Abstract
The relationship between biological sex and aldosterone (Aldo) on blood pressure (BP) is unclear. We hypothesized that sex would modify the interaction between Aldo and vascular responses to salt intake and angiotensin-II (AngII). To test this hypothesis, in 1,592 subjects from the well-controlled Hypertensive Pathotype cohort, we compared responses of women and men to chronic [BP and Aldo levels in response to dietary salt] and acute [BP, renal plasma flow (RPF) and Aldo responses to AngII infusion] manipulations. Women had a 30% higher salt sensitivity of BP than men (p<0.0005) regardless of age or hypertension status, a greater BP response to AngII and a15% greater Aldo response to AngII on both restricted and liberal salt diets (p<0.005). Further, there was an interaction (p=0.003) between sex and Aldo on BP response to AngII. Women also had a greater (p<0.01) increment in RPF in response to AngII than men. To assess potential mechanisms for this sex effect, we compared Aldo responses to AngII or potassium from rat zona glomerulosa (ZG) cells and observed greater Aldo production in female than male ZG cells basally and in response to both agonists. (p<0.0001). In a rodent model of Aldo-mediated cardiovascular disease (CVD) induced by increased AngII and low NO, circulating Aldo levels (p<0.01), myocardial damage (p<0.001) and proteinuria (p<0.05) were greater in female than male rats despite having similar BP responses. Thus, increased Aldo production likely contributes to sex differences in CVD, suggesting that women may be more responsive to mineralocorticoid receptor blockade than men.
Keywords: aldosterone, gender, sex, mineralocorticoid receptor, angiotensin, salt sensitive hypertension, blood pressure
INTRODUCTION
Less than 50% of hypertensive patients in the US have their blood pressure (BP) under control. These numbers worsen in older women. Furthermore, hypertension is more common in young males when compared to premenopausal females; a difference that disappears following menopause1. Indeed, premenopausal women are protected from cardiovascular diseases (CVD) while postmenopausal women have the same risk for these diseases, as do men. However, women appear to be at greater risk than men for developing hypertension-related CVD, i.e., heart failure with preserved ejection fraction –a condition with limited effective treatment options2. Thus, understanding the mechanisms driving disordered BP control and its outcomes in males and females may lead to more personalized and targeted therapy to effectively and better control BP and reduce adverse cardiovascular outcomes.
One such mechanistic pathway is the Renin-Angiotensin-Aldosterone System (RAAS). This system plays a critical role in BP regulation both via its effect on sodium and volume homeostasis and vascular reactivity and structure. For example, there is abundant evidence showing that in humans and rodents, chronic activation of the mineralocorticoid receptor (MR) and disordered angiotensin II (AngII) and/or aldosterone (Aldo) levels lead to an increase in BP and cardiovascular and renal damage. However, the impact of biological sex on RAAS activation is controversial. Early reports showed that plasma renin activity (PRA) was higher in men than in women regardless of age and ethnic heritage3, 4. Of interest, there are reports showing that men and women have similar mean BP responses to acute AngII infusion 5–7. In contrast, more recent studies showed that men had greater diastolic and mean BP responses to acute AngII than did women8.
Inconsistencies regarding Aldo levels among men and women also have been observed. For example, women from the Framingham Offspring Study showed higher Aldo levels than did men 9. Aldo levels in women are associated with changes in the estrous cycle. There is evidence that Aldo levels are higher in women than men during the luteal phase of the menstrual cycle, but not during the periovulatory period or during menses 10. Indeed, Aldo secretion rates during the follicular phase were comparable to those in men but significantly increased during the luteal phase in women11. However, these studies did not control for sodium intake or posture, major influences on RAAS activity. In contrast, Miller et al. reported plasma Aldo levels that were lower in women than men on a liberal sodium (200 mmol) intake that were measured while subjects were supine6. However, these women had a greater increase in Aldo response to AngII infusion than men6. Ruilope et al. reported opposite results: women had a greater Aldo response to upright posture than did men, again with dietary salt controlled12. Interestingly, these investigators did not find any difference in PRA responses to standing up. Reports by members of our group have shown that Aldo levels are higher during the luteal phase on high- but not low-sodium balance, whereas renin and AngII do not differ between phases13. Furthermore, Katz et al. reported that plasma Aldo levels were higher in women than in men on restricted sodium diets without control for phase of the menstrual cycle14. Finally, in a small population, we reported that Aldo responses to AngII were greater in women than men with hypertension and likely familially associated15, 16. This difference narrows in older subjects and is likewise not observed in hypertensives of African descent17. Thus, there remains controversy concerning the relationships between biological sex, Aldo/MR activation and cardiovascular damage. In part, the reported discrepancies are likely secondary to small sample sizes and failure to control for environmental factors, such as, salt intake, posture and time of day that have important effects on RAAS activity.
To address this controversy, we hypothesized that females, regardless of menopausal and disease status, will have greater salt sensitivity of BP than men and that, the primary mechanism underlying this difference is altered Aldo production. We used two approaches to test this hypothesis. First, we studied a large cohort of subjects participating in the Hypertensive Pathotype (HyperPATH) Consortium where subjects are well characterized and environmental, menopausal state and dietary factors that can affect Aldo and BP are tightly controlled18–20. We contrasted AngII stimulated Aldo levels and salt sensitivity of BP in women and men. Second, we assessed sex differences in a chronic rodent model that produces vascular and renal damage in response to increased AngII and low NO levels and characterized the effects of MR blockade on these injuries. These comparative studies of male and female rats allowed for sex-based analyses of AngII function and vascular damage in a model of chronic MR activation.
METHODS
HyperPATH Cohort and Study Protocol
Our studies were performed on extensively characterized subjects from the HyperPATH consortium. This is an ongoing study aimed at investigating the pathophysiologic and genotypic mechanisms involved in hypertension and CVD that has been previously described18–20. We studied 1592 individuals (682 women and 910 men), with similar protocols (Figure S1). The Human Subjects Committees approved these protocols and informed written consent was obtained from each subject. Subjects were consented well before the development of guidelines to promote openness. Therefore, requests for select de-identified study data and analytic methods will be considered on a case-by-case basis from qualified researchers with institutional review board approvals and executed institutional data transfer agreements. The dataset will be available from Dr. Gordon H. Williams, Principal Investigator for the HyperPATH consortium, by email request (gwilliams@bwh.harvard.edu). In this study, menopausal status was estimated by age <51 (premenopausal) and ≥51 years (postmenopausal) of age. In a subset of each age group, we verified a difference in menopausal status by estradiol and Follicle-stimulating hormone (FSH) levels. Women on hormonal contraceptives or hormone replacement therapy were excluded. Hormones and electrolytes in serum and urine were measured in the Brigham and Women’s Hospital’s Research Assay Core Laboratory as previously reported18–20. Details are included online Data Supplement Methods.
Clinical Statistical Analyses
Baseline analyses included Student t test and X2. Continuous variables are presented as mean ± standard deviation or mean ± SEM. Categorical variables are presented as a percentage of the total sample. Normal distribution of dependent variable was tested using Shapiro-Wilk test. Logarithmic transformation was performed for variables that were not normally distributed. The nominal p value was adjusted to 0.01 because of multiple comparisons. All statistical analyses were performed using STATA 14.1, adjusting for the following confounders: disease state (normotensive or hypertensive), age, sex, site, serum cortisol, PRA, serum potassium (K+) and urinary K+ excretion. The adjustments differed based on the primary endpoints being assessed as noted below. These covariates (fixed effects) were chosen for their clinical importance and significance in univariate analysis, whereas identity was the random effect. Multiple linear regression was used to analyze the relationship between primary endpoints (e.g., salt sensitivity of BP) and biological sex. For salt sensitivity of BP and Renal Plasma Flow (RPF) the regression was adjusted for known cardiovascular risk factors [age, BMI, disease state (hypertensive or normotensive) and race] and site. For analyses of the relationship between Aldo and sex four more adjustments were added: plasma cortisol [surrogate for ACTH], PRA, serum K+ and urine K+. In addition, in all subjects where the variables were available, we performed an interaction analysis between sex and Aldo on systolic BP where the data were obtained under the similar conditions. To maximize the potential to see an effect, we used data obtained from a “stress test”: restricted salt diet, supine and in response to an AngII infusion. The Aldo component was dichotomized by using the median Aldo increments (16.1 ng/dL). SBP response to AngII was the dependent variable using the same adjustments as noted above. We also compared female and male subjects using Propensity Score analyses (STATA PSMATCH2). We used this approach to allow us to assemble female and male study populations that are balanced on the baseline covariates of age, race, body mass index, site and disease state.
Rodents
Male and female wistar rats were maintained on a liberal salt diet and water ad libitum. As previously reported, injury was caused by treating rats with an inhibitor of NOS, N[omega]-nitro-L-arginine-methyl-ester (L-NAME), for 14 days, plus AngII on days 11 through 1421, 22. Adrenal glands were excised during euthanization, and adrenal zona glomerulosa (ZG) cells were isolated as previously reported23. Details are included online Data Supplement.
RESULTS
Increased Salt Sensitivity of Blood Pressure in Women Compared to Men
In the HyperPATH cohort, women and men had similar mean age, body mass index, PRA, serum K+ and urinary Aldo (Table S1). Admission BP, serum cortisol, serum Aldo, upright PRA and urinary sodium and K+ excretion were higher among men. However, salt sensitivity of BP was greater in female than males (Adjusted β=+3.40, 95% Confidence Interval (CI; 1.85,4.94) [Fig 1A]). This difference was evident even when subjects were stratified by hypertension status (Fig 1B) or those older than or equal to 51 years of age or younger than 51 years of age (Fig 1C). These differences in BP responses to salt intake maybe due, in part, to alterations in adrenal or renal vascular responses to RAAS activation24. Although we used age as a surrogate for menopausal state, hormonal data support this conclusion. In a subgroup analyses, the older women had greater FSH levels (54.8 ± 3.2 mIU/mL versus 14.3 ± 1.8 mIU/mL) and lower estradiol levels (30.4 ± 7.0 pg/mL versus 58.7 ± 4.8 pg/mL) than the younger women group.
Figure 1. (A) Women have increased Salt Sensitivity of Blood Pressure (BP) when compared to men.
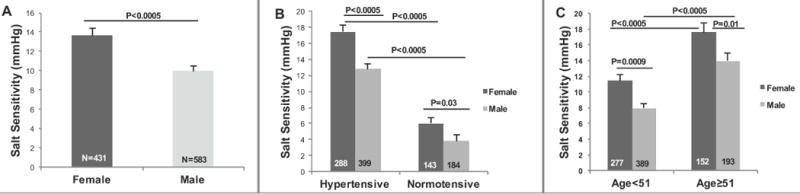
Salt Sensitivity = Baseline Systolic BP (SBP) on a liberal salt diet – Baseline SBP on a restricted salt diet. (B) Salt Sensitivity of BP is higher in women than men in both hypertensive and normotensive state. (C) Salt Sensitivity of BP increased with age in both males and females, but the females still had significantly greater salt sensitive BP than males regardless of age. The P values are from Multiple Linear Regression models that were adjusted for age, sex, body mass index, site and disease state. Values represent the means ± SEM. A propensity score was also calculated for the total population (Coefficient= 3.12; p= 0.001; 95% Confidence Intervals (CI)= 1.21, 5.02).
Sex Affects Aldosterone Regulation, Renal-Vascular Hemodynamics and Blood Pressure Responses to Acute RAAS–Activation in Human Subjects
To begin to understand the mechanism for sex differences in salt sensitivity of BP, we compared the acute Aldo responses to AngII infusion in males versus females in our cohort. In response to AngII, the adrenal gland produces and releases the steroid hormone Aldo, which can be measured in the serum. Sex exerted a strong influence on adrenal responsiveness to AngII (Fig 2) both in subjects maintained on a liberal salt diet or following a restricted salt diet (Fig 2A–B) with women having significantly greater Aldo responses on both. When stratified by age, the sex difference was evident only in subjects younger than 51 years of age, regardless of salt intake, with younger women having a significantly greater Aldo response than younger men (Fig S2 and Fig S3). However, this difference between men and women disappeared in subjects ≥51 years of age where the Aldo responses were similar. We also studied RPF response to AngII infusion in our cohort. RPF response to AngII was greater in women than men on a liberal salt diet (Fig 3A). This sex difference was amplified when subjects were salt restricted (Fig 3B). As with the adrenal response, the differences between men and women was not evident in subjects ≥51 years of age where the RPF responses were similar in both sexes (Fig S4). We then studied systolic BP responses to AngII in our cohort. Women had similar BP rise in response to AngII infusion when compared to men on both restricted salt (Fig S5) or liberal salt diets. However, sex differences in the BP responses to AngII were evident when stratified by age and by hypertensive versus normotensive subjects (Fig 4). Specifically, hypertensive women had greater BP responses to AngII than men in both age groups. In contrast, normotensive women <51 years of age had lower BP responses to AngII than did men; a difference that disappeared in subjects ≥51 years of age where the BP responses were similar in both sexes while young normotensive males had a greater response than young females to AngII. Interaction between sex and Aldo on systolic BP was also assessed. This analysis was performed using data where both BP and Aldo were “stressed”. The stress test was the Aldo and BP response to AngII infusion on a restricted salt diet. The Aldo levels were dichotomized above and below the median of the increment in Aldo in response to AngII (16.1 ng/dL). In the fully adjusted model, there was a significant interaction (p=0.003) between sex and Aldo on the systolic BP response to AngII as documented in figure legend 4. Finally, propensity scores were calculated on the data in Figs 1–4 to test for equivalency in the two sex groups. The differences between the sexes were still highly significantly different as documented in the figure legends.
Figure 2. Aldosterone (Aldo) responses to angiotensin II (AngII) are greater in women than men on either a Liberal or Restricted Salt Diet.
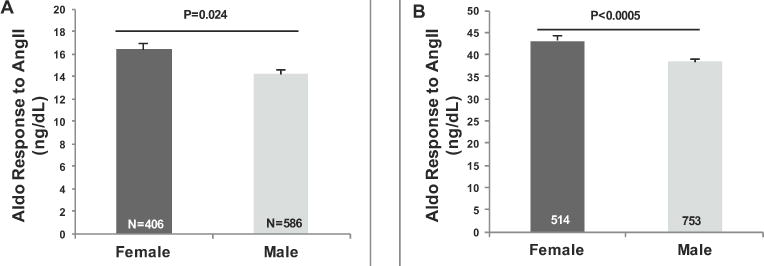
Aldo responses were determined on a Liberal Salt (A) or restricted salt (B) diet. The P values are from Multiple Linear Regression models that were adjusted for age, sex, body mass index, site, disease state, PRA, plasma K+, urine K+ and plasma cortisol. Values represent the means ± SEM. Propensity scores were also calculated for the total population: A) liberal salt (Coefficient= 3.24; p< 0.000; 95% CI= 1.83, 4.66); B) restricted salt (Coefficient= 7.10; p< 0.000; 95% CI= 4.28, 9.91).
Fig 3. (A) Delta PAH (baseline liberal salt PAH - PAH following stimulation by AngII) by gender. (B) Delta PAH (baseline salt restricted PAH-PAH following stimulation by AngII) by gender.
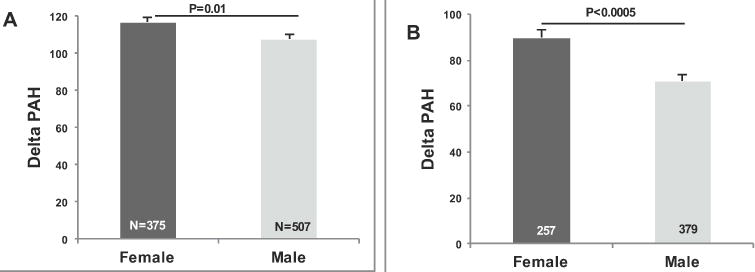
The P values are from Multiple Linear Regression models that were adjusted for age, sex, body mass index, site and disease state. Values represent the means ± SEM. Propensity scores were also calculated for the total population: A) liberal salt (Coefficient= 12.6; p< 0.004; 95% CI= 4.1, 21.2); B) restricted salt (Coefficient= 16.4; p< 0.000; 95% CI= 7.4, 25.3).
Figure 4. Systolic Blood Pressure (SBP) responses to Angiotensin II (AngII) increased with age and are higher in women than men in hypertensive group on a salt-restricted diet while in normotensive group only women with age<51 years old have higher response than men.
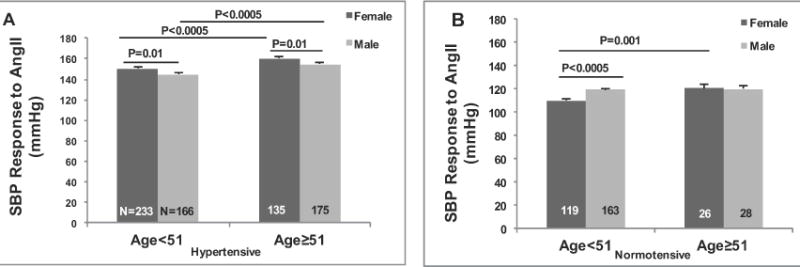
Interaction analyses between Aldo and sex on SBP response to AngII in the total population was shown to be highly significant (Coefficient= -7.41; p= 0.003; 95% CI= 12.24, 2.57). The p values are from Multiple Linear Regression models that were adjusted for age, sex, body mass index, site and disease state. For the interaction analyses the same adjustments were made except for sex. Values represent the means ± SEM. A propensity score was also calculated for the total population (Coefficient= 5.13; p< 0.000; 95% CI= 3.14, 7.13).
Rodent studies
Mechanism responsible for the sex difference
To characterize the differential effects of AngII on Aldo levels in women versus men, we then turned to an isolated rat adrenal ZG cell preparations. Aldo levels basally and in responses to AngII and K+ were significantly greater in females than males (Fig 5). Further, 24-hour urine Aldo levels were significantly greater (p<0.05) in females (1.1 ± 0.1 ng) than males (0.6 ± 0.1 ng). These data support our hypothesis that females produce more Aldo than males and thereby have an increased risk of Aldo mediated cardiovascular and renal damage.
Figure 5. Acutely isolated ex vivo adrenal Zona Glomerulosa (ZG) cells from female rats have greater Aldosterone (Aldo) responses to agonists than male rats.
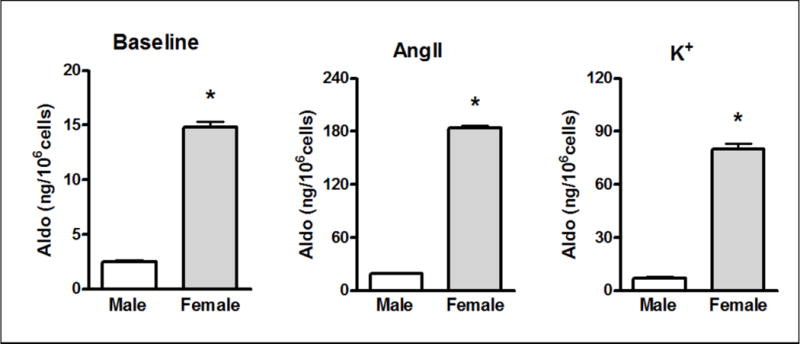
p<0.001, Values are means ± SEM n=9 per group.
Cardiac and renal damage
We have previously reported that in the L-NAME/AngII rat model, MR blockade prevents the cardiovascular and renal damage, suggesting that an inappropriately activated MR is the principle mechanism even though the Aldo levels are not increased21, 22. Thus, we assessed the effect of sex on the level of damage. Sex-based comparisons revealed greater cardiac and renal damage in female compared to male rats as measured by myocardial necrosis and renal protein excretion respectively (Fig 6A and 6B).
Figure 6. Compared to Male Rats, Female Rats Have Greater Myocardial Injury (A), Elevated Proteinuria (B) but Similar Blood Pressures Levels (C) and Higher Aldosterone Levels (D) Following Treatment with L-NAME/AngII.
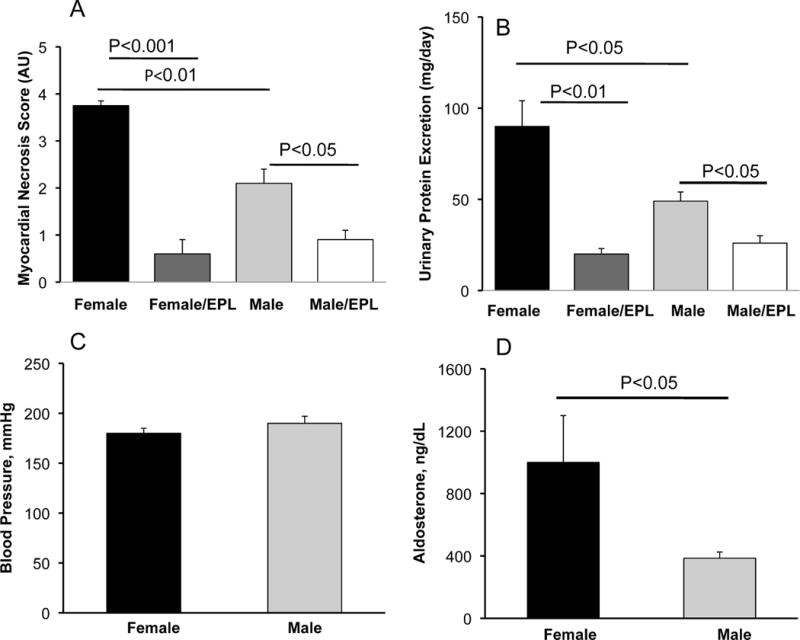
Values represent the means ± SEM n=6 per group.
These sex differences in end-organ damage were not secondary to female rats receiving more L-NAME/AngII (males and females weighed the same) nor to differences in BP (male and female rats had similar BPs responses to treatment–Fig 6C). However, the Aldo response to L-NAME/AngII was significantly greater in the female compared to the male rats (Fig 6D). Further, cardiac injury and proteinuria were reduced to the same extent in male and female rats in animals treated with eplerenone (Fig 6A and 6B).
DISCUSSION
Our human and animal data support the hypothesis that females, regardless of menopausal and disease status, will have greater salt sensitivity of BP than men and that the primary mechanism underlying this difference is altered Aldo production. Women, whether young or old, hypertensive or normal had a significantly greater degree of salt sensitivity of BP than men. Further, the mechanisms underlying these differences are likely secondary to differences in two-major sodium/volume modulating mechanisms –the kidney and Aldo responses. Both vascular [as estimated by RPF] and Aldo responses to stimulation with AngII in humans and Aldo responses to AngII and K+ in adrenal ZG cells and AngII in vivo in rats are greater in females than males. Finally, the potential pathophysiologic significance of these findings is exemplified by the greater degree of cardiovascular and renal damage in females observed in the L-NAME/AngII rodent model that was largely prevented by MR blockade.
Salt sensitivity of BP increased with age in both men and women in our cohort; confirming reports by others 25. However, we observed that salt sensitivity of BP was greater in women than men in both young and older individuals. Our results extend previous reports in young African American women, the DASH-Sodium trial and the GenSalt Chinese cohort study 26–28. Each of these studies suggested that women had greater salt sensitivity of BP than men26–28. We, and others, have shown that increased salt intake leads to increased RPF; a relationship that is lost in salt sensitive forms of hypertension24, 29. We explored whether RPF response to AngII would be affected by sex and found significant differences in our cohort. This would suggest that RAAS-mediated renal vascular hemodynamics might contribute to salt sensitivity of BP and CVD among women.
Sex differences in BP levels have been reported previously in rodents. A recent review by Blenck et al. summarized the literature in hypertensive rats30. In three different L-NAME/AngII models, BP was higher in males in one, higher in females in another and no difference in a third. In studies of Spontaneously Hypertensive Rats, males have higher BP than females30. In addition, Xue et al. reported that chronic AngII increases BP to a higher level in male than female mice.31 However, in agreement with our findings, Sartori-Valinotti et al. reported that female rats had greater BP lowering response to ACE inhibition and greater response to chronic AngII infusion than did male rats32. In addition, Calhoun et al. reported that male WKY rats have lower BP than aged-matched female rats33. There are differences between Xue’s study and ours that could account for these differences –Xue’s study used mice not rats, and the mice were gonadectomized.
Sex differences in Aldo/MR activation also have been reported in pre-clinical rodent models. In contrast to our results, Macova et al. and Wang et al. reported that estrogen treatment reduces adrenal and circulating Aldo levels34, 35. However, ovariectomy (OVX) did not change Aldo levels in either model and Wang et al.’s used the mRen2. Lewis rat. Studies in Stroke Prone Spontaneously Hypertensive rats show that OVX reduced strokes and renal injury, while estrogen replacement restored damage 36. In addition, in aged hypertensive mRen2. Lewis rats, OVX reduced proteinuria and renal injury on a high-salt diet37. Furthermore, there is evidence that estrogen increases heart damage in both lean and obese OVX Dahl Salt Sensitive rats38. These results suggest that in models of volume-sensitive hypertension, estrogen may promote rather than prevent cardiovascular damage.
What are the potential mechanisms that underlie our findings? Several mechanisms may be involved. First, we have reported that MR and estrogen receptor (ER)-α are part of a protein complex in cells that is necessary for ER-α inhibition of MR-mediated transcription39. Second, we recently documented that via a novel rapid-short negative feedback loop, MR activation of isolated adrenal ZG cells was associated with reduced Aldo secretion40. We posit that ER-α blocks the MR and disrupts the short negative feedback loop leading to increased Aldo secretion by ZG cells. These findings are consistent with our observations that isolated ZG cells from female rats have greater baseline and agonist-stimulated Aldo secretion than ZG cells isolated from aged-matched male rats. Furthermore, these studies are consistent with our data showing that women had greater in vivo AngII stimulated Aldo production than men. Lastly, we observed important end-organ consequences of the biological sex differences in Aldo (e.g. greater cardiac and renal damage in female than male rats). Third, regarding younger individuals, Aldo may also be involved in those pre-menopausal women who do develop hypertension. We recently documented that polymorphic variants in the ER are associated with increased salt sensitive BP but only in premenopausal females, and not in men or post-menopausal females41. Thus, even in young women, those who develop hypertension likely may have it mediated by disordered Aldo.
Our results suggest that women may derive more clinical benefit than men from therapeutic MR blockade. Clinical trials to assess this hypothesis are needed as we and others42, 44, 45, 46 are unaware of clinical trials specifically designed to determine whether the clinical response to MR blockade differs by sex 42–46. However, the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) provided an opportunity to assess the impact of sex on BP responses to MR blockade in 1,411 participants who received MR blockade as a fourth line anti-hypertensive agent44. Again, this trial was not specifically designed to test the impact of biological sex. In ASCOT, women had significantly greater decreases in diastolic blood pressure as compared to men, a result that is consistent with our findings. In addition, post-hoc analyses of the Eplerenone Post–Acute Myocardial Infarction Heart Failure Efficacy and Survival (EPHESUS) trial42 data showed that MR blockade significantly decreased all-cause mortality in women, but not men; however, a direct comparison of the effect of sex showed no statistical difference. These results highlight the need for trials to assess the impact of sex on the clinical responses to MR blockade. Thus, our results are important as they provide the scientific rationale for such trials.
This study is likely the largest (1592 subjects) that has assessed the effect of biological sex on changes in BP and Aldo in response to substantial, fixed changes in salt intake and their acute responses to AngII. Of importance, we controlled several environmental variables that could confound the interpretations of these results. Our studies were performed under standardized conditions of time of day, fixed sodium intake, withdrawal of interfering BP medications, and overnight supine posture as previously documented18, 19. However, there are limitations to our study. First, even though the human population was one of the largest reported of Aldo/MR activation and the study design tightly controlled many critically important variables, this report still was a cross-sectional observational study. Thus, the cause and effect relationship to BP, Aldo and RPF is limited by study design. Second, in our rodent studies we did not measure RPF nor did we assess the cause and effect relationships in other animal models of RAAS activation. Third, recent studies by Caroccia et al. showed that in the adrenocortical carcinoma cell line, HAC15 that express ER-α, -β and GPER-1, estrogen treatment did not affect Aldo production47,48. Yet, simultaneous blockade of ER-α and -β in these cells revealed the existence of a GPER-1–sensitive estrogen-stimulated Aldo production pathway. However, the in vivo and pathophysiological effects of this pathway have not been described and we did not assess the potential effects of GPER-1 in our studies. Fourth, progesterone is known to act as an Aldo antagonist. However, since the difference between men and women persists after the age of 51 when progesterone levels are low in women, this does not seem to be a confounding factor. Fifth, to directly compare the responses in younger versus older men to those in women, an age cutoff of 51 was used, as this is the mean age of menopause in American women. Estradiol/FSH levels would have been informative to stratify for menopausal status but were not available for most of the subjects.
Supplementary Material
PERSPECTIVES.
The present study shows evidence of chronic and acutely increased Aldo/MR activation in female human and rats when compared to males. In the present study, these sex differences were present in both hypertensive and normotensive subjects, suggesting that the differences in Aldo may predispose women, compared to men, to Aldo mediated CVD. This assumption was supported by a positive interaction between Aldo and sex on systolic BP response to AngII. Finally, since the differential effect was present even in older individuals, it is likely that the difference is not mediated simply by differences in estrogen levels per se.
NOVELTY AND SIGNIFICANCE.
What Is New?
Women, whether pre- or post-menopausal, hypertensive or normotensive, have greater degree of salt sensitivity of blood pressure and higher aldosterone levels than men.
Sex and aldosterone significantly interact to modify blood pressure.
Female versus male rats have increased aldosterone production, myocardial damage and proteinuria, which is prevented by mineralocorticoid receptor blockade.
What Is Relevant?
Salt sensitivity of blood pressure is a risk factor for cardiovascular morbidity and mortality.
Differences in aldosterone levels may predispose women to greater salt sensitivity of blood pressure and cardiovascular damage.
Summary.
Females have greater evidence of cardiovascular damage mediated, in part, by increased aldosterone levels supporting the hypothesis that chronic and acute aldosterone production may contribute to sex differences in the incidence cardiovascular diseases.
Acknowledgments
SOURCES OF FUNDING
This work was supported in part by the National Institute of Health National Heart, Lung and Blood Institute Grants R01HL096518 (JRR), R01HL11476 (GHW), R01HL104032 (LHP), the American Heart Association: 14GRNT20500000 (LHP) and by fellowships from the Ministry of Rural and Regional Development Malaysia [MARA] (MZS).
Footnotes
CONFLICT OF INTEREST
The authors have declared that no conflict of interest exists.
References
- 1.Garcia M, Mulvagh SL, Merz CN, Buring JE, Manson JE. Cardiovascular Disease in Women: Clinical Perspectives. Circ Res. 2016;118:1273–1293. doi: 10.1161/CIRCRESAHA.116.307547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gori M, Lam CS, Gupta DK, Santos AB, Cheng S, Shah AM, Claggett B, Zile MR, Kraigher-Krainer E, Pieske B, Voors AA, Packer M, Bransford T, Lefkowitz M, McMurray JJ, Solomon SD. Sex-specific cardiovascular structure and function in heart failure with preserved ejection fraction. Eur J Heart Fail. 2014;16:535–542. doi: 10.1002/ejhf.67. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan NM, Kem DC, Holland OB, Kramer NJ, Higgins J, Gomez-Sanchez C. The intravenous furosemide test: a simple way to evaluate renin responsiveness. Ann Intern Med. 1976;84:639–645. doi: 10.7326/0003-4819-84-6-639. [DOI] [PubMed] [Google Scholar]
- 4.James GD, Sealey JE, Muller F, Alderman M, Madhavan S, Laragh JH. Renin relationship to sex, race and age in a normotensive population. J Hypertens Suppl. 1986;4:S387–389. [PubMed] [Google Scholar]
- 5.Gandhi SK, Gainer J, King D, Brown NJ. Gender affects renal vasoconstrictor response to Ang I and Ang II. Hypertension. 1998;31:90–96. doi: 10.1161/01.hyp.31.1.90. [DOI] [PubMed] [Google Scholar]
- 6.Miller JA, Anacta LA, Cattran DC. Impact of gender on the renal response to angiotensin II. Kidney Int. 1999;55:278–285. doi: 10.1046/j.1523-1755.1999.00260.x. [DOI] [PubMed] [Google Scholar]
- 7.Miller JA, Cherney DZ, Duncan JA, Lai V, Burns KD, Kennedy CR, Zimpelmann J, Gao W, Cattran DC, Scholey JW. Gender differences in the renal response to renin-angiotensin system blockade. J Am Soc Nephrol. 2006;17:2554–2560. doi: 10.1681/ASN.2005101095. [DOI] [PubMed] [Google Scholar]
- 8.Toering TJ, van der Graaf AM, Visser FW, Buikema H, Navis G, Faas MM, Lely AT. Gender differences in response to acute and chronic angiotensin II infusion: a translational approach. Physiol Rep. 2015;3 doi: 10.14814/phy2.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kathiresan S, Larson MG, Benjamin EJ, Corey D, Murabito JM, Fox CS, Wilson PW, Rifai N, Meigs JB, Ricken G, Lifton RP, Levy D, Vasan RS. Clinical and genetic correlates of serum aldosterone in the community: the Framingham Heart Study. Am J Hypertens. 2005;18:657–665. doi: 10.1016/j.amjhyper.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Clark BA, Elahi D, Epstein FH. The influence of gender, age, and the menstrual cycle on plasma atrial natriuretic peptide. J Clin Endocrinol Metab. 1990;70:349–352. doi: 10.1210/jcem-70-2-349. [DOI] [PubMed] [Google Scholar]
- 11.Gray MJ, Strausfeld KS, Watanabe M, Sims EA, Solomon S. Aldosterone secretory rates in the normal menstrual cycle. J Clin Endocrinol Metab. 1968;28:1269–1275. doi: 10.1210/jcem-28-9-1269. [DOI] [PubMed] [Google Scholar]
- 12.Ruilope LM, Garcia-Robles R, Mancheno E, Rodriguez FJ, Barrientos A, Torres J, Radicio JL, Oriol-Bosch A. Response of plasma aldosterone to orthostatism during follicular and luteal phases of the normal menstrual cycle. Rev Esp Fisiol. 1977;33:341–345. [PubMed] [Google Scholar]
- 13.Bentley-Lewis R, Adler GK, Perlstein T, Seely EW, Hopkins PN, Williams GH, Garg R. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocrinol Metab. 2007;92:4472–4475. doi: 10.1210/jc.2007-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katz FH, Romfh P, Zimmering PE, Kelly WG. Radioimmunoassay of plasma aldosterone: effect of sexual differences and increasing duration of upright posture. Steroids Lipids Res. 1972;3:90–100. [PubMed] [Google Scholar]
- 15.Fisher ND, Ferri C, Bellini C, Santucci A, Gleason R, Williams GH, Hollenberg NK, Seely EW. Age, gender, and non-modulation. A sexual dimorphism in essential hypertension. Hypertension. 1997;29:980–985. doi: 10.1161/01.hyp.29.4.980. [DOI] [PubMed] [Google Scholar]
- 16.Giacche M, Vuagnat A, Hunt SC, Hopkins PN, Fisher ND, Azizi M, Corvol P, Williams GH, Jeunemaitre X. Aldosterone stimulation by angiotensin II : influence of gender, plasma renin, and familial resemblance. Hypertension. 2000;35:710–716. doi: 10.1161/01.hyp.35.3.710. [DOI] [PubMed] [Google Scholar]
- 17.Fisher ND, Hurwitz S, Jeunemaitre X, Price DA, Williams GH, Hollenberg NK. Adrenal response to angiotensin II in black hypertension: lack of sexual dimorphism. Hypertension. 2001;38:373–378. doi: 10.1161/01.hyp.38.3.373. [DOI] [PubMed] [Google Scholar]
- 18.Vaidya A, Underwood PC, Hopkins PN, Jeunemaitre X, Ferri C, Williams GH, Adler GK. Abnormal aldosterone physiology and cardiometabolic risk factors. Hypertension. 2013;61:886–893. doi: 10.1161/HYPERTENSIONAHA.111.00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garza AE, Rariy CM, Sun B, Williams JS, Lasky-Su J, Baudrand R, Yao T, Moize B, Hafiz WM, Romero JR, Adler GK, Ferri C, Hopkins PN, Pojoga LH, Williams GH. Variants in striatin gene are associated with salt-sensitive blood pressure in mice and humans. Hypertension. 2015;65:211–217. doi: 10.1161/HYPERTENSIONAHA.114.04233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baudrand R, Gupta N, Garza AE, Vaidya A, Leopold JA, Hopkins PN, Jeunemaitre X, Ferri C, Romero JR, Williams J, Loscalzo J, Adler GK, Williams GH, Pojoga LH. Caveolin 1 Modulates Aldosterone-Mediated Pathways of Glucose and Lipid Homeostasis. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez DV, Rocha R, Matsumura M, Oestreicher E, Ochoa-Maya M, Roubsanthisuk W, Williams GH, Adler GK. Cardiac damage prevention by eplerenone: comparison with low sodium diet or potassium loading. Hypertension. 2002;39:614–618. [PubMed] [Google Scholar]
- 22.Rocha R, Stier CT, Jr, Kifor I, Ochoa-Maya MR, Rennke HG, Williams GH, Adler GK. Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology. 2000;141:3871–3878. doi: 10.1210/endo.141.10.7711. [DOI] [PubMed] [Google Scholar]
- 23.Braley LM, Menachery AI, Yao T, Mortensen RM, Williams GH. Effect of progesterone on aldosterone secretion in rats. Endocrinology. 1996;137:4773–4778. doi: 10.1210/endo.137.11.8895346. [DOI] [PubMed] [Google Scholar]
- 24.Rydstedt LL, Williams GH, Hollenberg NK. Renal and endocrine response to saline infusion in essential hypertension. Hypertension. 1986;8:217–222. doi: 10.1161/01.hyp.8.3.217. [DOI] [PubMed] [Google Scholar]
- 25.Weinberger MH. Hypertension in African Americans: the role of sodium chloride and extracellular fluid volume. Semin Nephrol. 1996;16:110–116. [PubMed] [Google Scholar]
- 26.He J, Gu D, Chen J, Jaquish CE, Rao DC, Hixson JE, Chen JC, Duan X, Huang JF, Chen CS, Kelly TN, Bazzano LA, Whelton PK. Gender difference in blood pressure responses to dietary sodium intervention in the GenSalt study. J Hypertens. 2009;27:48–54. doi: 10.1097/hjh.0b013e328316bb87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vollmer WM, Sacks FM, Ard J, Appel LJ, Bray GA, Simons-Morton DG, Conlin PR, Svetkey LP, Erlinger TP, Moore TJ, Karanja N. Effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH-sodium trial. Ann Intern Med. 2001;135:1019–1028. doi: 10.7326/0003-4819-135-12-200112180-00005. [DOI] [PubMed] [Google Scholar]
- 28.Wilson DK, Bayer L, Sica DA. Variability in salt sensitivity classifications in black male versus female adolescents. Hypertension. 1996;28:250–255. doi: 10.1161/01.hyp.28.2.250. [DOI] [PubMed] [Google Scholar]
- 29.Hollenberg NK, Moore T, Shoback D, Redgrave J, Rabinowe S, Williams GH. Abnormal renal sodium handling in essential hypertension. Relation to failure of renal and adrenal modulation of responses to angiotensin II. Am J Med. 1986;81:412–418. doi: 10.1016/0002-9343(86)90291-3. [DOI] [PubMed] [Google Scholar]
- 30.Blenck CL, Harvey PA, Reckelhoff JF, Leinwand LA. The Importance of Biological Sex and Estrogen in Rodent Models of Cardiovascular Health and Disease. Circ Res. 2016;118:1294–1312. doi: 10.1161/CIRCRESAHA.116.307509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol. 2005;288:H2177–2184. doi: 10.1152/ajpheart.00969.2004. [DOI] [PubMed] [Google Scholar]
- 32.Sartori-Valinotti JC, Iliescu R, Yanes LL, Dorsett-Martin W, Reckelhoff JF. Sex differences in the pressor response to angiotensin II when the endogenous renin-angiotensin system is blocked. Hypertension. 2008;51:1170–1176. doi: 10.1161/HYPERTENSIONAHA.107.106922. [DOI] [PubMed] [Google Scholar]
- 33.Calhoun DA, Zhu ST, Chen YF, Oparil S. Gender and dietary NaCl in spontaneously hypertensive and Wistar-Kyoto rats. Hypertension. 1995;26:285–289. doi: 10.1161/01.hyp.26.2.285. [DOI] [PubMed] [Google Scholar]
- 34.Macova M, Armando I, Zhou J, Baiardi G, Tyurmin D, Larrayoz-Roldan IM, Saavedra JM. Estrogen reduces aldosterone, upregulates adrenal angiotensin II AT2 receptors and normalizes adrenomedullary Fra-2 in ovariectomized rats. Neuroendocrinology. 2008;88:276–286. doi: 10.1159/000150977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, Jessup JA, Zhao Z, Da Silva J, Lin M, MacNamara LM, Ahmad S, Chappell MC, Ferrario CM, Groban L. Characterization of the cardiac renin angiotensin system in oophorectomized and estrogen-replete mRen2.Lewis rats. PLoS One. 2013;8:e76992. doi: 10.1371/journal.pone.0076992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stier CT, Jr, Chander PN, Rosenfeld L, Powers CA. Estrogen promotes microvascular pathology in female stroke-prone spontaneously hypertensive rats. Am J Physiol Endocrinol Metab. 2003;285:E232–239. doi: 10.1152/ajpendo.00029.2003. [DOI] [PubMed] [Google Scholar]
- 37.Yamaleyeva LM, Pendergrass KD, Pirro NT, Gallagher PE, Groban L, Chappell MC. Ovariectomy is protective against renal injury in the high-salt-fed older mRen2. Lewis rat. Am J Physiol Heart Circ Physiol. 2007;293:H2064–2071. doi: 10.1152/ajpheart.00427.2007. [DOI] [PubMed] [Google Scholar]
- 38.Murase T, Hattori T, Ohtake M, Nakashima C, Takatsu M, Murohara T, Nagata K. Effects of estrogen on cardiovascular injury in ovariectomized female DahlS.Z-Lepr(fa)/Lepr(fa) rats as a new animal model of metabolic syndrome. Hypertension. 2012;59:694–704. doi: 10.1161/HYPERTENSIONAHA.111.180976. [DOI] [PubMed] [Google Scholar]
- 39.Barrett Mueller K, Lu Q, Mohammad NN, Luu V, McCurley A, Williams GH, Adler GK, Karas RH, Jaffe IZ. Estrogen receptor inhibits mineralocorticoid receptor transcriptional regulatory function. Endocrinology. 2014;155:4461–4472. doi: 10.1210/en.2014-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chong C, Hamid A, Yao T, Garza A, Pojoga L, Adler GK, Romero JR, Williams GH. Regulation of aldosterone secretion by mineralocorticoid receptor-mediated signaling. J Endocrinol. 2017;232:525. doi: 10.1530/JOE-16-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manosroi W, Tan JW, Rariy CM, Sun B, Goodarzi MO, Saxena AR, Williams JS, Pojoga LH, Lasky-Su J, Cui J, Guo X, Taylor KD, Chen YI, Xiang AH, Hsueh WA, Raffel LJ, Buchanan TA, Rotter JI, Williams GH, Seely EW. The Association of Estrogen Receptor-beta Gene Variation With Salt-Sensitive Blood Pressure. J Clin Endocrinol Metab. 2017;102:4124–4135. doi: 10.1210/jc.2017-00957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosano GM, Lewis B, Agewall S, Wassmann S, Vitale C, Schmidt H, Drexel H, Patak A, Torp-Pedersen C, Kjeldsen KP, Tamargo J. Gender differences in the effect of cardiovascular drugs: a position document of the Working Group on Pharmacology and Drug Therapy of the ESC. Eur Heart J. 2015;36:2677–2680. doi: 10.1093/eurheartj/ehv161. [DOI] [PubMed] [Google Scholar]
- 43.Wu AH, Pitt B, Anker SD, Vincent J, Mujib M, Ahmed A. Association of obesity and survival in systolic heart failure after acute myocardial infarction: potential confounding by age. Eur J Heart Fail. 2010;12:566–573. doi: 10.1093/eurjhf/hfq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chapman N, Dobson J, Wilson S, Dahlof B, Sever PS, Wedel H, Poulter NR. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension. 2007;49:839–845. doi: 10.1161/01.HYP.0000259805.18468.8c. [DOI] [PubMed] [Google Scholar]
- 45.Gudmundsdottir H, Hoieggen A, Stenehjem A, Waldum B, Os I. Hypertension in women: latest findings and clinical implications. Ther Adv Chronic Dis. 2012;3:137–146. doi: 10.1177/2040622312438935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Engberding N, Wenger NK. Management of hypertension in women. Hypertens Res. 2012;35:251–260. doi: 10.1038/hr.2011.210. [DOI] [PubMed] [Google Scholar]
- 47.Caroccia B, Seccia TM, Campos AG, Gioco F, Kuppusamy M, Ceolotto G, Guerzoni E, Simonato F, Mareso S, Lenzini L, Fassina A, Rossi GP. GPER-1 and estrogen receptor-beta ligands modulate aldosterone synthesis. Endocrinology. 2014;155:4296–4304. doi: 10.1210/en.2014-1416. [DOI] [PubMed] [Google Scholar]
- 48.Caroccia B, Seccia TM, Barton M, Rossi GP. Estrogen Signaling in the Adrenal Cortex: Implications for Blood Pressure Sex Differences. Hypertension. 2016;68:840–848. doi: 10.1161/HYPERTENSIONAHA.116.07660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


