz SL. Chronic estradiol replacement impairs performance on an operant delayed spatial alternation task in young, middle-aged, and old rats. Hormones and behavior. 2009;56(4):382–390. doi: 10.1016/j.yhbeh.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SG, Juraska JM. Spatial and nonspatial learning across the rat estrous cycle. Behavioral neuroscience. 1997;111(2):259. doi: 10.1037/0735-7044.111.2.259. [DOI] [PubMed] [Google Scholar]
- Wide JK, Hanratty K, Ting J, Galea LA. High level estradiol impairs and low level estradiol facilitates non-spatial working memory. Behavioural brain research. 2004;155(1):45–53. doi: 10.1016/j.bbr.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Winocur G, Greenwood CE. Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiology of aging. 2005;26(1):46–49. doi: 10.1016/j.neurobiolaging.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Yamada K, Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. Journal of pharmacological sciences. 2003;91(4):267–270. doi: 10.1254/jphs.91.267. [DOI] [PubMed] [Google Scholar]
- Zhan C, Yang J. Protective effects of isoliquiritigenin in transient middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Pharmacological Research. 2006;53(3):303–309. doi: 10.1016/j.phrs.2005.12.008. [DOI] [PubMed] [Google Scholar]