Abstract
Background.
We previously developed a prognostic index for assessing local-regional recurrence (LRR) risk in patients undergoing breast conservation therapy (BCT) after neoadjuvant chemotherapy. The prognostic index assigns a point for each of the following variables: clinical N2/N3 disease, lymphovascular invasion, residual pathologic tumor size >2 cm, and multifocal residual disease on pathology. The current study was undertaken to evaluate this prognostic index in an independent cohort.
Methods.
We identified 551 patients treated from 2001 to 2005 with neoadjuvant chemotherapy, mastectomy or BCT, and radiation. These patients were not used in the original development of the prognostic index. Outcomes were stratified by prognostic index. The 5-year LRR-free survival was calculated using the Kaplan-Meier method, and differences were compared using the log-rank test.
Results.
For patients undergoing BCT, the 5-year LRR-free survival rates were 92, 92, 84, and 69% when the prognostic index was 0 (n = 91), 1 (n = 82), 2 (n = 38), or 3–4 (n = 13) (P = 0.01). The 5-year LRR-free survival rates were similar between patients undergoing mastectomy or BCT when the prognostic index score was 0, 1, or 2. When the prognostic index score was 3–4, the 5-year LRR-free survival was significantly lower for patients treated with BCT compared with mastectomy (69 vs. 93%, P = 0.007).
Conclusion.
The previously developed prognostic index was successful in stratifying patients with respect to LRR in an independent cohort undergoing BCT after neoadjuvant chemotherapy. The prognostic index can be used to identify patients at high risk for LRR who may be considered for more extensive surgery or enrollment into clinical trials evaluating novel strategies for local-regional control.
Neoadjuvant chemotherapy is being increasingly used in breast cancer patients presenting with operable disease. For patients presenting with large tumors requiring mastectomy, the use of neoadjuvant chemotherapy has been demonstrated to downsize tumors, thereby facilitating breast conserving therapy (BCT).1–3 For patients who are BCT candidates at presentation, neoadjuvant chemotherapy has been shown to decrease the volume of resection allowing for more favorable cosmetic outcomes.4 Treatment with neoadjuvant chemotherapy has the added advantage of allowing for in vivo assessment of tumor response to systemic therapy.5
The increasing use of BCT following neoadjuvant chemotherapy has led to an interest in identifying appropriate candidates such that local-regional control is not compromised. Some studies have reported local-regional recurrence (LRR) rates as high as 21% in patients undergoing BCT following neoadjuvant chemotherapy.6–10 Other studies have reported more favorable outcomes. Taken together, these studies suggest that there is a need for selection criteria that can help to identify appropriate candidates for this approach. Our group previously addressed this issue in a cohort of 340 patients treated with BCT after neoadjuvant chemotherapy. We identified 4 clinicopathologic variables that were predictive of LRR on multivariate analysis: clinical N2 or N3 disease, the presence of lymphovascular invasion (LVI), residual pathologic tumor size >2 cm, and a multifocal residual pattern of disease.8 The MD Anderson Prognostic Index was developed by assigning 1 point for each of the 4 variables and using the summed total to give an overall score of 0–4. This prognostic index was able to stratify patients undergoing BCT after neoadjuvant chemotherapy with respect to LRR.11 We also compared rates of LRR as a function of the prognostic index for patients treated with BCT or mastectomy plus irradiation. Since the initial publication detailing this prognostic index, there have been several changes in our practice pattern with respect to treating patients in the neoadjuvant setting. These include increased use of neoadjuvant chemotherapy in patients with clinical stage II disease, administration of a greater number of chemotherapy cycles prior to surgery, and the increased use of regimens combining a taxane and anthracycline. The current study was undertaken to evaluate the prognostic index in a more contemporary cohort of patients.
METHODS
Patients and Multimodality Treatment
A prospectively maintained database of breast cancer patients treated at The University of Texas MD Anderson Cancer Center was used to identify patients with nonmetastatic disease who were treated with neoadjuvant chemotherapy, surgery, and postoperative radiation between January 2001 and December 2005. Clinicopatho-logic data were reviewed, and patients with data available for all four components of the prognostic index were included. This resulted in the identification of 551 patients, 224 treated with BCT, and 327 undergoing mastectomy with postmastectomy radiation therapy (PMRT). None of the patients were part of the original cohort treated from 1987 to 2000 that was used to develop the prognostic index. The MD Anderson institutional review board approved this study.
Patients were assessed at presentation using physical examination, mammography, ultrasound of the breast and regional nodal basins, and staging studies as clinically indicated to exclude metastatic disease. Patients were clinically staged according to the 6th edition of the American Joint Committee on Cancer Breast Cancer Staging System.12 All patients were treated with a neoadjuvant chemotherapy regimen that was anthracycline-based, taxane-based, or combination anthracycline/taxane-based. Neoadjuvant therapy also included a hormonal agent in four patients. The decision to offer BCT was made by the treating surgeon after assessment of the clinical response to neoadjuvant chemotherapy. BCT involved excision of the residual primary tumor and did not require resection of the prechemotherapy tumor volume. All patients underwent surgical evaluation of their axilla to include a complete axillary lymph node dissection in patients presenting with clinically node-positive disease. Patients who presented with clinically node-negative disease underwent sentinel lymph node biopsy with complete axillary lymph node dissection if the sentinel lymph node was positive or at the discretion of the attending surgeon. All patients were treated with adjuvant whole breast or chest wall radiation therapy. Typically, 50 Gy was delivered in 25 fractions to the breast or chest wall using tangential fields, followed by a 10-Gy boost to the tumor bed or chest wall scar using an appositional electron field. Regional nodal radiation was added at the discretion of the radiation oncologist. Adjuvant chemotherapy, given to patients who did not receive their entire chemotherapy course prior to surgery, was administered after surgery and before radiation. There were 42 patients (19%) in the BCT group and 93 patients (28%) in the mastectomy group who received adjuvant chemotherapy. Patients with hormone receptor positive disease were routinely offered adjuvant hormonal therapy. Hormonal agents were administered to 122 patients (54%) undergoing BCT and 222 patients (68%) undergoing mastectomy.
Prognostic Index and Statistical Analysis
The prognostic index was developed from prior analyses that identified four independent risk factors predictive of higher rates of LRR in patients undergoing BCT following receipt of neoadjuvant chemotherapy: clinical N2-N3 disease, LVI, residual pathologic tumor size >2 cm, and a multifocal pattern of residual disease.11 Each patient received a score on a scale of 0–4 according to the presence of each risk factor, assigning 1 point per risk factor.
The distribution of patient characteristics between patients undergoing BCT and those undergoing mastectomy was compared using t test for continuous variables and the χ2 test for categorical variables using the Yates’ correction when indicated. LRR-free survival was measured from the date of diagnosis to the date of first LRR defined as disease recurrence in the ipsilateral breast or chest wall, or in the ipsilateral axillary, supraclavicular, infraclavicular, or internal mammary lymph nodes. All LRR were counted as events regardless of whether they were the first site of failure or occurred concomitantly or after the diagnosis of distant metastases. Disease-specific survival (DSS) was measured from the date of diagnosis to the date of death from breast cancer. Patients who did not experience any of these events were censored at last follow-up or at the time of death. LRR-free survival and DSS were calculated using the Kaplan-Meier method, and differences were compared using the log-rank test. All calculations were performed with Stata software (Stata/SE 11 for Windows/Mac; Stata Corp., College Station, TX). A P value ≤ 0.05 was considered statistically significant.
RESULTS
Clinicopathologic characteristics of the study cohort are shown in Table 1. When compared with BCT patients, a greater percentage of mastectomy patients had more advanced clinical stage as well as larger residual tumor sizes after neoadjuvant chemotherapy and more pathologically positive lymph nodes. The median follow-up for the entire cohort was 62 months (range 8–114 months), 63 months for the BCT group and 61 months for the mastectomy group. The differences in clinicopathologic features are reflected in the 5-year DSS rates, which were 88% for the BCT patients versus 76% for the mastectomy patients (P = 0.005).
Table 1.
Distribution of clinicopathologic characteristics
| Factor | BCT (n = 224) | Mastectomy (n = 327) | P value |
|---|---|---|---|
| Age | 0.4 | ||
| Median | 51 | 50 | |
| Range | 28–76 | 24–80 | |
| Clinical tumor size (cm) (mean) | 3.42 | 4.62 | <0.001 |
| Clinical stage | <0.001 | ||
| I | 13 (6%) | 8 (2%) | |
| II | 160 (71%) | 140 (43%) | |
| III | 51 (23%) | 179 (55%) | |
| Histology | <0.01 | ||
| IDC | 201 (90%) | 255 (78%) | |
| ILC | 7 (3%) | 35 (11%) | |
| Mixed IDC/ILC | 11 (5%) | 29 (9%) | |
| Other | 5 (2%) | 8 (2%) | |
| Grade | 0.07 | ||
| I | 9 (4%) | 19 (6%) | |
| II | 64 (29%) | 120 (37%) | |
| III | 150 (67%) | 188 (57%) | |
| Unknown | 1 (<1%) | 0 | |
| ER | <0.01 | ||
| Positive | 131 (58%) | 230 (70%) | |
| Negative | 93 (42%) | 97 (30%) | |
| PR | 0.10 | ||
| Positive | 102 (46%) | 172 (53%) | |
| Negative | 122 (54%) | 155 (47%) | |
| HER | 0.22 | ||
| Positive | 27 (12%) | 52 (16%) | |
| Negative | 194 (87%) | 273 (83%) | |
| Unknown | 3 (1%) | 2 (1%) | |
| Margin status | 0.04 | ||
| Negative | 209 (93%) | 316 (97%) | |
| Close (<2 mm)a | 14 (6%) | 6 (2%) | |
| Positive | 1 (<1%) | 5 (1%) | |
| Residual tumor size (cm) | <0.001 | ||
| 0 | 22 (10%) | 25 (8%) | |
| 0–2 | 144 (64%) | 118 (36%) | |
| >2 | 58 (26%) | 184 (56%) | |
| Positive lymph nodes | <0.001 | ||
| 0 | 126 (56%) | 67 (20%) | |
| 1–3 | 58 (26%) | 101 (31%) | |
| 4–9 | 28 (13%) | 98 (30%) | |
| ≥10 | 12 (5%) | 61 (19%) | <0.001 |
ER estrogen receptor, PR progesterone receptor
Close included invasive cancer or ductal carcinoma in situ within 2 mm of the margin
With respect to LRR, the 5-year LRR-free survival rates were 90% in the BCT group and 92% in the mastectomy group (P = 0.54). In the BCT group, 23 patients experienced a LRR: an IBTR in 14 patients, an ipsilateral axillary nodal recurrence in 1 patient, regional lymph node recurrences in six patients, and both an IBTR and regional nodal recurrence in two patients. The median time to LRR in the BCT group was 23 months (range 8–63 months). In the mastectomy group, 27 patients experienced a LRR: 12 in the chest wall, six in the ipsilateral axilla, eight in regional lymph nodes, and both the axilla as well as other regional nodes in 1. The median time to LRR in the mastectomy group was 17 months (range 8–92 months).
The distribution of patients according to their prognostic index is shown in Table 2. As would be anticipated based on clinicopathologic factors, mastectomy patients had a greater percentage with a prognostic index of 3 or 4. Consistent with our previous work, the prognostic index was able to stratify BCT patients with respect to 5-year LRR-free survival rates (Fig. 1). The 5-year LRR-free survival rates were 92% (95% confidence interval [95% CI], 83–96) for a prognostic index of 0, 92% (95% CI, 84–96) for a prognostic index of 1, 84% (95% CI, 68–93) for a prognostic index of 2, and 68% (95% CI, 36–87) for a prognostic index of 3 or 4 (P = 0.01). When LRR was analyzed as a function of the type of surgery according to the prognostic index, the 5-year LRR rates were similar between patients undergoing BCT or mastectomy who had a score of 0, 1, or 2 (Fig. 2a, b, c). For patients with a prognostic index of 3 or 4, the 5-year LRR-free survival rate was significantly higher for those treated with mastectomy compared with those treated with BCT (94 vs. 68%; P = 0.007) (Fig. 2d). The LRR risk for the BCT group was 4-fold higher for those patients with a prognostic index of 3 or 4 compared with those with a score of 0 (hazard ratio [HR] = 4.05, 95% CI = 1.2–13.9).
Table 2.
Distribution of prognostic index
| BCT (n = 224) | Mastectomy (n = 327) | P value | |
|---|---|---|---|
| Prognostic index | <0.001 | ||
| 0 | 91 (41%) | 37 (11%) | |
| 1 | 82 (37%) | 107 (33%) | |
| 2 | 38 (17%) | 124 (38%) | |
| 3 | 12 (5%) | 47 (14%) | |
| 4 | 1 (<1%) | 12 (4%) | |
| Prognostic index factorsa | |||
| cN2-N3 disease | 32 (14%) | 74 (23%) | |
| LVI | 84 (38%) | 206 (63%) | |
| Pathologic tumor size >2 cm | 58 (26%) | 184 (56%) | |
| Pathologic multifocal disease | 24 (11%) | 80 (24%) |
Percentages do not add up to 100% because several patients had multiple factors or no factors
FIG. 1.
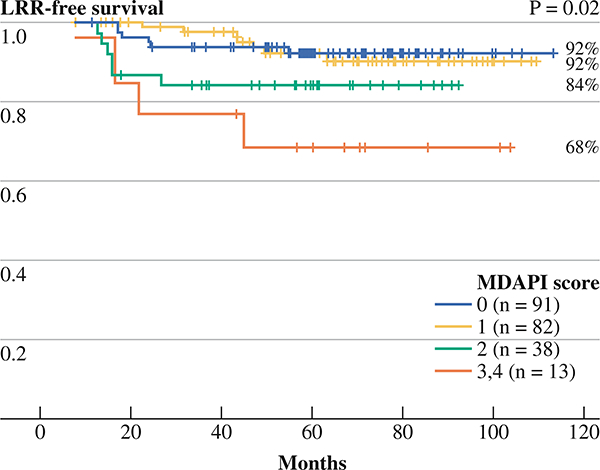
The 5-year rates of local-regional recurrence-free survival of 224 breast conservation patients stratified according to the prognostic index
FIG. 2.
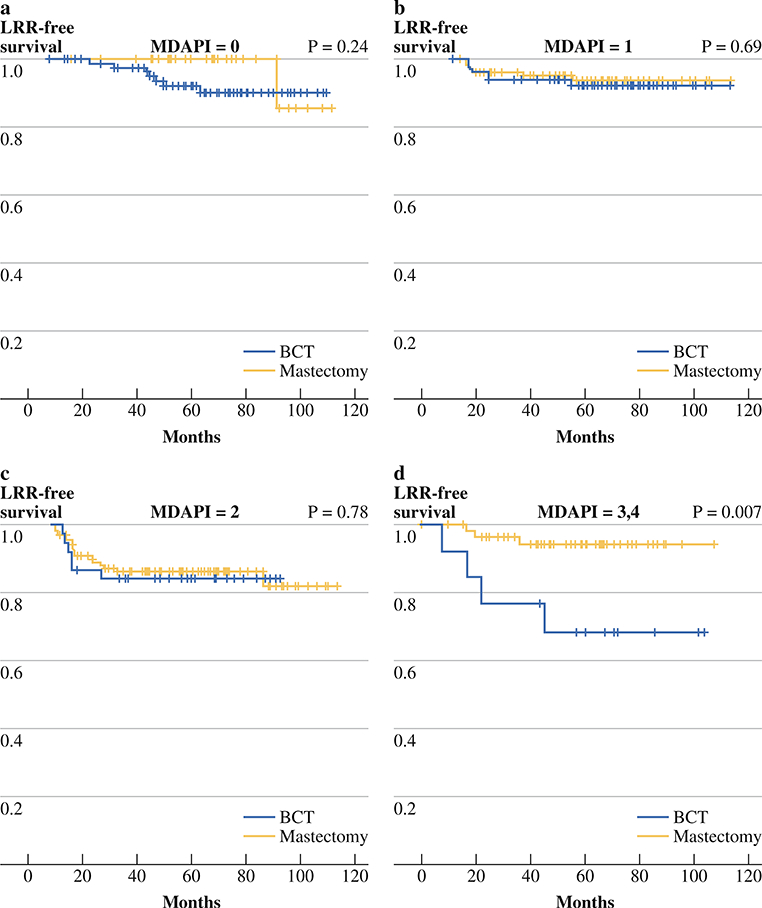
a The 5-year rates of local-regional recurrence-free survival of breast conservation therapy (BCT) (n = 91) and mastectomy (n = 37) patients with prognostic index of 0, b The 5-year rates of local-regional recurrence-free survival of breast conservation therapy (BCT) (n = 82) and mastectomy (n = 107) patients with prognostic index of 1, c The 5-year rates of local-regional recurrence-free survival of breast conservation therapy (BCT) (n = 38) and mastectomy (n = 124) patients with prognostic index of 2, d The 5-year rates of local-regional recurrence-free survival of breast conservation therapy (BCT) (n = 13) and mastectomy (n = 59) patients with prognostic index of 3 or 4
DISCUSSION
The MD Anderson prognostic index consists of four factors determined to be statistically significant predictors of LRR in patients undergoing BCT following neoadjuvant chemotherapy. This prognostic index was previously shown to stratify BCT patients into subgroups with distinct risk for LRR. In our current practice, neoadjuvant chemotherapy is more commonly used in patients with earlier clinical stage disease, more patients receive an increased number of cycles of chemotherapy preoperatively, and there has been greater use of combination chemotherapy regimens including both anthracyclines and taxanes. Even with these changes in practice, we found that the prognostic index is still effective in stratifying patients undergoing BCT with respect to LRR risk.
Historically, neoadjuvant chemotherapy has been used in the setting of locally advanced or inoperable breast cancer. However, the indications for use of neoadjuvant chemotherapy have expanded, and it is now administered to a significant number of women presenting with operable disease. Neoadjuvant chemotherapy was evaluated in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 trial that was designed to determine whether 4 cycles of doxorubicin and cyclophosphamide (AC) given preoperatively improved disease-free survival and overall survival compared with AC given in the adjuvant setting. Results from the NSABP B-18 trial demonstrated no statistical difference between the two groups with respect to these endpoints.13 Secondary aims of the NSABP B-18 trial evaluated the effectiveness of neoadjuvant chemotherapy in downstaging the primary breast tumor, compared BCT rates, and determined rates of ipsilateral breast tumor recurrence (IBTR) between the two groups. With respect to these secondary outcomes, patients randomized to receive neoadjuvant chemotherapy were more likely to undergo BCT. In a report of the 9-year results of the trial, there was no significant difference in the IBTR rate in patients undergoing BCT after neoadjuvant chemotherapy (11%) than in those who were randomized to receive adjuvant chemotherapy (8%).14 Through 16 years of follow-up, the IBTR rates for patients undergoing BCT after receiving neoadjuvant chemotherapy was 13 vs. 10% in patients receiving adjuvant chemotherapy, again, a difference that was not statistically significant. Regional recurrence rates for the entire cohort as the site of first treatment failure were low, 4% for the neoadjuvant chemotherapy group and 5% in the adjuvant therapy group.13 In the current study, we demonstrated an overall favorable 5-year LRR-free survival rate of 90% for patients undergoing BCT following neoadjuvant chemotherapy. Our data are not directly comparable to the NSABP B-18 study; however, taken together these data confirm that BCT is an appropriate option for select patients receiving neoadjuvant chemotherapy.
Although the majority of patients selected for BCT after neoadjuvant chemotherapy do well with respect to LRR, all patients do not have equal LRR risk. The prognostic index is therefore an important tool that can better stratify patients undergoing BCT with respect to risk of developing a LRR. Almost 80% of patients undergoing BCT had a prognostic index of 0 or 1, and the 5-year LRR-free survival rates were 92%, which were comparable to the 5-year LRR-free rates of 94% in our original publication of patients with a prognostic index of 0 or 1.11 For patients with a prognostic index of 2, the 5-year LRR-free survival rate was 84% and for the small subset of patients (n = 13) with a prognostic index of 3 or 4, the 5-year LRR-free survival rate was 69%. In our previous study, the 5-year LRR-free survival rates for patients with a prognostic index of 2 and 3–4 were 83 and 58%, respectively.11 Despite the differences in our practice patterns between the two study cohorts, the results are very similar. This suggests that the prognostic index, which accounts for several risk factors contributing to LRR risk, may have importance in its ability to identify patients with overall unfavorable biology.
We also confirmed our previous finding that in patients with a prognostic index of 3–4, mastectomy with PMRT was associated with a significantly lower rate of LRR.15 It should be noted that the small number of patients with a prognostic index of 3–4 precludes determination of whether this difference in LRR translates to a difference in disease-specific survival. For this reason, despite the fact that this independent cohort does confirm that having a completion mastectomy would significantly reduce the risk of LRR, we would caution against interpreting these data to suggest that all patients with a prognostic index of 3 or 4 should undergo mastectomy. Validation of the prognostic index in a larger, external cohort would be required to further evaluate the effect of LRR on disease-specific survival for patients with a high prognostic index.
In addition to the small number of patients with a prognostic index of 3 or 4, this study has other limitations. This was a retrospective analysis in which the surgical treatment was not randomized, but rather was determined by the patient and the attending surgeon based on multiple considerations. A possible selection bias exists in that there were undoubtedly patients who underwent mastectomy who were appropriate candidates for BCT but elected for more extensive surgery. In addition, all patients, including those undergoing mastectomy, received postoperative radiation. It is possible that omission of PMRT could have resulted in higher rates of LRR than those reported for a given prognostic index. Currently, at our institution, when a patient undergoes mastectomy after neoadjuvant chemotherapy, PMRT is considered for those with clinical stage III disease, clinical T3N0 disease, clinical T1,2 N1 disease with residual tumor size larger than 2 cm, residual nodal disease after chemotherapy, the presence of LVI, or age under 40. Finally, it should be noted that the time period of this study predates the use of trastuzumab in the neoadjuvant setting for patients with HER2-overexpressing tumors. Although the effect of adding trastuzumab to standard neoadjuvant chemotherapy regimens on the success of performing BCT has not been specifically addressed, pathologic complete response (pCR) rates of up to 65% have been reported, suggesting that the majority of women would have downsizing of their primary tumor that might facilitate BCT.16 In addition, we have previously reported that patients receiving trastuzumab-based neoadjuvant chemotherapy regimens who achieve a pCR have significantly improved recurrence-free survival compared with those achieving less than a pCR.17 We would hypothesize that the use of trastuzumab would affect the rates of LRR, and future work will evaluate factors associated with LRR after BCT in patients receiving trastuzumab in the neoadjuvant setting.
Despite the limitations of this study, we have confirmed that the prognostic index can stratify patients undergoing BCT after neoadjuvant chemotherapy with respect to their LRR risk. The most recent report of the Early Breast Cancer Trialists’ Collaborative group (EBCTG) demonstrated that for every four local recurrences avoided by 5 years, one breast cancer related death was avoided at 15 years.18 Although the data reported by the EBCTG did not include patients treated in the neoadjuvant setting, it is likely that local regional control is important for this population as well. Therefore, the prognostic index may be valuable in counseling patients regarding their local therapy options. Those with a favorable prognostic index can be assured that they have low LRR rates. Patients with a prognostic index of 3 or 4 can be counseled that they may be at increased risk for LRR and may be considered for more extensive surgery or participation in clinical trials such as those evaluating novel therapeutics or treatment strategies such as the use of radiosensitizers aimed at reducing LRR.
REFERENCES
- 1.Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–85. [DOI] [PubMed] [Google Scholar]
- 2.Makris A, Powles TJ, Ashley SE, Chang J, Hickish T, Tidy VA, et al. A reduction in the requirements for mastectomy in a randomized trial of neoadjuvant chemoendocrine therapy in primary breast cancer. Ann Oncol. 1998;9:1179–84. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz GF, Birchansky CA, Komarnicky LT, Mansfield CM, Cantor RI, Biermann WA, et al. Induction chemotherapy followed by breast conservation for locally advanced carcinoma of the breast. Cancer. 1994;73:362–9. [DOI] [PubMed] [Google Scholar]
- 4.Boughey JC, Peintinger F, Meric-Bernstam F, Perry AC, Hunt KK, Babiera GV, et al. Impact of preoperative versus postoperative chemotherapy on the extent and number of surgical procedures in patients treated in randomized clinical trials for breast cancer. Ann Surg. 2006;244:464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hortobagyi GN, Blumenschein GR, Spanos W, Montague ED, Buzdar AU, Yap HY, et al. Multimodal treatment of locoregionally advanced breast cancer. Cancer. 1983;51:763–8. [DOI] [PubMed] [Google Scholar]
- 6.Rouzier R, Extra JM, Carton M, Falcou MC, Vincent-Salomon A, Fourquet A, et al. Primary chemotherapy for operable breast cancer: incidence and prognostic significance of ipsilateral breast tumor recurrence after breast-conserving surgery. J Clin Oncol. 2001;19:3828–35. [DOI] [PubMed] [Google Scholar]
- 7.Calais G, Berger C, Descamps P, Chapet S, Reynaud-Bougnoux A, Body G, et al. Conservative treatment feasibility with induction chemotherapy, surgery, and radiotherapy for patients with breast carcinoma larger than 3 cm. Cancer. 1994;74:1283–8. [DOI] [PubMed] [Google Scholar]
- 8.Chen AM, Meric-Bernstam F, Hunt KK, Thames HD, Oswald MJ, Outlaw ED, et al. Breast conservation after neoadjuvant chemotherapy: the MD Anderson cancer center experience. J Clin Oncol. 2004;22:2303–12. [DOI] [PubMed] [Google Scholar]
- 9.Bonadonna G, Valagussa P, Brambilla C, Ferrari L, Moliterni A, Terenziani M, et al. Primary chemotherapy in operable breast cancer: eight-year experience at the Milan Cancer Institute. J Clin Oncol. 1998;16:93–100. [DOI] [PubMed] [Google Scholar]
- 10.Mauriac L, MacGrogan G, Avril A, Durand M, Floquet A, Debled M, et al. Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: a unicentre randomized trial with a 124-month median follow-up. Institut Bergonie Bordeaux Groupe Sein (IBBGS). Ann Oncol. 1999;10:47–52. [DOI] [PubMed] [Google Scholar]
- 11.Chen AM, Meric-Bernstam F, Hunt KK, Thames HD, Outlaw ED, Strom EA, et al. Breast conservation after neoadjuvant chemotherapy. Cancer. 2005;103:689–95. [DOI] [PubMed] [Google Scholar]
- 12.Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, Morrow M, eds. American Joint Committee on Cancer: AJCC Cancer Staging Manual. 6th ed. New York, NY: Springer-Verlag, 2002. [Google Scholar]
- 13.Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol. 2008;26:778–85. [DOI] [PubMed] [Google Scholar]
- 14.Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National surgical adjuvant breast and bowel project B-18. J Natl Cancer Inst Monogr. 2001:96–102. [DOI] [PubMed] [Google Scholar]
- 15.Huang EH, Strom EA, Perkins GH, Oh JL, Chen AM, Meric-Bernstam F, et al. Comparison of risk of local-regional recurrence after mastectomy or breast conservation therapy for patients treated with neoadjuvant chemotherapy and radiation stratified according to a prognostic index score. Int J Radiat Oncol Biol Phys. 2006;66:352–7. [DOI] [PubMed] [Google Scholar]
- 16.Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–85. [DOI] [PubMed] [Google Scholar]
- 17.Mittendorf EA, Wu Y, Scaltriti M, Meric-Bernstam F, Hunt KK, Dawood S, et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res. 2009;15:7381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–106. [DOI] [PubMed] [Google Scholar]


