Tellurium nanoparticles are used for broadband solar energy harvesting and efficient photothermal conversion.
Abstract
Nanophotonic materials for solar energy harvesting and photothermal conversion are urgently needed to alleviate the global energy crisis. We demonstrate that a broadband absorber made of tellurium (Te) nanoparticles with a wide size distribution can absorb more than 85% solar radiation in the entire spectrum. Temperature of the absorber irradiated by sunlight can increase from 29° to 85°C within 100 s. By dispersing Te nanoparticles into water, the water evaporation rate is improved by three times under solar radiation of 78.9 mW/cm2. This photothermal conversion surpasses that of plasmonic or all-dielectric nanoparticles reported before. We also establish that the unique permittivity of Te is responsible for the high performance. The real part of permittivity experiences a transition from negative to positive in the ultraviolet-visible–near-infrared region, which endows Te nanoparticles with the plasmonic-like and all-dielectric duality. The total absorption covers the entire spectrum of solar radiation due to the enhancement by both plasmonic-like and Mie-type resonances. It is the first reported material that simultaneously has plasmonic-like and all-dielectric properties in the solar radiation region. These findings suggest that the Te nanoparticle can be expected to be an advanced photothermal conversion material for solar-enabled water evaporation.
INTRODUCTION
Energy and water shortages are two of the most serious global challenges, and many researchers have devoted themselves to search materials for solar energy harvesting because solar energy is theoretically inexhaustible (1–4). It is well known that the spectrum of solar radiation is located in the range from 295 to 2500 nm. To make the most of solar radiation, optical materials for efficient solar energy harvesting should exhibit strong and broadband absorption. Among a large number of materials, plasmonic and high-index all-dielectric nanostructures have been extensively investigated for solar energy conversion (5–10). On the one hand, the optical absorption can be largely enhanced by localized surface plasmon resonances, which are a collective oscillation of free electrons in nanostructures made of metals or highly doped semiconductors, such as Au (5), Ag (6), Al (7), and TiO1.67 (8). Local heating through the internal decay of hot electrons inside metallic nanoparticles (11) has been applied in photothermal therapy (12), steam generation (5, 7, 13, 14), and photovoltaic device design (15, 16). On the other hand, recently, it has been experimentally and theoretically demonstrated that high-index all-dielectric nanoparticles can be heated as effective as plasmonic ones depending on Mie-type resonances based on the phase retardation effect, although their absorption coefficients are much smaller than those of metals (9, 10, 17, 18). High-index all-dielectric nanoparticles, such as Si and Ge nanoparticles, have been used for photovoltaics (19, 20) and water evaporation (9), respectively. Although the aforementioned plasmonic and all-dielectric nanostructures have their respective advantages in solar photothermal conversion, to our best knowledge, there are no nanophotonic materials that can combine the advantages of these two materials to more efficiently harvest solar energy.
In this contribution, we design a novel broadband perfect absorber based on self-assembly of tellurium (Te) nanoparticles. Te nanoparticles with a size distribution from 10 to 300 nm were prepared by nanosecond laser ablation in liquid (ns-LAL) (21). Perfect absorption (more than 85%) can be achieved in the entire spectrum of solar radiation (300 to 2000 nm). Especially in the ultraviolet (UV) region (300 to 400 nm), the absorptivity reaches a value of more than 95%. We establish that this good performance is attributed to the unique optical response of Te nanoparticles. Within the spectrum of solar radiation, the real part of permittivity of Te changes from negative to positive, which endows it with optical duality, enabling it to convert from a plasmonic-like material to a high-index all-dielectric material with increasing size. When a Te nanoparticle is smaller than 120 nm, it performs like a plasmonic nanoparticle whose resonance dominated by interband transition is located around 300 nm. As the size increases from 10 to 120 nm, the plasmonic-like resonance can be adjusted from 300 to 400 nm. It is worth noting that ground-state free carriers are absent in Te nanoparticles and that the negative permittivity is caused by interband transitions. Hence, we called this quasi-static resonance in Te nanoparticles plasmonic-like resonance to distinguish it from plasmonic resonance based on metal materials or doped semiconductors. When the Te nanoparticle is larger than 120 nm, both electric and magnetic Mie-type resonances are excited, which demonstrates that it converts to an all-dielectric material. Te has one of the highest refractive indices (6.3 to 4.8) in the visible (vis)–near-infrared (NIR) region so that Te slab or thin films can achieve large surface reflectance (19), making it promising for application as an efficient broadband backreflector (mirror) or the back surface of a solar cell. In addition, Te nanoparticles can support spectrally separated Mie-type resonances due to its high refractive index. Electromagnetic calculations based on Mie theory show that both plasmonic-like and Mie-type resonances can enhance the absorption. As the sizes of Te nanoparticles increase from 10 to 300 nm, the enhanced absorption covering the whole solar radiation spectrum can be realized. Hence, the wide size distribution of Te nanoparticles enables their aggregation to efficiently harvest solar radiation. Moreover, it is important that Te has a smaller bandgap (0.35 eV) (22) than Si (1.12 eV) and Ge (0.66 eV), which enables broader optical absorption up to 3500 nm.
Further, the strong and broadband absorption of Te nanoparticles has been used for solar-enabled photothermal conversion. The temperature of a perfect absorber made of Te nanoparticles can rapidly increase from 29° to 85°C within 100 s irradiated by a halogen tungsten lamp whose spectrum is similar to sunlight. In addition, the water evaporation rate of a Te nanoparticle solution (10 μg/ml) can be improved by three times that of pure water under the simulated solar radiation of 78.9 mW/cm2. The above photothermal conversion performance has surpassed that of Al nanoparticles (2.4 times improvement) (7), Ge nanoparticles (2.5 times improvement) (9), and layered BiInSe3@Nickel foam (2.5 times improvement) (23). Meanwhile, we have demonstrated that Te nanoparticles or species are almost nonexistent in the steam during long-term steam generation by the determination of Te in the evaporated water using inductively coupled plasma atomic emission spectrometry (ICP-AES). In other words, the Te nanoparticle acts only as a photothermal conversion material, and no pollution is produced during the process of water evaporation. These results suggest that the Te nanoparticle can be expected to be an advanced photothermal conversion material for efficient solar energy–driven water evaporation.
RESULTS
Figure 1 shows the typical morphology and structure characterization of Te nanoparticles prepared by ns-LAL. It can be seen that the synthesized nanoparticles have a wide size distribution, as shown in Fig. 1A. The top and tilted scanning electron microscopy (SEM) images in fig. S1 (A and B) show that they are quasi-spherical. Other detailed microscopic morphology and structure of Te nanoparticles are analyzed by transmission electron microscopy (TEM). Figure 1B is the TEM image of a Te nanoparticle (about 150 nm). Figure 1 (C to F) represents its corresponding high-resolution TEM (HRTEM) image, selected-area electron diffraction (SAED) pattern, and energy-dispersive x-ray spectrum (EDS). The HRTEM analysis of the lattice fringes indicates that there are several crystal orientations coexisting in one nanoparticle. Figure 1 (C and D) shows that the interplanar spacing of the nanocrystal is 0.235 and 0.206 nm, which are in good agreement with the value of (102) and (111) of the hexagonal Te structure (24). Besides, fig. S1 (C and F) displays the TEM of another two Te nanoparticles (about 200 and 25 nm, respectively). The interplanar spacing of the nanocrystal shown in fig. S1 (D and G) (0.176 and 0.198 nm, respectively) corresponds to crystal faces (103) and (003). The x-ray diffraction (XRD) pattern shown in Fig. 1G confirms these results. In addition, Fig. 1H depicts the Raman spectrum taken for the synthesized Te nanoparticles. The characteristic vibration peaks at 93.8, 118.9, and 138.5 cm−1 were observed at room temperature, which are consistent with the previous studies of other research groups (25).
Fig. 1. Typical morphology and structure characterization results of Te nanoparticles prepared by ns-LAL.
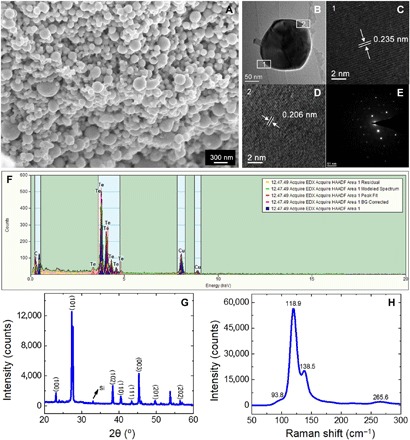
(A) SEM image of Te nanoparticles. (B) TEM image of a Te nanoparticle. (C and D) Corresponding HRTEM micrographs. (E and F) SAED and EDS patterns of the Te nanoparticle. (The Cu signal in EDS pattern originated from the Cu grid.) (G) XRD pattern of Te nanoparticles deposited on a Si substrate. (H) Raman spectrum of Te nanoparticles.
To understand the broadband perfect absorption of the self-assembled Te nanoparticles, we investigated the optical response of Te nanoparticles. First, we measured the scattering spectra of individual Te nanoparticles. Actually, optical response of the Te nanoparticle as a high-index all-dielectric material in the mid-infrared region has been reported by Ginn et al. (26). However, to date, the optical behavior of Te nanoparticles in the UV-vis–NIR region (300 to 2000 nm) is still unknown. As shown in Fig. 2A (solid curves), Te nanoparticles with diameters of 106.0, 120.6, 137.5, 156.1, and 178.4 nm were marked by SEM, and their backward scattering spectra were measured using a dark-field optical microscope. It can be seen that the peak located around 700 nm (orange arrow) has a distinct red shift as the particle size increases, while the peak located around 450 nm has a less obvious shift (green arrow). After exposure to air for about 2 months, the scattering spectra of the same Te nanoparticles are nearly unchanged, as shown in Fig. 2A (dashed curves). This result demonstrates that the Te nanoparticles have not been oxidized because if a Te nanoparticle is oxidized, then its scattering behavior will be changed because of the change of refractive index. For comparison, the scattering spectra of TeO2 nanoparticles are also simulated, as shown in fig. S2. It can be seen that the scattering behaviors of Te and TeO2 nanoparticles are totally different. Scattering results of Te nanoparticles are confirmed by finite-difference time-domain (FDTD) simulations shown in Fig. 2B. The discrepancies between actual experimental and simulated results are derived from imperfect geometry and the difference of permittivity between single crystal and polycrystal. In addition, Fig. 2C shows the corresponding simulated absorption spectra of Te nanoparticles. As the size increases, the absorption peaks located around 300 nm (green arrow) derived from plasmonic-like resonance remain quasi-static, while the peaks (orange arrow) derived from Mie-type resonances show red shifts. Further, when the Te nanoparticles are self-assembled to form oligomers, their scattering spectra are changed. As presented in fig. S3 (A to D), the scattering intensity of the oligomers are largely enhanced compared with the individual nanoparticles. The absorption cross section (Cabs) of Te nanoparticles with a size distribution shown in fig. S3E indicates that both plasmonic-like and Mie-type resonances can enhance the absorption. Although the spectrum of an individual Te nanoparticle is rather narrow, the overall absorption of particle aggregation becomes broadband due to the wide size distribution of the synthesized Te nanoparticles. When Te nanoparticles are combined to a dimer, the absorption mode becomes stronger and broadening (fig. S3F), which is beneficial to more efficient light harvesting.
Fig. 2. Scattering spectra of individual Te nanoparticles.
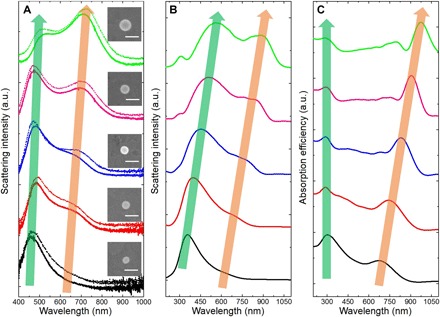
(A) Solid curves: Experimental backward scattering spectra of Te nanoparticles with diameters of 106.0 nm (black), 120.6 nm (red), 137.5 nm (blue), 156.1 nm (pink), and 178.4 nm (green). Dashed curves: Measurement results of the same Te nanoparticles after exposure to air for about 2 months. Insets: Corresponding SEM images of Te nanoparticles, respectively. Scale bar, 200 nm. a.u., arbitrary units. (B) Simulated backward scattering spectra of Te nanoparticles with diameters of 100 nm (black curve), 120 nm (red curve), 140 nm (blue curve), 160 nm (pink curve), and 180 nm (green curve). (C) Corresponding simulated absorption spectra of Te nanoparticles in (B).
To provide a better understanding of the unique optical properties of Te, we fundamentally investigated its dielectric function. Figure S4 shows the real part (n) and imaginary part (k) of the refractive index of Te, Au, and Si. It can be seen that Te is a low-loss, high-index all-dielectric material like Si in the NIR region, but it has a large extinction coefficient like Au in the UV-vis region. Figure 3A depicts a comparison of the real part of permittivity [that is, Re(ε)] of these three materials. As a plasmonic material, Au has a negative Re(ε) in the whole UV-vis–NIR region, and its plasmonic resonance is located at the wavelength (around 500 nm in the air) determined by Re(εAu) = −2εm, where εm is the dielectric function of the embedding medium (27). While as a high-index all-dielectric material, the Re(ε) of Si is positive, and Mie-type resonances can explain its optical behaviors. However, different from these two materials, the Re(ε) of Te is negative at 300 < λ < 490 nm (green area in Fig. 3A) while positive at λ > 490 nm. In other words, Te is of optical duality that exhibits as a plasmonic-like material at wavelength 300 < λ < 490 nm while a high-index all-dielectric material at λ > 490 nm. Its plasmonic-like resonance is located around 300 nm in the air.
Fig. 3. Optical duality of Te nanoparticles.
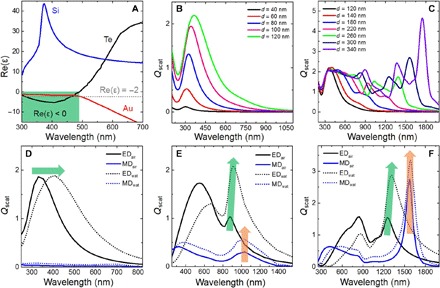
(A) Real part of permittivity of Te compared with Au (a plasmonic material) and Si (an all-dielectric material). (B) Plasmonic-like behavior of Te nanoparticles with diameters smaller than 120 nm. (C) All-dielectric behavior of Te nanoparticles with diameters ranging from 120 to 340 nm. (D to F) Contributions of ED and MD to the scattering efficiency of Te nanoparticles embedded in air and water with diameters of 100, 200, and 300 nm, respectively.
The optical duality is completely reflected in the scattering spectra of Te nanoparticles. As depicted in Fig. 3B, when Te nanoparticles are smaller than 120 nm, the plasmonic-like resonance dominates their scattering spectra. The spectra have a slight shift and broadening as the size increases, which are similar to the case of Au nanoparticles shown in fig. S5A. The scattering behaviors of individual Te nanoparticles larger than 120 nm, as presented in Fig. 3C, are similar to that of Si nanoparticles (shown in fig. S5B). When the particle size increases from 120 to 340 nm, the Mie-type resonances of Te nanoparticles can be moved from the vis to the NIR region. Considering that Mie theory is a useful tool to illustrate the optical duality of Te nanoparticles, Fig. 3 (D to F) shows multipolar contributions for scattering efficiency of three Te nanoparticles (100, 200, and 300 nm, respectively) surrounded by air (n = 1) and water (n = 1.33). The electric dipole (ED) contribution dominates the optical response of the smaller Te nanoparticle (100 nm), and the ED resonance peak exhibits a red shift and broadening with increasing the ambient refractive index (Fig. 3D). This property makes it possible for applications in wavelength-based sensors (28). When the size increases to 200 nm in diameter, the Mie-type magnetic resonance begins to arise, although the magnetic dipole (MD) contribution is not big (Fig. 3E). As the size of Te nanoparticle further increases to 300 nm, the MD contribution surpasses ED contribution, and both of them govern the optical response (Fig. 3F). The difference between plasmonic and Mie-type resonances is that the former is wavelength-sensitive but the latter is wavelength-insensitive to the change of ambient refractive index [green and orange arrows in Fig. 3 (D to F)], which is consistent with our previous study (29).
We carried out the solar heating experiment to investigate plasmonic-like and Mie-type resonances, enhanced optical absorption, and broadband photothermal effect of the Te nanoparticles. The SEM image (Fig. 4A) and the size distribution diagram (Fig. 4B) show that Te nanoparticles synthesized by ns-LAL have a relatively wide size distribution from 10 to 300 nm. The self-assembled Te nanoparticle absorber deposited on an Si wafer is shown in Fig. 4C (right) compared with the bare Si wafer (left). The weight of the Te nanoparticle layer is 0.35 mg, as measured by an electronic balance. As presented in Fig. 4D, the measured absorptivity (red curve) of the absorber exceeds 85% from 300 to 2000 nm, covering the whole solar radiation spectrum (blue area). It is even larger than 95% at the UV region (300 to 400 nm). Since the UV radiation is harmful to humans, the Te nanoparticle absorber can be used as an outer coating to prevent it. This perfect absorption performance is due to the wide size distribution and the enhancement by both plasmonic-like and Mie-type resonances. To carry out the solar heating experiment, we radiated the Te nanoparticle absorber using a halogen tungsten lamp whose radiation spectrum is similar to solar radiation and recorded the thermal radiation using a Fluke thermal imager. We used the bare Si wafer as a reference. As shown in Fig. 4F, the temperature of the Si wafer increased from 29° to 47°C, and it remained constant after 100 s under radiation. However, perfect broadband absorption endows the Te nanoparticle layer with much more efficient heating. Its temperature increased rapidly from 29° to more than 85°C within 100 s and then reached a steady state. The heating performance of the Te nanoparticle layer exceeds that of the TiO1.67 nanoparticle layer (a perfect absorber reported before) (10) whose temperature reached 80°C within the same time depending on plasmonic resonances. Figure 4 (G and H) shows thermal images of the bare Si wafer and Te nanoparticle absorber to give an intuitionistic description.
Fig. 4. Photothermal effect of a Te nanoparticle layer deposited on the Si substrate.
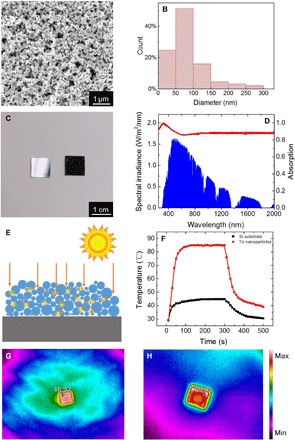
(A) SEM image of self-assembly of Te nanoparticles. (B) Size distribution based on the SEM image in (A) (statistic data from 500 particles). (C) Photograph of a bare Si wafer (left) and Te nanoparticle layer deposited on Si substrate (right). (D) Absorption spectrum (red curve) of the Te nanoparticle absorber. The blue area is the solar radiation spectrum. (E) Schematic diagram of Te nanoparticle layer irradiated by sunlight. (F) Time-dependent temperature variation of the bare Si wafer (black curve) and Te nanoparticle absorber (red curve). (G and H) Steady-state thermal images of the bare Si wafer and Te nanoparticle absorber, respectively.
For nonmagnetic materials, the absorbed electromagnetic power can be simply calculated by the following equation (30)
| (1) |
where ω is the angular frequency, Im(ε) is the imaginary part of the permittivity, and |E| is the amplitude of the electric field. It is clear that strong field enhancements and the large imaginary part of the permittivity are the two important factors that contribute to strong absorption according to this equation. As depicted in Fig. 5A, Im(ε) of Te is compared with that of Si, Ge, and Au. Although Im(ε) of Si and Ge is larger at λ < 400 nm and Im(ε) of Au is larger at λ > 1200 nm, they are smaller than Te in the main solar radiation spectrum (blue area in Fig. 4D). In addition, Fig. 5 (C to H) shows the electric field enhancements at different wavelengths in a Te nanoparticle oligomer. The value reaches dozens of times from 300 to 2000 nm, which is comparable to the Au nanoparticle oligomer (fig. S6).
Fig. 5. Perfect absorption of the Te nanoparticle layer.
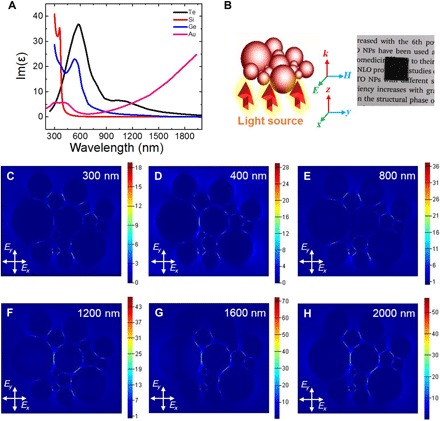
(A) Imaginary part of permittivity of Te compared with Au, Si, and Ge. (B) Schematic diagram of Te nanoparticle oligomer containing particles ranging from 25 to 300 nm. Inset: Perfect Te nanoparticle absorber. (C to H) Electric field enhancements of the oligomer at a broadband range from 300 to 2000 nm.
The Te nanoparticle is a material that converts sunlight to heat energy, and it can be used for photothermal water evaporation. Although Te has been considered to be mildly toxic, it plays an important role in biological systems (31, 32). According to the previous work, the content of Te in the human body is more than 0.5 g. It is inconceivable that Te is the fourth most abundant trace element after Fe, Zn, and Rb in the human body and is unusually abundant in human food and plants (31). Fränzle and Markert (32) even suggested that Te could be an essential nutrient according to certain criteria. Recently, a series of Te-containing polymers have been used as stimuli-responsive biomaterials (33). The field of biological Te chemistry has also become a major player in protein chemistry, imaging, and diagnostics, as well as in the search for new and more potent antibiotics and anticancer agents (34).
We radiated the Te nanoparticles dispersed in pure water using a solar simulator and recorded the vaporized amount of water. Before the radiation, we measured the UV-vis absorption spectrum of the Te nanoparticles colloid solution (5 μg/ml) with gray color (inset in Fig. 6A). The curve shown in Fig. 6A has two broad typical absorption peaks around 300 nm (green arrow) and 600 nm (orange arrow), respectively. According to the previous work (35), the absorption peak around 300 nm is due to the allowed direct transition from the valence band (p-bonding triplet) to the conduction band (p-antibonding triplet), and another broad absorption peak around 600 nm can be assigned to a forbidden direction transition. Note that the plasmonic-like resonance is located around 300 nm, which matches well to our absorption results. Meanwhile, we have demonstrated that Te nanoparticles or species are almost nonexistent in the steam during long-term steam generation by the determination of Te in the evaporated water using ICP-AES (detection limit, ~0.05 μg/ml), which is shown in Fig. 6B. The concentration of Te in the evaporated water is so low (under the detection limit) that it cannot be detected. Namely, the Te nanoparticle acts only as a photothermal conversion material, and it is pollution-free during the process of water evaporation. Te nanoparticles are insoluble in water, and of the chalcogens, Te has the highest melting and boiling points, at 722.66 and 1261.15 K, respectively (36). As presented in Fig. 6C, we measured four kinds of Te nanoparticle colloid solutions with different concentrations under the same irradiation at 78.9 mW/cm2. The vaporized weight rises with increasing concentration, and the maximum evaporation rate reaches three times that of pure water, surpassing the performance of Al nanoparticles (2.4 times) (7), Ge nanoparticles (2.5 times) (9), and layered BiInSe3@Nickel foam (2.5 times) (23) reported previously. Likewise, the evaporation rate increases with rising irradiation, which is shown in Fig. 6D. The water evaporation experiment demonstrates that the Te nanoparticle is a promising material for solar desalination.
Fig. 6. Te nanoparticles used for water evaporation.
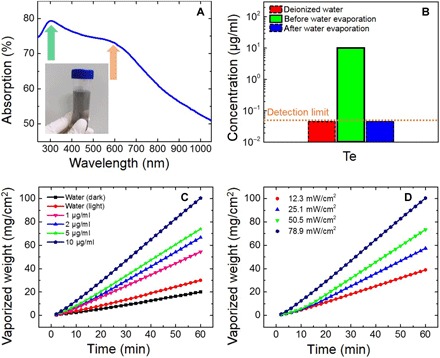
(A) Measured UV-vis–NIR absorption spectrum of Te nanoparticle colloid solution (5 μg/ml). Inset: Gray Te nanoparticle colloid solution synthesized by ns-LAL. (B) ICP-AES analysis results of Te in the water before and after evaporation. The deionized water is used for reference. The dashed boxes indicate that the concentration is lower than the detection limit of ~0.05 μg/ml. (C) Vaporized weight of Te nanoparticle solutions with different concentrations under the illumination of simulated sunlight at 78.9 mW/cm2. (D) Vaporized weight of Te nanoparticle solutions (10 μg/ml) under different illuminations of simulated sunlight.
To prove that the Te nanoparticles have not been oxidized during long-term steam generation, after working in steam generation for about 2 months, we again analyzed Te nanoparticles by SEM, XRD, TEM, and EDS, as shown in fig. S7. Compared with the “fresh” results shown in Fig. 1, there is little change between them. Besides, after long-term steam generation, the Te nanoparticles can still work efficiently. As shown in fig. S8, the same water evaporation experiment was taken for Te nanoparticles colloid solutions, which were exposed to steam generation for about 2 months. Compared with the results in Fig. 6, the performance is maintained. We think that the inoxidizability of Te nanoparticles is related to the synthetic method. Actually, ns-LAL is a preparation method based on high temperature (~1000 K) and high pressure (~GPa).
The materials used for efficient solar-enabled water evaporation should have (i) large optical absorption in the entire solar radiation spectrum, which we demonstrated as mentioned above, and (ii) high light-to-heat conversion efficiency. For plasmonic nanoparticles, the resonances can be damped radiatively by re-emission of a photon or nonradiatively through the creation of hot electron-hole pairs via Landau damping (11). The internal decay of hot electrons through the emission of phonons by interacting with the crystal lattice inside a metallic nanoparticle can lead to significant heating of the nanoparticle itself and its surroundings. However, for semiconductor nanopaticles, the mechanism for light-to-heat conversion was rarely discussed (37). In general, semiconductor nanoparticles with a large surface-to-volume ratio possess abundant dangling bonds and defects at their surface. This introduces surface states, resulting in the emergence of an electric level in the bandgap, which is similar to the intermediate band very recently discussed by Gaspari et al. (38). When the nanoparticle is excited, the excitation energy is transferred entirely to the carriers (electrons and holes), leading to the creation of nonequilibrium carrier densities with specific momentum states and elevated carrier temperatures initially. As the system evolves toward equilibrium, these carriers relax by intraband carrier-carrier scattering or interband recombining. The former results in Coulomb thermalization, forming a hot gas of thermalized carriers that couple with phonons and transfer their excess energy to the lattice (39). This results in the efficient heating of the nanoparticles. The dynamics of photoexcited electrons can be characterized by a pump-and-probe technique. Figure S9 shows an intuitive diagram illustrating the flow of energy in a photoexcited semiconductor.
CONCLUSION
In summary, we have designed a broadband perfect absorber made of Te nanoparticles with a wide size distribution synthesized by ns-LAL. Perfect absorption (more than 85%) can be achieved in the entire spectrum of solar radiation. The temperature of the fabricated absorber irradiated by sunlight rapidly increased from 29° to 85°C within 100 s. In addition, the water evaporation rate can be improved by three times that of pure water under the illumination of simulated sunlight of 78.9 mW/cm2 by dispersing Te nanoparticles into water (10 μg/ml). This good performance can be derived from the unique optical duality of Te nanoparticles. The real part of permittivity of Te transforms from negative to positive at the UV-vis–NIR region, which endows Te nanoparticles with optical duality. As the size increases from 10 to 300 nm, the Te nanoparticle gradually converts from a plasmonic-like material to a high-index all-dielectric material. Both the plasmonic-like and Mie-type resonances can strongly extend and enhance optical absorption. To the best of our knowledge, it is the first reported material that can simultaneously exhibit the properties of plasmonic-like and high-index all-dielectric materials in the solar radiation region. Thus, these findings demonstrated that the Te nanoparticle is a promising nanophotonic material for solar energy harvesting, solar desalination, and photovoltaic device design.
MATERIALS AND METHODS
Material preparation and characterization
Te nanoparticles were prepared by ns-LAL (21). The laser pulse [second harmonic produced using a Q-switched neodymium-doped yttrium aluminium garnet (Nd: YAG) laser device] with a wavelength of 532 nm, a pulse width of 10 ns, a repetition frequency of 10 Hz, and a single-pulse energy of 80 mJ was focused on the Te target (3-mm thickness, 1.2-cm diameter, 99.9% purity). The Te target was fixed at the bottom of a quartz chamber in advance and covered by deionized water (1-cm liquid layer). The spot size was smaller than 1 mm. A continuous nitrogen flow prevented the sample from oxidizing. After 10 min of laser ablation, the Te sample suspended in solution was collected. After ultrasonic agitation (59 kHz, 10 min), several drops of the colloidal suspension (2 μg/ml) were transferred to a piece of clean Si wafer placed on the heating platform (50°C, constant). During the evaporation process, the Te nanoparticles were self-assembled to form a thin layer. Finally, the samples were analyzed using a dark-field microscope and an Auriga-4523 field-emission (FE) scanning electron microscope (Zeiss) operated at 5 kV. TEM images were recorded using an FEI Tecnai G2 F30 transmission electron microscope equipped with an FE gun to identify the morphology and structure of the samples. The TEM sample was pipetted onto a carbon support film on a copper grid. The Raman spectrum of the samples deposited on Si substrate was recorded using a Renishaw inVia Reflex Laser Raman spectrometer (with laser irradiation at 325 nm). The absorption spectra were obtained by measuring the diffuse reflectance recorded (A% = 1 − R%) by an UV-vis–NIR spectrophotometer with an integration sphere (Lambda 950, PerkinElmer Company).
Dark-field scattering measurement
First, the Te nanoparticle colloidal suspension prepared by ns-LAL was diluted with nine volumes of deionized water. Then, one drop of the solution was transferred to a piece of clean indium tin oxide glass. After the evaporation of water, the Te nanoparticles were marked by SEM. Any two nano-objects were separated with a distance of at least 1 μm to avoid interference between them. The backward scattering spectra of individual Te nanoparticles and their oligomers were obtained using a dark-field optical microscope (BX51, Olympus) integrated with a quartz tungsten halogen lamp (100 W), a monochromator (SpectraPro 2300i, Acton), and a charge-coupled device (CCD) camera (Pixis 400BR_eXcelon, Princeton Instruments). The CCD camera was thermoelectrically cooled to −70°C during the measurements. It should be noted that the quantum efficiency of the CCD dropped quickly near the UV range, so the measured scattering spectrum intensities decreased to nearly zero at λ = 400 nm. In the scattering measurements, an objective lens (100×; numerical aperture, 0.80) was used to illuminate Te nanoparticles with white excitation light. All the scattering experiments were carried out under the same temperature (25°C) in the dark environment. The scattering measurement process was discussed in our previous work (40).
Numerical simulations
FDTD (FDTD Solutions 8.15.736, Lumerical Solutions Inc.) method was used to calculate the scattering spectra and the near-field distributions. The Te and TeO2 nanoparticles were illuminated with total-field scattered-field source (200 to 2000 nm), and the mesh size was set to 2 nm. The dielectric function of Te was obtained from Palik (41). It was obtained by measuring optical absorption edge and reflectivity spectrum of single-crystal Te grown by the Czochralski process. The dielectric function of TeO2 was obtained from Uchida (42). Unless otherwise specified, the spectra of the Te and TeO2 nanoparticles were calculated in the free space.
Photothermal and evaporation measurement
The Te nanoparticle layers deposited on the Si substrate and the pure Si wafer (1 cm × 1 cm) were irradiated by a halogen tungsten lamp whose spectrum is similar to sunlight. Their temperature was recorded using a thermal imager (Fluke TiS55) every 10 s. As for the water evaporation experiment, the Te nanoparticle colloid solution was poured into a quartz chamber (transparent; 1.8 cm × 1.8 cm × 1 cm), which was placed on an electronic balance, and the sunlight with tunable intensity was incident vertically from the top. Note that we placed another empty quartz chamber between the sample chamber and the electronic balance to eliminate the influence of the thermal effect of the electronic balance during the evaporation measurement. The vaporized weight of the water was recorded by the electronic balance every 2 min. The concentration of the Te nanoparticle colloidal suspension could be changed by dilution or condensation. Note that, in our evaporation measurements, the dark evaporation was included in the measured vapor mass.
Supplementary Material
Acknowledgments
We thank C. Jin and J. Yao from School of Materials Science and Engineering, Sun Yat-sen University for helpful discussions. Funding: The National Basic Research Program of China (2014CB931700), National Natural Science Foundation of China (91233203), Natural Science Foundation of Guangdong Province (2016A030313339), and State Key Laboratory of Optoelectronic Materials and Technologies supported this work. Author contributions: G.Y. designed the experiments. C.M. performed the experiments and calculations. J.Y., Y.H., and C.W. performed the data analysis. C.M. and G.Y. wrote the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/8/eaas9894/DC1
Fig. S1. Typical morphology and structure characterization of Te nanoparticles prepared by ns-LAL.
Fig. S2. Scattering spectra of individual TeO2 nanoparticles.
Fig. S3. Scattering and absorption spectra of Te nanoparticle oligomers.
Fig. S4. The real part and imaginary part of the refractive index of Te, Au, and Si.
Fig. S5. The scattering spectra of Au nanoparticles and Si nanoparticles.
Fig. S6. The electric field enhancements of the Au nanoparticle oligomer.
Fig. S7. Typical morphology and structure characterization of Te nanoparticles after working in steam generation for about 2 months.
Fig. S8. Water evaporation using Te nanoparticles that have been working in steam generation for about 2 months.
Fig. S9. An intuitive diagram illustrating the flow of energy in a photoexcited semiconductor.
REFERENCES AND NOTES
- 1.Elimelech M., Phillip W. A., The future of seawater desalination: Energy, technology, and the environment. Science 333, 712–717 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Li X., Xu W., Tang M., Zhou L., Zhu B., Zhu S., Zhu J., Graphene oxide-based efficient and scalable solar desalination under one sun with a confined 2D water path. Proc. Natl. Acad. Sci. U.S.A. 113, 13953–13958 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu N., Hu X., Xu W., Li X., Zhou L., Zhu S., Zhu J., Mushrooms as efficient solar steam-generation devices. Adv. Mater. 29, 1606762 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Zhou L., Zhuang S., He C., Tan Y., Wang Z., Zhu J., Self-assembled spectrum selective plasmonic absorbers with tunable bandwidth for solar energy conversion. Nano Energy 32, 195–200 (2017). [Google Scholar]
- 5.Guo A., Fu Y., Wang G., Wang X., Diameter effect of gold nanoparticles on photothermal conversion for solar steam generation. RSC Adv. 7, 4815–4824 (2017). [Google Scholar]
- 6.Politano A., Argurio P., Di Profio G., Sanna V., Cupolillo A., Chakraborty S., Arafat H. A., Curcio E., Photothermal membrane distillation for seawater desalination. Adv. Mater. 29, 1603504 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Zhou L., Tan Y., Wang J., Xu W., Yuan Y., Cai W., Zhu S., Zhu J., 3D self-assembly of aluminium nanoparticles for plasmon-enhanced solar desalination. Nat. Photonics 10, 393–398 (2016). [Google Scholar]
- 8.Yan J., Liu P., Ma C., Lin Z., Yang G., Plasmonic near-touching titanium oxide nanoparticles to realize solar energy harvesting and effective local heating. Nanoscale 8, 8826–8838 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Ishii S., Chen K., Okuyama H., Nagao T., Resonant optical absorption and photothermal process in high refractive index germanium nanoparticles. Adv. Opt. Mater. 5, 1600902 (2017). [Google Scholar]
- 10.Abujetas D. R., Paniagua-Domínguez R., Sánchez-Gil J. A., Unraveling the Janus role of Mie resonances and leaky/guided modes in semiconductor nanowire absorption for enhanced light harvesting. ACS Photonics 2, 921–929 (2015). [Google Scholar]
- 11.Brongersma M. L., Halas N. J., Nordlander P., Plasmon-induced hot carrier science and technology. Nat. Nanotechnol. 10, 25–34 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Stern J. M., Stanfield J., Kabbani W., Hsieh J.-T., Cadeddu J. A., Selective prostate cancer thermal ablation with laser activated gold nanoshells. J. Urol. 179, 748–753 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Zhou L., Tan Y. L., Ji D. X., Zhu B., Zhang P., Xu J., Gan Q., Yu Z., Zhu J., Self-assembly of highly efficient, broadband plasmonic absorbers for solar steam generation. Sci. Adv. 2, e1501227 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin H., Lin G., Bai L., Zeiny A., Wen D., Steam generation in a nanoparticle-based solar receiver. Nano Energy 28, 397–406 (2016). [Google Scholar]
- 15.Atwater H. A., Polman A., Plasmonics for improved photovoltaic devices. Nat. Mater. 9, 205–213 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Wang H., Chen Q., Wen L., Song S., Hu X., Xu G., Titanium-nitride-based integrated plasmonic absorber/emitter for solar thermophotovoltaic application. Photonics Res. 3, 329–334 (2015). [Google Scholar]
- 17.Zograf G. P., Petrov M. I., Zuev D. A., Dmitriev P. A., Milichko V. A., Makarov S. V., Belov P. A., Resonant nonplasmonic nanoparticles for efficient temperature-feedback optical heating. Nano Lett. 17, 2945–2952 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Bezares F. J., Long J. P., Glembocki O. J., Guo J., Rendell R. W., Kasica R., Shirey L., Owrutsky J. C., Caldwell J. D., Mie resonance-enhanced light absorption in periodic silicon nanopillar arrays. Opt. Express 21, 27587–27601 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Brongersma M. L., Cui Y., Fan S. H., Light management for photovoltaics using high-index nanostructures. Nat. Mater. 13, 451–460 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Wang B., Leu P. W., Enhanced absorption in silicon nanocone arrays for photovoltaics. Nanotechnology 23, 194003 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Xiao J., Liu P., Wang C. X., Yang G. W., External field-assisted laser ablation in liquid: An efficient strategy for nanocrystal synthesis and nanostructure assembly. Prog. Mater. Sci. 87, 140–220 (2017). [Google Scholar]
- 22.He Z., Yang Y., Liu J.-W., Yu S.-H., Emerging tellurium nanostructures: Controllable synthesis and their applications. Chem. Soc. Rev. 46, 2732–2753 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Yao J. D., Zheng Z. Q., Yang G. W., Alloying-assisted phonon engineering of layered BiInSe3@nickel foam for efficient solar-enabled water evaporation. Nanoscale 9, 16396–16403 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Qian H.-S., Yu S.-H., Gong J.-Y., Luo L.-B., Fei L.-f., High-quality luminescent tellurium nanowires of several nanometers in diameter and high aspect ratio synthesized by a poly (vinyl pyrrolidone)-assisted hydrothermal process. Langmuir 22, 3830–3835 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Song J.-M., Lin Y.-Z., Zhan Y.-J., Tian Y.-C., Liu G., Yu S.-H., Superlong high-quality tellurium nanotubes: Synthesis, characterization, and optical property. Cryst. Growth Des. 8, 1902–1908 (2008). [Google Scholar]
- 26.Ginn J. C., Brener I., Peters D. W., Wendt J. R., Stevens J. O., Hines P. F., Basilio L. I., Warne L. K., Ihlefeld J. F., Clem P. G., Sinclair M. B., Realizing optical magnetism from dielectric metamaterials. Phys. Rev. Lett. 108, 097402 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Hillenbrand R., Taubner T., Keilmann F., Phonon-enhanced light–matter interaction at the nanometre scale. Nature 418, 159–162 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Kabashin A. V., Evans P., Pastkovsky S., Hendren W., Wurtz G. A., Atkinson R., Pollard R., Podolskiy V. A., Zayats A. V., Plasmonic nanorod metamaterials for biosensing. Nat. Mater. 8, 867–871 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Yan J., Liu P., Lin Z., Yang G., New type high-index dielectric nanosensors based on the scattering intensity shift. Nanoscale 8, 5996–6007 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Li W., Guler U., Kinsey N., Naik G. V., Boltasseva A., Guan J., Shalaev V. M., Kildishev A. V., Refractory plasmonics with titanium nitride: Broadband metamaterial absorber. Adv. Mater. 26, 7959–7965 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Cohen B. L., Anomalous behavior of tellurium abundances. Geochim. Cosmochim. Acta 48, 203–205 (1984). [Google Scholar]
- 32.Fränzle S., Markert B., The Biological System of the Elements (BSE). Part II: A theoretical model for establishing the essentiality of chemical elements. The application of stoichiometric network analysis to the biological system of the elements. Sci. Total Environ. 249, 223–241 (2000). [DOI] [PubMed] [Google Scholar]
- 33.Wang L., Cao W., Xu H., Tellurium-containing polymers: Towards biomaterials and optoelectronic materials. ChemNanoMat 2, 479–488 (2016). [Google Scholar]
- 34.Ba L. A., Döring M., Jamier V., Jacob C., Tellurium: An element with great biological potency and potential. Org. Biomol. Chem. 8, 4203–4216 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Liu J.-W., Xu J., Hu W., Yang J.-L., Yu S.-H., Systematic synthesis of tellurium nanostructures and their optical properties: From nanoparticles to nanorods, nanowires, and nanotubes. ChemNanoMat 2, 167–170 (2016). [Google Scholar]
- 36.W. M. Haynes, D. R. Lide, T. J. Bruno, CRC Handbook of Chemistry and Physics (CRC Press, 2014). [Google Scholar]
- 37.Ghosh S., Avellini T., Petrelli A., Kriegel I., Gaspari R., Almeida G., Bertoni G., Cavalli A., Scotognella F., Pellegrino T., Manna L., Colloidal CuFeS2 nanocrystals: Intermediate Fe d-band leads to high photothermal conversion efficiency. Chem. Mater. 28, 4848–4858 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaspari R., Della Valle G., Ghosh S., Kriegel I., Scotognella F., Cavalli A., Manna L., Quasi-static resonances in the visible spectrum from all-dielectric intermediate band semiconductor nanocrystals. Nano Lett. 17, 7691–7695 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Othonos A., Probing ultrafast carrier and phonon dynamics in semiconductors. J. Appl. Phys. 83, 1789–1830 (1998). [Google Scholar]
- 40.Ma C., Yan J., Huang Y., Yang G., Directional scattering in a germanium nanosphere in the visible light region. Adv. Opt. Mater. 5, 1700761 (2017). [Google Scholar]
- 41.E. D. Palik, Handbook of Optical Constants of Solids (Academic Press, 1998). [Google Scholar]
- 42.Uchida N., Optical properties of single-crystal paratellurite (TeO2). Phys. Rev. B 4, 3736–3745 (1971). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/8/eaas9894/DC1
Fig. S1. Typical morphology and structure characterization of Te nanoparticles prepared by ns-LAL.
Fig. S2. Scattering spectra of individual TeO2 nanoparticles.
Fig. S3. Scattering and absorption spectra of Te nanoparticle oligomers.
Fig. S4. The real part and imaginary part of the refractive index of Te, Au, and Si.
Fig. S5. The scattering spectra of Au nanoparticles and Si nanoparticles.
Fig. S6. The electric field enhancements of the Au nanoparticle oligomer.
Fig. S7. Typical morphology and structure characterization of Te nanoparticles after working in steam generation for about 2 months.
Fig. S8. Water evaporation using Te nanoparticles that have been working in steam generation for about 2 months.
Fig. S9. An intuitive diagram illustrating the flow of energy in a photoexcited semiconductor.


